Abstract
The mangrove biome is a highly productive system globally, with flora and fauna adapted to significant saline influence, where salt flats coexist alongside these systems, emerging over sands and muds with high salinity and sparse vegetation. The objective of this research is to describe, for the first time in Ecuador, the diversity of epiphytic lichens in salt flats in the southern region of Ecuador. Two salt flats were selected where Avicennia germinans and Laguncularia racemosa were the dominant trees with the shrub Batis maritima. A total of 30 species of epiphytic lichens were recorded, with the families Arthoniaceae, Graphidaceae, and Ramalinaceae having the highest number of species, and crustose lichens with photobiont type Trentepohlia showed high richness. The salt flats in the southern region of Ecuador have a high richness of epiphytic lichen species, and the species composition is similar to mangroves, highlighting the importance of their conservation as biodiversity refuges for lichens and consequently other flora and fauna groups. Therefore, epiphytic lichens in salt flats can be used as model organisms to assess their conservation in tropical areas.
1. Introduction
The mangrove biome is a highly productive system on a global level [1,2,3]. Thus, these ecosystems are recognized as one of the most diverse and important ecosystems in the world [4,5], and provide the habitat and resources for a variety of flora and fauna species [6,7,8]. However, mangroves have been affected by significant human threats such as the overexploitation of forest resources, salt extraction, pollution, and land use changes [9,10,11,12]. One of the main threats is the shrimp industry, where ponds are constructed in salt flat areas for shrimp production that lack an environmental management system, leading to increasing pollution [13,14]. Among the great biodiversity of mangroves, less-studied groups such as lichens, bryophytes, bromeliads, and fungi are characteristic and diverse elements of these systems [8,15,16,17,18].
In this context, salt flats are ecosystems that coexist with mangroves and emerge over sands and muds, where the salt concentration in the soil is very high, sometimes showing sparse vegetation [19,20], but most studies have been conducted on mangroves rather than salt flats [21]. Nevertheless, lichens, also known as manglicolous lichens [8], have been previously studied in temperate zones [22,23,24] with limited studies in the Neotropics [25,26]. Additionally, in Ecuador, studies have only been conducted in the few localities of mangroves on the Galapagos Islands [27], and for continental Ecuador, studies have been carried out in dry forests [28]. However, although lichen specimens have been sampled from mangroves by researchers, more detailed and distributional studies based on systematic sampling have not been completed to date for the salt flats considered here, despite these areas being amongst the better-conserved salt flats areas of Ecuador.
Epiphytes are important organisms in mangroves [29], and the diversity and composition of the communities is determined by several factors related to the host tree traits (e.g., tree species, bark roughness, and tree size) and microclimatic factors [29,30,31,32,33]. Thus, we expect to find a higher diversity of epiphytic lichens in salt flats because they are ecosystems that coexist with mangroves. Studies on diversity in salt flats are scarce, describing a limited number of species, for example, in vascular plants [20,34], beetles [35], and microbial diversity [36]. For lichen diversity, there is a study where the genera of crustose Arthonia, Caloplaca, and Lecanora were registered [37]. Therefore, the objective of this research was to describe, for the first time in Ecuador, the diversity of epiphytic lichens in two salt flats of Reserva Ecológica Arenillas.
2. Materials and Methods
2.1. Study Area
This study was conducted in the salt flats of the Reserva Ecológica Arenillas, located in the province of El Oro in southern Ecuador (Figure 1). The area has a humid tropical climate in the coastal zone with temperatures ranging from 25 °C to 35 °C., with an area of 5791.85 km2 (weather station Huaquillas for a recorded period of 45 years, 1969–2014).
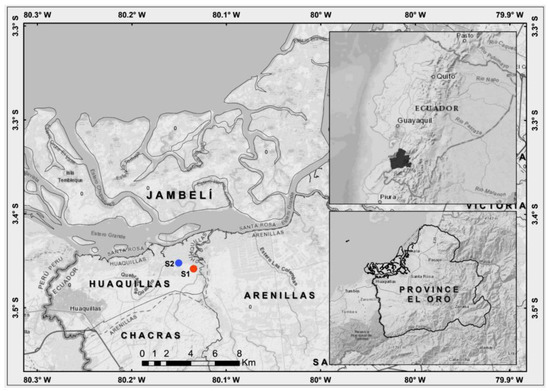
Figure 1.
