Abstract
A partial mitochondrial DNA Cytochrome Oxidase subunit I (mtCOI) gene haplotype variant of the coconut rhinoceros beetle (CRB) Oryctes rhinoceros, classed as ‘CRB-G (clade I)’, has been the focus of much research since 2007, with reports of invasions into new Pacific Island locations (e.g., Guam, Hawaii, Solomons Islands). For numerous invasive species, inference of invasion biology via whole genome is superior to assessments via the partial mtCOI gene. Here, we explore CRB draft mitochondrial genomes (mitogenomes) from historical and recent collections, with assessment focused on individuals associated within the CRB-G (clade I) classification. We found that all Guam CRB individuals possessed the same mitogenome across all 13 protein-coding genes and differed from individuals collected elsewhere, including ‘non-Guam’ individuals designated as CRB-G (clade I) by partial mtCOI assessment. Two alternative ATP6 and COIII partial gene primer sets were developed to enable distinction between CRB individuals from Guam that classed within the CRB-G (clade I) haplotype grouping and CRB-G (Clade I) individuals collected elsewhere. Phylogenetic analyses based on concatenated ATP6–COIII genes showed that only Guam CRB-G (clade I) individuals clustered together, and therefore Guam was not the source of the CRB that invaded the other locations in the Pacific assessed in this study. The use of the mtCOI and/or mtCOIII genes for initial molecular diagnosis of CRB remained crucial, and assessment of more native CRB populations will further advance our ability to identify the provenance of CRB invasions being reported within the Pacific and elsewhere.
1. Introduction
The mitochondrial DNA (mtDNA) genome (mitogenome) is largely maternally inherited and generally consists of 13 protein-coding genes (PCG’s), 22 tRNA genes, 2 rRNA genes, and an AT-rich region that is low in nucleotide complexity (e.g., [1]). Due to the maternal inheritance nature, the mitogenome in general does not undergo recombination (e.g., [2,3]). Hebert et al. [4] demonstrated the use of the partial mtDNA cytochrome oxidase subunit I (mtCOI) gene sequence to aid in species diagnostics, and this system helped transform understanding of species diversity [5,6,7]. Multiple partial mtCOI databases (e.g., BOLD; GenBank) subsequently provided considerable contributions to disentangling species status (e.g., [8,9]). However, the use of partial mtCOI is not without its limitations: association with endosymbionts, effect of selective sweep, and impact of pseudogenes, amongst other factors, can all lead to inaccurate interpretations [10,11]. Analysis of population-wide partial mtCOI gene diversity has also found that the 5′ gene region typically has low nucleotide diversity (i.e., conserved nucleotide sequence) in some arthropod groups, such as Hemiptera, Lepidoptera, and Coleoptera (e.g., [12,13,14,15]). Subsequently, an over-reliance on partial mtCOI gene sequences has resulted in some misidentification of species status (e.g., [11]), and some misunderstandings of population dynamics (e.g., [16] versus [14,17]; see also review by [18]). Examples of confounded interpretations include the invasion history of Spodoptera frugiperda (Lepidoptera: Noctuidae) [19,20,21] and population expansion patterns of Helicoverpa (Lepidoptera: Noctuidae) species ([22] versus [23]).
As technology advances and costs decrease, it is now easier and cheaper than ever before to obtain greater genetic data from specimens to provide richer information content for analysis and interpretation. Combining sequence data from multiple mtDNA genes, full mitochondrial DNA genomes (mitogenomes) and/or whole genomes are now regularly being shown to provide an analysis superior to the use of single genes (e.g., partial mtCOI) alone for all applications. Examples include identifications of species (e.g., [24]), sub-species (e.g., [25,26,27]), hybrids (e.g., [25,26,28]), populations [14,17], patterns like demographic expansion (e.g., [29,30]), and pest incursion histories (e.g., [31,32,33]).
Of specific relevance to the current understanding of CRB invasion biology and reported resistance/increased tolerance of some CRB-G populations to the OrNV biological control agent (e.g., [34,35,36,37]) are OrNV detection inconsistencies in CRB-G (clade I), as defined by the partial mtCOI marker (e.g., Palauan CRB populations [35]; Solomon Islands CRB populations [34,37]), as well as between detected mtCOI signatures of CRB-G (e.g., on Guam, Taiwan, Palau, Hawaii) versus the presence of Guam-only signatures when analysed using nuclear markers [36]. These inconsistencies emerging in the CRB research bear significant resemblances to recent examples found in other invasive species, such as Bemisia tabaci (e.g., [26,38]), Helicoverpa armigera (e.g., [25]), Spodoptera frugiperda (e.g., [14,17]), and Tuta absoluta [32], that demonstrated mtCOI marker limitations and the benefit of re-assessment using alternative mitochondrial and/or nuclear genome-based marker systems.
Here, we examine draft-full mitogenomes of the coconut rhinoceros beetle (CRB; Oryctes rhinoceros), a pest that causes economic yield losses to coconut and oil palm [39,40]. These mitogenomes were generated through whole-genome sequencing (WGS) from multiple geographically distinct locations to develop additional molecular markers for tracking and monitoring genetically distinct populations of this species. Particular attention is given to the CRB-G (clade I) group determined using partial mtCOI gene assessment [35] because of the current biosecurity focus on this mitochondrial haplotype variant due to its reported resistance to some isolates of the Oryctes rhinoceros Nudivirus (OrNV) biological control agent [35], and new incursions of CRB associated with the CRB-G (clade I) grouping across the Pacific region [35,41]. Specifically, we test whether or not the Guam CRB-G (clade I) was the source population for the CRB-G (clade I) populations detected in Solomon Islands and in Palau, as suggested in some publications (e.g., [42,43]). We do this by identifying two partial mitochondrial gene regions that more confidently differentiated CRB (clade I) individuals that invaded Guam from other CRB individuals, including other individuals classed as CRB-G (clade I) using the partial mtCOI gene, but which were collected on other Pacific islands (e.g., Solomon Islands, Palau). We discuss the benefits of mitogenomes as resources for developing alternative diagnostic markers and assess efficacies of the partial mtCOI gene as the current preferred standard diagnostic DNA marker to distinguish CRB populations.
