Unraveling Functional Diversity Patterns in Hyporheic Zones: A Trait-Based Approach Applied to Copepods from the Rio Gamberale Creek
Abstract
1. Introduction
2. Materials and Methods
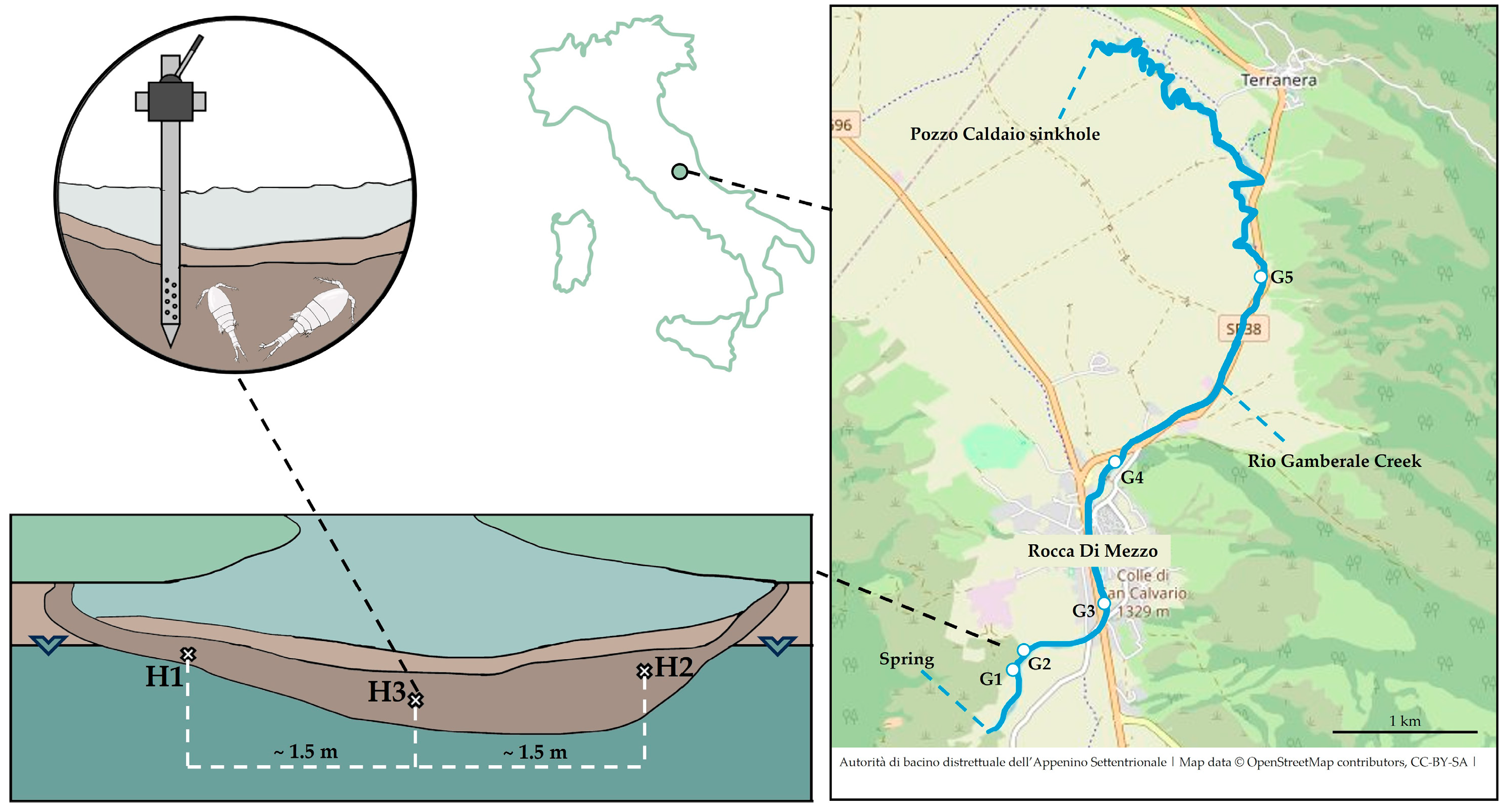
2.1. Study Area
2.2. Sampling Methods
2.3. Functional Traits of Hyporheic Copepods
- Life history: i. juveniles-to-adults ratio (J/A), ii. males-to-females ratio (M/F);
- Morphological: i. average biomass (B), ii. sexual dimorphism (SD);
- Behavioral: i. body flexibility (BF);
- Physiological: i. diet (D), ii. feeding habits (FH), iii. ecology (E), iv. thermal preference (TP).
