Diversity of Freshwater Calanoid Copepods (Crustacea: Copepoda: Calanoida) in North-Eastern China
Abstract
1. Introduction
2. Materials and Methods
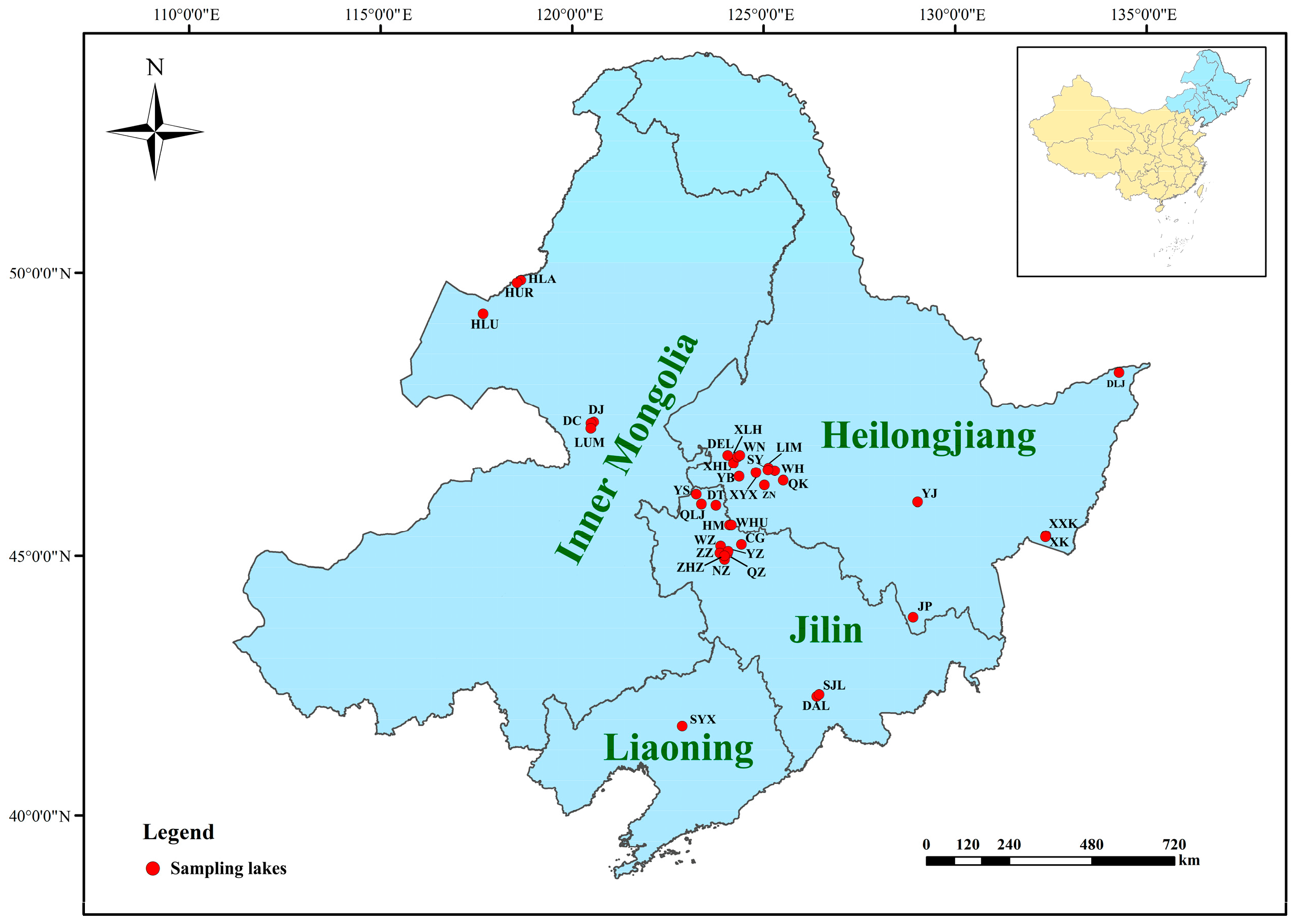
2.1. Study Area
2.2. Sampling, Preservation, and Identification
2.3. Data Analysis
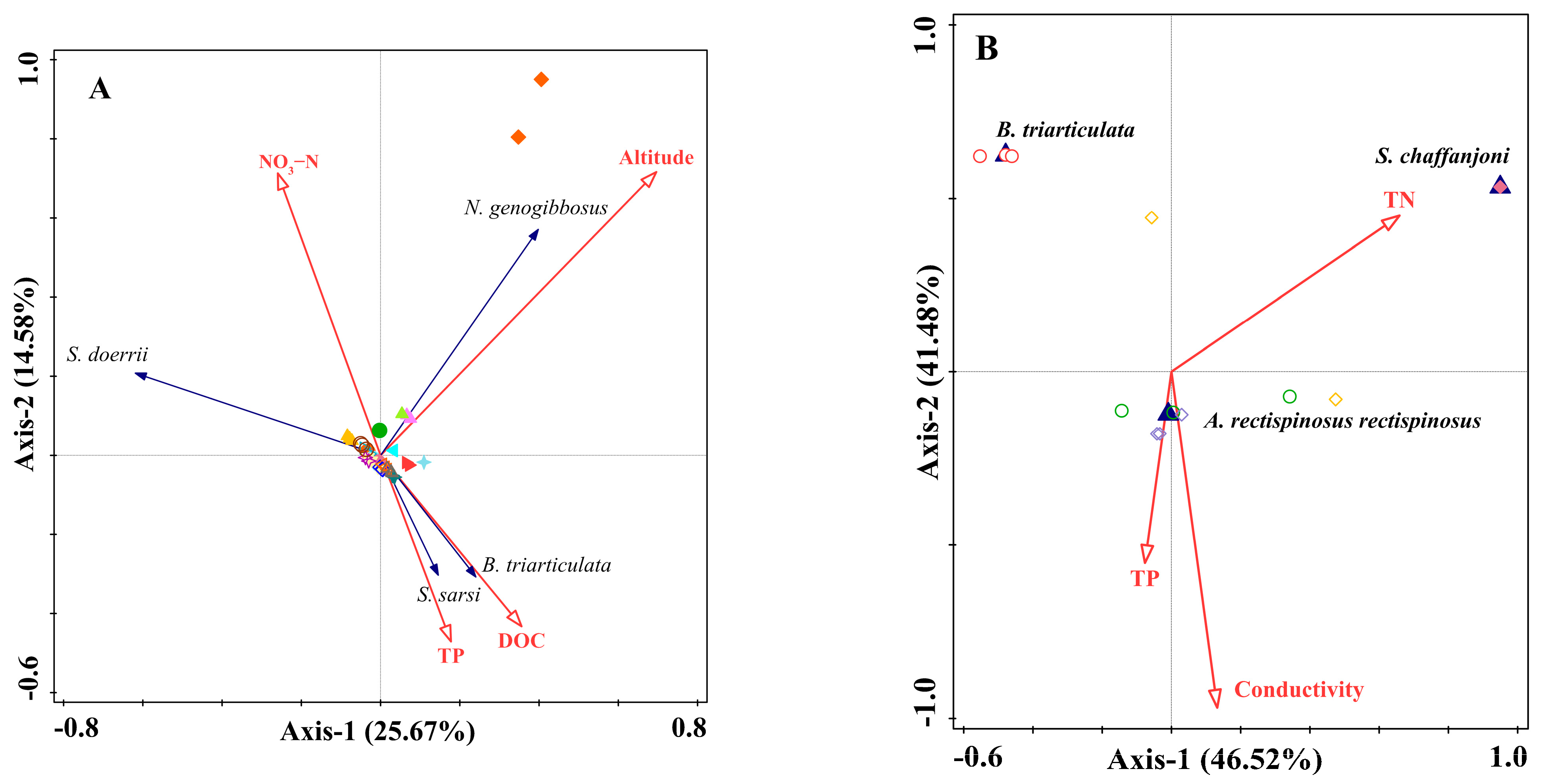
3. Results
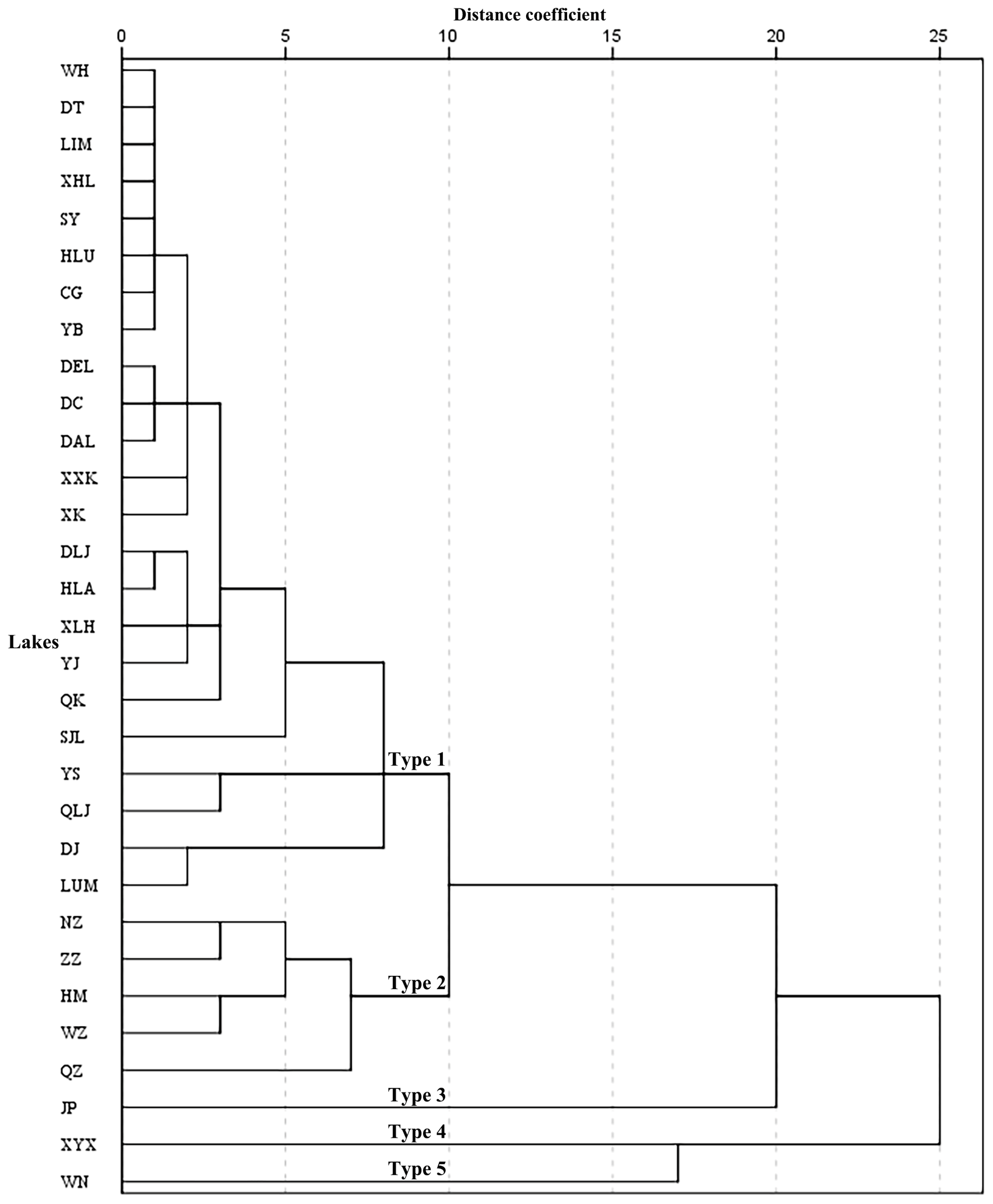
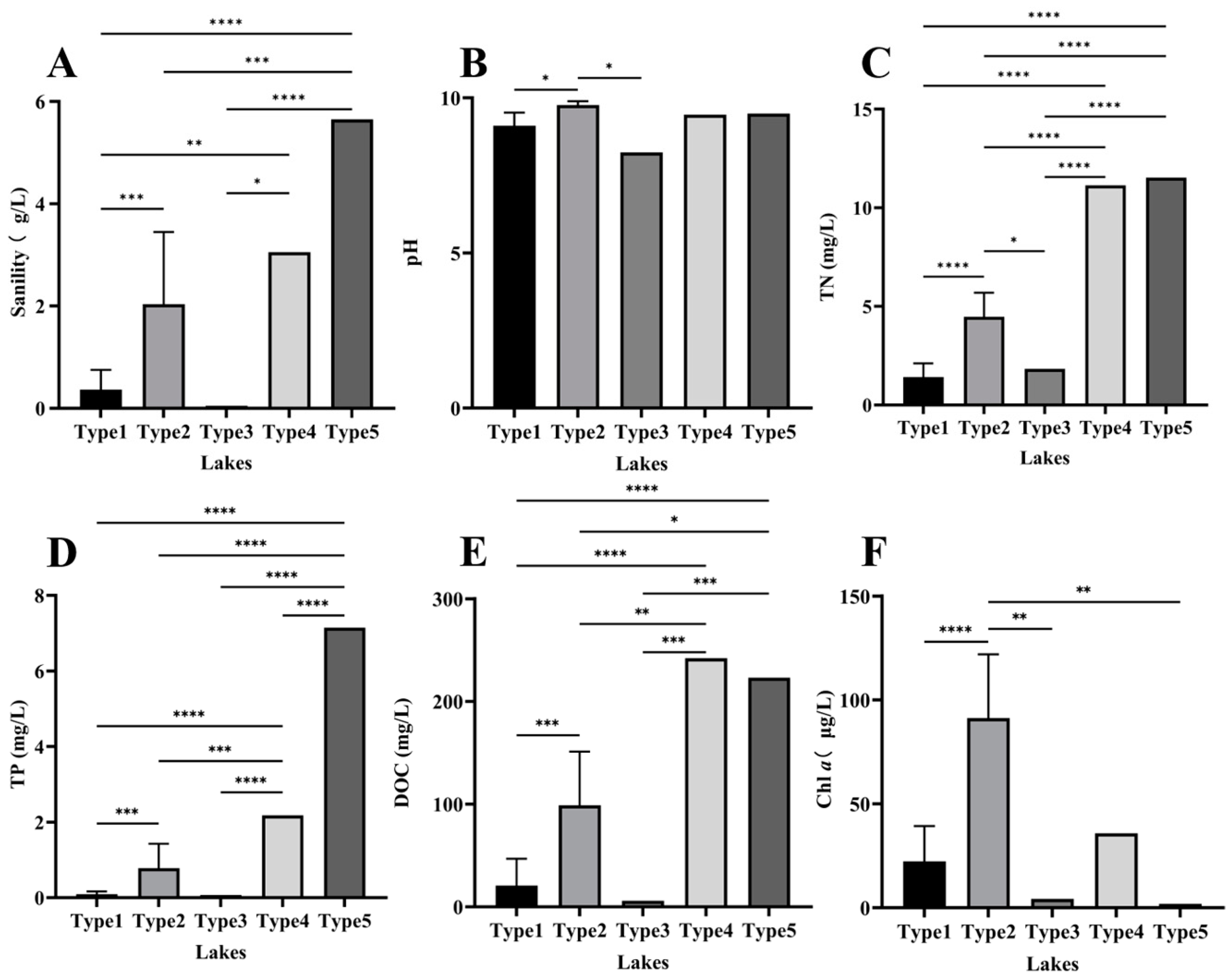
3.1. Lake Chemistry and Cluster Analysis
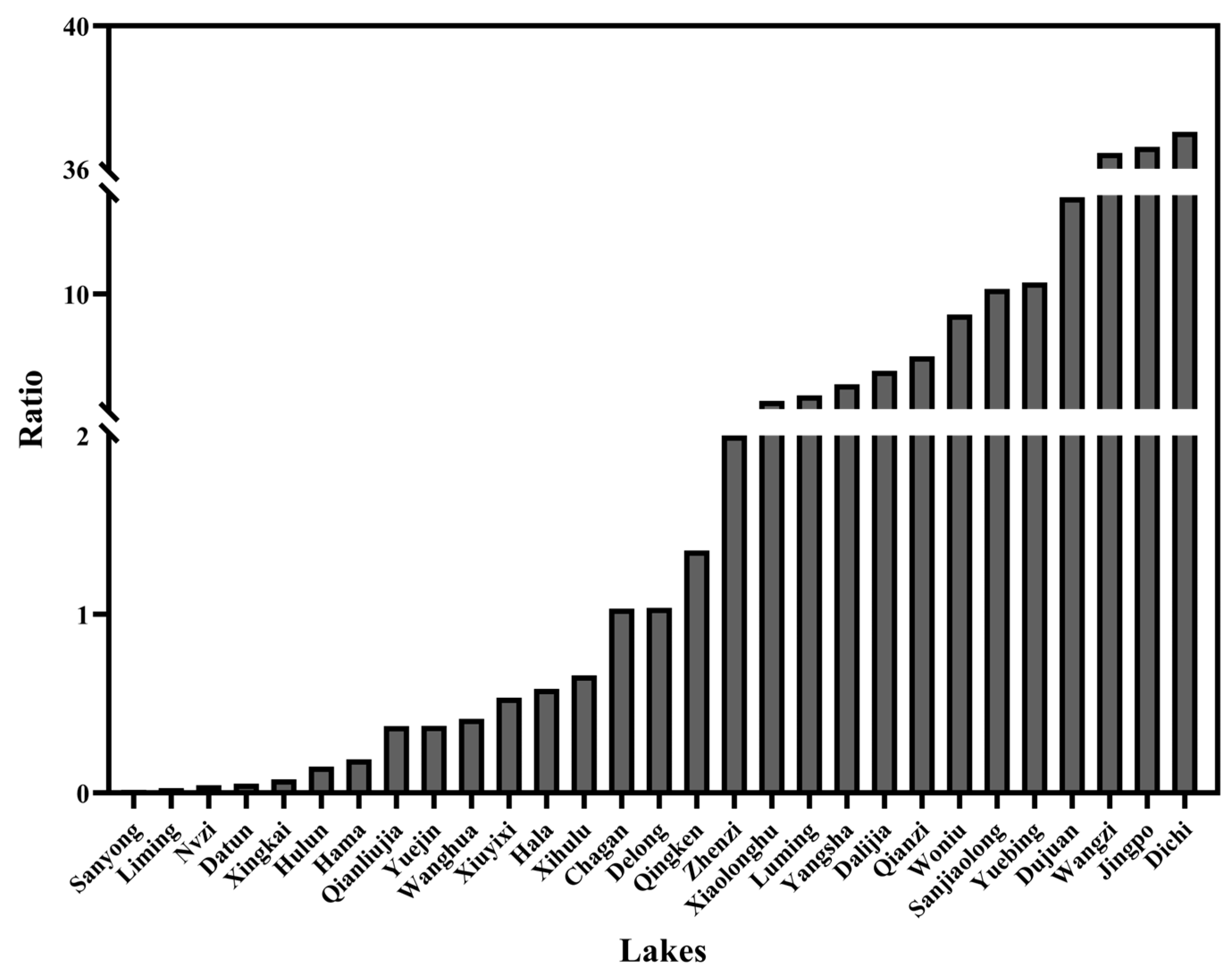
3.2. Calanoid Copepod Taxonomy and Distribution
3.3. Environmental Variables and Correlation Analysis
4. Discussion
- Family Temoridae Giesbrecht, 1893
- Family Diaptomidae Baird, 1850
- Family Centropagidae Giesbrecht, 1893
- The Relationship between Calanoid Species and environmental variables
| Species | This Study | Shen and Song (1979) [7] | Yu et al. (2003–2018) [6,8,9,65,66] | Distribution | |
|---|---|---|---|---|---|
| Family Temoridae Giesbrecht, 1893 | |||||
| 1 | Epischura chankensis Rylov, 1928 | √ | √ | China and Russia [77]. | |
| 2 | Heterocope appendiculata Sars G.O., 1863 | √ | China and Russia [34], Ukraine [78], Turkey [79], Germany [80], Iceland [81], Finland [82], Sweden [83], and Poland [84]. | ||
| 3 | Heterocope soldatovi Rylov, 1922 | √ | China and Far-Eastern Russia [45]. | ||
| Family Centropagidae Giesbrecht, 1893 | |||||
| 4 | Boeckella triarticulata (Thomson G.M., 1883) | √ | √ | China, New Zealand [85], Australia [27], Eastern Mongolia, Far-Eastern Russia, and Italy [4,69]. | |
| 5 | Sinocalanus doerrii (Brehm, 1909) | √ | √ | China and the United States [86]. | |
| 6 | Sinocalanus tenellus (Kikuchi, 1928) | √ | China [66], Japan [87], and South Korea [88]. | ||
| Family Diaptomidae Baird, 1850 | |||||
| 7 | Arctodiaptomus wierzejskii (Richard, 1888) | √ | √ | China, Mongolia [89], Russia [45], Tunisia [90], and France [91]. | |
| 8 | Acanthodiaptomus pacificus (Burckhardt, 1913) | √ | √ | √ | China, Japan [33], and South Korea [92]. |
| 9 | Arctodiaptomus rectispinosus Kikuchi K., 1940 | √ | China. | ||
| 10 | Arctodiaptomus stewartianus (Brehm, 1925) | √ | China and India [93]. | ||
| 11 | Eudiaptomus graciloides (Lilljeborg, 1888) | √ | China and European lakes [94]. | ||
| 12 | Mongolodiaptomus birulai (Rylov, 1922) | √ | √ | China, Vietnam, and the Philippines [95]. | |
| 13 | Heliodiaptomus kikuchii Kiefer, 1932 | √ | China, the Korean Peninsula, Japan, and Indonesia [33,96]. | ||
| 14 | Neodiaptomus schmackeri (Poppe and Richard, 1892) | China, Japan [33], South Korea [92], Kazakhstan [50], India [97], and Thailand [98]. | |||
| 15 | Neutrodiaptomus genogibbosus Shen, 1956 | √ | √ | China and Kazakhstan [50]. | |
| 16 | Neutrodiaptomus incongruens (Poppe, 1888) | √ | China, Russia [52], and Kazakhstan [50]. | ||
| 17 | Neutrodiaptomus pachypoditus (Rylov, 1925) | √ | √ | √ | China, Japan [33], and Russia [52]. |
| 18 | Metadiaptomus asiaticus (Uljanin, 1875) | √ | China, Mongolia [51], and Russia [50,99]. | ||
| 19 | Sinodiaptomus chaffanjoni (Richard, 1897) | √ | √ | √ | China [7,32]. |
| 20 | Sinodiaptomus sarsi (Rylov, 1923) | √ | √ | Eastern Asia [6,33,92], Turkey [100], Romania [101], Kazakhstan [50], and Ukraine [102]. | |
| 21 | Tropodiaptomus oryzanus Kiefer, 1937 | √ | China [32], South Korea [92], Vietnam, Cambodia, and Thailand [3,103]. |
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boxshall, G.A.; Defaye, D. Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia 2007, 595, 195–207. [Google Scholar] [CrossRef]
