Abstract
Although the afforestation of former arable lands is a common global land-use conversion, its impact on soil microbial communities at the aggregate scale has not been adequately addressed. In this study, soil samples were categorized into large macroaggregates (LM, >2 mm), small macroaggregates (SM, 2–0.25 mm), and microaggregates (MI, <0.25 mm) to assess the changes in microbial composition, diversity, network complexity, and network stability within soil aggregates after the afforestation of a former dryland in northwestern China. The results revealed that afforestation enhanced the relative abundance of Actinobacteriota, Chloroflexi, Ascomycota, and Mortierellomycota within the soil aggregates, suggesting that these phyla may have greater advantages in microbial communities post-afforestation. The Shannon–Wiener and Pielou indices for bacterial communities showed no significant differences between land-use types across all aggregate fractions. However, the alpha diversity of fungal communities within the LM and SM significantly increased after afforestation. Bray–Curtis dissimilarity indices showed that afforestation altered bacterial beta diversity within the LM and MI but had a minimal impact on fungal beta diversity across all three aggregate fractions. The topological features of cross-kingdom microbial co-occurrence networks within the soil aggregates generally exhibited a decreasing trend post-afforestation, indicating a simplification of microbial community structure. The reduced robustness of microbial networks within the LM and SM fractions implies that afforestation also destabilized the structure of microbial communities within the macroaggregates. The composition of the soil microbial communities correlated closely with soil carbon and nitrogen contents, especially within the two macroaggregate fractions. The linkages suggests that improved resource conditions could be a key driver behind the shifts in microbial communities within soil aggregates following afforestation. Our findings indicate that the impact of afforestation on soil microbial ecology can be better understood by soil aggregate fractionation.
1. Introduction
Afforestation, a common form of land-use change worldwide, has contributed to a 123 million ha increase in the global forest area from 1990 to 2020 [1]. Like natural forests, planted forests provide various ecological services, such as water conservation, disaster risk reduction, and climate change mitigation [2,3]. The improved soil environment is one of the major reasons for these benefits after afforestation, especially in arable lands with low soil quality [4]. Empirical evidence has shown that afforestation promotes carbon sequestration, nutrient mineralization, and water retention in former arable soils [4,5,6]. Such variations can further influence the structure of soil microorganisms, which play vital roles in regulating soil functions [7,8]. However, the effects of arable land afforestation on soil microbial composition and diversity differ considerably across individual studies. For instance, previous studies have reported that the afforestation of former arable lands has increased, reduced, or had a minimal impact on soil microbial diversity [9,10,11]. The inconsistent findings suggest that the variations and influencing factors of soil microbial communities are complex under the background of afforestation.
Over the past decade, network analysis has been increasingly applied to quantify the inter-taxa associations within soil microbial communities [12,13]. Recently, there has been a growing concern regarding the complexity and stability of these associations with soil microbial co-occurrence networks [14]. In general, complex soil microbial networks indicate that the microbiome has an active metabolism and a rapid growth rate, whereas stable soil microbial networks imply that the existing microbial species possess a robust capacity to maintain their structure under external disturbances [14,15]. Therefore, co-occurrence network analysis provides a novel perspective in soil microbial community research. Although recent evidence has revealed that soil microbial networks undergo changes after converting arable lands to planted forests, the results differ greatly between studies in the limited literature. For example, a study conducted in subtropical China found that the afforestation of former arable lands significantly decreased the complexity of the soil microbial networks [16]. In contrast, Wang et al. observed that planted forests had more complex soil microbial networks than arable lands in the North China Plain [17]. These divergent observations demonstrate that more empirical studies are needed to elucidate a general response of soil microbial networks to the afforestation of former arable lands.
In general, soil microorganisms are spatially organized communities because soil structure can affect the distribution and availability of microbial substrates [18,19]. As basic building components of soil structure, soil aggregates can serve as microhabitats for microorganisms and offer a microbially relevant scale to investigate how afforestation influences the ecology of soil microbial communities [18,19]. Using 0.25 mm as the threshold, soil aggregates can be separated into macroaggregates (>0.25 mm) and microaggregates (<0.25 mm) [20]. The former generally harbor more abundant labile carbon and oxygen than the latter [20,21]. In addition, a part of microaggregates can be wrapped within macroaggregates during soil aggregation [22]. As a result, the turnover of soil aggregates likely plays a key role in shaping the spatial heterogeneity of microbial composition, diversity, and inter-taxa associations [18,19]. Ample evidence indicates that the afforestation of former arable lands can alter the distribution and stability of soil aggregates [23,24]. Unfortunately, the dynamics of the microbial communities within soil aggregates have received much less attention after arable land afforestation. This limits our understanding of the potential biodiversity and functions of soil microbial communities for ecosystem sustainability and their feedback to environmental change.
As a major type of rainfed arable land worldwide, dryland farming system is crucial to agricultural production in semi-arid regions [25]. The recorded history of farming spans approximately 8000 years in China, where more than half of the total arable land area is currently covered by dryland [26]. However, long-term dryland farming in China has led to numerous environmental issues (e.g., soil erosion), particularly in hilly and mountainous regions [27]. In this context, a large-scale ecological restoration project named “Grain for Green” was implemented in 1999 to rehabilitate the degraded drylands across China. Afforestation is an important restoration measure of this project, and its impacts on soil aggregates in drylands have been well-documented in previous studies [24,28,29]. Taking the Loess Plateau as an example, several studies found that the afforestation of former drylands increased the contents of carbon and nutrients within soil aggregates [28,29]. These factors have been identified as key factors influencing soil microbial characteristics in this region [30]. It is thus speculated that the structure of microbial communities within soil aggregates may undergo changes after the afforestation of former drylands, which have not been adequately addressed and needs empirical evidence for verification.
In this study, we investigated the variations in microbial composition, diversity, network complexity, and network stability across different soil aggregate fractions after 23 years of afforestation in a dryland of northwestern China. The impacts of afforestation on the physicochemical properties of the soil aggregates were also assessed to identify the driving factors of soil microbial communities at the aggregate scale. Our hypotheses were as follows: (i) afforestation would increase the diversity, network complexity, and network stability of microbial communities within soil aggregates; (ii) the responses of microbial communities to afforestation would differ between macroaggregates and microaggregates; and (iii) the contents of carbon, nitrogen, and phosphorus would be key factors mediating microbial communities within soil aggregates. The findings of our study can provide insight into the impacts of afforestation on soil microbial ecology across diverse microhabitats in semi-arid regions.
2. Materials and Methods
2.1. Study Area
The study site is located in the Yongdeng County of Gansu Province, northwestern China (36.72° N, 103.06° E, 2489 m above sea level). The area experiences a temperate continental climate, with a mean annual temperature of 5.9 °C. The mean annual precipitation, sunshine duration, and frost-free period are 290 mm, 2659 h, and 121 d, respectively. Dryland is one of the main land-use types in this area, covering an area of approximately 7.1 × 105 ha. As a transitional zone between the Loess Plateau and the Tibetan Plateau, this area has a hilly topography, characterized by a large number of loess hills and stony mountains. Therefore, soil erosion has been a longstanding, severe environmental issue, posing a threat to sustainable development in this area [31]. Since 2000, some drylands in Yongdeng County have undergone afforestation with shrubs (e.g., Caragana korshinskii) after the implementation of the “Grain for Green” project.