2.4. Statistical Analysis
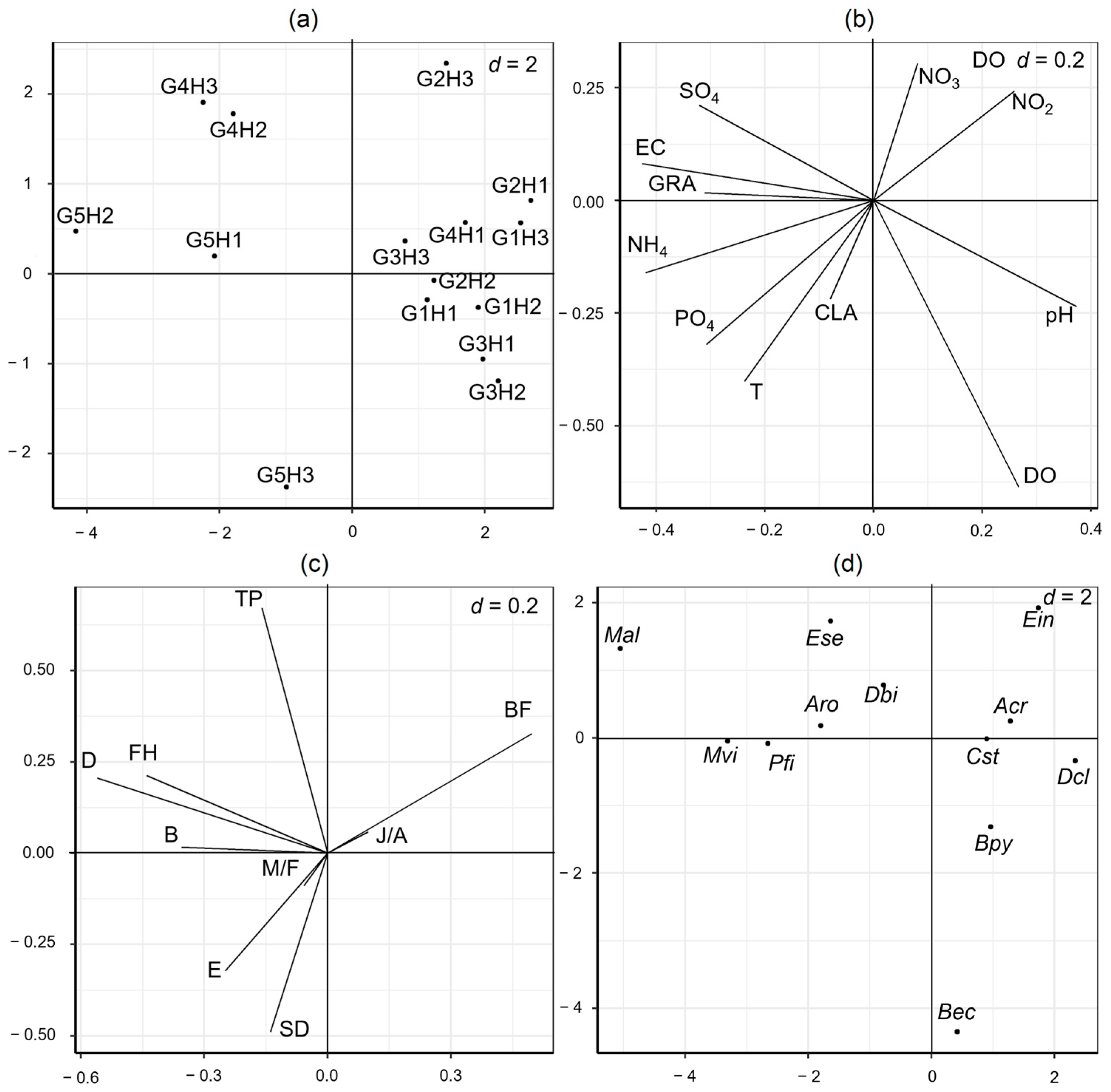
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orghidan, T. Ein neuer Lebensraum des unterirdischen Wassers, der hyporheische Biotop. Arch. Hydrobiol. 1959, 55, 392–414. [Google Scholar]
- Gibert, J. Groundwater systems and their boundaries: Conceptual framework and prospects in groundwater ecology. SIL Proc. 1991, 24, 1605e1608. [Google Scholar] [CrossRef]
- Boulton, A.J.; Findlay, S.; Marmonier, P.; Stanley, E.H.; Valett, H.M. The functional significance of the hyporheic zone in streams and rivers. Annu. Rev. Ecol. Syst. 1998, 29, 59e81. [Google Scholar] [CrossRef]
- Malard, F.; Tockner, K.; Dole-Olivier, M.-J.; Ward, J.V. A landscape perspective of surface-subsurface hydrological exchanges in river corridors. Freshw. Biol. 2002, 47, 621e640. [Google Scholar] [CrossRef]
- Hancock, P.J.; Boulton, A.J.; Humphreys, W.F. Aquifers and hyporheic zones: Towards an ecological understanding of groundwater. Hydrogeol. J. 2005, 13, 98e111. [Google Scholar] [CrossRef]
- Ward, A.S. The evolution and state of interdisciplinary hyporheic research. WIREs Water 2016, 3, 83e103. [Google Scholar] [CrossRef]
- Brunke, M.; Gonser, T. The ecological significance of exchange processes between rivers and groundwater. Freshw. Biol. 1997, 37, 1–33. [Google Scholar] [CrossRef]
- Williams, D.D.; Febria, C.M.; Wong, J.C.Y. Ecotonal and other properties of the hyporheic zone. Fundam. Appl. Limnol. 2010, 176, 349e364. [Google Scholar] [CrossRef]
- Robertson, A.; Brancelj, A.; Stein, H.; Hahn, H.J. Classifying groundwater ecosystems. In Groundwater Ecology and Evolution, 2nd ed.; Malard, F., Griebler, C., Rétaux, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 2; pp. 39–60. [Google Scholar] [CrossRef]
- Danielopol, D.L.; Hartmann, G. Ostracoda. In Stygofauna mundi. A Faunistic, Distributional and Ecological Synthesis of the World Fauna Inhabiting Subterranean Waters; Botosaneanu, L., Ed.; E.J. Brill & Dr W. Backhuys: Leiden, The Netherlands, 1986; pp. 265–294. [Google Scholar]
- Rouch, R. Copepoda: Les Harpacticoides souterrains des eaux douces continentales. In Stygofauna mundi. A Faunistic, Distributional and Ecological Synthesis of the World Fauna Inhabiting Subterranean Waters; Botosaneanu, L., Ed.; E.J. Brill & Dr W. Backhuys: Leiden, The Netherlands, 1986; pp. 321–355. [Google Scholar]