- Kobayashi, T.; Miller, J.; Bayly, I.A.E.; Tang, C.; Hunter, S.J.; Ralph, T.J.; Stone, L. Latitude and elevation as factors controlling occurrence of calanoid copepods in marginal lotic waters in New South Wales, Australia. Ecol. Res. 2018, 33, 1103–1111. [Google Scholar] [CrossRef]
- Sanoamuang, L.; Dabseepai, P. Diversity, distribution, and habitat occurrence of the Diaptomid copepods (Crustacea: Copepoda: Diaptomidae) in freshwater ecosystems of Thailand. Water 2021, 13, 2381. [Google Scholar] [CrossRef]
- Dussart, B.; Defaye, D. World Directory of Crustacea Copepoda of Inland Waters. Vol. I—Calaniformes; Backhuys Publishers: Leiden, The Netherlands, 2002; pp. 1–276. [Google Scholar]
- Boonmak, P.; Sanoamuang, L. Diversity of freshwater calanoid copepods (Crustacea: Copepoda: Calanoida) in southern Vietnam with an updated checklist for the country. Diversity 2022, 14, 523. [Google Scholar] [CrossRef]
- Li, H.M.; Dumont, H.J.; Han, B.-P.; Lin, Q. Updated checklist and distribution of the diaptomid copepods (Copepoda, Calanoida, Diaptomidae) of China. Crustaceana 2018, 91, 335–352. [Google Scholar] [CrossRef]
- Shen, C.J.; Song, D.X. Fauna Sinica: Crustacean—Freshwater Copepoda; Science Press: Beijing, China, 1979; pp. 53–158. [Google Scholar]
- Jiang, Z.F.; Dong, C.Z.; Zhan, P.Y.; Zhao, J.W.; Zhao, C.G.; Tang, F.J.; Han, Y. The population structure of zooplankton in Daxingkai Lake. J. Dalian Fish. Univ. 2003, 18, 292–295. [Google Scholar] [CrossRef]
- Yu, H.X.; Wang, Y.; Ma, C.X.; Zuo, Y.D. Community structure of crustacean zooplankton in Jingpo Lake. J. Northeast. For. Univ. 2008, 36, 66–68. [Google Scholar]
- Ji, S.C.; Li, Y.; Zhao, W.; Chen, H.X.; Xie, Z.G.; Zhang, J.W.; Wei, J.; Cai, Z.L. Study on the community structure of zooplankton in Biliuhe Reservoir and basin. J. Biol. 2018, 35, 68–73. [Google Scholar] [CrossRef]
- Hall, C.J.; Burns, C.W. Effects of salinity and temperature on survival and reproduction of Boeckella hamata (Copepoda: Calanoida) from a periodically brackish lake. J. Plankton Res. 2001, 23, 97–103. [Google Scholar] [CrossRef]
- Rhyne, A.L.; Ohs, C.L.; Stenn, E. Effects of temperature on reproduction and survival of the calanoid copepod Pseudodiaptomus pelagicus. Aquaculture 2009, 292, 53–59. [Google Scholar] [CrossRef]
- Rajakaruna, H.; Lewis, M. Temperature cycles affect colonization potential of calanoid copepods. J. Theor. Biol. 2017, 419, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.R.; Krishnan, K.A. Grazing behaviour of tropical calanoid copepods and its effect on phytoplankton community structure. Environ. Monit. Assess. 2021, 193, 495. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, S. Seasonal variations in body length and weight and ingestion rate of Centropages tenuiremis Tompson and Scott. Acta Oceanol. Sin. 1998, 20, 104–109. [Google Scholar]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining body size: A third universal response to warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef]
- Havens, K.E.; Beaver, J.R. Composition, size, and biomass of zooplankton in large productive Florida lakes. Hydrobiologia 2010, 668, 49–60. [Google Scholar] [CrossRef]
- Beaver, J.R.; Tausz, C.E.; Renicker, T.R.; Ordosch, D.M. Distributions and range expansions of rare or invasive species of planktonic calanoid copepods (Copepoda: Calanoida) within lakes and reservoirs in the continental United States. J. Crustac. Biol. 2019, 39, 533–539. [Google Scholar] [CrossRef]