2.2. Field Sampling
In September 2023, a dryland and a C. korshinskii plantation in the study area were selected based on the space-for-time substitution approach. The C. korshinskii plantation was converted from dryland in 2000. The two land-use types are adjacent and are similar in soil type (Ustochnept, USDA soil taxonomy), soil texture (silt loam, USDA soil textural classification), and landform. Within each land-use type, we randomly established three 50 m × 50 m plots as pseudo-replications. This is a commonly used sampling design in restoration studies [32]. Three 1 m × 1 m quadrats were selected for soil sampling within each plot. Three intact soil samples (0 to 20 cm) were collected from each quadrat using a sterilized shovel and then mixed thoroughly to obtain a composite soil sample. A portion of the soil sample was passed through a sieve set (from up to down: 2 mm and 0.25 mm) after manual oscillation for 5 min to obtain three classes of soil aggregates: large macroaggregates (>2 mm), small macroaggregates (2–0.25 mm), and microaggregates (<0.25 mm) [33]. The screened aggregate samples were stored in cryogenic vials and immediately placed into liquid nitrogen for soil DNA extraction. The remaining soil samples were kept in an aluminum box to prevent the breakdown of soil aggregates during transportation. In the laboratory, the soil samples were passed through an 8 mm sieve to remove any visible plant debris, roots, and stones before measuring the distribution, stability, and chemical properties of the soil aggregates.
2.3. Determination of Distribution, Stability, and Chemical Properties of Soil Aggregates
A total of 200 g of air-dried soil was sieved according to the previously described procedure to determine the distribution and stability of the soil aggregates [33]. The screened aggregate samples were dried at 65 °C to obtain their dry weight. The mass proportion of each aggregate fraction was calculated using the following equation:
where ωi is the mass proportion of a specific aggregate fraction (%), mi is the dry weight of a specific aggregate fraction (g), and M is the total dry weight of the three aggregate fractions (g). Aggregate stability was indicated by the mean weight diameter (MWD) (mm), which was computed based on the following equation:
where xi is the average diameter of a specific aggregate fraction (mm).
The chemical properties of the soil aggregates, including their pH, total organic carbon (TOC), total nitrogen (TN), total phosphorus (TP), alkali-hydrolyzable nitrogen (AN), and available phosphorus (AP) were determined to explore the key factors regulating microbial community composition within each aggregate fraction. A PHS-3E pH meter (INESA Scientific Instrument Co., Ltd., Shanghai, China) was used to measure the soil pH at a 1:2.5 (w:v) soil to water ratio. The TOC content was determined according to the H2SO4-K2Cr2O7 oxidation method. The TN and AN contents were measured using the Kjeldahl method and the semi-micro-Kjeldahl digestion method, respectively. The TP and AP contents were analyzed according to the molybdate antimony anticolorimetric method [34].
2.4. DNA Extraction, PCR Amplification, and High-Throughput Sequencing
Soil DNA was extracted based on the CTAB method. We used 1% agarose gel to assess DNA concentration and quality. The obtained DNA was diluted to 1 ng·μL−1 using deionized water. The primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′) were utilized to amplify the V3-V4 region of the bacterial 16S rRNA genes, while the fungal ITS1 genes were amplified using the primers ITS5F (5′-GGAAGTAAAAGTC-GTAACAAGG-3′) and ITS2R (5′-GCTGCGTTCTTCAT-CGATGC-3′). The PCR amplification was conducted in a 15 µL reaction system, including 10 ng of template DNA, 0.2 µM of each primer, and Phusion® High-Fidelity PCR Master Mix (New England Biolabs Inc., Ipswich, MA, USA) under the following conditions: initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, extension at 72 °C for 30 s, and a final step at 72 °C for 5 min. The PCR products were mixed with 1X buffer, quantified by electrophoresis on 2% agarose gel, and purified before sequencing. A TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina Inc., San Diego, CA, USA) was used to generate the sequencing library according to the reference instructions. The samples were sequenced on an Illumina NovaSeq platform with a 250 bp paired-end model at Novogene Co., Ltd. in Beijing, China. Paired-end reads were assigned to samples using FLASH software (Version 1.2.11) after truncating the barcode and primer sequence. The denoising of the effective tags was performed using the DADA2 module in QIIME2 software (Version 202202) to obtain initial amplicon sequence variants (ASVs). The bacterial and fungal ASVs were annotated according to the Silva database and Unite database, respectively.
2.5. Data Analysis
We assessed the diversity of microbial communities within the soil aggregates by employing both alpha and beta metrics. Alpha diversity reflects the richness and evenness of species within a specific habitat or ecosystem, whereas beta diversity quantifies the dissimilarity in species composition across distinct habitats. In this study, the alpha diversity of microbial communities was indicated by the Shannon–Wiener and Pielou indices, both of which were calculated using the QIIME2 software. Principal coordinates analysis (PCoA) based on the Bray–Curtis distance matrix and Bray–Curtis dissimilarity were used to analyze the differences in the microbial beta diversity between land-use types and aggregate fractions. The differences in the mass proportion, chemical properties, and microbial characteristics between aggregate fractions were examined using one-way ANOVA. An independent-sample t-test was used to check the differences in the mass proportion, chemical properties, and microbial characteristics between land-use types. The interactions between the land-use type and aggregate fraction on the alpha diversity of microbial communities were quantified using two-way ANOVA. The significance level was set at p < 0.05. A Mantel test was performed to reveal the relationship between the microbial community composition and chemical properties for each aggregate fraction using the “linkET” R package (Version 0.0.7.4).
The cross-kingdom co-occurrence networks of bacterial and fungal taxa were constructed to assess the inter-taxa associations of microbial communities for each aggregate fraction. First, ASVs with a relative abundance of less than 0.01% and an occurrence frequency of less than 1/6 were removed. Second, the “corAndPvalue” function from the “WGCNA” R package (Version 1.72.5) was employed to calculate the Spearman correlation coefficient matrix of the filtered ASVs. The p values were adjusted using the “BH” method in the “p.adjust” function. Third, p values > 0.001 or |r| < 0.6 were excluded in the following analysis. Finally, the topological features of the microbial networks, including the number of nodes, number of edges, average degree, average path length, and modularity, were obtained using the “igraph” R package (Version 2.0.3) [35]. The co-occurrence networks of the microbial communities was visualized using Gephi software (Version 0.10.1). The stability of microbial networks was quantified by their weighted and unweighted robustness, which was calculated after randomly removing 50% of the taxa [14]. For each aggregate fraction, the difference in the robustness of the microbial networks between land-use types was tested using a two-sided t-test. All the statistical analyses were conducted using R software (Version 4.4.1).
3. Results
3.1. Distribution, Stability, and Chemical Properties of Soil Aggregates
As shown in Table 1, small macroaggregates (SM) were the main fraction of soil aggregate in both land-use types. There was no significant difference in the mass proportion between large macroaggregates (LM) and microaggregates (MI) in the dryland (p > 0.05), while in the C. korshinskii plantation, the mass proportion of MI was significantly higher compared to that of LM (p < 0.05). The mass proportions of both the LM and SM did not significantly differ between the two land-use types (p > 0.05). In contrast, the mass proportion of the MI significantly increased from 28.89% to 36.03% after land-use change (p < 0.05). Although afforestation induced a reduction in the MWD of the soil aggregates, no significant difference was observed between the two land-use types (p > 0.05).

