Bacterial Community of Heermann’s Gull (Larus heermanni): Insights into Their Most Common Species and Their Functional Role during the Breeding Season in the Gulf of California
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Area, Sampling, DNA Extraction, and Molecular Sexing
2.2. Isolation of Metagenomic DNA and Massive Semiconductor Sequencing
2.3. Library Analysis
2.4. Bioinformatic and Reference Operational Taxonomic Units (OTUs)
2.5. Search and Identification of Chimeric Sequences
2.6. Taxonomic Assignment
2.7. Alpha and Beta Diversity Analyses
2.8. Metabolic Predictions
2.9. Identification of Pathogens of Birds and Humans
2.10. Determination of Correlations Between Species
3. Results
3.1. Partial 16S rRNA Gene Sequencing and Library Preprocessing
3.2. Taxonomic Bacteriome Composition
3.3. Richness, Alpha, and Beta Diversity
3.4. Assessed Metabolic Predictions
3.5. Identification of Pathogenic Bacteria
3.6. Correlations between Members of HG Gut Bacteriome
4. Discussion
4.1. HG Gut Bacteriome Diversity
4.2. HG Gut Bacteriome Diversity Unassociate with Sex
4.3. Funtional Roles of the HG Gut Bacteriome Based on Metabolic Recostruction of Dominant Species
4.4. Search for Pathogens in Different Animals
4.5. Correlations bewteen Dominant Members of HG Gut Bacteriome
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- White, J.; Mirleau, P.; Danchin, E.; Mulard, H.; Hatch, S.A.; Heeb, P.; Wagner, R.H. Sexually transmitted bacteria affect female cloacal assemblages in a wild bird. Ecol. Lett. 2010, 13, 1515–1524. [Google Scholar] [CrossRef] [PubMed]
- Knell, R.J. Sexually transmitted disease and parasite-mediated sexual selection. Evolution 1999, 53, 957–961. [Google Scholar] [CrossRef]
- Pedroso, A.; Maurer, J.; Cheng, Y.; Lee, M. Remodeling the intestinal ecosystem toward better performance and intestinal health. J. Appl. Poult. Res. 2012, 21, 432–443. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Escallón, C.; Belden, L.K.; Moore, I.T. The Cloacal Microbiome Changes with the Breeding Season in a Wild Bird. Integr. Org. Biol. 2019, 1, oby009. [Google Scholar] [CrossRef] [PubMed]
- Noguera, J.C.; Aira, M.; Pérez-Losada, M.; Domínguez, J.; Velando, A. Glucocorticoids modulate gastrointestinal microbiome in a wild bird. R. Soc. Open Sci. 2018, 5, 171743. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef]
- Benskin, C.M.; Wilson, K.; Jones, K.; Hartley, I.R. Bacterial pathogens in wild birds: A review of the frequency and effects of infection. Biol. Rev. Camb. Philos. Soc. 2009, 84, 349–373. [Google Scholar] [CrossRef]
- Poiani, A. Do Cloacal Pathogenic Microbes Behave as Sexually Transmitted Parasites in Birds? Open Ornithol. J. 2010, 3, 72–85. [Google Scholar] [CrossRef]
- Loehle, C. Social Barriers to Pathogen Transmission in Wild Animal Populations. Ecology 1995, 76, 326–335. [Google Scholar] [CrossRef]
- Thrall, P.H.; Antonovics, J.; Bever, J.D. Sexual transmission of disease and host mating systems: Within-season reproductive success. Am. Nat. 1997, 149, 485–506. [Google Scholar] [CrossRef]
- Monaghan, P.; Shedden, C.B.; Ensor, K.; Fricker, C.R.; Girdwood, R.W.A. Salmonella Carriage by Herring-Gulls in the Clyde Area of Scotland in Relation to Their Feeding Ecology. J. Appl. Ecol. 1985, 22, 669–680. [Google Scholar] [CrossRef]
- Fuirst, M.; Veit, R.R.; Hahn, M.; Dheilly, N.; Thorne, L.H. Effects of urbanization on the foraging ecology and microbiota of the generalist seabird Larus argentatus. PloS ONE 2018, 13, e0209200. [Google Scholar] [CrossRef]
- Lu, J.; Santo Domingo, J.W.; Lamendella, R.; Edge, T.; Hill, S. Phylogenetic diversity and molecular detection of bacteria in gull feces. Appl. Environ. Microbiol. 2008, 74, 3969–3976. [Google Scholar] [CrossRef]
- Contreras-Rodríguez, A.; Aguilera-Arreola, M.G.; Osorio, A.R.; Martin, M.D.; Guzmán, R.L.; Velarde, E.; Ruiz, E.A. Detection of Potential Human Pathogenic Bacteria Isolated From Feces of Two Colonial Seabirds Nesting on Isla Rasa, Gulf of California: Heermann’s Gull (Larus heermanni) and Elegant Tern (Thalasseus elegans). Trop. Conserv. Sci. 2019, 12, 1–8. [Google Scholar] [CrossRef]
- Swayne, D.E. Diseases of Poultry, 14th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; p. 1451. [Google Scholar]
- Velarde, E. Breeding biology of Heermann’s Gulls on Isla Rasa, Gulf of California, Mexico. Auk 1999, 116, 513–519. [Google Scholar] [CrossRef]
- Alvarez-Borrego, S. Physical Oceanography. In A New Island Biography of the Sea of Cortes; Chase, T.J., Cody, M.L., Ezcurra, E., Eds.; Oxford University Press: Oxford, UK, 2002; pp. 41–59. [Google Scholar]
- Bird International. Larus heermanni. The IUCN Red List of Threatened Species 2020. Available online: https://www.iucnredlist.org/species/22694296/178958787 (accessed on 14 November 2023).