- Sutherland, J.W.; Quinn, S.O.; Bloomfield, J.A.; Siegfried, C.A. Lake acidification and the biology of adirondack Lakes: Crustacean zooplankton communities. Lake Reserv. Manag. 2009, 1, 380–384. [Google Scholar] [CrossRef]
- Wærvagen, S.B.; Nilssen, J.P. Life histories and seasonal dynamics of common boreal pelagic copepods (Crustacea, Copepoda) inhabiting an oligotrophic Fennoscandian lake. J. Limnol. 2010, 69, 311–332. [Google Scholar] [CrossRef][Green Version]
- Cai, M.; Johansson, L.S.; Søndergaard, M.; Lauridsen, T.L.; Chen, F.; Shu, T.; Jeppesen, E. Copepods as environmental indicator in lakes: Special focus on changes in the proportion of calanoids along nutrient and pH gradients. Aquat. Ecol. 2021, 55, 1241–1252. [Google Scholar] [CrossRef]
- Torke, B. The distribution of calanoid copepods in the plankton of Wisconsin Lakes. Hydrobiologia 2001, 453, 351–365. [Google Scholar] [CrossRef]
- Escalante, P.R.D.l.R.; Kies, F. Calanoid copepods in central Chilean and Chilean Patagonian lakes (33–55° S, Chile), probable ecological key role in pelagic environments. Crustaceana 2017, 90, 1793–1802. [Google Scholar] [CrossRef]
- Lu, M.; Xie, P. Impacts of filter-feeding fishes on the long-term changes of crustacean zooplankton in a eutrophic subtropical Chinese lake. J. Freshw. Ecol. 2001, 16, 219–228. [Google Scholar] [CrossRef]
- Xie, P.; Yang, Y. Long-term changes of copepoda community (1957–1996) in a subtropical Chinese lake stocked densely with planktivorous filter-feeding silver and bighead carp. J. Plankton Res. 2000, 22, 1757–1778. [Google Scholar] [CrossRef][Green Version]
- Santer, B. Life cycle strategies of free-living copepods in fresh waters. J. Mar. Syst. 1998, 15, 327–336. [Google Scholar] [CrossRef]
- Maly, E.J.; Maly, M.P. Predation, competition, and co-occurrences of Boeckella and Calamoecia (Copepoda: Calanoida) in Western Australia. Hydrobiologia 1997, 354, 41–50. [Google Scholar] [CrossRef]
- Islam, M.; Hibino, M.; Tanaka, M. Distribution and dietary relationships of the Japanese temperate bass Lateolabrax japonicus juveniles with two contrasting copepod assemblages in estuarine nursery grounds in the Ariake Sea, Japan. J. Fish Biol. 2006, 68, 569–593. [Google Scholar] [CrossRef]
- McGann, B.N.; Strecker, A.L. Zooplankton recovery from a whole-lake disturbance: Examining roles of abiotic factors, biotic interactions, and traits. Ecosphere 2022, 13, e3983. [Google Scholar] [CrossRef]
- Lynch, M. Predation, competition, and zooplankton community structure: An experimental study. Limnol. Oceanogr. 1979, 24, 253–272. [Google Scholar] [CrossRef]
- Cao, J.L.; Xu, Q.G.; Xi, B.D.; Li, X.P.; Yang, L.Y.; Jiang, L.W.; Wei, Z.M.; Wu, X.H. Regional Heterogeneity of Lake Eutrophication Effects in China. Environ. Sci. 2012, 33, 1777–1783. [Google Scholar] [CrossRef]
- Li, H.M.; Han, B.P.; Guo, F.F.; Dumont, H.J. Re-allocation of two south Chinese species of Argyrodiaptomus Brehm, 1933 to Sinodiaptomus Kiefer, 1932, and biogeography of the genus Sinodiaptomus (Copepoda, Calanoida, Diaptomidae). Crustaceana 2014, 87, 328–339. [Google Scholar] [CrossRef]
- Makino, W.; Tanabe, A.S.; Urabe, J. The fauna of freshwater calanoid copepods in Japan in the early decades of the 21st Century: Implications for the assessment and conservation of biodiversity. Limnol. Oceanogr. 2018, 63, 758–772. [Google Scholar] [CrossRef]