Map of the study area for lichens in the salt flats of the Reserva Ecológica Arenillas in southern Ecuador. Salt flat 1 = S1 and Salt flat 2 = S2.
2.2. Sampling Design and Data Collection
Two sampling areas were selected (Figure 2). Due to the complexity of the terrain in each salt flat, we randomly selected 20 host trees and shrubs. For each host, we recorded the presence or absence of epiphytic lichens. In both salt flats, the tree species Avicennia germinans and Laguncularia racemosa and the shrub Batis maritima were the most common. Additionally, herbaceous species such as Sporobolus pyramidatus, Sesuvium edmonstonei, S. portulacastrum, and Isocarpha microcephala were more abundant in both salt flats. Various published keys were used to identify the lichen specimens based on morphological, anatomical, and chemical characteristics. Traditional microscopy techniques were employed, along with spot tests using ultraviolet (UV) light, potassium hydroxide (K), commercial bleach (C), and Lugol’s solution (I). For lichen species’ nomenclature, we consulted MycoBank (www.mycobank.org, accessed on 8 February 2023) and the LIAS database (1995–2016), which provides a global information system for lichenized and non-lichenized ascomycetes (www.lias.net, accessed on 8 February 2023).

Figure 2.
Sampling zones: (A) Salt flat 1, (B) Salt flat 2.
3. Results and Discussion
A total of 30 species were recorded in two salt flats, distributed in 15 genera and 10 families (Table 1, Figure A1). The number of reported species is similar compared to the study by Sethy et al. [23] and Logesh et al. [22] in the mangroves of India, where 29 and 21 species of lichens were reported, respectively. However, it is higher than the 11 species of cyanolicens reported by Rangsiruji et al. [16] in the mangroves of Thailand and 19 species reported by Crespo and Atienza [37] in the salt flats of Spain.

Table 1.
Lichen species in salt flats. + = Species presence.
The most representative families in these ecosystems are Arthoniaceae and Ramalinaceae with five species, followed by Graphidaceae with four species (Figure 3). These families are very numerous in dry ecosystems such as mangroves [22,23]. Additionally, Lücking et al. [38] describe, Graphidaceae with 2161 species, Ramalinaceae with 916 species, and Arthoniaceae with 717 species as among the most diverse families worldwide.
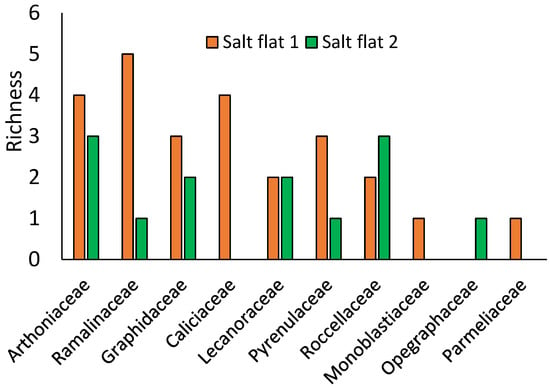
Figure 3.
Representative lichen families in two salt flats.
The most representative genus was Arthonia, where Crespo and Atienza [37] describe that Arthonia is very abundant in salt flats ecosystems of Spain. In our study, representative genera such as Dirinaria, Graphis, Lecanora, Pyrenula, Ramalina, and Rocella are very common in salt flats; similarly, previous studies indicate that these lichen genera are very characteristic in trees and shrubs with saline soils such as mangroves [8,22,23,24,25,26]. In addition, the genera Anisomeridium, Bactrospora, Opegrapha, Parmotrema, Ramalina, Roccella, and Pyxine were recorded for the first time in the studied salt flats.
Similarly, the growth type was dominated by crustaceous lichens with 21 species, followed by fruticose and foliose with 5 and 4 species, respectively (Figure 4). In agreement with our results in salt flats, crustose lichens are the most dominant in mangrove ecosystems [22,23,39]. Supporting our results, Benítez et al. [28] noted that 90% (110 species) of epiphytic lichens in the dry forests of the Reserva Ecológica Arenillas were crustaceous, followed by 11 and 1 species of foliose and fruticose lichens, respectively. In terms of foliose lichens, Rangsiruji et al. [16], shown the presence of 11 species in the study area, where the thallus foliose were abundant; however, the study is focused in cyanobacterial lichens.
