Diet Composition Explains Interannual Fluctuations in Reproductive Performance in a Lowland Golden Eagle Population
Abstract
1. Introduction
2. Materials and Methods
3. Results
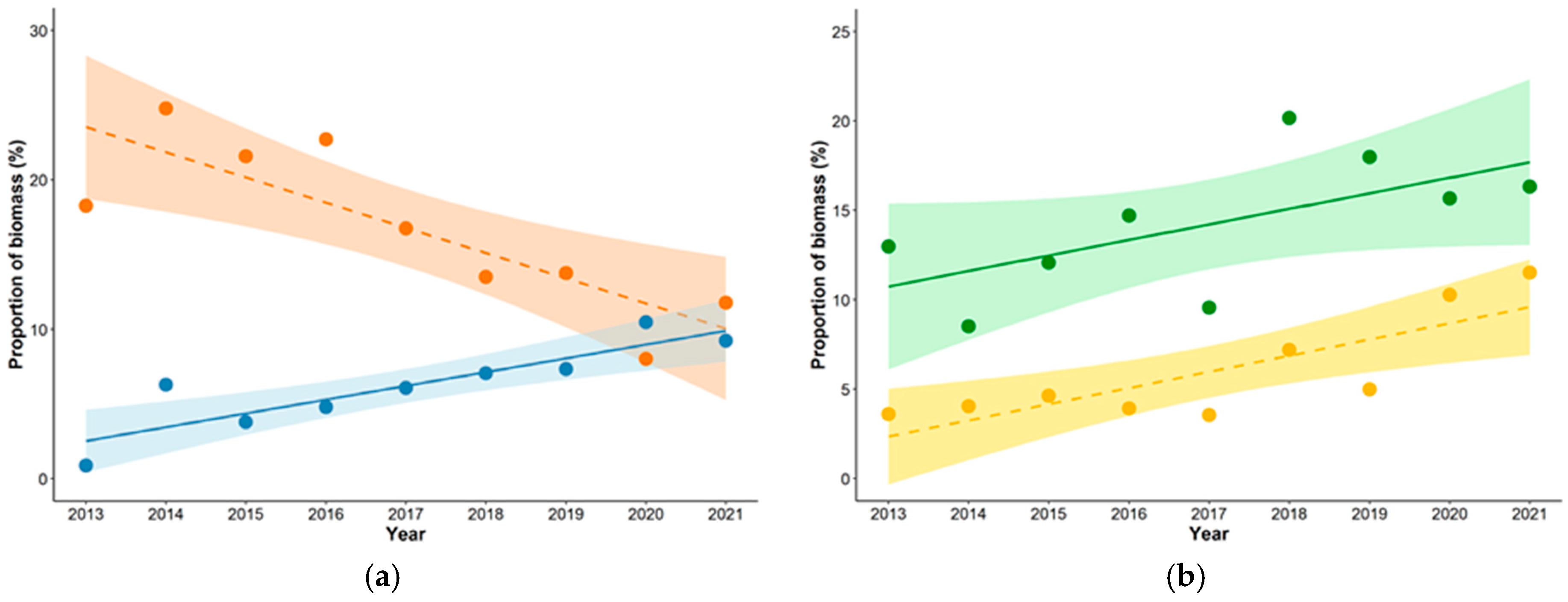
3.1. Diet Composition
3.2. Reproductive Performance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mills, L.S. Conservation of Wildlife Populations: Demography, Genetics, and Management; Wiley-Blackwell: New York, NY, USA, 2006; ISBN 978-1-4051-2146-0. [Google Scholar]
- Fryxell, J.M.; Sinclair, A.R.E.; Caughley, G. Wildlife Ecology, Conservation, and Management; Wiley-Blackwell: Oxford, UK, 2014; ISBN 978-1-118-29107-8. [Google Scholar]
- Williams, G.E. Natural Selection, the Costs of Reproduction, and a Refinement of Lack’s Principle. Am. Nat. 1966, 100, 687–690. [Google Scholar] [CrossRef]
- Newton, I. Population Limitation in Birds; Academic Press: Cambridge, MA, USA, 1998; ISBN 978-0-08-087923-9. [Google Scholar]
- Newton, I. Population Ecology of Raptors; T & AD Poyser: Berkhamsted, UK, 1979; ISBN 978-0-85661-023-3. [Google Scholar]
- Erikstad, K.E.; Fauchald, P.; Tveraa, T.; Steen, H. On the Cost of Reproduction in Long-Lived Birds: The Influence of Environmental Variability. Ecology 1998, 79, 1781–1788. [Google Scholar] [CrossRef]
- Hanski, I.; Hansson, L.; Henttonen, H. Specialist Predators, Generalist Predators, and the Microtine Rodent Cycle. J. Anim. Ecol. 1991, 60, 353–367. [Google Scholar] [CrossRef]
- Korpimäki, E.; Hongisto, K.; Masoero, G.; Laaksonen, T. The Difference between Generalist and Specialist: The Effects of Wide Fluctuations in Main Food Abundance on Numbers and Reproduction of Two Co-Existing Predators. J. Avian Biol. 2020, 51, e02508. [Google Scholar] [CrossRef]
- Whitfield, D.P.; Reid, R.; Haworth, P.F.; Madders, M.; Marquiss, M.; Tingay, R.; Fielding, A.H. Diet Specificity Is Not Associated with Increased Reproductive Performance of Golden Eagles Aquila Chrysaetos in Western Scotland. Ibis 2009, 151, 255–264. [Google Scholar] [CrossRef]
- Watson, J. The Golden Eagle; T & AD Poyser: London, UK, 1997; ISBN 0-85661-099-2. [Google Scholar]
- Bedrosian, G.; Watson, J.W.; Steenhof, K.; Kochert, M.N.; Preston, C.R.; Woodbridge, B.; Williams, G.E.; Keller, K.R.; Crandall, R.H. Spatial and Temporal Patterns in Golden Eagle Diets in the Western United States, with Implications for Conservation Planning. J. Raptor Res. 2017, 51, 347–367. [Google Scholar] [CrossRef]
- Schweiger, A.; Fünfstück, H.-J.; Beierkuhnlein, C. Availability of Optimal-Sized Prey Affects Global Distribution Patterns of the Golden Eagle Aquila Chrysaetos. J. Avian Biol. 2015, 46, 81–88. [Google Scholar] [CrossRef]
- Nyström, J.; Ekenstedt, J.; Angerbjörn, A.; Thulin, L.; Hellström, P.; Dalén, L. Golden Eagles on the Swedish Mountain Tundra—Diet and Breeding Success in Relation to Prey Fluctuations. Ornis Fenn. 2006, 83, 145–152. [Google Scholar]