- Mancilla-Morales, M.D.; Velarde, E.; Aguilar, A.; Contreras-Rodriguez, A.; Ezcurra, E.; Rosas-Rodriguez, J.A.; Sonanez-Organis, J.G.; Ruiz, E.A. Strong Philopatry, Isolation by Distance, and Local Habitat Have Promoted Genetic Structure in Heermann’s Gull. Diversity 2022, 14, 108. [Google Scholar] [CrossRef]
- Longmire, L.J.; Maltbie, M.; Baker, J.R. Use of "Lysis buffer" in DNA isolation and Its implication for museum collections. Mus. Tex. Tech Univ. 1997, 163, 1–3. [Google Scholar]
- Fridolfsson, A.K.; Ellegren, H. A simple and universal method for molecular sexing of non-ratit birds. J. Avian Biol. 1999, 30, 116–121. [Google Scholar] [CrossRef]
- Herlemann, D.P.; Labrenz, M.; Jürgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.M.; Comeau, A.M.; Langille, M.G.I. Processing a 16S rRNA Sequencing Dataset with the Microbiome Helper Workflow. In Microbiome Analysis: Methods in Molecular Biology; Beiko, R., Hsiao, W., Parkinson, J., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1849, pp. 131–141. [Google Scholar] [CrossRef]
- Leggett, R.M.; Ramirez-Gonzalez, R.H.; Clavijo, B.J.; Waite, D.; Davey, R.P. Sequencing quality assessment tools to enable data-driven informatics for high throughput genomics. Front. Genet. 2013, 4, 288. [Google Scholar] [CrossRef] [PubMed]
- Rincón-Molina, C.I.; Hernández-García, J.A.; Rincón-Rosales, R.; Gutiérrez-Miceli, F.A.; Ramírez-Villanueva, D.A.; González-Terreros, E.; Peña-Ocaña, B.A.; Palomeque-Domínguez, H.; Dendooven, L.; Ruíz-Valdiviezo, V.M. Structure and Diversity of the Bacterial Communities in the Acid and Thermophilic Crater-Lake of the Volcano “El Chichón”, Mexico. Geomicrobiol. J. 2019, 36, 97–109. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Chao, A.; Lee, S.-M.; Chen, T.-C. A Generalized Good’s Nonparametric Coverage Estimator. Chin. J. Math. 1988, 16, 189–199. [Google Scholar]
- Huson, D.H.; Auch, A.F.; Qi, J.; Schuster, S.C. MEGAN analysis of metagenomic data. Genome Res. 2007, 17, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Lee, S.-M. Estimating the Number of Classes via Sample Coverage. J. Am. Stat. Assoc. 1992, 87, 210–217. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1949. [Google Scholar]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta diversity as the variance of community data: Dissimilarity coefficients and partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis; Palaeontologia Electronica: Oslo, Norway, 2001. [Google Scholar]
- Abdi, H. Bonferroni and Sidák Corrections for Multiple Comparisons. In Encyclopedia of Measurement and Statistics; Salkind, C.S.N., Ed.; SAGE Publications: Dallas, TX, USA, 2007; pp. 103–107. [Google Scholar]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Vega Thurber, R.L.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef]
- Cockerham, S.; Lee, B.; Orben, R.A.; Suryan, R.M.; Torres, L.G.; Warzybok, P.; Bradley, R.; Jahncke, J.; Young, H.S.; Ouverney, C.; et al. Microbial Ecology of the Western Gull (Larus occidentalis). Microb. Ecol. 2019, 78, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Kreisinger, J.; Cízková, D.; Kropácková, L.; Albrecht, T. Cloacal Microbiome Structure in a Long-Distance Migratory Bird Assessed Using Deep 16sRNA Pyrosequencing. PLoS ONE 2015, 10, e0137401. [Google Scholar] [CrossRef] [PubMed]
- Musitelli, F.; Ambrosini, R.; Rubolini, D.; Saino, N.; Franzetti, A.; Gandolfi, I. Cloacal microbiota of barn swallows from Northern Italy. Ethol. Ecol. Evol. 2018, 30, 362–372. [Google Scholar] [CrossRef]
- Godon, J.J.; Arulazhagan, P.; Steyer, J.P.; Hamelin, J. Vertebrate bacterial gut diversity: Size also matters. BMC Ecol. 2016, 16, 12. [Google Scholar] [CrossRef] [PubMed]
- Reese, A.T.; Dunn, R.R. Drivers of Microbiome Biodiversity: A Review of General Rules, Feces, and Ignorance. Mbio 2018, 9, 10–1128. [Google Scholar] [CrossRef]
- Bodawatta, K.H.; Sam, K.; Jonsson, K.A.; Poulsen, M. Comparative Analyses of the Digestive Tract Microbiota of New Guinean Passerine Birds. Front. Microbiol. 2018, 9, 1830. [Google Scholar] [CrossRef]
- Hernández-Becerril, D.U. Biodiversity of marine planktonic algae (Cyanobacteria, Prasinophyceae, Euglenophyta, Chrysophyceae, Dictyochophyceae, Eustigmatophyceae, Parmophyceae, Raphidophyceae, Bacillariophyta, Cryptophyta, Haptophyta, Dinoflagellata) in Mexico. Rev. Mex. Biodivers. 2014, 85, 44–53. [Google Scholar] [CrossRef]
- Velarde, E.; Cartron, J.L.E.; Drummond, H.; Anderson, D.W.; Rebon Gallardo, F.; Palacios, E.; Rodriguez, C. Nesting seabirds of the Gulf of California’s offshore islands: Diversity, ecology and conservation. In Biodiveristy, Ecosystemes, and Conservation in Northern Mexico; Cartron, J.L.E., Ceballos, G., Felger, R.S., Eds.; Oxford University Press: New York, NY, USA, 2005; pp. 452–470. [Google Scholar]
- Renouf, M.; Hendrich, S. Is a Putative Bacterial Species Associated with the Degradation of the Isoflavone Genistein in Human Feces. J. Nutr. 2011, 141, 1120–1126. [Google Scholar] [CrossRef]
- Hehemann, J.H.; Correc, G.; Barbeyron, T.; Helbert, W.; Czjzek, M.; Michel, G. Transfer of carbohydrate-active enzymes from marine bacteria to Japanese gut microbiota. Nature 2010, 464, 908–912. [Google Scholar] [CrossRef]
- Martens, E.C.; Lowe, E.C.; Chiang, H.; Pudlo, N.A.; Wu, M.; McNulty, N.P.; Abbott, D.W.; Henrissat, B.; Gilbert, H.J.; Bolam, D.N.; et al. Recognition and Degradation of Plant Cell Wall Polysaccharides by Two Human Gut Symbionts. PLoS Biol. 2011, 9, e1001221. [Google Scholar] [CrossRef]
- Espinoza-Avalos, J. Macroalgas marinas del Golfo de California. In Biodiversidad Marina y Costera de México; Salazar-Vallejo, S., González, N., Eds.; Comisión Nacional Para el Conocimiento y Aprovechamiento de la Biodiversidad: Mexico City, Mexico, 1993; pp. 328–357. [Google Scholar]
- Ferreira-Halder, C.V.; Faria, A.V.D.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, V.; Wang, J.; Van Parys, A.; Haesebrouck, F.; Joossens, M.; Falony, G.; Raes, J.; Ducatelle, R.; Van Immerseel, F. The Probiotic Butyricicoccus pullicaecorum Reduces Feed Conversion and Protects from Potentially Harmful Intestinal Microorganisms and Necrotic Enteritis in Broilers Front. Microbiol. 2016, 7, 1416. [Google Scholar] [CrossRef]
- Liu, X.; Mao, B.; Gu, J.; Wu, J.; Cui, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Blautia—A new functional genus with potential probiotic properties? Gut Microbes 2021, 13, 1875796. [Google Scholar] [CrossRef]
- Yu, X.; Åvall-Jääskeläinen, S.; Koort, J.; Lindholm, A.; Rintahaka, J.; Von Ossowski, I.; Palva, A.; Hynönen, U. A comparative characterization of different host-sourced Lactobacillus ruminis strains and their adhesive, inhibitory, and immunomodulating functions. Front. Microbiol. 2017, 5, 657. [Google Scholar] [CrossRef]
- Singh, T.P.; Kaur, G.; Kapila, S.; Malik, R.K. Antagonistic activity of Lactobacillus reuteri strains on the adhesion characteristics of selected pathogens. Front. Microbiol. 2017, 8, 486. [Google Scholar] [CrossRef]
- Liu, H.; Ji, H.F.; Zhang, D.Y.; Wang, S.X.; Wang, J.; Shan, D.C.; Wang, Y.M. Effects of Lactobacillus brevis preparation on growth performance, fecal microflora and serum profile in weaned pigs. Livest. Sci. 2015, 178, 251–254. [Google Scholar] [CrossRef]
- Buckles, E.; Ruiz, J.; Korich, J.; Torres, A.; Banda, A.; Mondal, S.; Lucio-Martínez, B. Atlas of Avian Diseases. Available online: https://partnersah.vet.cornell.edu/avian-atlas/#/disease/Avian_Encephalomyelitis (accessed on 22 May 2024).