Table 1.
Mass proportion (MP) and mean weight diameter (MWD) of soil aggregates in different land-use types. Values in parentheses are standard errors.
The soil pH increased with aggregate size, and the pH of the LM was significantly higher compared to that of the MI in both land-use types (p < 0.05). A significant reduction in the soil pH was detected in the MI following afforestation (p < 0.05). The soil TOC content decreased with aggregate size in the dryland, while it did not significantly differ between aggregate fractions in the C. korshinskii plantation (p > 0.05). The TOC content of the aggregates generally showed an increasing tendency after afforestation, especially for the LM and SM (p < 0.05). After 23 years of afforestation, only the TN content of the LM showed a significant increase (p < 0.05). In comparison, afforestation did not change the TP content in each aggregate fraction (p > 0.05). In the dryland, the C:N ratio of the aggregates decreased following the order: MI > LM > SM; there was a significant difference detected between the SM and MI (p < 0.05). By contrast, there was no significant difference in the C:N ratio between aggregate fractions in the C. korshinskii plantation (p > 0.05). Only the C:N ratio of the SM showed a significant variation after afforestation (p < 0.05). The AN content was generally low in our study site, varying between 1.07 mg·kg−1 and 4.98 mg·kg−1 across land-use types and aggregate fractions. Similarly to the TOC content, the AN level of the two macroaggregate fractions (LM and SM) was significantly improved by afforestation (p < 0.05). On the contrary, the AP content of the aggregates generally showed a decreasing trend after the conversion of land-use type, particularly for the SM (p < 0.05) (Table 2).

Table 2.
Chemical properties of soil aggregates in different land-use types. Values in the parentheses are standard errors.
3.2. Composition of Microbial Communities Within Soil Aggregates
In both land-use types, Actinobacteriota was the dominant bacterial phyla across all aggregate fractions, exhibiting a relative abundance that ranged from 25.92% to 44.90%. Gemmatimonadota, Chloroflexi, Acidobacteriota, and Proteobacteria were the other four important phyla of soil bacterial communities in both land-use types (Figure 1a). After afforestation, the relative abundances of Actinobacteriota and Chloroflexi within the aggregates showed increasing trends, while that of Gemmatimonadota decreased (p < 0.05). In contrast, afforestation had a minimal impact on the relative abundances of Acidobacteriota and Proteobacteria (p > 0.05) (Figure 1c). At the phyla level, the fungal communities within the aggregates were dominated by Ascomycota and Mortierellomycota in both land-use types (Figure 1b). The relative abundance of Ascomycota decreased with aggregate size, whereas that of Basidiomycota showed an increasing trend. Afforestation enhanced the relative abundance of Ascomycota within the LM (p < 0.01) and the relative abundance of Mortierellomycota across all aggregate fractions (p < 0.05). Although land-use change decreased the relative abundance of Basidiomycota by 21.49–66.04% across different aggregate fractions, such variations were not statistically significant (Figure 1d).
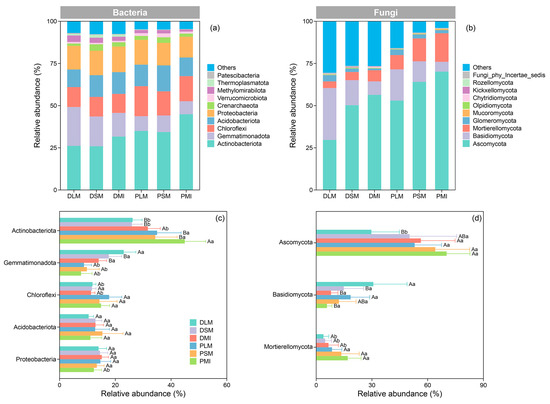
Figure 1.
The characteristics of microbial community compositions ((a,c): bacteria; (b,d): fungi) within the soil aggregates of different land-use types. Different uppercase letters indicate significant differences among aggregate fractions within a specific land-use type, whereas different lowercase letters denote significant differences between land-use types within a specific aggregate fraction. DLM, DSM, and DMI represent large macroaggregates, small macroaggregates, and microaggregates in the dryland, respectively, while PLM, PSM, and PMI represent large macroaggregates, small macroaggregates, and microaggregates in the C. korshinskii plantation, respectively.
3.3. Diversity of Microbial Communities Within Soil Aggregates
In the dryland, the Shannon–Wiener and Pielou indices for bacterial communities were significantly lower within the LM than within the other two aggregate fractions (p < 0.05). However, they did not exhibit significant differences among aggregate fractions in the C. korshinskii plantation (p > 0.05). Land-use change had a minimal impact on bacterial alpha diversity across all aggregate fractions (Figure 2a,b). In the dryland, fungal alpha diversity generally increased with aggregate size, and a significant difference was observed between the LM and MI (p < 0.05). By contrast, the alpha diversity of fungal communities did not significantly vary among aggregate fractions in the C. korshinskii plantation (p > 0.05). After 23 years of afforestation, the fungal alpha diversity showed an increasing trend within both the LM and SM (p < 0.05) but exhibited no significant change within the MI (p > 0.05). In addition, the alpha diversity of both bacterial and fungal communities was significantly influenced by the interaction between the land-use type and aggregate fraction (p < 0.05) (Figure 2c,d).
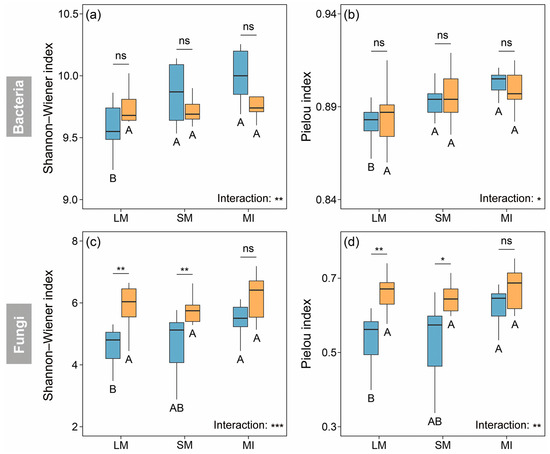
Figure 2.
Shannon–Wiener and Pielou indices of bacterial (a,b) and fungal communities (c,d) within soil aggregates of different land-use types. Different uppercase letters denote significant differences among aggregate fractions, while asterisks indicate significant differences between land-use types. * p < 0.05; ** p < 0.01; *** p < 0.001; ns, non-significant.
The results of PCoA revealed that the beta diversity of both bacterial and fungal communities was mainly affected by the land-use type, rather than the aggregate fraction (Figure 3a,b). Based on the Bray–Curtis dissimilarity, we detected that the bacterial beta diversity within the SM significantly differed from that within the other two aggregate fractions in the C. korshinskii plantation (p < 0.05). By contrast, the beta diversity of the fungal communities differed significantly between the MI and the other two macroaggregate fractions (p < 0.05). Afforestation significantly altered the beta diversity of bacterial communities within the LM and MI (p < 0.05), whereas it did not change the beta diversity of fungal communities across all the aggregate fractions (p > 0.05) (Figure 3c,d).