- Sheveleva, N.G.; Chertoprud, E.; Lazareva, V.I.; Bayanov, N. The Genus Heterocope Sars 1863 (Copepoda, Calanoida) in Russia: Morphology and Distribution. Biol. Bull. 2021, 48, 120–139. [Google Scholar] [CrossRef]
- Gui, Z.; Xue, B.; Yao, S.; Wei, W. Responses of lakes in the Songnen Plain to climate change. J. Lake Sci. 2010, 22, 852–861. [Google Scholar]
- Administration, S.E.P. Water and Wastewater Monitoring and Analysis Methods, 4th ed.; China Environmental Science Press: Beijing, China, 2002; pp. 200–284. [Google Scholar]
- Lin, S.J.; He, L.J.; Huang, P.S.; Han, B.P. Comparison and improvement on the extraction method for chlorophyll a in phytoplankton. Ecol. Sci. 2005, 24, 9–11. [Google Scholar]
- Dumont, H.J.; Van de Velde, I.; Dumont, S. The dry weight estimate of biomass in a selection of cladocera, copepoda and rotifera from the plankton, periphyton and benthos of continental waters. Oecologia 1975, 19, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, J.; Jeppesen, E.; Chen, Y.; Liu, X.; Zhang, W. Horizontal distribution of pelagic crustacean zooplankton biomass and body size in contrasting habitat types in Lake Poyang, China. Environ. Sci. Pollut. Res. 2019, 26, 2270–2280. [Google Scholar] [CrossRef]
- Ning, L.M.; Wang, X.L.; Zhu, M.Y. Application of cluster analysis in the classification of Jianghan Lake Group. Resour. Environ. Yangtze Basin 2007, 16, 118–122. [Google Scholar]
- Gong, L.J.; Yang, X.F.; Xiong, B.X.; Zhou, M.; Chen, X.L. Study of water physical and chemical indexes of lakes in Wuhan city in summer by means of principal component analysis and cluster analysis. Resour. Environ. Yangtze Basin 2009, 18, 550–554. [Google Scholar]
- Wang, S.; Xie, P.; Wu, S.; Wu, A. Crustacean zooplankton distribution patterns and their biomass as related to trophic indicators of 29 shallow subtropical lakes. Limnologica 2007, 37, 242–249. [Google Scholar] [CrossRef]
- Guo, N.; Zhang, M.; Yu, Y.; Qian, S.; Li, D.; Kong, F. Crustacean zooplankton communities in 13 lakes of Yunnan-Guizhou plateau: Relationship between crustacean zooplankton biomass or size structure and trophic indicators after invasion by exotic fish. Ann. De Limnol.-Int. J. Limnol. 2009, 45, 279–288. [Google Scholar] [CrossRef][Green Version]
- Rylov, V.M. Heterocope soldatovi n. sp., a new species of freshwater crustacea (Copepoda, Calanoida). Ezhegodnik’ Zool. Muzeya Ross. Akad. 1922, 23, 164–178. [Google Scholar]
- Garibian, P.G.; Chertoprud, E.S.; Sinev, A.Y.; Korovchinsky, N.M.; Kotov, A.A. Cladocera and copepoda (Crustacea: Branchiopoda) of the Lake Bolon and its basin (Far East of Russia). Arthropoda Sel. 2019, 28, 37–63. [Google Scholar] [CrossRef]
- Cui, S.; Li, Y.; Liu, L.; Wang, Q.; Chen, F. Changes in astaxanthin and fatty acid concentrations during the developmental process in the calanoid Arctodiaptomus walterianus in an alpine lake at low latitudes. J. Plankton Res. 2021, 43, 314–324. [Google Scholar] [CrossRef]
- Wei, C.J.; Xiong, D.N.; Wang, Y.L.; Feng, W.S.; Miao, R.L.; Gong, Y.C. First record of Mongolodiaptomus mekongensis in China and its phylogenetic analysis. Acta Hydrobiol. Sin. 2023, 47, 1640–1648. [Google Scholar] [CrossRef]
- Ueda, H.; Tomikawa, K.; Ohtsuka, S. Redescription of the freshwater calanoid copepod Neutrodiaptomus formosus with key to females of diaptomid species in Japan. Plankton Benthos Res. 2020, 15, 178–184. [Google Scholar] [CrossRef]