- Dole-Olivier, M.-J.; Galassi, D.M.P.; Marmonier, P.; Creuzé des Châtelliers, M. The biology and ecology of lotic microcrustaceans. Freshw. Biol. 2000, 44, 63–91. [Google Scholar] [CrossRef]
- Galassi, D.M.P.; Huys, R.; Reid, J.W. Diversity, ecology and evolution of groundwater copepods. Freshw. Biol. 2009, 54, 691–708. [Google Scholar] [CrossRef]
- Mermillod-Blondin, F.; Hose, G.C.; Simon, K.S.; Korbel, K.; Avramov, M.; Vander Vorste, R. Role of invertebrates in groundwater ecosystem processes and services. In Groundwater Ecology and Evolution, 2nd ed.; Malard, F., Griebler, C., Rétaux, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 11; pp. 263–281. [Google Scholar] [CrossRef]
- Dole-Olivier, M.-J. The hyporheic refuge hypothesis reconsidered: A review of hydrological aspects. Mar. Freshw. Res. 2011, 62, 1281–1302. [Google Scholar] [CrossRef]
- Stubbington, R. The hyporheic zone as an invertebrate refuge: A review of variability in space, time, taxa and behavior. Mar. Freshw. Res. 2012, 63, 293–311. [Google Scholar] [CrossRef]
- Gandy, C.J.; Smith, J.W.N.; Jarvis, A.P. Attenuation of mining-derived pollutants in the hyporheic zone: A review. Sci. Total Environ. 2007, 273, 435–446. [Google Scholar] [CrossRef]
- Lewandowski, J.; Arnon, S.; Banks, E.; Batelaan, O.; Betterle, A.; Broecker, T.; Coll, C.; Drummond, J.D.; Gaona Garcia, J.; Galloway, J.; et al. Is the Hyporheic Zone Relevant beyond the Scientific Community? Water 2019, 11, 2230. [Google Scholar] [CrossRef]
- Descloux, S.; Datry, T.; Usseglio-Polatera, P. Trait-based structure of invertebrates along a gradient of sediment colmation: Benthos versus hyporheos responses. Sci. Total Environ. 2014, 466, 265–276. [Google Scholar] [CrossRef]
- Gagic, V.; Bartomeus, I.; Jonsson, T.; Taylor, A.; Winqvist, C.; Fischer, C.; Slade, E.M.; Steffan-Dewenter, I.; Emmerson, M.; Potts, S.G.; et al. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. B 2015, 282, 20142620. [Google Scholar] [CrossRef]
- Šimek, K.; Pitsch, G.; Salcher, M.M.; Sirová, D.; Shabarova, T.; Adamec, L.; Posch, T. Ecological traits of the algae-bearing Tetrahymena utriculariae (Ciliophora) from traps of the aquatic carnivorous plant Utricularia reflexa. J. Eukaryot. Microbiol. 2017, 64, 336–348. [Google Scholar] [CrossRef]