2. Material and Methods
2.1. Samples
CRB samples collected between 2019 and 2022 were sourced from Guam, Palau, Indonesia, Malaysia, and Philippines (Table 1; Figure S1). The gut of each specimen was dissected, preserved in 100% ethanol, and stored at −20 °C until needed for DNA extraction. Additionally, a historic specimen collected from Guam (04-Or5; circa 2014) was used as a reference to enable matching of more recently collected CRB individuals from Guam that classed within the CRB-G (clade I) haplotype grouping sensu Marshall et al. [35]. A related Oryctes nasicornis mitogenome (GenBank OK484312; [44]) was included to provide comparison of inter-specific nucleotide distance with CRB. Nucleotide positions followed annotation of MT457815 [45].

Table 1.
Details of Oryctes rhinoceros (CRB) and Oryctes nasicornis samples used in this study, including GenBank accession numbers of publicly available, assembled, and annotated mitogenomes and single nucleotide polymorphisms (SNPs), differentiating CRB-G (clade I) that invaded Guam from other CRB using the mitochondrial cytochrome oxidase subunit I (mtCOI), ATP synthase membrane subunit 6 (ATP6), and cytochrome oxidase subunit III (COIII).
2.2. Whole-Genome Sequencing (WGS)
We used a Qiagen Blood and Tissue DNA extraction kit (Duesseldorf, Germany) and the manufacturer’s protocol to extract genomic DNA. The extracted DNA was eluted in 200 μL EB and kept frozen until used for WGS. We assessed the quality of the extracted DNA using Qubit 2.0 (Life Technologies, Foster City, CA, USA) prior to sequencing. WGS was carried out by the Australian Genome Resource Facility (AGRF) in Melbourne, Australia, or by AZENTA Life Sciences in China. The WGS data returned an average of 25x coverage, 150 bp paired-end reads/sample, assuming a genome size of approximately 350 Mbp.
2.3. Mitogenome Assembly and Annotation
We assembled all mitogenomes by importing the raw sequence reads into Geneious Prime 2022.2.2 (Build 18 August 2022 14:34) (Biomatters Ltd., Auckland, New Zealand) and used the published mitogenome (MT457815, [45]) as the reference sequence. We used Geneious Mapper with ‘Low Sensitivity/Fastest’ option and selecting no fine tuning (i.e., None (fast/read mapping)) during the mitogenome-assembling process. Although we received pair-ended reads for all samples, mitogenomes were assembled using forward reads only due to the high genome coverage for each sample. All assembled mitogenomes were initially annotated using a MITOS programme and selecting invertebrate mitochondrial genetic code [48]. As a final quality assessment, the annotated CRB mitogenomes were visually inspected. The mtCOI, ATP6, and COIII genes used in this study, as well as the assembled and annotated mitogenomes, are available from the CSIRO data repository [49,50].
2.4. Mitogenome Identity Assessment
The non-recombination nature of the mitogenome implies that CRB individuals classified as CRB-G (clade I) based on the partial mtCOI gene assessment method [35] (e.g., Solomon Islands MT457815, Taiwan MW632131) would share mitogenome identity with our reference Guam specimen (i.e., 04-Or5; Table 1) if a single source of invasion entered into Guam and subsequently spread from to other locations. To assess this, randomly selected CRB specimens from Guam (i.e., NZ-20-738; Guam-01_GDoA, Guam-02_GDoA, Guam-09_GDoA, Guam-13_GDoA, Guam-17_GDoA; Table 1) that were collected in more recent times (2020 and 2021) were compared with the representative historical Guam individual (04-Or5) to visually assess and confirm mitogenome identity. This was then followed by comparison with all other CRB individuals, including CRB-G-typed individuals collected from elsewhere (Table 1). Individuals were compared based on the partial mtCOI sequence analysis (described in [35]), as well as sequence similarity of other mitochondrial genes assessed by this work.
2.5. Alternative CRB Marker Development to Identify the Original CRB Population That Invaded Guam
To identify candidate mitochondrial genes as alternative DNA markers specific to individuals from Guam, all mitogenomes generated from this study, as well as publicly available CRB mitogenomes from GenBank, were downloaded and aligned within GenBank using MAFFT V7.490 [51,52] with default setting options (i.e., algorithm: FFT-NS-2; Scoring matrix: 200 PAM/k = 2; Gap open penalty: 1.53; Offset value: 0.123). We visually identified candidate polymorphic sites unique to individuals from Guam (i.e., 04-Or5, NZ-20-738, Guam-01_GDoA, Guam-02_GDoA, Guam-09_GDoA, Guam-13_GDoA, Guam-17_GDoA) but absent in all other CRB individuals (Table 1). The SNPs identified were analysed for potential restriction endonucleases to develop polymerase-chain reaction (PCR) restriction fragment length polymorphism (RFLP) solutions (PCR–RFLP) for a simple and easy-to-use approach to confidently differentiate CRB-G (clade I) that invaded Guam from all other genetically distinct CRB, including CRB from elsewhere, classed as CRB-G (clade I) by partial COI assessment. Design and analysis of PCR-primers were performed through the Primer Analysis Software version 7.60 (Molecular Biology Insights, Inc., Cascade, CO, USA). Primers were optimised for minimal false-primer-annealing sites, minimal primer dimer and duplex formation, and minimal/no hairpin structure, with a Ta (theoretical annealing temperature) of ≥60 °C (calculated as Ta = 4(G + C) + 2(A + T)) and an optimal amplicon length of between 500 and 600 bp to facilitate ease of Sanger sequencing. The candidate restriction endonuclease was initially selected for a single cut site with in silico analysis of all different mitochondrial DNA haplotypes in Enzyme X version 3.3 (http://nucleobytes.com/enzymex/). We reconfirmed primer efficacies and RFLP conditions by randomly selecting and analysing DNA from specimens collected from Guam and elsewhere, as well as by PCR-Sanger sequencing to confirm primer amplification accuracy. We used the restriction digestion conditions as recommended by the manufacturer of the BmpI restriction enzyme (New England BioLabs; Ipswich, MA, USA). Visualisation of the RFLP was on a 1.5% 1x TAE agarose gel.