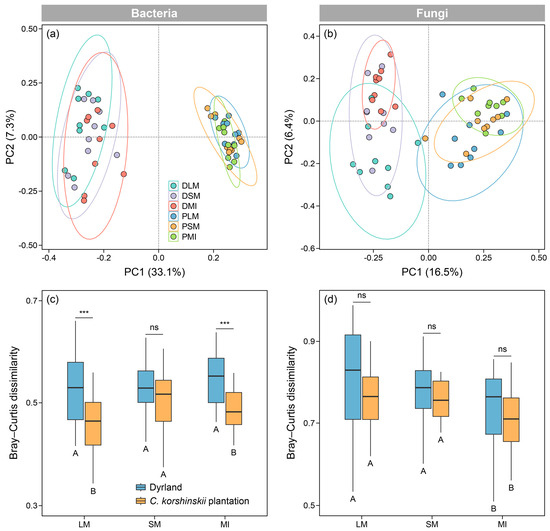
Figure 3.
Beta diversity of bacterial (a,c) and fungal communities (b,d) within soil aggregates of different land-use types as revealed by principal coordinates analysis and Bray–Curtis dissimilarity. DLM, DSM, and DMI denote large macroaggregates, small macroaggregates, and microaggregates in dryland, respectively, while PLM, PSM, and PMI represent large macroaggregates, small macroaggregates, and microaggregates in C. korshinskii plantation, respectively. Different uppercase letters indicate significant differences between aggregate fractions, while asterisks indicate significant differences between land-use types. *** p < 0.001; ns, non-significant.
3.4. Characteristics of Microbial Co-Occurrence Networks Within Soil Aggregates
In the dryland, the microbial networks within the LM had a higher number of nodes and edges compared to those within the other two aggregate fractions. By contrast, in the C. korshinskii plantation, the number of nodes and edges of the microbial networks decreased in the following order: SM > MI > LM. Among the three aggregate fractions, the highest average degree of the microbial networks was detected within the LM in the dryland and within the MI in the C. korshinskii plantation. Similar trends were observed for the average path length of the microbial networks. The modularity of the microbial networks exhibited low variability across the land-use types and aggregate fractions. After afforestation, the number of nodes and edges of the microbial networks decreased by 5.96–52.79% and 17.50–76.13%, respectively, across different aggregate fractions. The average degree of the microbial networks also showed a decreasing trend after land-use change for each aggregate fraction (Table 3). As illustrated in Figure 4, the microbial networks in the dryland had more modules compared to those in the C. korshinskii plantation across all three aggregate fractions, especially within the LM.

Table 3.
Cross-kingdom network topological features of microbial taxa within soil aggregates of different land-use types.
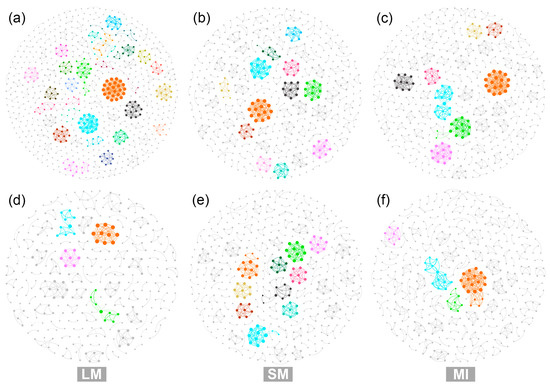
Figure 4.
Cross-kingdom co-occurrence networks of microbial taxa within soil aggregates of different land-use types ((a–c): dryland; (d–f): C. korshinskii plantation). Different colors represent different modules, and modules with <5 nodes are represented in gray. LM, SM, and MI represent large macroaggregates, small macroaggregates, and microaggregates, respectively.
Across different land-use types and aggregate fractions, the weighted robustness of the microbial networks varied between 0.217 and 0.291, which was generally lower than the unweighted robustness (0.341 to 0.380). In the dryland, the robustness of the microbial networks increased with aggregate size, whereas an opposite trend was observed in the C. korshinskii plantation. After afforestation, the weighted robustness of the microbial networks within the LM significantly decreased by 34.10% (p < 0.001). Meanwhile, the unweighted robustness of the microbial networks within both the LM and SM also showed a decreasing trend (p < 0.05). In contrast, although the robustness of the microbial networks within the MI decreased after afforestation, such variations were not statistically significant (p > 0.05) (Figure 5).
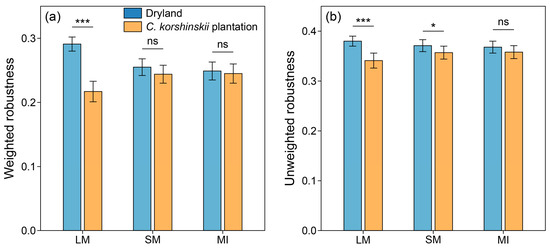
Figure 5.
Weighted (a) and unweighted robustness (b) of microbial cross-kingdom co-occurrence networks within the soil aggregates of different land-use types. Error bars represent the standard deviations of the mean. * p < 0.05; *** p < 0.001; ns, non-significant.
3.5. Factors Influencing Microbial Communities Within Soil Aggregates
The results of the Mantel test showed that both bacterial and fungal compositions within the LM were positively correlated with the TOC content, AN content, and AP content (p < 0.05). The bacterial composition within the LM was also positively related to the mass proportion of aggregates and TN content (p < 0.05). The bacterial composition within the SM was positively correlated to the soil pH and the contents of TOC, AN, and AP (p < 0.05), whereas that within the MI showed a significant positive correlation with the TN content (p < 0.05). However, there was no significant relationship between the fungal composition and chemical properties for both the SM and MI (p > 0.05). Furthermore, we observed that the microbial composition across all three aggregate fractions was positively associated with the alpha diversity of the microbial communities (p < 0.05) (Figure 6).
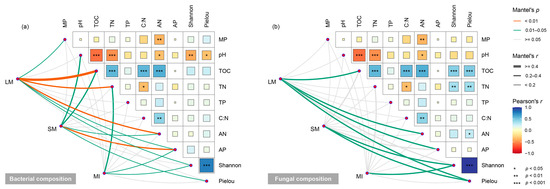
Figure 6.
Factors that influenced the compositions of bacterial (a) and fungal communities (b) within the soil aggregates as revealed by the Mantel test. LM, large macroaggregates; SM, small macroaggregates; MI, microaggregates; MP, mass proportion; TOC, total organic carbon; TN, total nitrogen; TP, total phosphorus; C:N, carbon to nitrogen ratio; AN, alkali-hydrolyzable nitrogen; AP, available phosphorus. * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Discussion
4.1. Impacts of Afforestation on Distribution, Stability, and Chemical Properties of Soil Aggregates
Using the dry-sieving approach, we found that the afforestation of the former dryland did not alter the mass proportion of the two macroaggregates, while it significantly increased that of the MI by 24.71%. However, the changed aggregate distribution had a minimal impact on the aggregate stability (Table 1). The results imply that the conversion of the dryland to a C. korshinskii plantation did not contribute to soil aggregation. In general, the formation of soil aggregates is primarily regulated by binding agents, which can be divided into organic and inorganic forms. The organic binding agents of soil aggregates mainly consist of roots, hyphae, and soil organic matter, whereas the major inorganic binding agents include clay particles, polyvalent metal cations, oxides, and hydroxides of Fe and Al [36]. In the present study, afforestation led to a significant increase in the TOC content for both the LM and SM (Table 2), suggesting an accumulation of soil organic matter. A well-developed root system could also be expected in the C. korshinskii plantation [37]. However, such increases did not lead to an improved soil aggregate stability, suggesting that organic binding agents played a limited role in soil aggregate dynamics. Our findings contradict those of most previous studies, which have indicated that the afforestation of former arable lands improve soil aggregate stability by promoting the formation of macroaggregates [23,38]. In southwestern China, Guo et al. employed the dry-sieving method to separate soil aggregates and observed that the afforestation of former arable land did not alter soil aggregate stability, which was positively correlated with the contents of Fe/Al oxides [39]. Consequently, variations in inorganic binding agents should be paid more attention in further studies to better understand the controlling factors of soil aggregate stability in the context of afforestation.