- Kirkwood, J.K.; Macgregor, S.K. Infectious Diseases of Garden Birds—Minimising the Risks. Available online: https://www.ufaw.org.uk/animal-welfare-publications/infectious-diseases-of-garden-birds---minimising-the-risks (accessed on 22 May 2024).
- Canturri, A.; Doria-Torra, G.; Casanova, I.; Martínez, J.; Domingo, M.; Majó, N. Espondilitis enterocócica Asociada a Enterococcus cecorum en Reproductores y Aves de Engorde. Available online: https://www.wpsa-aeca.es/articulo.php?id_articulo=4070 (accessed on 22 May 2024).
- Zhao, C.; Tang, N.; Wu, Y.; Zhang, Y.; Wu, Z.; Li, W.; Qin, X.; Zhao, J.; Zhang, G. First reported fatal Morganella morganii infections in chickens. Vet. Microbiol. 2012, 156, 452–455. [Google Scholar] [CrossRef]
- Saidenberg, A.B.S.; Teixeira, R.H.F.; Astolfi-Ferreira, C.S.; Knöbl, T.; Ferreira, A.J.P. Serratia marcescens infection in a swallow-tailed hummingbird. J. Wildlife Dis. 2007, 43, 107–110. [Google Scholar] [CrossRef][Green Version]
- Bjornsdottir-Butler, K.; McCarthy, S.A.; Dunlap, P.V.; Benner, R.A. Photobacterium angustum and Photobacterium kishitanii, Psychrotrophic High-Level Histamine-Producing Bacteria Indigenous to Tuna. Appl. Environ. Microb. 2016, 82, 2167–2176. [Google Scholar] [CrossRef]
- Dhanashekar, R.; Akkinepalli, S.; Nellutla, A. Milk-borne infections. An analysis of their potential effect on the milk industry. Germs 2012, 2, 101–109. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, J.W.; Lee, M.K.; Kim, J.G. Septicemia progressing to fatal hepatic dysfunction in an cirrhotic patient after oral ingestion of Photobacterium damsela: A case report. Infection 2009, 37, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Hammerum, A.M. Enterococci of animal origin and their significance for public health. Clin. Microbiol. Infec. 2012, 18, 619–625. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, L.M.; Daborn, C.J. The epidemiology of Mycobacterium bovis infections in animals and man: A review. Tuber. Lung Dis. 1995, 76, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Management of human and animal bite wounds: An overview. Adv. Skin Wound Care 2005, 18, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Asante, J.; Noreddin, A.; El Zowalaty, M.E. Systematic Review of Important Bacterial Zoonoses in Africa in the Last Decade in Light of the ‘One Health’ Concept. Pathogens 2019, 8, 50. [Google Scholar] [CrossRef]
- Grim, K.D.; Doherty, C.; Rosen, T. Serratia marcescens bullous cellulitis after iguana bites. J. Am. Acad. Dermatol. 2010, 62, 1075–1076. [Google Scholar] [CrossRef]
- Howell, J.M.; Dalsey, W.C. Aerobic-Bacteria Cultured from the Mouth of the American Opossum (Didelphis virginiana) with Reference to Bacteria Associated with Bite Infections. J. Clin. Microbiol. 1990, 28, 2360–2361. [Google Scholar] [CrossRef]
- Sachett, J.A.G.; da Silva, I.M.; Alves, E.C.; Oliveira, S.S.; Sampaio, V.S.; do Vale, F.F.; Romero, G.A.S.; dos Santos, M.C.; Marques, H.O.; Colombini, M.; et al. Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from snakebites in the Brazilian Amazon: A randomized controlled clinical trial. PLoS Neglect. Trop. Dis. 2017, 11, e0005745. [Google Scholar] [CrossRef]
- Henke, M.T.; Kenny, D.J.; Cassilly, C.D.; Vlamakis, H.; Xavier, R.J.; Clardy, J. Ruminococcus gnavus, a member of the human gut microbiome associated with Crohn’s disease, produces an inflammatory polysaccharide. Proc. Natl. Acad. Sci. USA 2019, 116, 12672–12677. [Google Scholar] [CrossRef]
- Bonwitt, J.; Tran, M.; Droz, A.; Gonzalez, A.; Glover, W.A. Wound Infection Associated with Marine Environment Exposure, Washington, USA. Emerg. Infect. Dis. 2018, 24, 1942–1944. [Google Scholar] [CrossRef]
- Vela, A.I.; Collins, M.D.; Latre, M.V.; Mateos, A.; Moreno, M.A.; Hutson, R.; Domínguez, L.; Fernández-Garayzábal, J.F. Psychrobacter pulmonis sp nov., isolated from the lungs of lambs. Int. J. Syst. Evol. Microbiol. 2003, 53, 415–419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kozinska, A.; Pazdzior, E.; Pekala, A.; Niemczuk, W. Acinetobacter johnsonii and Acinetobacter lwoffii—The emerging fish pathogens. J. Vet. Res. 2014, 58, 193–199. [Google Scholar] [CrossRef]