- Kattge, J.; Bönisch, G.; Díaz, S.; Lavorel, S.; Prentice, I.C.; Leadley, P.; Tautenhahn, S.; Werner, G.D.A.; Aakala, T.; Abedi, M.; et al. TRY plant trait database—Enhanced coverage and open access. Glob. Chang. Biol. 2020, 26, 119–188. [Google Scholar] [CrossRef]
- Magliozzi, C.; Usseglio-Polatera, P.; Meyer, A.; Grabowski, R.C. Functional traits of hyporheic and benthic invertebrates reveal importance of wood-driven geomorphological processes in rivers. Funct. Ecol. 2019, 33, 1758–1770. [Google Scholar] [CrossRef]
- Lourenço-de-Moraes, R.; Campos, F.S.; Ferreira, R.B.; Beard, K.H.; Solé, M.; Llorent, G.A.; Bastos, R.P. Functional traits explain amphibian distribution in the Brazilian Atlantic Forest. J. Biogeogr. 2020, 47, 275–287. [Google Scholar] [CrossRef]
- Várbíró, G.; Borics, G.; Novais, M.H.; Morais, M.M.; Rimet, F.; Bouchez, A.; Tapolczai, K.; Bácsi, I.; Usseglio-Polatera, P.; B-Béres, V. Environmental filtering and limiting similarity as main forces driving diatom community structure in Mediterranean and continental temporary and perennial streams. Sci. Total Environ. 2020, 741, 140459. [Google Scholar] [CrossRef]
- Hébert, M.-P.; Beisner, B.E.; Maranger, R. Linking zooplankton communities to ecosystem functioning: Toward an effect-trait framework. J. Plankton Res. 2016, 39, 3–12. [Google Scholar] [CrossRef]
- Premate, E.; Fišer, C. Functional trait dataset of European groundwater Amphipoda: Niphargidae and Typhlogammaridae. Sci. Data 2024, 11, 188. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Fiasca, B.; Di Cicco, M.; Cifoni, M.; Galassi, D.M.G. Taxonomic and functional trait variation along a gradient of ammonium contamination in the hyporheic zone of a Mediterranean stream. Ecol. Indic. 2021, 132, 108268. [Google Scholar] [CrossRef]
- Sliva, L.; Williams, D.D. Responses of hyporheic meiofauna to habitat manipulation. Hydrobiologia 2005, 548, 217–232. [Google Scholar] [CrossRef]
- Peralta-Maraver, I.; Galloway, J.; Posselt, M.; Arnon, S.; Reiss, J.; Lewandowski, J.; Robertson, A.L. Environmental filtering and community delineation in the streambed ecotone. Sci. Rep. 2018, 8, 15871. [Google Scholar] [CrossRef]