We found that the chemical properties were relatively stable within the MI after the 23 years of afforestation. By comparison, afforestation was beneficial to C and N accumulations within both the LM and SM (Table 2). This is due to the fact that macroaggregates mainly store labile organic matter [20], which can be derived from the litter and roots of C. korshinskii after afforestation [40]. The increased soil microbial residues may be another reason for the accrual of C and N in the macroaggregates, as empirical evidence has shown that microbially derived organic matter is usually associated with soil mineral particles [41,42], which have small sizes and can be incorporated within macroaggregates [22]. However, the relative importance of these two potential mechanisms in the sequestration of C and N within macroaggregates remains unclear and should be quantified in the future. Similarly to our results, some studies have also pointed out that the afforestation of former arable lands increased C and N contents within macroaggregates [28,43]. These findings have demonstrated the key role of macroaggregates in soil C and N sequestration after afforestation. In contrast, afforestation did not change the TP content across all the aggregate fractions (Table 2). These results are in agreement with those of a previous study, supporting the statement that the soil P pool is generally stable due to the relatively fixed sources in semi-arid regions [28]. Furthermore, we detected that nutrient availability within the macroaggregates was also influenced by afforestation. In particular, the AN and AP contents within the macroaggregates showed an increasing and a decreasing trend after the 23 years of afforestation, respectively (Table 2). The dynamics of the soil AP are in agreement with the global response of soil AP to the afforestation of arable lands [44]. Our observations imply that P may be a major limiting nutrient in the study area, given its low availability.
4.2. Impacts of Afforestation on Composition and Diversity of Microbial Communities Within Soil Aggregates
The variations in the microbial relative abundance at the phyla level suggest that the microbial community composition within the soil aggregates was altered by afforestation (Figure 1). For bacterial communities, afforestation improved the predominance of Actinobacteriota across all three aggregate fractions. It also enhanced the relative abundance of Chloroflexi, whereas it decreased that of Gemmatimonadota at the aggregate scale (Figure 1c). Actinobacteriota and Chloroflexi are typically considered oligotrophic bacteria because of their high viability under resource-limited conditions [45,46]. By contrast, Gemmatimonadota prefers nutrient-rich soils and is often classified into a copiotrophic group [47]. However, there is growing evidence that some microorganisms belonging to a given phyla should not be strictly assigned into oligotrophic or copiotrophic categories due to significant taxonomic variation [48,49]. This explains why the relative abundance of Actinobacteriota and Chloroflexi increased, while that of Gemmatimonadota decreased, with the C and N contents in our study. Similar variations in bacterial abundance have also been reported after the afforestation of former arable lands in sub-humid regions [17,47]. The changes in the relative abundance of fungal communities suggest that Ascomycota became more dominant across all aggregate fractions following afforestation (Figure 1b). These findings align with previous studies, which have shown that the soils of the Loess Plateau feature Ascomycota as the predominant fungal phyla [50,51]. This prevalence is attributed to the fact that Ascomycota can survive in environments with limited resources [50]. Compared to the dryland, the C. korshinskii plantation may provide a greater amount of substrates for the Ascomycota through litter return and root deposition. Such variations are also responsible for the increased abundance of Mortierellomycota, as empirical evidence has suggested that this group prefers nutrient-rich soils [52]. In addition to the impacts of afforestation, we observed that the predominant phyla of both the bacterial and fungal communities were significantly affected by aggregate size (Figure 1). Specifically, Actinobacteriota and Ascomycota were more prevalent within the MI than within the LM and SM. The observations partly support our second hypothesis. Liu et al. also pointed out that the relative abundance of Ascomycota decreased with aggregate size in plantations that had been converted from natural forests in tropical regions [53]. This can possibly be attributed to the fact that the substrates within MI are physically and chemically protected [23], which restricts the growth of microbial taxa with a low abundance [54]. The high resistance to external changes in MI may also form a stable environment that facilitates the expansion of predominant phyla [54]. Nevertheless, these speculations remain untested and require empirical verification in future studies.
Based on the Shannon–Wiener and Pielou indices of soil microbial communities, we observed that the impact of afforestation on microbial alpha diversity varied depending on the microbial kingdom and aggregate size. The conversion from dryland to a C. korshinskii plantation did not affect the alpha diversity of bacterial communities across all three aggregate fractions, but it significantly increased the alpha diversity of fungal communities within the two macroaggregate fractions (Figure 2). The observations partly confirm our first and second hypotheses. The increased fungal alpha diversity within the LM and SM might be attributed to the fact that afforestation provided more labile substrates for a diverse range of fungal species by promoting the accrual of TOC, TN, and AN contents within the macroaggregates. The results support our third hypothesis that soil C and nutrients would be key factors mediating microbial communities within the soil aggregates. As an important binding agent for the macroaggregates, the extensive root system in the C. korshinskii plantation could also have influenced the fungal alpha diversity within the LM and SM through multiple pathways, such as root deposition and soil pH regulation [8,55]. In the dryland, the alpha diversity of both the bacterial and fungal communities generally increased with the decrease in aggregate size (Figure 2). This is possibly ascribed to the fact that agricultural activities (e.g., tillage and seeding) accelerated the turnover of the soil aggregates. Due to their high stability under external disturbances, MI might serve as more suitable microhabitats for microbial metabolism compared to macroaggregates [18,19]. Since the alpha diversity of the microbial communities was also significantly affected by the interaction between the land-use type and aggregate fraction, we suggest that dividing bulk soil into different aggregate fractions can be a promising way to further assess the impact of afforestation on belowground biodiversity. In a recent study, Zhang et al. found a decrease in the alpha diversity of bacterial communities within soil aggregates after the afforestation of former dryland [56]. Furthermore, they pointed out that the bacterial alpha diversity remained statistically consistent across aggregate fractions in both the dryland and planted forests [56]. By contrast, a study conducted in subtropical China observed that the alpha diversity of bacterial communities decreased as aggregate size increased, while fungal communities showed minimal responses to aggregate size in a 30-year-old planted forest [57]. These conflicting findings suggest that the impact of afforestation on microbial alpha diversity within soils is complex.
As indicated by the Bray–Curtis dissimilarities, we found that afforestation significantly altered the bacterial beta diversity within the LM and MI, whereas it had no significant impact on the fungal beta diversity across all three aggregate fractions (Figure 3). Since the bacterial beta diversity did not significantly differ between aggregate fractions in the dryland, such variations indicate that afforestation enhanced the spatial heterogeneity of bacterial diversity within the soils. A possible explanation is the high mobility of soil bacteria, which allows them to select habitats in a changing environment [58]. This assertion is partly supported by previous studies [53,59]. For instance, Li et al. observed considerable changes in bacterial beta diversity at the aggregate level after land-use conversion [59]. Liu et al. reported that land-use type, rather than aggregate size, was the main influencing factor of soil fungal beta diversity [53]. Our results again serve to emphasize that soil aggregates can act as microhabitats for soil microorganisms.