- Rayner, N.A. Distribution and biogeography of the Paradiaptominae (Copepoda: Calanoida: Diaptomidae). Afr. J. Aquat. Sci. 2000, 25, 93–97. [Google Scholar] [CrossRef]
- Krupa, E.; Aubakirova, M. Checklist and distribution of calanoida (Crustacea: Copepoda) in Kazakhstan (Central Asia). Water 2021, 13, 2015. [Google Scholar] [CrossRef]
- Afonina, E.Y.; Tashlykova, N.A. Plankton community and the relationship with the environment in saline lakes of Onon-Torey plain, Northeastern Mongolia. Saudi J. Biol. Sci. 2018, 25, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Melnik, N.G.; Sheveleva, N.G.; Pomazkova, G.I. Distribution of planktonic copepods of Lake Baikal. J. Mar. Syst. 1998, 15, 149–153. [Google Scholar] [CrossRef]
- Podshivalina, V.N.; Sheveleva, N.G. First record of Sinodiaptomus sarsi (Copepoda: Calanoida) from the East European Plain. Zoosystematica Ross. 2020, 29, 60–69. [Google Scholar] [CrossRef]
- Bayly, I.A.E. The Non-Marine Centropagidae (Copepoda: Calanoida) of the World; SPB Academic Publishing: Hague, The Netherlands, 1992. [Google Scholar]
- Bradford-Grieve, J.M. Colonization of the pelagic realm by calanoid copepods. Hydrobiologia 2002, 485, 223–244. [Google Scholar] [CrossRef]
- Adamowicz, S.J.; Menu-Marque, S.; Halse, S.A.; Topan, J.C.; Zemlak, T.S.; Hebert, P.D.N.; Witt, J.D.S. The evolutionary diversification of the Centropagidae (Crustacea, Calanoida): A history of habitat shifts. Mol. Phylogenetics Evol. 2010, 55, 418–430. [Google Scholar] [CrossRef] [PubMed]
- Bayly, I.A.E.; Boxshall, G.A. An all-conquering ecological journey: From the sea, calanoid copepods mastered brackish, fresh, and athalassic saline waters. Hydrobiologia 2009, 630, 39–47. [Google Scholar] [CrossRef]
- Boxshall, G.A.; Jaume, D. Making waves: The repeated colonization of fresh water by copepod crustaceans. Adv. Ecol. Res. 2000, 31, 61–79. [Google Scholar] [CrossRef]
- Quinlan, K.; Bayly, I.A.E. A new species of Boeckella (Copepoda: Calanoida) from arid Western Australia, an updated key, and aspects of claypan ecology. Rec. West. Aust. Mus. 2017, 32, 191–206. [Google Scholar] [CrossRef][Green Version]
- Alfonso, G.; Belmonte, G. Expanding distribution of Boeckella triarticulata (Thomson, 1883) (Copepoda: Calanoida: Centropagidae) in Southern Italy. Aquat. Invasions 2008, 3, 247–251. [Google Scholar] [CrossRef]
- Figuerola, J.; Green, A.J. How frequent is external transport of seeds and invertebrate eggs by waterbirds? A study in Doñana, SW Spain. Fundam. Appl. Limnol. 2002, 155, 557–565. [Google Scholar] [CrossRef]
- Figuerola, J.; Green, A.J.; Black, K.; Okamura, B. Influence of gut morphology on passive transport of freshwater bryozoans by waterfowl in Doñana (southwestern Spain). Can. J. Zool. 2004, 82, 835–840. [Google Scholar] [CrossRef][Green Version]
- Green, A.J.; Figuerola, J. Recent advances in the study of long-distance dispersal of aquatic invertebrates via birds. Divers. Distrib. 2005, 11, 149–156. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, Z.; Sun, G.-Q.; Sun, X.-D.; Wang, Y.-M.; Huang, B. Determination of original infection source of H7N9 avian influenza by dynamical model. Sci. Rep. 2014, 4, 4846. [Google Scholar] [CrossRef]
- An, H.; Xie, Z.G.; Wang, M.R.; Cai, Z.L.; Liu, L.; Zhao, W. Community structure analysis on plankton in Biliuhe Reservoir in spring of 2016. Jilin Water Resour. 2018, 429, 39–44. [Google Scholar] [CrossRef]