- Bruno, M.C.; Doretto, A.; Boano, F.; Ridolfi, L.; Fenoglio, S. Role of the hyporheic zone in increasing the resilience of mountain streams facing intermittency. Water 2020, 12, 2034. [Google Scholar] [CrossRef]
- Edie, S.M.; Jablonski, D.; Valentine, J.W. Contrasting responses of functional diversity to major losses in taxonomic diversity. Proc. Natl. Acad. Sci. USA 2018, 115, 732–737. [Google Scholar] [CrossRef]
- Scorzini, A.R.; Leopardi, M. River basin planning: From qualitative to quantitative flood risk assessment: The case of Abruzzo Region (central Italy). Nat. Hazards 2017, 88, 71–93. [Google Scholar] [CrossRef]
- Regione Abruzzo, 2010. Piano di Tutela delle Acque. Relazione Generale e Allegati. Available online: http://www.regione.abruzzo.it/pianoTutelaacque/index (accessed on 25 November 2023).
- Malard, F.; Bernard, C.; Lyon, U.; Claude, M.-J.D.-O.; Lyon, B.U.; Stoch, F. Sampling Manual for the Assessment of Regional Groundwater Biodiversity. 2002. Available online: https://www.researchgate.net/publication/267567541 (accessed on 12 January 2024).
- Bou, C.; Rouch, R. Un nouveau champ de recherches sur le faune aquatique souterraine. CR Acad. Sci. 1967, 265, 369–370. [Google Scholar]
- Borutzkii, E.V. Freshwater Harpacticoida. Fauna USSR. Crustac. 1952, 4, 425. [Google Scholar]
- Dussart, B.H. Les Copépodes des Eaux Continentales d’Europe Occidentale. Tome I: Calanoides et Harpacticoides; Dussart, B.H., Ed.; N. Boubee & Cie: Paris, France, 1967; p. 500. [Google Scholar]
- Dussart, B.H. Les Copépodes des Eaux Continentales d’Europe Occidentale. Tome II. Cyclopoïdes et Biologie Quantitative; Dussart, B.H., Ed.; N. Boubee & Cie: Paris, France, 1969; p. 292. [Google Scholar]
- Boxshall, G.A.; Halsey, S.H. An Introduction to Copepod Diversity; Ray Society: Andover, UK, 2004. [Google Scholar]
- Wells, J.B.J. An annotated checklist and keys to the species of Copepoda Harpacticoida (Crustacea). Zootaxa 2007, 1568, 1–872. [Google Scholar] [CrossRef]
- Irsa CNR, APAT. Metodi Analitici per le Acque. Manuali e Linee Guida, 29 (2003), 1153. ISBN 88-448-0083-7. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/metodi-analitici-per-le-acque (accessed on 6 May 2024).