- Steenhof, K.; Kochert, M.N.; Mcdonald, T.L. Interactive Effects of Prey and Weather on Golden Eagle Reproduction. J. Anim. Ecol. 1997, 66, 350–362. [Google Scholar] [CrossRef]
- Tjemberg, M. Prey Abundance and Reproductive Success of the Golden Eagle Aquila Chrysaetos in Sweden. Ecography 1983, 6, 17–23. [Google Scholar] [CrossRef]
- Tjernberg, M. Diet of the Golden Eagle Aquila Chrysaetos during the Breeding Season in Sweden. Ecography 1981, 4, 12–19. [Google Scholar] [CrossRef]
- Sulkava, S.; Huhtala, K.; Rajala, P.; Tornberg, R. Changes in the Diet of the Golden Eagle Aquila Chrysaetos and Small Game Populations in Finland in 1957–96. Ornis Fenn. 1999, 76, 1–16. [Google Scholar]
- Sulkava, S.; Huhtala, K.; Rajala, P. Diet and Breeding Success of the Golden Eagle in Finland 1958–82. Ann. Zool. Fenn. 1984, 21, 283–286. [Google Scholar]
- Mcintyre, C.L.; Schmidt, J.H. Ecological and Environmental Correlates of Territory Occupancy and Breeding Performance of Migratory Golden Eagles Aquila Chrysaetos in Interior Alaska. Ibis 2012, 154, 124–135. [Google Scholar] [CrossRef]
- Moss, E.H.R.; Hipkiss, T.; Oskarsson, I.; Häger, A.; Eriksson, T.; Nilsson, L.-E.; Halling, S.; Nilsson, P.-O.; Hörnfeldt, B. Long-Term Study of Reproductive Performance in Golden Eagles in Relation to Food Supply in Boreal Sweden. J. Raptor Res. 2012, 46, 248–257. [Google Scholar] [CrossRef]
- Schmidt, J.H.; McIntyre, C.L.; Roland, C.A.; MacCluskie, M.C.; Flamme, M.J. Bottom-up Processes Drive Reproductive Success in an Apex Predator. Ecol. Evol. 2018, 8, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Randla, T. Estonian Birds of Prey; Valgus: Tallinn, Estonia, 1976. [Google Scholar]
- Zastrov, M. Om Kungsörnens (Aquila Chr. chrysaetos L.) Utbredning Och Biologi i Estland. Vår Fågelvärld 1946, 5, 64–80. [Google Scholar]
- Ivanovsky, V.V. Trophic Links Between Birds of Prey and Their Preys and Their Competitors in Wetlands During Nesting Period. Raptors Conserv. 2019, 38, 15–22. [Google Scholar] [CrossRef]
- Ivanovsky, V.V. Birds of Prey of Belarussian Lakeland; VGU P.M. Masherova: Vicebsk, Belarus, 2012. [Google Scholar]
- Bergmanis, U. Klinšu Ērgla Aquila Chrysaetos Atgriešanās Teiču Purvā. Putni Dabā 2000, 10, 6–11. [Google Scholar]
- Lõhmus, A.; Leivits, M.; Pēterhofs, E.; Zizas, R.; Hofmanis, H.; Ojaste, I.; Kurlavičius, P. The Capercaillie (Tetrao Urogallus): An Iconic Focal Species for Knowledge-Based Integrative Management and Conservation of Baltic Forests. Biodivers. Conserv. 2017, 26, 1–21. [Google Scholar] [CrossRef]
- Veeroja, R.; Männil, P.; Jõgisalu, I.; Kübarsepp, M. Status of Game Populations in Estonia and Proposal for Hunting in 2023; Estonian Environment Agency: Tartu, Estonia, 2023. [Google Scholar]