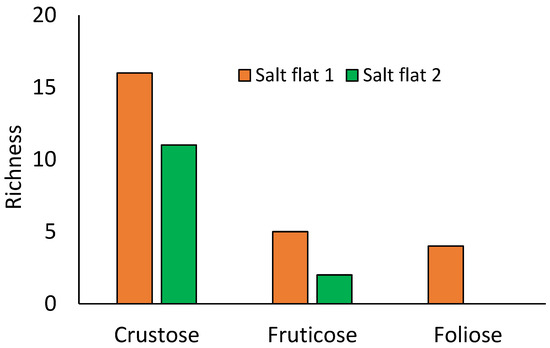
Figure 4.
Lichen species by growth forms in two salt flats.
In terms of species diversity in our study, Anisomeridium biforme, Arthonia antillarum, Arthonia aff. conferta, Coniocarpon cinnabarinum, Dirinaria confluens, Dirinaria picta, Pyrenula cerina, and Pyrenula ochraceoflava were reported. Similar to our findings in salt flats, other studies have also reported these species growing in mangroves [23,26,39,40,41]. Although our study reports the diversity of lichens in salt flats, other groups such as vascular plants, beetles, and bacteria [36,42,43] should be considered, as other countries have reported a high diversity of these organisms in these ecosystems.
Our findings suggest that salt flats serve as biodiversity refuges with high epiphytic lichen richness. However, other factors influencing lichen diversity in similar ecosystems, such as mangroves and dry forests, were not examined in this study. Variables such as topography, disturbance, forest type, microclimatic conditions (e.g., humidity, temperature, light), and host tree characteristics (e.g., species, bark type, pH, tree diameter) may influence epiphytic lichen diversity [8,28,44,45] in salt flats and should be investigated in future research.
4. Conclusions
The salt flats of the Reserva Ecológica Arenillas in southern Ecuador demonstrate a significant diversity of lichens, with 30 species. The growth type with the highest occurrence in salt flats was crustaceous, followed by fruticose and foliose lichens. This aligns with several studies in mangroves and dry forests, where crustose lichens were dominant. Therefore, epiphytic lichens in salt flats serve as valuable model organisms for evaluating conservation efforts in tropical mangroves, due to the fact that the species composition of salt flats are similar to mangroves. Additional sampling is necessary to explore environmental factors and their relationship with epiphytic lichens in these forests, as well as to gain a deeper understanding of lichen diversity in tropical salt flats.
Author Contributions
Conceptualization, Á.B.; methodology, Á.B. and M.V.; formal analysis, Á.B. and M.V.; investigation, Á.B. and M.V.; writing—original draft preparation, Á.B., M.V., D.C., N.C., M.R., C.N., T.O.-P. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Universidad Técnica Particular de Loja”, grant number POA VIN-56.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank the Ministerio del Ambiente, Agua y Transición Ecológica del Ecuador for granting access to the field sites and the necessary collecting permits.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

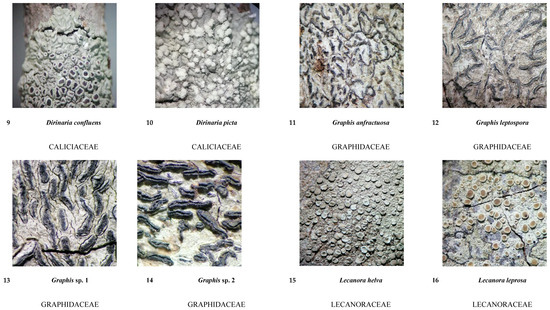

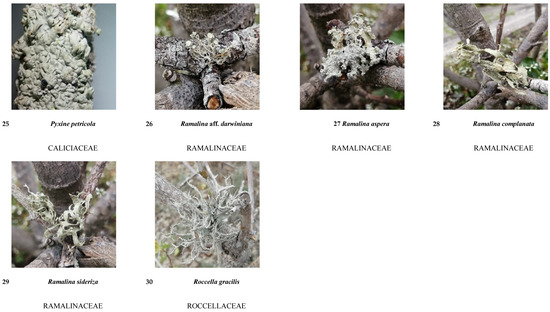
Figure A1.