2.6. Mitogenome Analysis
The mitogenomes from the GenBank database and those generated from this study were aligned to estimate pairwise nucleotide identity and distances (p-dist) between the following: (i) full mtCOI gene versus full ATP6 gene and (ii) full mtCOI versus full COIII genes. The related O. nasicornis mitogenome (0K484312) was included to provide comparison of inter-specific nucleotide distance with CRB. We also inferred phylogenies of the CRB individuals based on the widely used partial mtCOI gene region (676 bp) versus our proposed alternative mitochondrial ATP6 and COIII partial gene regions (excluding nucleotides at primer annealing sites, see [35]). The APT6 and COIII partial gene sequences were concatenated before phylogeny inference. We used IQ-Tree [53] and selected the ‘Auto’ option for estimating substitution models, and 1000 bootstrap alignments to estimate branch support using the ultrafast bootstrap approximation (UFBoot) [54] algorithm. We used Dendroscope 3 [55] for visualisation and post-analysis editing for both COI and ATP6 + COIII phylogenies. The 13 protein-coding genes (PCGs) from the samples’ mitochondrial genomes were extracted and concatenated for use in phylogenetic analysis using IQ-Tree and visualisation using Dendroscope 3 (version 3.5.7, built 30 January 2016) with procedures as described above.
3. Results
3.1. Mitochondrial Genome Analysis
Mitochondrial genomes were assembled and annotated from an average of 1472 fragments (mean standard deviation 997 fragments) per sample. Across all the mitochondrial COI, ATP6, and COIII gene sequences, nucleotide differences between CRB individuals were low (<2% difference), suggesting that all were the same species (i.e., O. rhinoceros) (Table S1). The fully assembled and annotated mitogenome from the Guam 04-Or5 specimen (collected in 2014) provided evidence that all Guam individuals examined here shared the same mitogenome (Table 1) that was uniquely identified only in Guam. Nucleotide differences within the mitochondrial ATP6 and COIII genes were identified in Guam individuals, but these nucleotide polymorphisms were absent from specimens collected elsewhere, including those classed as CRB-G (clade I) by partial COI assessment. For the ATP6_T4430C and COIII_C5390T SNPs identified from the partial ATP6 and COIII genes (see Table 1), there were, on average, 1876 and 1740 reads at each of these nucleotide sites to confirm further differentiation of CRB that invaded Guam from other CRB-G (clade I) members (i.e., equivalent to the diagnostic SNP for ATP6 and COIII being independently confirmed an average of 1876 and 1740 times, respectively).
Pairwise nucleotide analysis of the complete mtCOI gene sequence versus complete ATP6 gene sequence, and also the complete mtCOI gene sequence versus the complete COIII gene sequence, showed that the seven Guam CRB, one Solomon Islands CRB (MT457815), one Taiwan (MW632131), two Palau CRBs (Palau-03, Palau-04), and four Philippines CRB (Phil-01, -02, -03, -04) specimens analysed shared 100% identity across the complete mtCOI gene sequence. However, when the comparison included the full ATP6 and full COIII gene sequences, only the Guam individuals remained 100% identical to each other. CRB from Solomon Islands (MT457815), Taiwan (MW632131), Palau (Palau-03, Palau-04), and Philippines (Phil-01, -02, -03, -04) all had polymorphisms in these two alternative mitochondrial marker genes (Table 1).
3.2. Alternative Primers to Identify the Original Invasive CRB Population Present in Guam
Two alternative sets of primers were developed (Table 2) to distinguish CRB-G (clade I) that invaded Guam from other CRB, including those collected elsewhere, classed as CRB-G (clade 1) by partial COI assessment. One primer amplifies a partial ATP6 gene region of 494 bp length, and the other amplifies a partial COIII gene region of 469 bp length. The optimal PCR-annealing temperature for both ATP6 and COIII was 52 °C, with a 1.0 μM primer concentration for both ATP6 and COIII primer pairs, a 0.5 mM dNTPs concentration, and 1 unit of DNA polymerase in a 50 μL PCR volume.

Table 2.
PCR primer sets for ATP6 (for PCR–RFLP) and COIII were developed to differentiate CRB that invaded Guam from other CRB (including CRB classed as CRB-G (clade I)) using the partial mtCOI gene in other locations; sensu [35].
3.3. Phylogeny
Phylogenetic analysis was carried out using specimens (see Table 1) with available mitogenome DNA sequence data to allow for comparison using both the 13 PCGs from the full mitogenomes (Figure S2) and the mitochondrial gene regions from COI, COIII, and ATP6. Based on the widely used mtCOI partial gene (Figure 1a), three clades could be recognised. One clade (red branches) included seven Guam specimens, four Philippines (green circles), two individuals from Palau (yellow circles), one from Taiwan (aqua blue circle), and one from the Solomon Islands (purple circle). The other two major and minor clades (blue branches) do not contain any individuals from Guam but included six individuals from Indonesia (i.e., IND-J14, IND-J15, IND-J20, IND-H01, IND-H02, IND-H10); eight from Malaysia (i.e., six from the major clade (i.e., OP694174, OP694176, ON764800,MY-A-02, MY-A-04, MY-A-10; and two from the minor but evolutionary divergent clade (i.e., ON764799, ON764801)); and two from Palau (i.e., PALAU-01, PALAU-02). The phylogeny from partial ATP6- and COIII-concatenated sequences (Figure 1b; cladogram) showed different population demographic patterns, with all Guam individuals clustering together (red), whereas Philippines (green), Malaysia (khaki), and Indonesia (pink) largely clustered according to geography. CRB specimens from Palau (yellow circles) appeared to have multiple origins involving at least Philippines and Indonesia, whereas Taiwan (aqua blue circle) and the Solomon Islands (purple circle) appeared to have closer affinity with Philippines CRB individuals but with low (<50%) bootstrap node support values.