- Wang, X.; Ma, X.; Hu, W.; Yin, M. Genetic diversity and population differentiation of the freshwater copepod Sinocalanus tenellus (Calanoida, Centropagidae) in China. J. Limnol. 2018, 77, 300–307. [Google Scholar] [CrossRef]
- Yang, F.; Lv, X.; Lou, Y.; Lou, X.; Xue, B.; Yao, S.; Xiao, H. Ichthyofauna and its community diversity in volcanic barrier lakes of Northeast China. Chin. J. Appl. Ecol. 2012, 23, 3449–3457. [Google Scholar] [CrossRef]
- Hutchinson, G.E. A Treatise on Limnology, Vol. II.; Wiley: New York, NY, USA, 1967; p. 676. [Google Scholar]
- Ferrari, I.; Rossetti, G. New records of the centropagid Boeckella triarticulata (Copepoda: Calanoida) in Northern Italy: Evidence of a successful invasion? Aquat. Invasions 2006, 1, 219–222. [Google Scholar] [CrossRef]
- Ohman, M.D.; Hieche, H.J. Density-dependent mortality in an oceanic copepod population. Nature 2001, 412, 438–641. [Google Scholar] [CrossRef]
- Boersma, M.; Wesche, A.; Hirche, H.-J. Predation of calanoid copepods on their own and other copepods’ offspring. Mar. Biol. 2014, 161, 733–743. [Google Scholar] [CrossRef]
- Šorf, M.; Davidson, T.A.; Brucet, S.; Menezes, R.F.; Søndergaard, M.; Lauridsen, T.L.; Landkildehus, F.; Liboriussen, L.; Jeppesen, E. Zooplankton response to climate warming: A mesocosm experiment at contrasting temperatures and nutrient levels. Hydrobiologia 2014, 742, 185–203. [Google Scholar] [CrossRef]
- Li, Y.; Geng, M.; Yu, J.; Du, Y.; Xu, M.; Zhang, W.; Wang, J.; Su, H.; Wang, R.; Chen, F. Eutrophication decrease compositional dissimilarity in freshwater plankton communities. Sci. Total Environ. 2022, 821, 153434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yi, Y.; Cao, Y.; Yang, Z. Disentangling the effects of phosphorus loading on food web stability in a large shallow lake. J. Environ. Manag. 2023, 328, 116991. [Google Scholar] [CrossRef]
- Alimov, A.F. Relations between biological diversity in continental waterbodies and their morphometry and water mineralization. Inland Water Biol. 2008, 1, 1–6. [Google Scholar] [CrossRef]
- Zhao, W.; He, Z. Biological and ecological features of inland saline waters in North Hebei, China. Int. J. Salt Lake Res. 1999, 8, 267–285. [Google Scholar]
- Zaidykov, I.; Bukin, Y.; Naumova, E.; Kirilchik, S.; Sukhanova, L. Phylogenetic relationships and historical population reconstruction of Asian members of the genus Epischura (Copepoda, Calanoida). J. Great Lakes Res. 2020, 46, 12–16. [Google Scholar] [CrossRef]
- Samchyshyna, L. Copepoda calanoida of the Shatski Lakes (Ukraine). Vestn. Zool. 2001, 35, 47–51. [Google Scholar]
- Bozkurt, A.; Gu, S.E. Zooplankton composition and distribution in vegetated and unvegetated area of three reservoirs in Hatay, Turkey. J. Anim. Vet. Adv. 2009, 8, 984–994. [Google Scholar] [CrossRef]
- Santonja, M.; Minguez, L.; Gessner, M.O.; Sperfeld, E. Predator–prey interactions in a changing world: Humic stress disrupts predator threat evasion in copepods. Oecologia 2016, 183, 887–898. [Google Scholar] [CrossRef]
- Novichkova, A.; Chertoprud, E.; Gíslason, G.M. Freshwater crustacea (Cladocera, Copepoda) of Iceland: Taxonomy, ecology, and biogeography. Polar Biol. 2014, 37, 1755–1767. [Google Scholar] [CrossRef]
- Viljanen, M. Food and food selection of cisco (Coregonus albula L.) in a dysoligotrophic lake. Hydrobiologia 1983, 101, 129–138. [Google Scholar] [CrossRef]
- Pejler, B. Zooplanktic indicators of trophy and their food. Hydrobiologia 1983, 101, 111–114. [Google Scholar] [CrossRef]