Guide to lichen species in different salt flat areas.
References
- Kathiresan, K.; Bingham, B.L. Biology of mangroves and mangrove Ecosystems. Adv. Mar. Biol. 2001, 40, 81–251. [Google Scholar]
- Alongi, D.M. Present state and future of world’s mangrove forest. Environ. Conserv. 2002, 29, 331–349. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Sorrell, B.K.; Hancock, N.; Hua, Q.; Swales, A. Mangrove forest and soil development on a rapidly accreting shore in New Zealand. Ecosystems 2010, 13, 437–451. [Google Scholar] [CrossRef]
- Ronnback, P. The ecological basis for economic value of sea food production supported by mangrove ecosystems. Ecol. Econ. 1999, 29, 235–252. [Google Scholar] [CrossRef]
- Carugati, L.; Gatto, B.; Rastelli, E.; Lo Martire, M.; Coral, C.; Greco, S.; Danovaro, R. Impact of Mangrove Forests Degradation on Biodiversity and Ecosystem Functioning. Sci. Rep. 2018, 8, 13298. [Google Scholar] [CrossRef] [PubMed]
- Iftekhar, M.S.; Saenger, P. Vegetation dynamics in the Bangladesh Sundarbans mangroves: A review of forest inventories. Wetl. Ecol. Manag. 2008, 16, 291–312. [Google Scholar] [CrossRef]
- Pawar, P.R. Species diversity of birds in mangroves of Uran (Raigad), Navi Mumbai, Maharashtra, West coast of India. J. Exp. Sci. 2011, 2, 73–77. [Google Scholar]
- Reynolds, C.L.; Er, O.A.H.; Winder, L.; Blanchon, D.J. Distribution and community composition of lichens on mature mangroves (Avicennia marina subsp. australasica (Walp.) J. Everett) in New Zealand. PLoS ONE 2017, 12, e0180525. [Google Scholar] [CrossRef]
- Halpern, B.S.; Selkoe, K.A.; Micheli, F.; Kappel, C.V. Evaluating and ranking the vulnerability of global marine ecosystems to anthropogenic threats. Conserv. Biol. 2007, 21, 1301–1315. [Google Scholar] [CrossRef]
- Gilman, E.L.; Ellison, J.; Duke, N.C.; Field, C. Threats to mangroves from climate change and adaptation options: A review. Aquat. Bot. 2008, 89, 237–250. [Google Scholar] [CrossRef]
- Alongi, D.M. Mangrove forests: Resilience, protection from tsunamis, and responses to global climate change. Estuar. Coast. Shelf Sci. 2008, 76, 1–13. [Google Scholar] [CrossRef]
- Malik, A.; Fensholt, R.; Mertz, O. Mangrove exploitation effects on biodiversity and ecosystem services. Biodivers. Conserv. 2015, 24, 3543–3557. [Google Scholar] [CrossRef]
- de Lacerda, L.D.; Ward, R.D.; Godoy, M.D.P.; de Andrade Meireles, A.J.; Borges, R.; Ferreira, A.C. 20-years cumulative impact from shrimp farming on mangroves of Northeast Brazil. Front. For. Glob. Chang. 2021, 4, 653096. [Google Scholar] [CrossRef]
- Merecí-Guamán, J.; Casanoves, F.; Delgado-Rodríguez, D.; Ochoa, P.; Cifuentes-Jara, M. Impact of shrimp ponds on mangrove blue carbon stocks in Ecuador. Forests 2021, 12, 816. [Google Scholar] [CrossRef]
- Alias, S.A.; Zainuddin, N.; Jones, E.G. Biodiversity of marine fungi in Malaysian mangroves. Bot. Mar. 2010, 53, 545–554. [Google Scholar] [CrossRef]
- Rangsiruji, A.; Boonpragob, A.; Mongkolsuk, P.; Sodamuk, M.; Buaruang, K.; Binchai, S.; Lumbsch, H.T.; Parnmen, S. Diversity and phylogenetic survey of cyanobacterial lichens (Collematineae, Ascomycota) in mangrove forests of eastern Thailand. Bryologist 2017, 119, 123–130. [Google Scholar] [CrossRef]