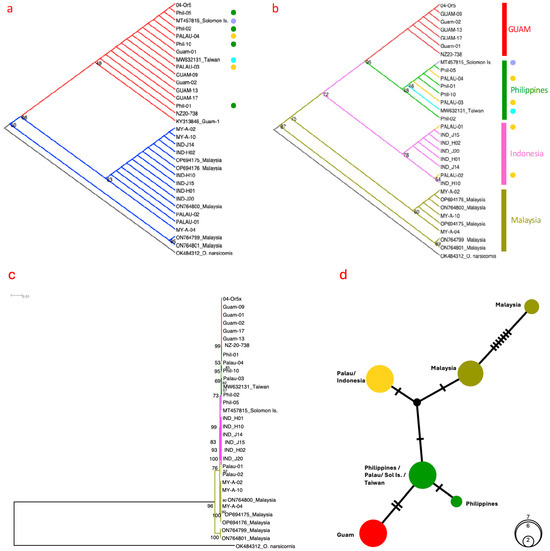
Figure 1.
Phylogenetic analysis using (a) partial mtCOI gene sequence (676 bp) and (b) concatenated partial APT6 (446 bp) and partial COIII (422 bp) gene sequences. A phylogram based on concatenated ATP6–COIII partial gene sequences and the haplotype network are also presented in (c) and (d), respectively. The Oryctes narsicornis sample (OK484312) was included as an outgroup.
Phylogenetic analysis based on both partial ATP6 and partial COIII genes (Figure 1b (cladogram); Figure 1c (phylogram)) and also on concatenation of the 13 PCGs (Figure S2) therefore demonstrated a high level of topology similarity but returned a different population-clustering pattern from the partial mtCOI gene phylogeny (Figure 1a). Together, the use of the concatenated sequences of the 13 PCGs, as well as the concatenated partial ATF6 and COIII gene regions, showed that Guam CRB individuals (red branches) clustered by themselves, whereas the Philippines, Malaysian, and Indonesian individuals clustered largely according to their geographical distributions. CRBs from Palau (yellow circles) appeared to have multiple origins, clustering with specimens collected from both the Philippines and Indonesia. However, branch node confidence values for Indonesia (54–78) and Philippines (46–48) were low, suggesting longer sequence lengths from both mitochondrial and inclusion of nuclear genes, as well as more samples from both native and introduced populations, are required for confident assessment. Notably, CRB populations in Malaysia appeared to be consisted of two diverse evolutionary lineages based on both COI and the concatenated ATP6–COIII partial genes, with the unique ON764799 and ON764801 individuals originating from both coconut palm and oil palm hosts from the state of Johor [47]. A concatenated ATP6–COIII partial gene haplotype network (Figure 1d) is also presented, showing the limited base substitutions between the haplotypes except between the more diverse Malaysian individuals separated by seven base substitutions.
4. Discussion
In this study, we characterised and reanalysed the draft mitogenomes of CRB individuals from both the native (i.e., Indonesia, Malaysia, Philippines, Taiwan) and exotic (i.e., Guam, Palau, Solomon Islands) ranges. This is also the first time the mitogenome of all recently collected Guam CRB individuals analysed in this study were found to share sequence identity with specimens historically collected from Guam by possessing the same mitogenome sequence across all 13 protein-coding genes [50], and specifically to the ATP6 and COIII genes that exhibited nucleotide differences with CRB from other locations, as also supported by the phylogeny from concatenation all 13 PCG sequences (Figure S2). This enabled the ATP6 and COIII protein-coding genes to be used as alternative DNA markers for differentiating Guam-specific CRB-G from the other tested CRB-G (clade I) individuals. The remaining individuals from elsewhere, however, including those designated as CRB-G (clade I) (based on the partial mtCOI assessment approach), did not share the same maternal lineage as the Guam CRB-G individuals. In other words, the multi-gene assessment (albeit with a limited number of specimens) provided strong supporting evidence that the CRB invasion into Guam was distinct from the CRB invasions detected in Solomon Islands and in Palau, and therefore Guam was not the source of the CRB that invaded these other locations. Increasing sampling of CRB from Guam, Palau, and Solomon Islands is needed to further increase confidence of the specificity of the ATP6 and COIII alternative markers to differentiate Guam CRB from other CRB-G (clade I) populations.