4.3. Impacts of Afforestation on Complexity and Stability of Microbial Co-Occurrence Networks Within Soil Aggregates
In this study, the complexity of the microbial networks was higher within the LM than within the other two aggregate fractions in the dryland. However, afforestation reduced the complexity of the microbial networks across all aggregate fractions, especially within the LM (Table 3). Hence, the microbial network complexity within the LM was the lowest among the three aggregate fractions in the C. korshinskii plantation. Our findings suggest that afforestation weakened the connections and interactions within the microbiome at the aggregate scale. Similarly, recent studies conducted in various regions in China also found a reduction in the complexity of soil microbial communities following afforestation [11,16]. A likely reason is that the litter and root deposition from C. korshinskii enriched the microbial substrates, thereby squeezing the ecological niche of the microbial communities by accelerating the growth of dominant microorganisms [16,60]. As a consequence, the death or dormancy of the less competitive microorganisms might have reduced the number of nodes, the number of edges, and the average degree of the microbial communities [16]. The significant increases in the TOC, TN, and AN contents within the LM partially support this speculation (Table 2). Due to their large size, LM typically exhibit a more rapid turnover rate than the other two aggregate fractions [61]. Given that the C. korshinskii plantation experienced fewer external disturbances than the dryland, a decreased turnover of LM could be anticipated after long-term afforestation [22,62]. This variation might have diminished the connections and interactions of microbial taxa between the LM and the other soil structural units, subsequently reducing the microbial network complexity within the LM.
Employing both weighted and unweighted robustness as indicators, we found that the 23 years of afforestation also weakened the stability of microbial networks within the two macroaggregate fractions (Figure 5). This result implies that the complexity and stability of the microbial networks showed a synergistic response to afforestation within the macroaggregates. Our findings align with MacArthur’s theory that ecosystem complexity fosters stability [63], yet they contrast with the theoretical analysis suggesting that increased complexity destabilizes ecosystems [64]. The detected reduction in the complexity and stability of microbial networks within the macroaggregates indicates that afforestation might lead to the instability or collapse of the microbial food web, potentially exerting a negative impact on soil multifunctionality [15]. On the Loess Plateau, Kong et al. observed that soil desiccation was a likely reason for the reduced stability of bacterial networks in deep soils after the afforestation of former dryland [11]. On the contrary, Zhang et al. pointed out that improved soil nutrient and water conditions after afforestation contributed to increased bacterial network stability within soil aggregates [56]. However, these studies did not consider microbial interactions across different kingdoms. Since the soil microbiome encompasses a diverse array of microorganisms, such as bacteria, fungi, archaea, and protozoa, we recommend incorporating more kingdoms into microbial co-occurrence network analysis to obtain a deeper understanding of inter-taxa associations within soil microbiome in future studies.
5. Conclusions
Our results showed that the responses of microbial composition, diversity, network complexity, and network stability to the afforestation of former dryland differed between aggregate sizes. Afforestation increased the relative abundances of dominant bacterial and fungal phyla across all aggregate fractions. The alpha diversity of the bacterial communities remained unchanged after afforestation, while fungal alpha diversity significantly increased within the two macroaggregate fractions. In contrast, fungal beta diversity within the soil aggregates showed a minimal response to afforestation, whereas bacterial beta diversity within the LM and MI significantly altered following afforestation. The number of nodes, number of edges, and average degree of the cross-kingdom microbial co-occurrence networks generally exhibited a decreasing trend post-afforestation, indicating a simplification of microbial community structure. The reduced robustness of microbial networks within the LM and SM implies that afforestation also destabilized the stability of microbial communities within the macroaggregates. The shifted soil microbial communities were associated with changes in the soil C and N contents, especially in the two macroaggregate fractions. Our findings suggest that dividing bulk soil into different aggregate fractions is a promising way to better understand the dynamics of soil microbial ecology in the context of land-use conversion.
Author Contributions
Conceptualization, L.B. and X.L.; methodology, D.Z. and W.W.; software, Y.W., Q.Y. and Y.Z.; validation, Y.W. and Q.Y.; formal analysis, Y.W., T.C. and S.K.; investigation, Y.W., T.C. and H.C.; resources, D.Z., L.B. and W.W.; data curation, S.K. and Y.Z.; writing—original draft preparation, D.Z. and L.B.; writing—review and editing, W.W. and X.L.; visualization, L.B. and Q.Y.; supervision, X.L.; project administration, L.B. and W.W.; funding acquisition, D.Z. and W.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Geological Survey Project (Grant No. DD20230523).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors are grateful to Ping Yue from the Northwest Institute of Eco-Environment and Resources of Chinese Academy of Sciences for his assistance during the field survey.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO. Global Forest Resources Assessment 2020: Main Report; FAO: Rome, Italy, 2020. [Google Scholar]
- La Manna, L.; Tarabini, M.; Gomez, F.; Rostagno, C.M. Changes in soil organic matter associated with afforestation affect erosion processes: The case of erodible volcanic soils from Patagonia. Geoderma 2021, 403, 115265. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Llena, M.; Cortijos-López, M.; Lasanta, T. Afforestation after land abandonment as a nature-based solution in Mediterranean mid-mountain areas: Implications and research gaps. Curr. Opin. Environ. Sci. Health 2023, 34, 100481. [Google Scholar] [CrossRef]
- Guo, Y.; Abdalla, M.; Espenberg, M.; Hastings, A.; Hallett, P.; Smith, P. A systematic analysis and review of the impacts of afforestation on soil quality indicators as modified by climate zone, forest type and age. Sci. Total Environ. 2021, 757, 143824. [Google Scholar] [CrossRef]