- Hundenborn, J.; Thurig, S.; Kommerell, M.; Haag, H.; Nolte, O. Severe Wound Infection with Photobacterium damselae ssp. damselae and Vibrio harveyi, following a Laceration Injury in Marine Environment: A Case Report and Review of the Literature. Case Rep. Med. 2013, 2013, 610632. [Google Scholar] [CrossRef] [PubMed]
- Drago, L. Prevotella Copri and Microbiota in Rheumatoid Arthritis: Fully Convincing Evidence? J. Clin. Med. 2019, 8, 1837. [Google Scholar] [CrossRef] [PubMed]
- Hatziioanou, D.; Gherghisan-Filip, C.; Saalbach, G.; Horn, N.; Wegmann, U.; Duncan, S.H.; Flint, H.J.; Mayer, M.J.; Narbad, A. Discovery of a novel lantibiotic nisin O from Blautia obeum A2-162, isolated from the human gastrointestinal tract. Microbiology 2017, 163, 1292–1305. [Google Scholar] [CrossRef]
- Hosseini, P.R.; Mills, J.N.; Prieur-Richard, A.-H.; Ezenwa, V.O.; Bailly, X.; Rizzoli, A.; Suzán, G.; Vittecoq, M.; García-Peña, G.E.; Daszak, P.; et al. Does the impact of biodiversity differ between emerging and endemic pathogens? The need to separate the concepts of hazard and risk. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160129. [Google Scholar] [CrossRef]
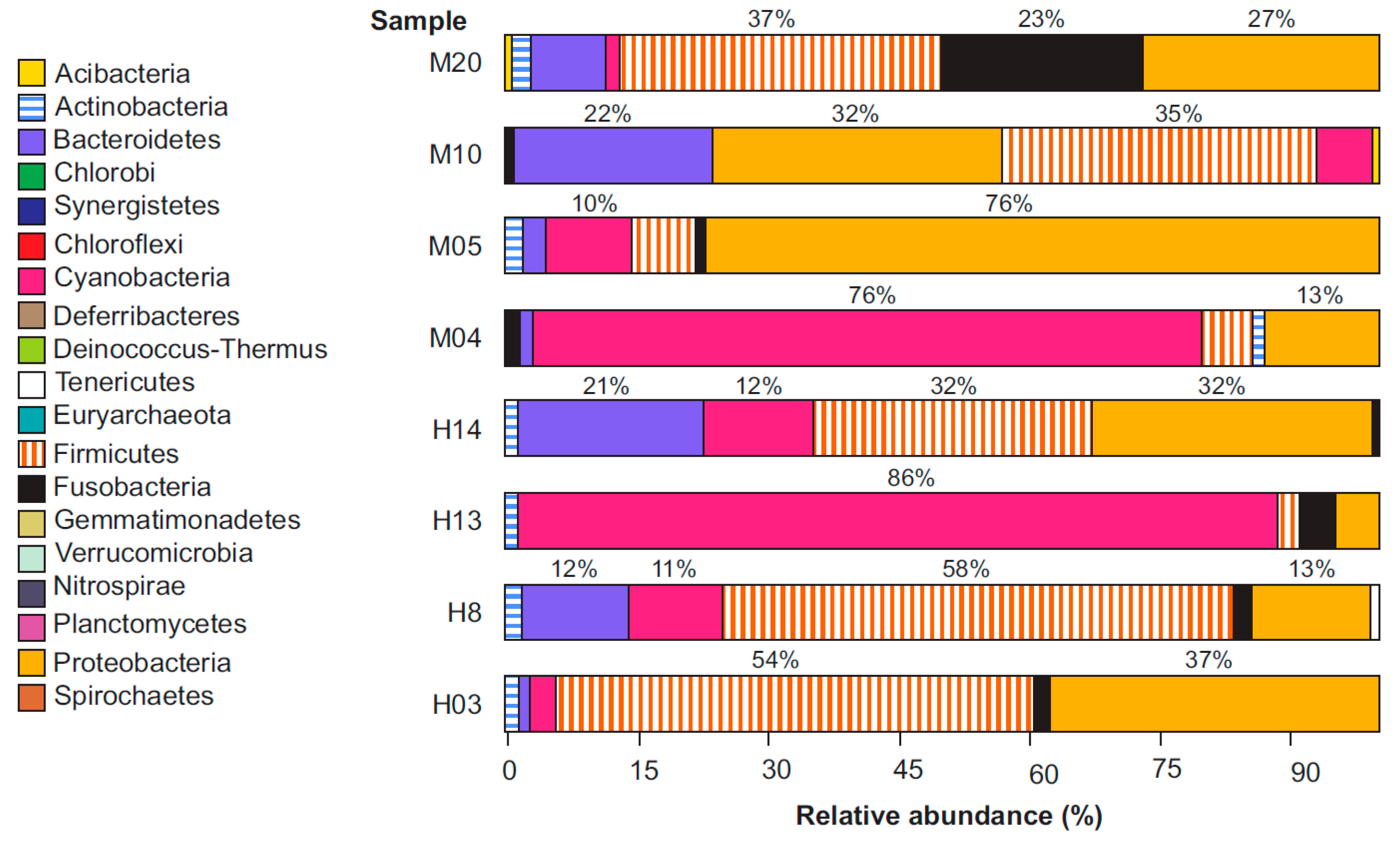
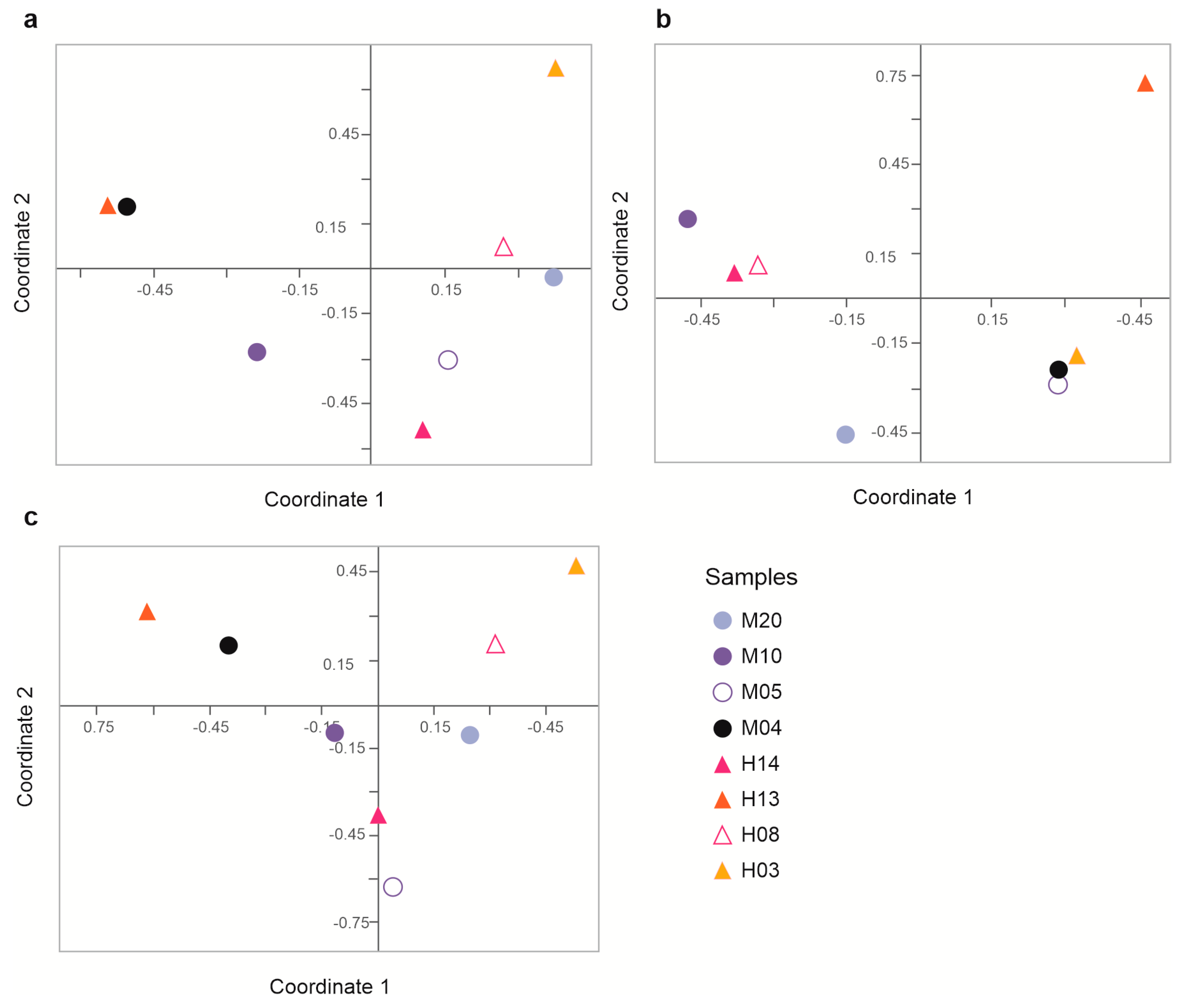
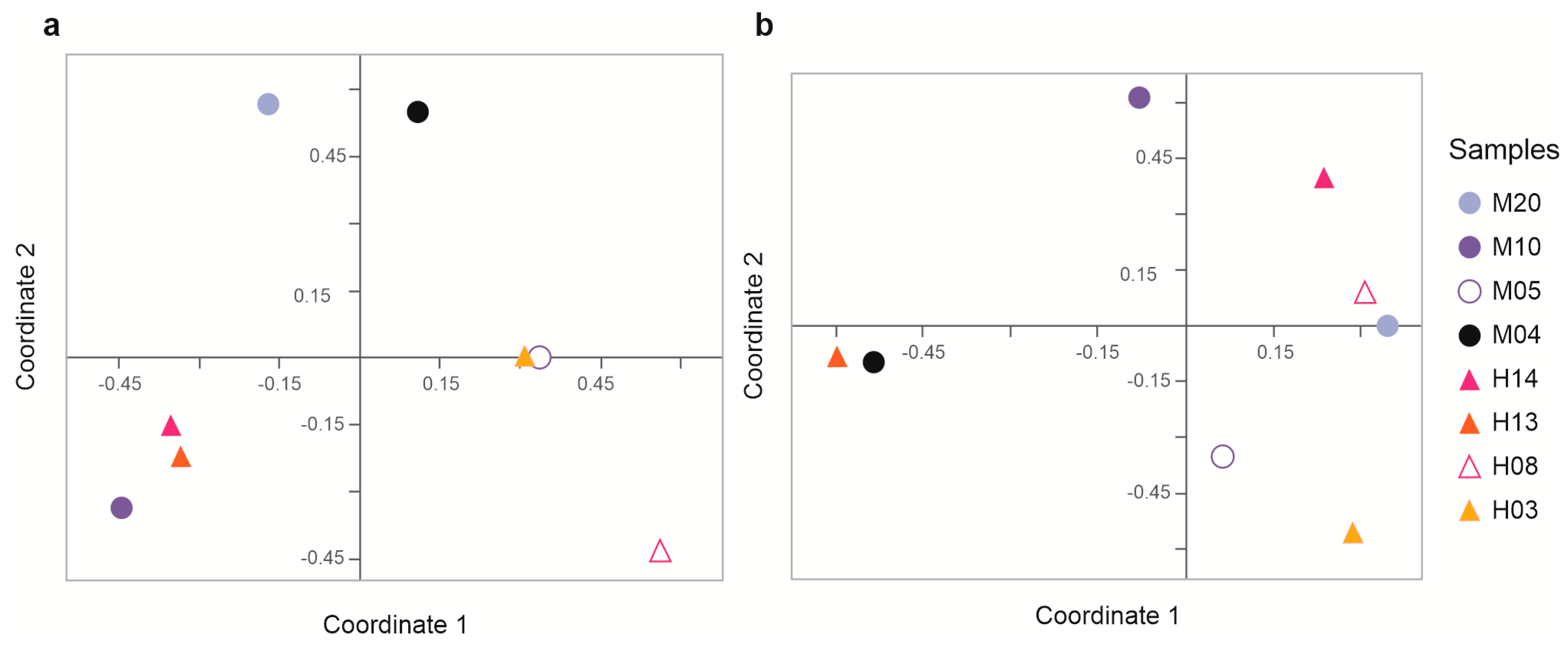
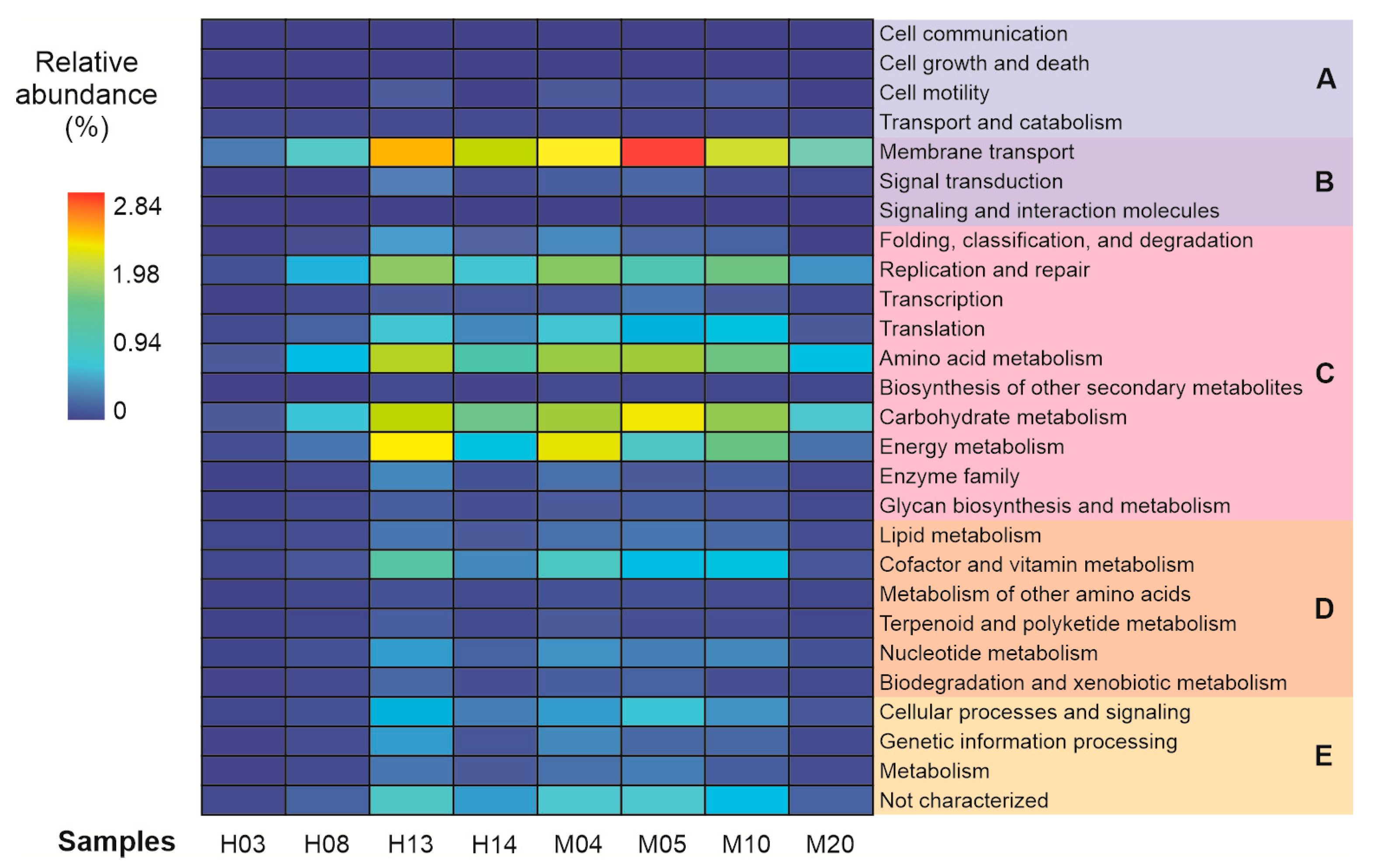
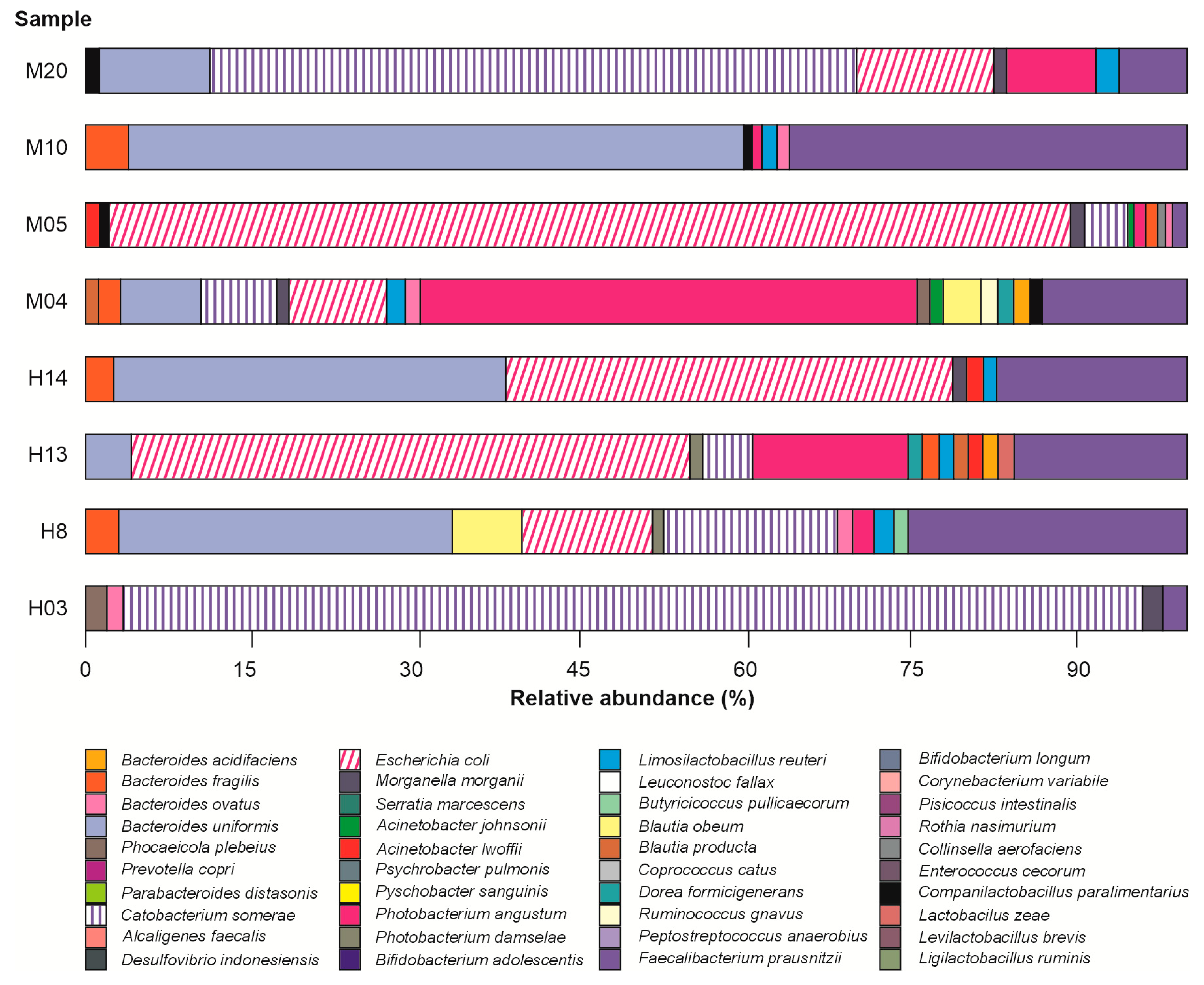
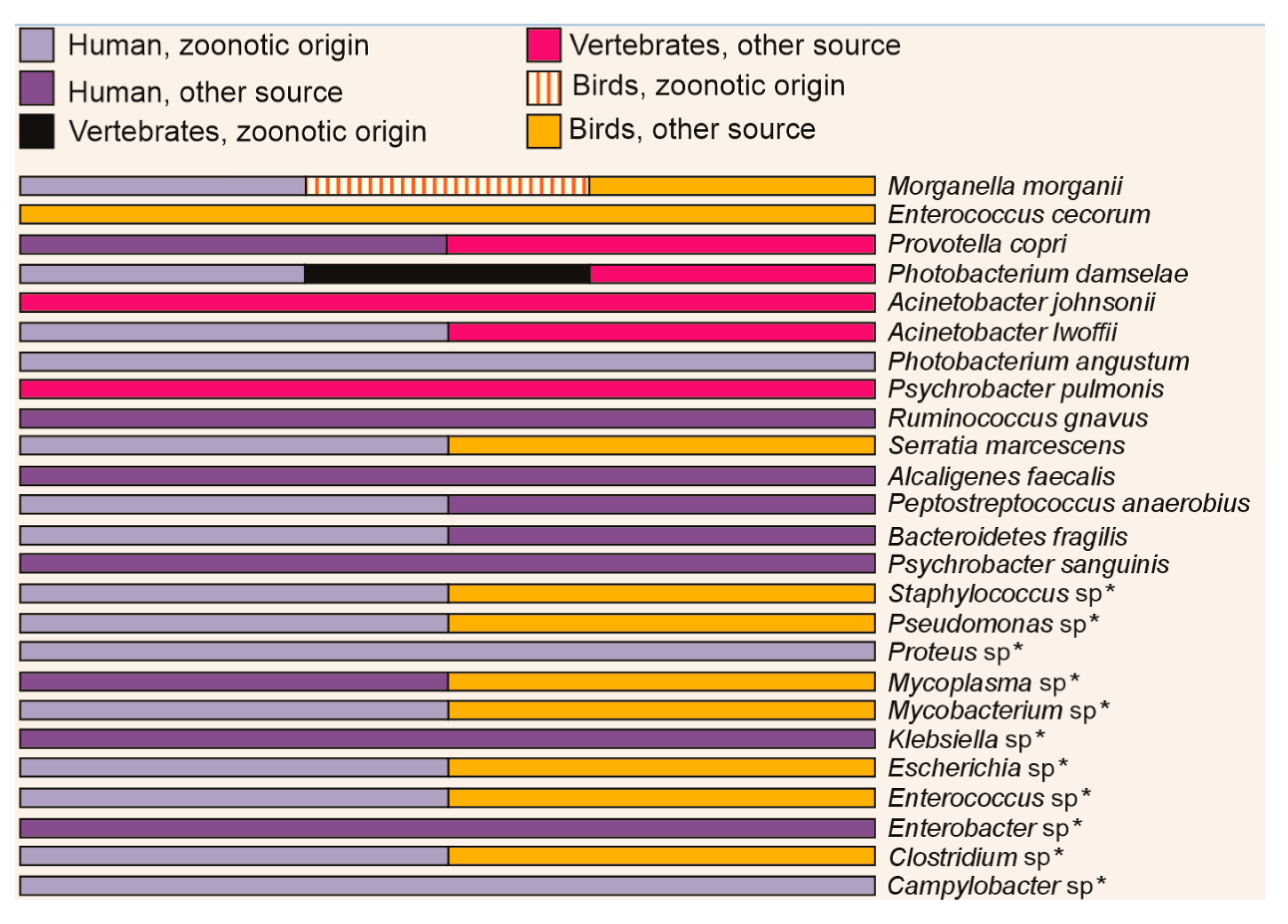
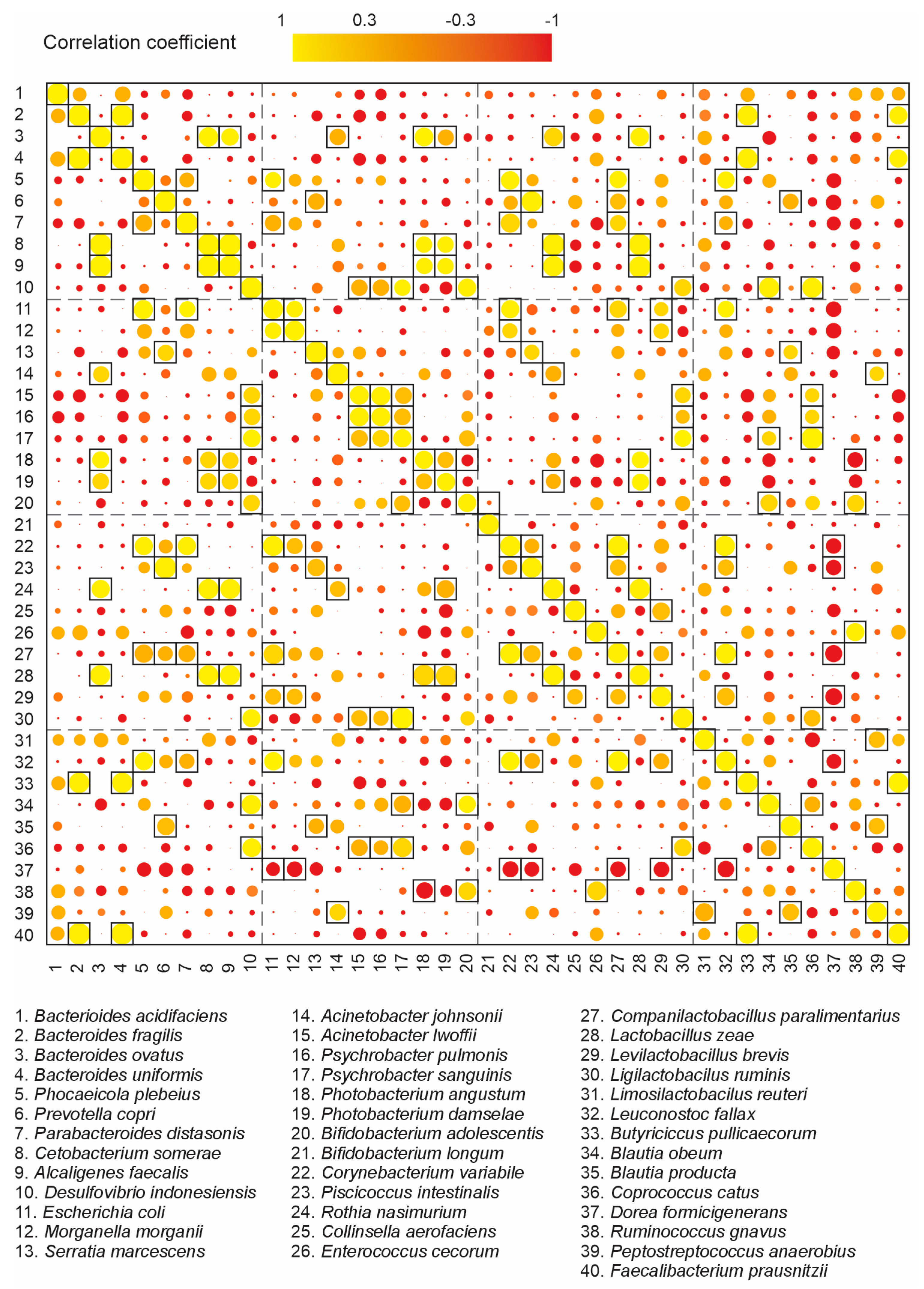
| Sample | Number of Sequences | Number of Sequences Recovered | % Sequences Recovered | Number of Bases (nt) | Mean of Readings (nt) | Quality (Q20)% | GC% |
|---|---|---|---|---|---|---|---|
| H3 | 197,304 | 139,342 | 70.62 | 5.89 × 10−7 | 422 | 87.13 | 51.31 |