For the PCR–RFLP primers focused on the partial ATP6 gene sequence, separation is based on a BpmI restriction site. Individuals classed as Guam CRB produced two fragments (i.e., 271 bp and 223 bp), whereas all other CRB remained uncut (i.e., 494 bp) (Table 2). A second primer set was developed based on the COIII gene that can also differentiate between Guam CRB-G from other CRB; however, this diagnostic method requires sequence analysis (such as through Sanger sequencing) to detect the presence of a ‘C’ or a ‘T’ base at nucleotide position 5390 (see Table 1). These identified SNPs to differentiate Guam CRB from other CRB-G (clade I) could be further explored for alternative detection methods, including DNA-sensing CRISPR Cas12a-based diagnostics which are sensitive to single SNP’s [56], and modified Loop-mediated isothermal amplification (LAMP) methods to detect unique SNPs (e.g., see [57] for review). Although these new markers improve the differentiation between CRB that invaded Guam and other CRB populations, assessment of more CRB individuals from native populations (e.g., Malaysia, Singapore, Sri Lanka, India, Bangladesh, Myanmar, Cambodia, Laos, Vietnam, southern China, Indonesia, Philippines, Thailand) will be needed to provide a more robust confirmation of CRB invasion histories. Also, for all work using molecular diagnostics of CRB, use of either the mtCOI or mtCOIII genes is recommended as an initial approach to first confirm that samples are O. rhinoceros. For example, while the T4430C SNP site within ATP6 from Guam specimens was a T, it was also a ‘T’ in O. narsicornis (see Table 1). Therefore, a direct PCR–RFLP without first confirming the species status could lead to misidentification of O. rhinoceros among other Oryctes species.
The CRB is a hitchhiker pest [58] and is continuing to disperse to new locations, being recently reported in the Marshall Islands [59] and multiple Hawaiian islands [60]. Notably, our results found that Palau CRB appear to have multiple origins (Figure 1b). The node confidence support estimates in Figure 1b displayed a range of values, with some of the individuals (e.g., from Palau, Indonesia) appearing low (less than 60), which limited the power of inference for better understanding the invasion history of this pest across its distributional ranges. It is likely that future detailed genetic assessments of CRB will provide the resolving power required to further elucidate CRB invasion histories.
5. Conclusions
Increasingly, WGS and multigene approaches have provided greater analytical power than partial genome assessments and are therefore rapidly becoming more widely adopted for the interrogation of demographic history and evolutionary relationships of some of the world’s most significant transboundary invasive plant pests [14,17,25,26,27,38,61], including CRB [34,36,45]. Given that the WGS/multigene approaches can provide more comprehensive evidence than single-gene analyses (e.g., partial mtCOI), and we have found exactly this result with this analysis. We suggest that a detailed study using these more detailed genetic assessments is needed to further improve the current understanding of CRB invasion biology.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/d16100634/s1, Figure S1: A map detailing countries (in bold) of Oryctes rhinoceros samples featured in the Figure 1 phylogeny. Country names in grey were provided as general reference. Map generated using Mapchart.net with editing in Microsoft PowerPoint for Mac Version 16.87; Figure S2: Phylogeny based on concatenated sequences from the 13 mitogenome protein coding genes of coconut rhinoceros beetle (CRB) Oryctes rhinoceros samples as detailed in Table 1. Branch node estimates are shown. Note that the overall topology of the phylogeny with respect to the clades was the same as shown for the partial mitochondrial ATP6 and COIII concatenated sequence phylogeny in Figure 1b; Table S1: Pairwise nucleotide comparisons of the full mitochondrial DNA cytochrome oxidase sub- unit I (mtCOI) gene (lower triangle), with the concatenated full ATP6 (ATP synthase membrane subunit 6) and full COIII (cytochrome oxidase subunit III) genes (top triangle, left and right values, respectively) for Oryctes rhinoceros (CRB) individuals.
Author Contributions
Conceptualization, W.T.T., B.D.H. and J.C.A.; methodology, W.T.T., D.Y.-C.C. and T.H.; formal analysis, W.T.T., S.D.G.M., D.Y.-C.C., T.H., A.D.P.-B. and R.V.R.; investigation, J.W.S., J.B.H.M., M.H., G.F.J.D., A.L.B., M.M. and M.F.; resources, M.M., G.F.J.D., A.L.B., J.W.S., M.H., J.B.H.M. and M.F.; data curation, W.T.T., A.D.P.-B. and R.V.R.; writing—original draft preparation, W.T.T., S.D.G.M., B.D.H., G.F.J.D., A.L.B. and J.B.H.M.; writing—review and editing, W.T.T., S.D.G.M., A.D.P.-B., G.F.J.D., A.L.B., J.W.S., M.H., J.B.H.M., M.M., R.V.R., T.H., D.Y.-C.C., J.C.A., M.F. and B.D.H.; visualization, W.T.T.; supervision, W.T.T. and B.D.H.; project administration, W.T.T. and B.D.H.; funding acquisition, W.T.T. and B.D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Australian Government Department of Foreign Affairs and Trade (DFAT) Administered (aid) Simple Grant Agreement grant number 77092.
Data Availability Statement
Data to this study can be downloaded from CSIRO Data Access Portal as detailed in references [49,50].