- De Sousa, M.M.; Colpo, K.D. Diversity and distribution of epiphytic bromeliads in a Brazilian subtropical mangrove. An. Acad. Bras. Ciências 2017, 89, 1085–1093. [Google Scholar] [CrossRef]
- Gomes, P.W.P.; Simões, M.C.; Tavares-Martins, A.C.C. Spatial Distribution and Substrate Preferences of Bryophyte Species in Mangrove Ecosystems of the East Coast of Marajó Island, Brazil. Cryptogam. Bryol. 2023, 44, 219–235. [Google Scholar]
- Delgadillo, J.; Peinado, M.; Parras, J.M.M.; Alcaraz, F.; De La Torre, A. Análisis fitosociológico de los saladares y manglares de Baja California, México. Acta Bot. Mex. 1992, 19, 1–35. [Google Scholar] [CrossRef][Green Version]
- Ladero, M.; Navarro, F.; Valle, C.J.; Marcos, B.; Ruiz, T.; Santos, M.T. Vegetación de los saladares Castellano-Leoneses. Stud. Bot. Univ. Salamanca 1984, 3, 17–62. [Google Scholar]
- Ashton, E.C.; Macintosh, D.J. Preliminary assessment of the plant diversity and community ecology of the sematan mangrove forest, Sarawak, Malaysia. For. Ecol. Manag. 2002, 166, 111–129. [Google Scholar] [CrossRef]
- Logesh, A.R.; Upreti, D.K.; Kalaiselvam, M.; Nayaka, S.; Kathiresan, K. Lichen flora of Pichavaram and Muthupet mangroves (Southeast Coast of India). Mycosphere 2012, 3, 884–888. [Google Scholar] [CrossRef]
- Sethy, P.P.; Pandit, G.S.; Sharma, B.O. Lichens on mangrove plants in Andaman Islands, India. Mycosphere 2012, 3, 476–484. [Google Scholar] [CrossRef]
- Panda, M.; Murthy, T.V.R.; Samal, R.N.; Lele, N.; Patnaik, A.K.; Mohan, P.K. A comparative study of manglicolous lichens and their distribution inside Bhitarkanika National Park (Odisha), India. Stud. Fungi 2017, 2, 1–13. [Google Scholar] [CrossRef]
- Álvarez-León, R.; Avendaño-Remolina, D.; Sanjuan-Muñoz, A.M. La relación entre Peltigera sp. Y Rhizophora mangle en Arroyo de Plata (Bolívar), Caribe colombiano. Luna Azul 2014, 38, 105–121. [Google Scholar] [CrossRef]
- García-Martínez, Y.A.; Heredia Abarca, G.; Guzmán-Guillermo, J.; Valenzuela, R.; Raymundo, T. Hongos asociados al mangle rojo Rhizophoramangle (Rhizophoraceae) en la Reserva de la Biosfera Isla Cozumel, Quintana Roo, México. Acta Bot. Mex. 2021, 128, e1792. [Google Scholar]
- Bungartz, F.; Lücking, R.; Aptroot, A. The family Graphidaceae (Ostropales, Lecanoromycetes) in the Galapagos Islands. Nova Hedwig. 2010, 90, 1–44. [Google Scholar] [CrossRef]
- Benítez, Á.; Aragón, G.; Prieto, M. Lichen Diversity on Tree Trunks in Tropical Dry Forests Is Highly Influenced by Host Tree Traits. Biodivers. Conserv. 2019, 28, 2909–2929. [Google Scholar] [CrossRef]
- Hayasaka, D.; Kimura, N.; Fujiwara, K.; Thawatchai, W.; Nakamura, T. Relationship between microenvironment of mangrove forests and epiphytic fern species richness along the Pan Yi river, Thailand. J. Trop. For. Sci. 2012, 24, 265–274. [Google Scholar]
- Yao, H.; Sun, X.; He, C.; Li, X.-C.; Guo, L.-D. Host identity is more important in structuring bacterial epiphytes than endophytes in a tropical mangrove forest. FEMS Microbiol. Ecol. 2020, 96, fiaa038. [Google Scholar] [CrossRef]
- Zhu, C.; Lin, Y.; Wang, Z.; Luo, W.; Zhang, Y.; Chu, C. Community assembly and network structure of epiphytic and endophytic phyllosphere fungi in a subtropical mangrove ecosystem. Front. Microbiol. 2023, 14, 1147285. [Google Scholar] [CrossRef] [PubMed]