| H08 | 124,918 | 111,485 | 89.25 | 4.69 × 10−7 | 420 | 86.16 | 51.51 |
| H13 | 98,613 | 95,371 | 96.71 | 3.90 × 10−7 | 409 | 82.86 | 54.79 |
| H14 | 112,096 | 95,164 | 84.9 | 3.98 × 10−7 | 418 | 86.26 | 51.67 |
| M04 | 109,037 | 107,053 | 98.18 | 4.40 × 10−7 | 410 | 75.76 | 54.98 |
| M05 | 98,797 | 86,156 | 87.21 | 3.65 × 10−7 | 423 | 86.45 | 53.61 |
| M10 | 197,294 | 160,066 | 81.13 | 6.65 × 10−7 | 415 | 82.6 | 51.53 |
| M20 | 99,380 | 90,803 | 91.37 | 3.82 × 10−7 | 420 | 84.11 | 51.3 |
| Estimator | Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H03 | H08 | H13 | H14 | Female Mean (SD) | M04 | M05 | M10 | M20 | Male Mean (SD) | Total Mean (SD) | |
| Species richness | 774 | 1003 | 350 | 1102 | 807.3 (334.4) | 828 | 782 | 934 | 1415 | 989.8 (290.6) | 898.5 (306) |
| Chao 1 | 1020.1 | 1345.9 | 553.3 | 1538.7 | 1114.5 (431) | 1173.1 | 1085.5 | 1387.8 | 1893.3 | 1384.9 (361.9) | 1249.7 (395.8) |
| ACE | 1099.7 | 1310.6 | 618.7 | 1557.3 | 1146.6 (398.5) | 1201.4 | 1077.1 | 1427.5 | 1849.8 | 1388.9 (339.8) | 1267.8 (366.5) |
| Good’s coverage | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 (0) | 0.99 | 0.99 | 0.98 | 0.98 | 0.99 (0) | 0.99 (0) |
| Shannon | 4.03 | 5.8 | 2.73 | 6.23 | 4.7 (1.6) | 3.9 | 4.95 | 5.84 | 6.35 | 5.3 (1.1) | 4.98 (1.3) |
| Simpson | 0.77 | 0.89 | 0.69 | 0.95 | 0.83 (0.1) | 0.84 | 0.88 | 0.95 | 0.93 | 0.9 (0) | 0.86 (0.1) |
| Phylogenetic diversity | 54.57 | 64.38 | 32.62 | 71.51 | 55.77 (16.9) | 70.68 | 51.98 | 59.98 | 88.6 | 67.8 (15.8) | 61.79 (16.5) |
| M10 | M05 | H08 | M20 | H03 | H14 | M04 | H13 | |
|---|---|---|---|---|---|---|---|---|
| M10 | - | 0.863 10,861.9 | 0.558 9622.4 | 0.785 8867.9 | 0.938 14,639.9 | 0.384 4926.2 | 0.581 7695.3 | 0.63 12,047.3 |
| M05 | 0.837 | - | 0.756 12,531.1 | 0.745 10,736.8 | 0.882 16,132.8 | 0.559 6806.8 | 0.837 13,560.1 | 0.855 16,625.8 |
| H08 | 0.634 | 0.766 | - | 0.532 6721.7 | 0.548 7195.5 | 0.519 9720.5 | 0.792 12,863.7 | 0.825 16,618.3 |
| M20 | 0.762 | 0.779 | 0.725 | - | 0.734 11,149.3 | 0.627 8122.5 | 0.877 12,954.8 | 0.926 16,881.5 |