- Rong, G.; Zhang, X.; Wu, H.; Ge, N.; Yao, Y.; Wei, X. Changes in soil organic carbon and nitrogen mineralization and their temperature sensitivity in response to afforestation across China’s Loess Plateau. Catena 2021, 202, 105226. [Google Scholar] [CrossRef]
- Kumar, N.; Khamzina, A.; Knöfel, P.; Lamers, J.P.A.; Tischbein, B. Afforestation of degraded croplands as a water-saving option in irrigated region of the aral sea basin. Water 2021, 13, 1433. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617. [Google Scholar] [CrossRef]
- Basu, S.; Kumar, G.; Chhabra, S.; Prasad, R. Role of soil microbes in biogeochemical cycle for enhancing soil fertility. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 149–157. [Google Scholar]
- Liu, J.; Le, T.H.; Zhu, H.; Yao, Y.; Zhu, H.; Cao, Y.; Zhao, Z. Afforestation of cropland fundamentally alters the soil fungal community. Plant Soil 2020, 457, 279–292. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Fan, X.D.; Ren, C.J.; Zhang, L.; Han, X.H.; Yang, G.H.; Wang, J.; Doughty, R. Changes of the organic carbon content and stability of soil aggregates affected by soil bacterial community after afforestation. Catena 2018, 171, 622–631. [Google Scholar] [CrossRef]
- Kong, W.; Wei, X.; Wu, Y.; Shao, M.; Zhang, Q.; Sadowsky, M.J.; Ishii, S.; Reich, P.B.; Wei, G.; Jiao, S.; et al. Afforestation can lower microbial diversity and functionality in deep soil layers in a semiarid region. Glob. Chang. Biol. 2022, 28, 6086–6101. [Google Scholar] [CrossRef] [PubMed]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Matchado, M.S.; Lauber, M.; Reitmeier, S.; Kacprowski, T.; Baumbach, J.; Haller, D.; List, M. Network analysis methods for studying microbial communities: A mini review. Comput. Struct. Biotechnol. J. 2021, 19, 2687–2698. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.M.; Guo, X.; Wu, L.; Zhang, Y.; Xiao, N.; Ning, D.; Shi, Z.; Zhou, X.; Wu, L.; Yang, Y.; et al. Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 2021, 11, 343–348. [Google Scholar] [CrossRef]
- Yang, Y.; Chai, Y.; Xie, H.; Zhang, L.; Zhang, Z.; Yang, X.; Hao, S.; Gai, J.; Chen, Y. Responses of soil microbial diversity, network complexity and multifunctionality to three land-use changes. Sci. Total Environ. 2023, 859, 160255. [Google Scholar] [CrossRef]
- Yu, P.; Tang, H.; Sun, X.; Shi, W.; Pan, J.; Liu, S.; Jia, H.; Ding, Z.; Tang, X.; Chen, M. Afforestation alters soil microbial community composition and reduces microbial network complexity in a karst region of Southwest China. Land Degrad. Dev. 2024, 35, 2926–2939. [Google Scholar] [CrossRef]
- Wang, M.; Masoudi, A.; Wang, C.; Yang, J.; Zhai, Y.; Wu, C.; Yu, Z.; Liu, J. Plantation type and afforestation age disclose variable influences on soil microbial compositions in man-made forests in the Xiong’an New Area, China. Land Degrad. Dev. 2022, 33, 3058–3073. [Google Scholar] [CrossRef]
- Upton, R.N.; Bach, E.M.; Hofmockel, K.S. Spatio-temporal microbial community dynamics within soil aggregates. Soil Biol. Biochem. 2019, 132, 58–68. [Google Scholar] [CrossRef]
- Bach, E.M.; Williams, R.J.; Hargreaves, S.; Yang, F.; Hofmockel, K.S. Greatest soil microbial diversity found in micro-habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Mechanisms of carbon sequestration in soil aggregates. Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Sey, B.K.; Manceur, A.M.; Whalen, J.K.; Gregorichm, E.G.; Rochette, P. Small-scale heterogeneity in carbon dioxide, nitrous oxide and methane production from aggregates of a cultivated sandy-loam soil. Soil Biol. Biochem. 2008, 40, 2468–2473. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Yu, P.; Liu, J.; Tang, H.; Ci, E.; Tang, X.; Liu, S.; Ding, Z.; Ma, M. The increased soil aggregate stability and aggregate-associated carbon by farmland use change in a karst region of Southwest China. Catena 2023, 231, 107284. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Q.; Wang, H.; Wang, W.; Zhong, Z.; Di, G. Effects of poplar shelterbelt plantations on soil aggregate distribution and organic carbon in northeastern China. Forests 2022, 13, 1546. [Google Scholar] [CrossRef]
- Stewart, B.A.; Thapa, S. Dryland farming: Concept, origin and brief history. In Innovations in Dryland Agriculture; Farooq, M., Siddique, K.H.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 3–29. [Google Scholar]
- Zhuo, Z.; Chen, Q.; Zhang, X.; Chen, S.; Gou, Y.; Sun, Z.; Huang, Y.; Shi, Z. Soil organic carbon storage, distribution, and influencing factors at different depths in the dryland farming regions of Northeast and North China. Catena 2022, 210, 105934. [Google Scholar] [CrossRef]
- Li, P.; Chen, J.; Zhao, G.; Holden, J.; Liu, B.; Chan, F.K.S.; Hu, J.; Wu, P.; Mu, X. Determining the drivers and rates of soil erosion on the Loess Plateau since 1901. Sci. Total Environ. 2022, 823, 153674. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Liu, X.; Xiao, L.; Shi, P.; Zhao, B. Effects of farmland conversion on the stoichiometry of carbon, nitrogen, and phosphorus in soil aggregates on the Loess Plateau of China. Geoderma 2019, 351, 188–196. [Google Scholar] [CrossRef]
- Rong, G.; Li, W.; Zhu, H.; Zhou, J.; Qiu, L.; Ge, N.; Wei, X.; Shao, M. Dynamics of new- and old-organic carbon and nitrogen in bulk soils and aggregates following afforestation on farmland. Catena 2020, 195, 104838. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, G.B.; Zhang, J.Y.; Xue, S. Long-term effects of vegetational restoration on soil microbial communities on the Loess Plateau of China. Restor. Ecol. 2016, 24, 794–804. [Google Scholar] [CrossRef]
- Adingo, S.; Yu, J.-R.; Liu, X.; Jing, S.; Li, X.; Zhang, X. Land-use change influence soil quality parameters at an ecologically fragile area of YongDeng County of Gansu Province, China. PeerJ 2021, 9, e12246. [Google Scholar] [CrossRef]
- Ju, W.; Fang, L.; Shen, G.; Delgado-Baquerizo, M.; Chen, J.; Zhou, G.; Ma, D.; Bing, H.; Liu, L.; Liu, J.; et al. New perspectives on microbiome and nutrient sequestration in soil aggregates during long-term grazing exclusion. Glob. Chang. Biol. 2024, 30, e17027. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, J.; Yang, L.; Gunina, A.; Yang, Y.; Peixoto, L.; Zeng, Z.; Zang, H.; Kuzyakov, Y. Diversified cropping systems benefit soil carbon and nitrogen stocks by increasing aggregate stability: Results of three fractionation methods. Sci. Total Environ. 2022, 824, 153878. [Google Scholar] [CrossRef]
- Liu, X.; Ma, J.; Ma, Z.W.; Li, L.H. Soil nutrient contents and stoichiometry as affected by land-use in an agro-pastoral region of northwest China. Catena 2017, 150, 146–153. [Google Scholar] [CrossRef]
- Li, X.W.; Li, X.L.; Shi, Y.; Zhao, S.J.; Liu, J.L.; Lin, Y.Y.; Li, C.L.; Zhang, C.H. Effects of microtopography on soil microbial communities in alpine meadows on the Qinghai-Tibetan Plateau. Catena 2024, 239, 107945. [Google Scholar] [CrossRef]