- Karpowicz, M.; Ejsmont-Karabin, J. Diversity and structure of pelagic zooplankton (Crustacea, Rotifera) in NE Poland. Water 2021, 13, 456. [Google Scholar] [CrossRef]
- Twombly, S.; Clancy, N.; Burns, C.W. Life history consequences of food quality in the freshwater copepod Boeckella Triarticulata. Ecology 1998, 79, 1711–1724. [Google Scholar] [CrossRef]
- Bryant, M.E.; Arnold, J.D. Diets of age-0 striped bass in the San Francisco Estuary, 1973–2002. Calif. Fish Game 2007, 93, 1–22. [Google Scholar]
- Uye, S.; Shimazu, T.; Yamamuro, M.; Ishitobi, Y.; Kamiya, H. Geographical and seasonal variations in mesozooplankton abundance and biomass in relation to environmental parameters in Lake Shinji-Ohashi River-Lake Nakaumi brackish-water system, Japan. J. Mar. Syst. 2000, 26, 193–207. [Google Scholar] [CrossRef]
- Chae, Y.J.; Oh, H.J.; Chang, K.H.; Kwak, I.S.; Jo, H.B. Application of next-generation sequencing for the determination of the bacterial community in the gut contents of brackish copepod species (Acartia hudsonica, Sinocalanus tenellus, and Pseudodiaptomus inopinus). Animals 2021, 11, 542. [Google Scholar] [CrossRef] [PubMed]
- Marrone, F.; Alonso, M.; Pieri, V.; Augugliaro, C.; Stoch, F. The crustacean fauna of Bayan Onjuul area (Tov Province, Mongolia) (Crustacea: Branchiopoda, Copepoda, Ostracoda). North-West. J. Zool. 2015, 11, 288–295. [Google Scholar]
- Marrone, F.; Stoch, F.; Turki, S.; Naselli-Flores, L. The Diaptomidae (Copepoda, Calanoida) of Tunisia and the role of spatial and environmental factors as drivers of their distribution patterns. Hydrobiologia 2023, 850, 4815–4829. [Google Scholar] [CrossRef]
- Pont, D.; Crivelli, A.J.; Guillot, F. The impact of three-spined sticklebacks on the zooplankton of a previously fish-free pool. Freshw. Biol. 2006, 26, 149–163. [Google Scholar] [CrossRef]
- Chang, C.Y.; Kim, H.S. The freshwater calanoida (Crustacea: Copedpoda) of Korea. Korean J. Syst. Zool. 1986, 2, 49–60. [Google Scholar]
- Verleyen, E.; Vyverman, W.; Sterken, M.; Hodgson, D.A.; De Wever, A.; Juggins, S.; Van de Vijver, B.; Jones, V.J.; Vanormelingen, P.; Roberts, D.; et al. The importance of dispersal related and local factors in shaping the taxonomic structure of diatom metacommunities. Oikos 2009, 118, 1239–1249. [Google Scholar] [CrossRef]
- Zeller, M. Diapause in the calanoid freshwater copepod Eudiaptomus graciloides. J. Plankton Res. 2004, 26, 1379–1388. [Google Scholar] [CrossRef]
- Sanoamuang, L.; Watiroyram, S. Mongolodiaptomus mekongensis, a new species of copepod (Copepoda, Calanoida, Diaptomidae) from temporary waters in the floodplain of the lower Mekong River Basin. Raffles Bull. Zool. 2018, 66, 782–796. [Google Scholar]
- Baek, S.Y.; Jang, K.H.; Choi, E.H.; Ryu, S.H.; Kim, S.K.; Lee, J.H.; Lim, Y.J.; Lee, J.; Jun, J.; Kwak, M.; et al. DNA barcoding of metazoan zooplankton copepods from South Korea. PLoS ONE 2016, 11, e0157307. [Google Scholar] [CrossRef] [PubMed]
- Ranga Reddy, Y. Neodiaptomus prateek n. sp., a new freshwater copepod from Assam, India, with critical review of generic assignment of Neodiaptomus spp. and a note on diaptomid species richness (Calanoida: Diaptomidae). J. Crustac. Biol. 2013, 33, 849–865. [Google Scholar] [CrossRef][Green Version]
- Lai, H.C.; Fernando, C.H. The freshwater calanoida (Crustacea, Copepoda) of Thailand. Hydrobiologia 1981, 76, 161–178. [Google Scholar] [CrossRef]