| H03 | 0.819 | 0.745 | 0.787 | 0.795 | - | 0.924 14,609.6 | 0.903 16,944.2 | 0.929 20,246.1 |
| H14 | 0.605 | 0.74 | 0.629 | 0.715 | 0.787 | - | 0.819 10,622.7 | 0.827 14,231.8 |
| M04 | 0.81 | 0.767 | 0.779 | 0.786 | 0.785 | 0.81 | - | 0.266 5956 |
| H13 | 0.847 | 0.793 | 0.819 | 0.858 | 0.797 | 0.829 | 0.804 | - |
| M10 | M05 | H08 | M20 | H03 | H14 | M04 | H13 | |
|---|---|---|---|---|---|---|---|---|
| M10 | - | 0.663 | 0.498 | 0.624 | 0.650 | 0.462 | 0.645 | 0.695 |
| M05 | 0.431 | - | 0.611 | 0.637 | 0.572 | 0.598 | 0.595 | 0.604 |
| H08 | 0.571 | 0.549 | - | 0.592 | 0.626 | 0.483 | 0.623 | 0.676 |
| M20 | 0.766 | 0.503 | 0.361 | - | 0.632 | 0.564 | 0.561 | 0.716 |
| H03 | 0.25 | 0.509 | 0.395 | 0.5 | - | 0.609 | 0.611 | 0.617 |
| H14 | 0.542 | 0.466 | 0.35 | 0.401 | 0.639 | - | 0.609 | 0.679 |
| M04 | 0.586 | 0.577 | 0.679 | 0.717 | 0.709 | 0.69 | - | 0.668 |
| H13 | 0.431 | 0.629 | 0.721 | 0.762 | 0.753 | 0.728 | 0.134 | - |
| Phyla | Species 1 | Species 2 | Correlation (Positive/Negative) |
|---|---|---|---|
| Firmicutes/Proteobacteria | Dorea formicigenerans | Morganella morganii | negative |
| Enterococcus cecorum | Photobacterium angustum | negative | |
| Ruminococcus gnavus | P. angustum | negative | |
| Blautia obeum | Photobacterium damselae | negative | |
| Firmicutes/Bacteroidetes | Butyricicoccus pullicaecorum | Bacteroides fragilis | positive |
| B. pullicaecorum | Bacteroides uniformis | positive | |
| B. pullicaecorum | Faecalibacterium prausnitzii | positive | |
| Firmicutes/Firmicutes | E. cecorum | Ruminococcus gnavus | positive |
| Peptostreptococcus anaerobius | Limosilactobacillus reuteri | positive | |
| Proteobacteria/Proteobacteria | Morganella morganii | Escherichia coli | positive |
| Acinetobacter lwoffii | Psychrobacter pulmonis | positive | |
| Bacteroidetes/Actinobacteria | Prevotella copri | Piscicoccus intestinalis | positive |
| P. copri | Corynebacterium variable | positive | |