- Fischer, H.; Wanner, S.C.; Pusch, M. Bacterial abundance and production in river sediments as related to the biochemical composition of particulate organic matter (POM). Biogeochemistry 2002, 61, 37–55. [Google Scholar] [CrossRef]
- Reiss, J.; Schmid-Araya, J.M. Existing in plenty: Abundance, biomass and diversity of ciliates and meiofauna in small streams. Freshw. Biol. 2008, 53, 652–668. [Google Scholar] [CrossRef]
- Warwick, R.M.; Gee, J.M. Community Structure of Estuarine Meiobenthos. Mar. Ecol. Prog. Ser. 1984, 18, 97–111. [Google Scholar] [CrossRef]
- Reiss, J.; Perkins, D.M.; Fussmann, K.E.; Krause, S.; Canhoto, C.; Romeijn, P.; Robertson, A.L. Groundwater flooding: Ecosystem structure following an extreme recharge event. Sci. Total Environ. 2019, 625, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Feller, R.J.; Warwick, R.M. Energetics. In Introduction to the Study of Meiofauna; Higgings, R.P., Thiel, H., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1988; pp. 181–196. [Google Scholar]
- Culver, D.C.; Pipan, T.; Fišer, Ž. Ecological and evolutionary jargon in subterranean biology. In Groundwater Ecology and Evolution, 2nd ed.; Malard, F., Griebler, C., Rétaux, S., Eds.; Academic Press: Cambridge, MA, USA, 2023; Chapter 4; pp. 89–110. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D.; Ter Braak, C.J.; Champely, S. Matching species traits to environmental variables: A new three-table ordination method. Environ. Ecol. Stat. 1996, 3, 143–166. [Google Scholar] [CrossRef]
- Shieh, S.H.; Wang, L.K.; Hsiao, W.F. Shifts in functional traits of aquatic insects along a subtropical stream in Taiwan. Zool. Stud. 2012, 51, 1051–1065. [Google Scholar]
- Dray, S.; Choler, P.; Dolédec, S.; Peres-Neto, P.R.; Thuiller, W.; Pavoine, S.; ter Braak, C.J.F. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 2014, 95, 14–21. [Google Scholar] [CrossRef]
- Johnson, M.D. Draftsman Displays, a Graphical Technique for Exploratory Data Analysis. Master’s Thesis, Naval Postgraduate School, Monterey, CA, USA, 1 June 1984. [Google Scholar]
- Dray, S.; Legendre, P. Testing the species traits–environment relationships: The fourth-corner problem revisited. Ecology 2008, 89, 3400–3412. [Google Scholar] [CrossRef]
- ter Braak, C.; Cormont, A.; Dray, S. Improved testing of species traits–environment relationships in the fourth corner problem. Ecology 2012, 93, 1525–1526. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: http://www.R-project.org/ (accessed on 25 November 2023).
- Mason, N.W.; Mouillot, D.; Lee, W.G.; Wilson, J.B. Functional richness, functional evenness and functional divergence: The primary components of functional diversity. Oikos 2005, 111, 112–118. [Google Scholar] [CrossRef]
- Schleuter, D.; Daufresne, M.; Massol, F.; Argillier, C. A user’s guide to functional diversity indices. Ecol. Monogr. 2010, 80, 469–484. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B.; Laliberté, M.E. Package “FD”: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0-12. 2014. Available online: https://CRAN.R-project.org/package=FD (accessed on 25 November 2023).