- Siqueiros Beltrones, D.A.; López Fuerte, F.O. Epiphytic diatoms associated with red mangrove (Rhizophora mangle) prop roots in Bahía Magdalena, Baja California Sur, Mexico. Rev. Biol. Trop. 2006, 54, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Isa, H.M.; Kamal, A.H.M.; Idris, M.H.; Rosli, Z.; Ismail, J. Biomass and habitat characteristics of epiphytic macroalgae in the Sibuti Mangroves, Sarawak, Malaysia. Trop. Life Sci. Res. 2017, 28, 1–21. [Google Scholar] [PubMed]
- Molina, J.; Pertiñez, C.; de la Cruz, M. Datos sobre la relación suelo-vegetación en los saladares de Cordobilla (Albacete, España). Rev. Estud. Albacet. 2001, 1, 217–232. [Google Scholar]
- Cartagena, A.; Viñolas, E.; Galante, E. Biodiversidad de tenebriónidos (Coleoptera: Tenebriónidae) en saladares ibéricos. Bull. Inst. Catalana Hist. Nat. 2002, 70, 91–104. [Google Scholar]
- Hazzouri, K.M.; Sudalaimuthuasari, N.; Saeed, E.E.; Kundu, B.; Al-Maskari, R.S.; Nelson, D.; AlShehhi, A.A.; Aldhuhoori, M.A.; Almutawa, D.S.; Alshehhi, F.R.; et al. Salt flat microbial diversity and dynamics across salinity gradient. Sci. Rep. 2022, 12, 11293. [Google Scholar] [CrossRef] [PubMed]
- Crespo, A.; Atienza, V. Sobre la flora y vegetación liquénica epifitica de las formaciones fruticosas de saladar. Lazaroa 1989, 11, 135–139. [Google Scholar]
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota—Approaching one thousand genera. Bryologist 2017, 119, 361–416. [Google Scholar] [CrossRef]
- Fajardo, W.T. Taxonomy and new distributional records of corticolous manglicolous microlichens in Bangrin Marine Protected Area, Philippines. Multidiscip. Sci. J. 2024, 6, 2024218. [Google Scholar] [CrossRef]
- Logesh, A.R.; Kalaiselvam, M.; Upreti, D.K.; Nayaka, S.; Karthiresan, K. Mangroves—An Abode for Unique Lichens Coastal Ecosystems of India; Special Publication; Annamalai University: Parangipettai, India, 2013; pp. 39–44. [Google Scholar]
- Lucban, M.C.; Paguirigan, J.A.G. Occurrence of manglicolous lichens in Calabarzon, Philippines. Stud. Fungi 2019, 4, 263–273. [Google Scholar] [CrossRef]
- Torres-Fernández del Campo, J.; Olvera-Vargas, M.; Figueroa-Rangel, B.L.; Cuevas-Guzmán, R.; Iñiguez-Dávalos, L.I. Patterns of Spatial Diversity and Structure of Mangrove Vegetation in Pacific West-Central Mexico. Wetlands 2018, 38, 919–931. [Google Scholar] [CrossRef]
- Jaramillo, J.J.; Rivas, C.A.; Oteros, J.; Navarro-Cerrillo, R.M. Forest Fragmentation and Landscape Connectivity Changes in Ecuadorian Mangroves: Some Hope for the Future? Appl. Sci. 2023, 13, 5001. [Google Scholar] [CrossRef]
- Benítez, Á.; Ortiz, J.; Matamoros-Apolo, D.; Bustamante, A.; López, F.; Yangua-Solano, E.; Gusmán-Montalván, E. Forest Disturbance Determines Diversity of Epiphytic Lichens and Bryophytes on Trunk Bases in Tropical Dry Forests. Forests 2024, 15, 1565. [Google Scholar] [CrossRef]
- Laakso, K.; López-Rodríguez, J.C.; Rivard, B.; Sánchez-Azofeifa, G.A. Using visible-near-infrared spectroscopy to classify lichens at a Neotropical Dry Forest. Ecol. Indic. 2020, 111, 105999. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).