Acknowledgments
Indonesian CRB samples were provided under Republic of Indonesia Ministry of Agriculture Agricultural Quarantine Agency Permit numbers 2021.1.2005.0.K13.E.00003 No. 4299301 and 2022.1.2005.0.K12.E.00002 No. 5896762. CRB samples from the Philippines were gathered under the Gratuitous Permit DENR8-GP No. 2022-02 (10 January 2022) provided by the Department of Environment and Natural Resources 8 of the Republic of the Philippines and conducted under the VSU-IP 2021-10 (BIO-CAMP) and VSU-IP 2022-2 (CRB) projects. All other CRB individuals sourced for this study did not require collection/export permits.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Crozier, R.H. From population genetics to phylogeny: Uses and limits of mitochondrial DNA. Syst. Bot. 1990, 3, 111–124. [Google Scholar] [CrossRef]
- Leducq, J.B.; Henault, M.; Charron, G.; Nielly-Thibault, L.; Terrat, Y.; Fiumera, H.L.; Shapiro, B.J.; Landry, C.R. Mitochondrial Recombination and Introgression during Speciation by Hybridization. Mol. Biol. Evol. 2017, 34, 1947–1959. [Google Scholar] [CrossRef] [PubMed]
- Saville, B.J.; Kohli, Y.; Anderson, J.B. mtDNA recombination in a natural population. Proc. Natl. Acad. Sci. USA 1998, 95, 1331–1335. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef]
- Hebert, P.D.; Penton, E.H.; Burns, J.M.; Janzen, D.H.; Hallwachs, W. Ten species in one: DNA barcoding reveals cryptic species in the neotropical skipper butterfly Astraptes fulgerator. Proc. Natl. Acad. Sci. USA 2004, 101, 14812–14817. [Google Scholar] [CrossRef]
- Rugman-Jones, P.F.; Hoddle, C.D.; Hoddle, M.S.; Stouthamer, R. The lesser of two weevils: Molecular-genetics of pest palm weevil populations confirm Rhynchophorus vulneratus (Panzer 1798) as a valid species distinct from R. ferrugineus (Olivier 1790), and reveal the global extent of both. PLoS ONE 2013, 8, e78379. [Google Scholar] [CrossRef]
- Tay, W.T.; Beckett, S.J.; De Barro, P.J. Phosphine resistance in Australian Cryptolestes species (Coleoptera: Laemophloeidae): Perspectives from mitochondrial DNA cytochrome oxidase I analysis. Pest Manag. Sci. 2016, 72, 1250–1259. [Google Scholar] [CrossRef]
- Behere, G.T.; Tay, W.T.; Russell, D.A.; Heckel, D.G.; Appleton, B.R.; Kranthi, K.R.; Batterham, P. Mitochondrial DNA analysis of field populations of Helicoverpa armigera (Lepidoptera: Noctuidae) and of its relationship to H. zea. BMC Evol. Biol. 2007, 7, 117. [Google Scholar] [CrossRef]
- De Barro, P.J.; Liu, S.S.; Boykin, L.M.; Dinsdale, A.B. Bemisia tabaci: A statement of species status. Annu. Rev. Entomol. 2011, 56, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hurst, G.D.; Jiggins, F.M. Problems with mitochondrial DNA as a marker in population, phylogeographic and phylogenetic studies: The effects of inherited symbionts. Proc. Biol. Sci. 2005, 272, 1525–1534. [Google Scholar] [CrossRef]
- Tay, W.T.; Elfekih, S.; Court, L.N.; Gordon, K.H.J.; Delatte, H.; De Barro, P.J. The Trouble with MEAM2: Implications of Pseudogenes on Species Delimitation in the Globally Invasive Bemisia tabaci (Hemiptera: Aleyrodidae) Cryptic Species Complex. Genome Biol. Evol. 2017, 9, 2732–2738. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Huang, J.R.; Dai, J.; Guo, Y.F.; Sun, J.T.; Hong, X.Y. Intraspecific mitochondrial genome comparison identified CYTB as a high-resolution population marker in a new pest Athetis lepigone. Genom. 2019, 111, 744–752. [Google Scholar] [CrossRef]
- Roe, A.D.; Sperling, F.A. Patterns of evolution of mitochondrial cytochrome c oxidase I and II DNA and implications for DNA barcoding. Mol. Phylogenet. Evol. 2007, 44, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Tay, W.T.; Rane, R.V.; Padovan, A.; Walsh, T.K.; Elfekih, S.; Downes, S.; Nam, K.; d’Alencon, E.; Zhang, J.; Wu, Y.; et al. Global population genomic signature of Spodoptera frugiperda (fall armyworm) supports complex introduction events across the Old World. Commun. Biol. 2022, 5, 297. [Google Scholar] [CrossRef] [PubMed]
- Vyskocilova, S.; Tay, W.T.; van Brunschot, S.; Seal, S.; Colvin, J. An integrative approach to discovering cryptic species within the Bemisia tabaci whitefly species complex. Sci. Rep. 2018, 8, 10886. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef]
- Rane, R.; Walsh, T.K.; Lenancker, P.; Gock, A.; Dao, T.H.; Nguyen, V.L.; Khin, T.N.; Amalin, D.; Chittarath, K.; Faheem, M.; et al. Complex multiple introductions drive fall armyworm invasions into Asia and Australia. Sci. Rep. 2023, 13, 660. [Google Scholar] [CrossRef]
- Tay, W.T.; Meagher, R.L., Jr.; Czepak, C.; Groot, A.T. Spodoptera frugiperda: Ecology, Evolution, and Management Options of an Invasive Species. Annu. Rev. Entomol. 2023, 68, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Cock, M.J.W.; Beseh, P.K.; Buddie, A.G.; Cafa, G.; Crozier, J. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci. Rep. 2017, 7, 4103. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Dhanani, I.; Asokan, R.; Mahadevaswamy, H.M.; Kalleshwaraswamy, C.M.; Sharanabasappa; Meagher, R.L. Genetic characterization of fall armyworm infesting South Africa and India indicate recent introduction from a common source population. PLoS ONE 2019, 14, e0217755. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Goergen, G.; Tounou, K.A.; Agboka, K.; Koffi, D.; Meagher, R.L. Analysis of strain distribution, migratory potential, and invasion history of fall armyworm populations in northern Sub-Saharan Africa. Sci. Rep. 2018, 8, 3710. [Google Scholar] [CrossRef]