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+for PRIMER: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008; p. 214. [Google Scholar]
- Di Lorenzo, T.; Cifoni, M.; Lombardo, P.; Fiasca, B.; Galassi, D.M.P. Ammonium threshold values for groundwater quality in the EU may not protect groundwater fauna: Evidence from an alluvial aquifer in Italy. Hydrobiologia 2015, 743, 139–150. [Google Scholar] [CrossRef]
- Di Marzio, W.D.; Cifoni, M.; Sáenz, M.E.; Galassi, D.M.P.; Di Lorenzo, T. The ecotoxicity of binary mixtures of Imazamox and ionized ammonia on freshwater copepods: Implications for environmental risk assessment in groundwater bodies. Ecotoxicol. Environ. Saf. 2018, 149, 72–79. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Toxic effects of ammonia, nitrite, and nitrate to decapod crustaceans: A review on factors influencing their toxicity, physiological consequences, and coping mechanisms. Rev. Fish Biol. Fish. 2013, 21, 1–21. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Shieh, L.W.; Chen, J.C. Changes in hemolymph oxyhemocyanin, acid–base balance, and electrolytes in Marsupenaeus japonicus under combined ammonia and nitrite stress. Aquat. Toxicol. 2013, 130, 132–138. [Google Scholar] [CrossRef]
- Di Marzio, W.D.; Castaldo, D.; Di Lorenzo, T.; Di Cioccio, A.; Sáenz, M.E.; Galassi, D.M.P. Developmental endpoints of chronic exposure to suspected endocrine-disrupting chemicals on benthic and hyporheic freshwater copepods. Ecotoxicol. Environ. Saf. 2013, 96, 86–92. [Google Scholar] [CrossRef]
- Lemes da Silva, A.L.; de Macedo-Soares, L.C.P.; Serra, S.R.Q.; Petrucio, M.M.; Feio, M.J. Changes in functional diversity of aquatic invertebrates across urbanization levels in a coastal island, Brazil. Hydrobiologia 2024, 1, 2731–2748. [Google Scholar] [CrossRef]
- Statzner, B.; Bêche, L.A. Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems? Freshw. Biol. 2010, 55, 80–119. [Google Scholar] [CrossRef]
- de Castro, D.M.P.; Dolédec, S.; Callisto, M. Land cover disturbance homogenizes aquatic insect functional structure in neotropical savanna streams. Ecol. Indic. 2018, 84, 573–582. [Google Scholar] [CrossRef]
- Kefford, B.J. The relationship between electrical conductivity and selected macroinvertebrate communities in four river systems of south-west Victoria, Australia. Int. J. Salt Lake Res. 1998, 7, 153–170. [Google Scholar] [CrossRef]
- Deschamps, M.M.; Boersma, M.; Meunier, C.L.; Kirstein, I.V.; Wiltshire, K.H.; Di Pane, J. Major shift in the copepod functional community of the southern North Sea and potential environmental drivers. ICES J. Mar. Sci. 2024, 81, 540–552. [Google Scholar] [CrossRef]
- Feng, J.; Mazzei, M.; Di Gregorio, S.; Niccolini, L.; Vitiello, V.; Ye, Y.; Guo, B.; Yan, X.; Buttino, I. Marine Copepods as a Microbiome Hotspot: Revealing Their Interactions and Biotechnological Applications. Water 2023, 15, 4203. [Google Scholar] [CrossRef]
- Krupa, E.G. Population densities, sex ratios of adults, and occurrence of malformations in three species of cyclopoid copepods in waterbodies with different degrees of eutrophy and toxic pollution. J. Mar. Sci. Technol. 2005, 13, 226–237. [Google Scholar]
- Ozga, V.A.; da Silva de Castro, V.; da Silva Castiglioni, D. Population structure of two freshwater amphipods (Crustacea: Peracarida: Hyalellidae) from southern Brazil. Nauplius 2018, 26, e2018025. [Google Scholar] [CrossRef]
- Di Lorenzo, T.; Murolo, A.; Fiasca, B.; Tabilio Di Camillo, A.; Di Cicco, M.; Galassi, D.M.P. Potential of A Trait-Based Approach in the Characterization of An N-Contaminated Alluvial Aquifer. Water 2019, 11, 2553. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Voß, K.; Schäfer, R.B. Taxonomic and functional diversity of stream invertebrates along an environmental stress gradient. Ecol. Indic. 2017, 81, 235–242. [Google Scholar] [CrossRef]