- Afonina, E.Y.; Tashlykova, N.A. Phytoplankton and zooplankton succession during the dry–refilling cycle: A case study in large, fluctuating soda lakes. Freshw. Biol. 2023, 68, 987–1006. [Google Scholar] [CrossRef]
- Ertunç, G. Sinodiaptomus sarsi (Rylov, 1923) (Copepoda, Calanoida) in Turkey. Hydrobiologia 1999, 392, 261–262. [Google Scholar] [CrossRef]
- Battes, K. A species on the rise in Europe: Sinodiaptomus sarsi (Rylov, 1923) (Copepoda, Calanoida), a new record for the Romanian crustacean fauna. BioInvasions Rec. 2020, 9, 320–332. [Google Scholar] [CrossRef]
- Svetlichny, L.; Samchyshyna, L. A new finding of the non-native copepod Sinodiaptomus sarsi (Copepoda, Calanoida, Diaptomidae) in Ukraine. Zoodiversity 2021, 55, 1–8. [Google Scholar] [CrossRef]
- Dang, P.D.; Khoi, N.V.; Nga, L.T.N.; Thanh, D.N.; Hai, H.T. Identification Handbook of Freshwater Zooplankton of the Mekong River and Its Tributaries; Mekong River Commission: Vientiane, Laos, 2015; p. 145. [Google Scholar]

| Species | Distribution | Type of Habitats | Frequency (%) |
|---|---|---|---|
| Family Temoridae Giesbrecht, 1893 | |||
| Heterocope soldatovi Rylov, 1922 | Dalijia | 1 | 3.0 |
| Family Centropagidae Giesbrecht, 1893 | |||
| Boeckella triarticulata (Thomson G.M., 1883) | Xingkai, Sanjiaolong, Yuejin, Xiaolonghu, Qianzi, Hala, Hulun | 1, 2 | 21.2 |
| Sinocalanus doerrii (Brehm, 1909) | Xingkai, Dalijia, Xiaoxingkai, Delong, Jingpo, Yuebing, Wanghua, Xihulu, Sanyong, Liming, Chagan, Yangsha, Qianliujia, Datun | 1 | 39.4 |
| Family Diaptomidae Baird, 1850 | |||
| Acanthodiaptomus pacificus (Burckhardt, 1913) | Dalong | 1 | 2.8 |
| Arctodiaptomus rectispinosus Kikuchi K., 1940 | Xiuyixi, Nvzi, Zhenzi | 2, 4 | 9.1 |
| Neutrodiaptomus genogibbosus Shen, 1956 | Dujuan, Luming, Dichi | 1 | 9.1 |
| Neutrodiaptomus pachypoditus (Rylov, 1925) | Jingpo | 3 | 3.0 |
| Metadiaptomus asiaticus (Uljanin, 1875) | Woniu | 5 | 3.0 |
| Sinodiaptomus chaffanjoni (Richard, 1897) | Hama | 2 | 3.0 |
| Sinodiaptomus sarsi (Rylov, 1923) | Qingken, Wanghua, Qianliujia, Wangzi | 1, 2 | 18.2 |
| Lake | Species | Body Length | Biomass | ||
|---|---|---|---|---|---|
| t | p | t | p | ||
| Lake XK | S. doerrii–B. triarticulata | −3.803 | 0.019 | 0.899 | 0.419 |
| Lake JP | S. doerrii–N. pachypoditus | −2.527 | 0.065 | 3.259 | 0.031 |
| Lake WH | S. doerrii–S. sarsi | −2.881 | 0.045 | 2.528 | 0.065 |
| Lake QLJ | S. doerrii–S. sarsi | 0.062 | 0.954 | -0.025 | 0.982 |
| Lake DLJ | S. doerrii–H. soldatovi | −0.356 | 0.740 | 4.293 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, R.; Liu, L.; Shu, S.; Li, Y.; Chen, F. Diversity of Freshwater Calanoid Copepods (Crustacea: Copepoda: Calanoida) in North-Eastern China. Diversity 2024, 16, 288. https://doi.org/10.3390/d16050288
Ding R, Liu L, Shu S, Li Y, Chen F. Diversity of Freshwater Calanoid Copepods (Crustacea: Copepoda: Calanoida) in North-Eastern China. Diversity. 2024; 16(5):288. https://doi.org/10.3390/d16050288
Chicago/Turabian StyleDing, Ruirui, Le Liu, Shusen Shu, Yun Li, and Feizhou Chen. 2024. "Diversity of Freshwater Calanoid Copepods (Crustacea: Copepoda: Calanoida) in North-Eastern China" Diversity 16, no. 5: 288. https://doi.org/10.3390/d16050288
APA StyleDing, R., Liu, L., Shu, S., Li, Y., & Chen, F. (2024). Diversity of Freshwater Calanoid Copepods (Crustacea: Copepoda: Calanoida) in North-Eastern China. Diversity, 16(5), 288. https://doi.org/10.3390/d16050288