- Amézketa, E. Soil Aggregate Stability: A Review. J. Sustain. Agric. 1999, 14, 83–151. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, W.W.; Wang, G.; Yang, S.Q.; Pereira, P. Effects of long-term afforestation and natural grassland recovery on soil properties and quality in Loess Plateau (China). Sci. Total Environ. 2021, 770, 144833. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, S.; Lu, X.; Ren, Z.; Wu, Q.; Xu, M.; Ren, C.; Yang, G.; Han, X. Organic carbon, nitrogen accumulation, and soil aggregate dynamics as affected by vegetation restoration patterns in the Loess Plateau of China. Catena 2021, 196, 104867. [Google Scholar] [CrossRef]
- Guo, L.K.; Shen, J.; Li, B.; Li, Q.Q.; Wang, C.Q.; Guan, Y.; D’Acqui, L.P.; Luo, Y.L.; Tao, Q.; Xu, Q.; et al. Impacts of Agricultural Land Use Change on Soil Aggregate Stability and Physical Protection of Organic C. Sci. Total Environ. 2020, 707, 136049. [Google Scholar] [CrossRef]
- Han, X.; Zhao, F.; Tong, X.; Deng, J.; Yang, G.; Chen, L.; Kang, D. Understanding soil carbon sequestration following the afforestation of former arable land by physical fractionation. Catena 2017, 150, 317–327. [Google Scholar] [CrossRef]
- Creamer, C.A.; Foster, A.L.; Lawrence, C.; McFarland, J.; Schulz, M.; Waldrop, M.P. Mineralogy dictates the initial mechanism of microbial necromass association. Geochim. Cosmochim. Acta 2019, 260, 161–176. [Google Scholar] [CrossRef]
- Xuan, M.; Ai, L.; Wu, F.; Zhang, X.; Ni, X. Biomarkers evidence shows a preferential occlusion of microbial necromass in mineral-associated and not particle organic matter. Geoderma 2024, 450, 117030. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, Q.; Wan, Y.; Yang, R.; Mou, J.; Li, Y.; Meng, L.; Zhu, T.; Elrys, A.S. Afforestation improves soil organic carbon and total nitrogen stocks mainly through increasing >2 mm aggregate fractions and stimulating carbon and nitrogen transformations within aggregates in subtropical karst region. Catena 2024, 243, 108220. [Google Scholar] [CrossRef]
- Deng, Q.; McMahon, D.E.; Xiang, Y.; Yu, C.-L.; Jackson, R.B.; Hui, D. A global meta-analysis of soil phosphorus dynamics after afforestation. New Phytol. 2017, 213, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Dragone, N.B.; Hoffert, M.; Strickland, M.S.; Fierer, N. The taxonomic and genomic attributes of oligotrophic soil bacteria. ISME Commun. 2024, 4, ycae081. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, A.C.H.; Hartmann, M.; Conz, R.F.; Six, J.; Solly, E.F. Prolonged water limitation shifts the soil microbiome from copiotrophic to oligotrophic lifestyles in Scots pine mesocosms. Environ. Microbiol. Rep. 2024, 16, e13211. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.C.; Wang, S.S.; Wang, J.X.; Qi, X.; Long, Q.X.; Huang, M.Z. The Shift of Soil Bacterial Community After Afforestation Influence Soil Organic Carbon and Aggregate Stability in Karst Region. Front. Microbiol. 2022, 13, 901126. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.; Di Lonardo, D.P.; Bodelier, P.L. Revisiting life strategy concepts in environmental microbial ecology. FEMS Microbiol. Ecol. 2017, 93, fix006. [Google Scholar] [CrossRef]
- He, B.; Li, Q.; Zou, S.; Bai, X.; Li, W.; Chen, Y. Dynamic changes of soil microbial communities during the afforestation of Pinus armandii in a karst region of Southwest China. Microb. Ecol. 2024, 87, 36. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.; Shan, Y.; Lu, X.; Cao, J. Effects of land-use patterns on soil microbial diversity and composition in the Loess Plateau, China. J. Arid. Land 2024, 16, 415–430. [Google Scholar] [CrossRef]
- Zeng, Q.; Liu, Y.; Xiao, L.; An, S. Climate and soil properties regulate soil fungal communities on the Loess Plateau. Pedobiologia 2020, 81, 150668. [Google Scholar] [CrossRef]
- Chen, W.; Yu, T.; Zhao, C.; Li, B.; Qin, Y.; Li, H.; Zhang, X. Development and determinants of topsoil bacterial and fungal communities of afforestation by aerial sowing in Tengger Desert, China. J. Fungi 2023, 9, 399. [Google Scholar] [CrossRef]
- Liu, C.G.; Jin, Y.Q.; Lin, F.M.; Jiang, C.; Zeng, X.L.; Feng, D.F.; Huang, F.Z.; Tang, J.W. Land use change alters carbon and nitrogen dynamics mediated by fungal functional guilds within soil aggregates. Sci. Total Environ. 2023, 902, 166080. [Google Scholar] [CrossRef]
- Trivedi, P.; Rochester, I.J.; Trivedi, C.; Van Nostrand, J.D.; Zhou, J.; Karunaratne, S.; Anderson, I.C.; Singh, B.K. Soil aggregate size mediates the impacts of cropping regimes on soil carbon and microbial communities. Soil Biol. Biochem. 2015, 91, 169–181. [Google Scholar] [CrossRef]
- Huang, K.; Guo, Z.; Zhao, W.; Song, C.; Wang, H.; Li, J.; Mumin, R.; Sun, Y.; Cui, B. Response of fungal communities to afforestation and its indication for forest restoration. For. Ecosyst. 2023, 10, 100125. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, H.; Liang, Y.; Liang, Y.; Zhao, Y.; Wang, Z.; Li, Y.; Liu, W.; Wang, X.; Yang, G.; et al. The Multifunctionality of Soil Aggregates Is Related to the Complexity of Aggregate Microbial Community during Afforestation. Catena 2024, 236, 107737. [Google Scholar] [CrossRef]
- Chen, P.; Wang, L.; Li, J.-Y.; Wan, W.; Zhang, R.-Q.; Liu, Y.; Li, Z.-G. Response of Soil Aggregate-Associated Fertility and Microbial Communities to Afforestation in the Degraded Ecosystem of the Danjiangkou Reservoir, China. Plant Soil 2024, 501, 171–189. [Google Scholar] [CrossRef]
- Yang, P.; van Elsas, J.D. Mechanisms and ecological implications of the movement of bacteria in soil. Appl. Soil Ecol. 2018, 129, 112–120. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Sun, M.; Xu, N.; Sun, G.; Zhao, M. Land use change from upland to paddy field in Mollisols drives soil aggregation and associated microbial communities. Appl. Soil Ecol. 2020, 146, 103351. [Google Scholar] [CrossRef]
- Guseva, K.; Darcy, S.; Simon, E.; Alteio, L.V.; Montesinos-Navarro, A.; Kaiser, C. From diversity to complexity: Microbial networks in soils. Soil Biol. Biochem. 2022, 69, 108604. [Google Scholar] [CrossRef]
- De Gryze, S.; Six, J.; Merckx, R. Quantifying water-stable soil aggregate turnover and its implication for soil organic matter dynamics in a model study. Eur. J. Soil Sci. 2006, 57, 693–707. [Google Scholar]
- Feng, H.; Wang, S.; Gao, Z.; Pan, H.; Zhuge, Y.; Ren, X.; Hu, S.; Li, C. Aggregate Stability and Organic Carbon Stock under Different Land Uses Integrally Regulated by Binding Agents and Chemical Properties in Saline-sodic Soils. Land Degrad. Dev. 2021, 32, 4151–4161. [Google Scholar] [CrossRef]
- MacArthur, R. Fluctuations of animal populations and a measure of community stability. Ecology 1955, 36, 533–536. [Google Scholar] [CrossRef]
- May, R. Stability and Complexity in Model Ecosystems; Princeton University Press: Princeton, NJ, USA, 1973. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).