- Leite, N.A.; Alves-Pereira, A.; Correa, A.S.; Zucchi, M.I.; Omoto, C. Demographics and genetic variability of the new world bollworm (Helicoverpa zea) and the old world bollworm (Helicoverpa armigera) in Brazil. PLoS ONE 2014, 9, e113286. [Google Scholar] [CrossRef] [PubMed]
- Arnemann, J.A.; Roxburgh, S.; Walsh, T.; Guedes, J.; Gordon, K.; Smagghe, G.; Tay, W.T. Multiple incursion pathways for Helicoverpa armigera in Brazil show its genetic diversity spreading in a connected world. Sci. Rep. 2019, 9, 19380. [Google Scholar] [CrossRef]
- Walsh, T.K.; Perera, O.; Anderson, C.; Gordon, K.; Czepak, C.; McGaughran, A.; Zwick, A.; Hackett, D.; Tay, W.T. Mitochondrial DNA genomes of five major Helicoverpa pest species from the Old and New Worlds (Lepidoptera: Noctuidae). Ecol. Evol. 2019, 9, 2933–2944. [Google Scholar] [CrossRef]
- Anderson, C.J.; Tay, W.T.; McGaughran, A.; Gordon, K.; Walsh, T.K. Population structure and gene flow in the global pest, Helicoverpa armigera. Mol. Ecol. 2016, 25, 5296–5311. [Google Scholar] [CrossRef]
- Elfekih, S.; Tay, W.T.; Polaszek, A.; Gordon, K.H.J.; Kunz, D.; Macfadyen, S.; Walsh, T.K.; Vyskocilova, S.; Colvin, J.; De Barro, P.J. On species delimitation, hybridization and population structure of cassava whitefly in Africa. Sci. Rep. 2021, 11, 7923. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, F.; Tay, W.T.; Robin, C.; Shi, Y.; Guan, F.; Yang, Y.; Wu, Y. Population genomics provides insights into lineage divergence and local adaptation within the cotton bollworm. Mol. Ecol. Resour. 2022, 22, 1875–1891. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Montoya, W.A.; Elfekih, S.; North, H.L.; Meier, J.I.; Warren, I.A.; Tay, W.T.; Gordon, K.H.J.; Specht, A.; Paula-Moraes, S.V.; Rane, R.; et al. Adaptive Introgression across Semipermeable Species Boundaries between Local Helicoverpa zea and Invasive Helicoverpa armigera Moths. Mol. Biol. Evol. 2020, 37, 2568–2583. [Google Scholar] [CrossRef] [PubMed]
- Iannucci, A.; Benazzo, A.; Natali, C.; Arida, E.A.; Zein, M.S.A.; Jessop, T.S.; Bertorelle, G.; Ciofi, C. Population structure, genomic diversity and demographic history of Komodo dragons inferred from whole-genome sequencing. Mol. Ecol. 2021, 30, 6309–6324. [Google Scholar] [CrossRef]
- Pearce, S.L.; Clarke, D.F.; East, P.D.; Elfekih, S.; Gordon, K.H.J.; Jermiin, L.S.; McGaughran, A.; Oakeshott, J.G.; Papanicolaou, A.; Perera, O.P.; et al. Genomic innovations, transcriptional plasticity and gene loss underlying the evolution and divergence of two highly polyphagous and invasive Helicoverpa pest species. BMC Biol. 2017, 15, 63. [Google Scholar] [CrossRef]
- Benelli, G.; Lucchi, A.; Anfora, G.; Bagnoli, B.; Botton, M.; Campos-Herrera, R.; Carlos, C.; Daugherty, M.P.; Gemeno, C.; Harari, A.R.; et al. European grapevine moth, Lobesia botrana. Part I: Biology and ecology. Entomol. Gen. 2023, 43, 261–280. [Google Scholar] [CrossRef]
- Li, X.W.; Fu, K.Y.; Guo, W.C.; Wang, T.Z.; Lu, Y.B. The complete mitochondrial genome of Tuta absoluta (Lepidoptera: Gelechiidae) and genetic variation in two newly invaded populations in China. J. Asia-Pac. Entomol. 2022, 25, 101988. [Google Scholar] [CrossRef]
- Otim, M.H.; Tay, W.T.; Walsh, T.K.; Kanyesigye, D.; Adumo, S.; Abongosi, J.; Ochen, S.; Sserumaga, J.; Alibu, S.; Abalo, G.; et al. Detection of sister-species in invasive populations of the fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) from Uganda. PLoS ONE 2018, 13, e0194571. [Google Scholar] [CrossRef]
- Etebari, K.; Hereward, J.; Sailo, A.; Ahoafi, E.M.; Tautua, R.; Tsatsia, H.; Jackson, G.V.; Furlong, M.J. Examination of population genetics of the Coconut Rhinoceros Beetle (Oryctes rhinoceros) and the incidence of its biocontrol agent (Oryctes rhinoceros nudivirus) in the South Pacific Islands. Curr. Res. Insect Sci. 2021, 1, 100015. [Google Scholar] [CrossRef]
- Marshall, S.D.G.; Moore, A.; Vaqalo, M.; Noble, A.; Jackson, T.A. A new haplotype of the coconut rhinoceros beetle, Oryctes rhinoceros, has escaped biological control by Oryctes rhinoceros nudivirus and is invading Pacific Islands. J. Invertebr. Pathol. 2017, 149, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Reil, J.B.; Doorenweerd, C.; San Jose, M.; Sim, S.B.; Geib, S.M.; Rubinoff, D. Transpacific coalescent pathways of coconut rhinoceros beetle biotypes: Resistance to biological control catalyses resurgence of an old pest. Mol. Ecol. 2018, 27, 4459–4474. [Google Scholar] [CrossRef]