- Schmera, D.; Heino, J.; Podani, J.; Dolédec, S. Functional diversity: A review of methodology and current knowledge in freshwater macroinvertebrate research. Hydrobiologia 2017, 787, 27–44. [Google Scholar] [CrossRef]
- Tabilio Di Camillo, A.; Galassi, D.M.P.; Fiasca, B.; Di Cicco, M.; Galmarini, E.; Vaccarelli, I.; Di Lorenzo, T. Variation in Copepod Morphological and Life History Traits along a Vertical Gradient of Freshwater Habitats. Environments 2023, 10, 199. [Google Scholar] [CrossRef]
- Brandl, Z. Freshwater Copepods and Rotifers: Predators and their Prey. Hydrobiologia 2005, 546, 475–489. [Google Scholar] [CrossRef]
- Fryer, G. The feeding mechanism of some freshwater cyclopoid copepods. Proc. Zool. Soc. Lond 1957, 129, 1–25. [Google Scholar] [CrossRef]
- Fryer, G. The food of some freshwater cyclopoid copepods and its ecological significance. J. Anim. Ecol. 1957, 26, 263–286. [Google Scholar] [CrossRef]
- Galassi, D.M.P. Groundwater copepods: Diversity patterns over ecological and evolutionary scales. Hydrobiologia 2001, 453, 227–253. [Google Scholar] [CrossRef]
- Sarvala, J. Patterns of benthic copepod assemblages in an oligotrophic lake. Ann. Zool. Fenn. 1986, 23, 101–130. [Google Scholar]
- Sarvala, J. Ecology and role of benthic copepods in northern lakes. J. Mar. Syst. 1998, 15, 75–86. [Google Scholar] [CrossRef]
| Environmental Variable 1 | Min | μ | SD | Max |
|---|---|---|---|---|
| T (°C) | 4.00 | 18.40 | 9.79 | 4.70 |
| EC (μS cm−1) | 420.00 | 942.00 | 511.80 | 107.19 |
| pH | 7.37 | 8.25 | 7.92 | 0.23 |
| DO (mg L−1) | 0.15 | 10.80 | 6.09 | 2.70 |
| GRA (%) | 0.00 | 3.20 | 0.53 | 0.86 |
| CLA (%) | 18.07 | 39.83 | 29.18 | 7.25 |
| POM (mg L−1) | 20.00 | 1202.00 | 347.40 | 275.65 |
| DOC (mg L−1) | 1.00 | 2.80 | 1.67 | 0.44 |
| NO2 (mg L−1) | 0.03 | 0.78 | 0.15 | 0.19 |
| NO3 (mg L−1) | 0.25 | 8.80 | 3.67 | 3.02 |
| NH4 (mg L−1) | 0.03 | 2.25 | 0.42 | 0.67 |
| SO4 (mg L−1) | 1.80 | 43.00 | 7.98 | 7.73 |
| Cl (mg L−1) | 0.25 | 17.00 | 4.38 | 4.47 |
| PO4 (mg L−1) | 0.03 | 0.25 | 0.16 | 0.10 |
| Ca (mg L−1) | 70.00 | 145.00 | 101.07 | 14.75 |
| K (mg L−1) | 0.50 | 5.00 | 1.31 | 1.00 |
| Na (mg L−1) | 3.80 | 3.80 | 3.80 | 3.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabilio Di Camillo, A.; Cerasoli, F.; Di Cicco, M.; Galassi, D.M.P.; Di Lorenzo, T. Unraveling Functional Diversity Patterns in Hyporheic Zones: A Trait-Based Approach Applied to Copepods from the Rio Gamberale Creek. Diversity 2024, 16, 289. https://doi.org/10.3390/d16050289
Tabilio Di Camillo A, Cerasoli F, Di Cicco M, Galassi DMP, Di Lorenzo T. Unraveling Functional Diversity Patterns in Hyporheic Zones: A Trait-Based Approach Applied to Copepods from the Rio Gamberale Creek. Diversity. 2024; 16(5):289. https://doi.org/10.3390/d16050289
Chicago/Turabian StyleTabilio Di Camillo, Agostina, Francesco Cerasoli, Mattia Di Cicco, Diana Maria Paola Galassi, and Tiziana Di Lorenzo. 2024. "Unraveling Functional Diversity Patterns in Hyporheic Zones: A Trait-Based Approach Applied to Copepods from the Rio Gamberale Creek" Diversity 16, no. 5: 289. https://doi.org/10.3390/d16050289
APA StyleTabilio Di Camillo, A., Cerasoli, F., Di Cicco, M., Galassi, D. M. P., & Di Lorenzo, T. (2024). Unraveling Functional Diversity Patterns in Hyporheic Zones: A Trait-Based Approach Applied to Copepods from the Rio Gamberale Creek. Diversity, 16(5), 289. https://doi.org/10.3390/d16050289