| Firmicutes/Actinobacteria | C. variabile | Levilactobacillus brevis | positive |
| Bacteroidetes/Bacteroidetes | Bacteroides fragilis | Bacteroides uniformis | positive |
| Proteobacteria/Fusobacteria | Acinetobacter johnsonii | Cetobacterium somerae | positive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz, E.A.; Contreras-Rodríguez, A.; Araiza, O.; Aguilera-Arreola, M.G.; Hernández-García, J.A.; Flores-Martínez, J.J.; Sánchez-Cordero, V.; Gomez-Lunar, Z. Bacterial Community of Heermann’s Gull (Larus heermanni): Insights into Their Most Common Species and Their Functional Role during the Breeding Season in the Gulf of California. Diversity 2024, 16, 617. https://doi.org/10.3390/d16100617
Ruiz EA, Contreras-Rodríguez A, Araiza O, Aguilera-Arreola MG, Hernández-García JA, Flores-Martínez JJ, Sánchez-Cordero V, Gomez-Lunar Z. Bacterial Community of Heermann’s Gull (Larus heermanni): Insights into Their Most Common Species and Their Functional Role during the Breeding Season in the Gulf of California. Diversity. 2024; 16(10):617. https://doi.org/10.3390/d16100617
Chicago/Turabian StyleRuiz, Enrico A., Araceli Contreras-Rodríguez, Oliva Araiza, Ma G. Aguilera-Arreola, Juan A. Hernández-García, José J. Flores-Martínez, Víctor Sánchez-Cordero, and Zulema Gomez-Lunar. 2024. "Bacterial Community of Heermann’s Gull (Larus heermanni): Insights into Their Most Common Species and Their Functional Role during the Breeding Season in the Gulf of California" Diversity 16, no. 10: 617. https://doi.org/10.3390/d16100617
APA StyleRuiz, E. A., Contreras-Rodríguez, A., Araiza, O., Aguilera-Arreola, M. G., Hernández-García, J. A., Flores-Martínez, J. J., Sánchez-Cordero, V., & Gomez-Lunar, Z. (2024). Bacterial Community of Heermann’s Gull (Larus heermanni): Insights into Their Most Common Species and Their Functional Role during the Breeding Season in the Gulf of California. Diversity, 16(10), 617. https://doi.org/10.3390/d16100617