- Tanaka, S.; Harrison, R.L.; Arai, H.; Katayama, Y.; Mizutani, T.; Inoue, M.N.; Miles, J.; Marshall, S.D.G.; Kitalong, C.; Nakai, M. Confirmation of Oryctes rhinoceros nudivirus infections in G-haplotype coconut rhinoceros beetles (Oryctes rhinoceros) from Palauan PCR-positive populations. Sci. Rep. 2021, 11, 18820. [Google Scholar] [CrossRef]
- Elfekih, S.; Etter, P.; Tay, W.T.; Fumagalli, M.; Gordon, K.; Johnson, E.; De Barro, P. Genome-wide analyses of the Bemisia tabaci species complex reveal contrasting patterns of admixture and complex demographic histories. PLoS ONE 2018, 13, e0190555. [Google Scholar] [CrossRef]
- Bedford, G.O. Biology, Ecology, and Control of Palm Rhinoceros Beetles. Annu. Rev. Entomol. 1980, 25, 309–339. [Google Scholar] [CrossRef]
- Indriyanti, D.R.; Utami, Z.T.; Setiati, N.; Soesilowati, E.; Slamet, M. Identification of insect pests that attack the coconut plants in Jepara regency. J. Phys. Conf. Ser. 2019, 1321, 032030. [Google Scholar] [CrossRef]
- Paudel, S.; Mansfield, S.; Villamizar, L.F.; Jackson, T.A.; Marshall, S.D.G. Can Biological Control Overcome the Threat From Newly Invasive Coconut Rhinoceros Beetle Populations (Coleoptera: Scarabaeidae)? A Review. Ann. Entomol. Soc. Am. 2021, 114, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Caasi, J.A.S.; Guerrero, A.L.; Yoon, K.; Aquino, L.J.C.; Moore, A.; Oh, H.; Rychtar, J.; Taylor, D. A mathematical model of invasion and control of coconut rhinoceros beetle Oryctes rhinoceros (L.) in Guam. J. Theor. Biol. 2023, 570, 111525. [Google Scholar] [CrossRef] [PubMed]
- Datt, N.; Gosai, R.C.; Ravuiwasa, K.; Timote, V. Key transboundary plant pests of Coconut [Cocos nucifera] in the Pacific Island countries—A biosecurity perspective. Plant Pathol. Quar. 2020, 10, 152–171. [Google Scholar] [CrossRef]
- Ayivi, S.P.G.; Tong, Y.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. The Mitochondrial Genomes of 18 New Pleurosticti (Coleoptera: Scarabaeidae) Exhibit a Novel trnQ-NCR-trnI-trnM Gene Rearrangement and Clarify Phylogenetic Relationships of Subfamilies within Scarabaeidae. Insects 2021, 12, 1025. [Google Scholar] [CrossRef]
- Filipovic, I.; Hereward, J.P.; Rasic, G.; Devine, G.J.; Furlong, M.J.; Etebari, K. The complete mitochondrial genome sequence of Oryctes rhinoceros (Coleoptera: Scarabaeidae) based on long-read nanopore sequencing. PeerJ 2021, 9, e10552. [Google Scholar] [CrossRef]
- Cheng, C.T.; Jeng, M.L.; Tsai, J.F.; Li, C.L.; Wu, L.W. Two mitochondrial genomes of Taiwanese rhinoceros beetles, Oryctes rhinoceros and Eophileurus chinensis (Coleoptera: Scarabaeidae). Mitochondrial DNA B Resour. 2021, 6, 2260–2262. [Google Scholar] [CrossRef]
- Anggraini, E.; Vadamalai, G.; Kong, L.L.; Mat, M.; Lau, W.H. Variants in the mitochondrial genome sequence of Oryctes rhinoceros (Coleoptera: Scarabaeidae) infected with Oryctes rhinoceros nudivirus in oil palm and coconut plantations. Sci. Rep. 2023, 13, 16850. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Juhling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Putz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Tay, W.T.; Popa-Baez, A.; Dulla, G.; Blas, A.; Sundalangi, J.; Meldy, H.; Bennette Millado, J.; Melzer, M.; Rane, R.; Hogarty, T.; et al. Mitochondrial COI, ATP6, and COIII Complete Sequence Database for Coconut Rhinoceros Beetles (Oryctes rhinoceros) from Native and Introduced Ranges; CSIRO: Canberra, Australia, 2024; Volume 1. [Google Scholar] [CrossRef]
- Tay, W.T.; Popa-Baez, A.; Dulla, G.; Blas, A.; Sundalangi, J.; Meldy, H.; Bennette Millado, J.; Melzer, M.; Rane, R.; Hogarty, T.; et al. Draft Mitochondrial Genome Protein Coding Genes of the Coconut Rhinoceros Beetles (Oryctes rhinoceros) from Native and Introduced Ranges; CSIRO: Canberra, Australia, 2024; Volume 1. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Huson, D.H.; Richter, D.C.; Rausch, C.; Dezulian, T.; Franz, M.; Rupp, R. Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinform. 2007, 8, 460. [Google Scholar] [CrossRef]
- Kaminski, M.M.; Abudayyeh, O.O.; Gootenberg, J.S.; Zhang, F.; Collins, J.J. CRISPR-based diagnostics. Nat. Biomed. Eng. 2021, 5, 643–656. [Google Scholar] [CrossRef]
- Gill, P.; Hadian Amree, A. AS-LAMP: A New and Alternative Method for Genotyping. Avicenna J. Med. Biotechnol. 2020, 12, 2–8. [Google Scholar] [PubMed]
- Hoffmann, B.D.; Tay, W.T.; Blas, A.L. Biosecurity interceptions of Coconut Rhinoceros Beetle Oryctes rhinoceros. Manag. Biol. Invasions 2024, 15, 437–443. [Google Scholar] [CrossRef]
- Annon. Rhino beetle takes root. Marshall Isl. J. 2023. Available online: https://marshallislandsjournal.com/rhino-beetle-takes-root/ (accessed on 17 March 2024).
- HDOA (Hawaii Department of Agriculture). Coconut Rhinoceros Beetle Information. Available online: https://hdoa.hawaii.gov/pi/main/crb/ (accessed on 10 April 2024).
- Anderson, C.J.; Oakeshott, J.G.; Tay, W.T.; Gordon, K.H.J.; Zwick, A.; Walsh, T.K. Hybridization and gene flow in the mega-pest lineage of moth, Helicoverpa. Proc. Natl. Acad. Sci. USA 2018, 115, 5034–5039. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).