- Randla, T.; Tammur, E. Population Trends and Breeding Success of the Golden Eagle Aquila Chrysaetos in Estonia, 1935–1991. In Eagle Studies; WWGBP: Berlin, Germany; London, UK; Paris, France, 1996; pp. 483–487. [Google Scholar]
- Elts, J.; Leito, A.; Leivits, A.; Luigujõe, L.; Mägi, E.; Nellis, R.; Nellis, R.; Ots, M.; Pehlak, H. Status and Numbers of Estonian Birds, 2008–2012. Hirundo 2013, 26, 80–112. [Google Scholar]
- Elts, J.; Leito, A.; Leivits, M.; Luigujõe, L.; Nellis, R.; Ots, M.; Tammekänd, I.; Väli, Ü. Status and Numbers of Estonian Birds, 2013–2017. Hirundo 2019, 32, 1–39. [Google Scholar]
- Lõhmus, A. Selection of Foraging Habitats by Birds of Prey in North-Western Tartumaa. Hirundo 2001, 14, 27–42. [Google Scholar]
- Sein, G.; Lõhmus, A. Nest-stand and Nest-tree Characteristics of the Golden Eagle in Estonia. Hirundo 2006, 19, 68–80. [Google Scholar]
- Svensson, L.; Mullarney, K.; Zetterström, D.; Grant, P.J. Bird Guide: The Most Complete Guide to the Birds of Britain and Europe, 2nd ed.; Harper Collins: London, UK, 2009; ISBN 978-0-00-726814-6. [Google Scholar]
- Macdonald, D.W.; Barrett, P. Mammals of Britain & Europe; Harper Collins: London, UK, 1993; ISBN 978-0-00-219779-3. [Google Scholar]
- Siivonen, L.; Sulkava, S. Pohjolan Nisäkkäät; Otava: Keuruu, Finland, 1994; ISBN 978-9-51-113004-8. [Google Scholar]
- Haase, A.; Schlusen, J.; Schwenk, K. Featherbase. Available online: https://www.featherbase.info (accessed on 3 December 2023).
- Schubert, S. Vogelfedern—Die Seite Als Bestimmungshilfe Für Mauserfedern Und Rupfungen. Available online: http://www.vogelfedern.de/ (accessed on 3 December 2023).
- Levins, R. Evolution in Changing Environments: Some Theoretical Explorations; Princeton University Press: Princeton, NJ, USA, 1968; ISBN 978-0-691-08062-8. [Google Scholar]
- Kumari, E. Birds of Estonian SSR; Eesti Riiklik Kirjastus: Tallinn, Estonia, 1954. [Google Scholar]
- Aul, J.; Ling, H.; Paaver, K. Mammals of Estonian SSR; Eesti Riiklik Kirjastus: Tallinn, Estonia, 1957. [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023.
- Högström, S.; Wiss, L.-E. Diet of the Golden Eagle Aquila chrysaetos (L.) in Gotland, Sweden during the Breeding Season. Ornis Fenn. 1992, 69, 39–44. [Google Scholar]
- Lõhmus, A. Vole-Induced Regular Fluctuations in the Estonian Owl Populations. Ann. Zool. Fenn. 1999, 36, 167–178. [Google Scholar]
- McIntyre, C.L. Patterns in Nesting Area Occupancy and Reproductive Success of Golden Eagles (Aquila Chrysaetos) in Denali National Park and Preserve, Alaska, 1988–1999. J. Raptor Res. 2002, 36, 50–54. [Google Scholar]
- McIntyre, C.L.; Adams, L.G. Reproductive Characteristics of Migratory Golden Eagles in Denali National Park, Alaska. Condor 1999, 101, 115–123. [Google Scholar] [CrossRef]
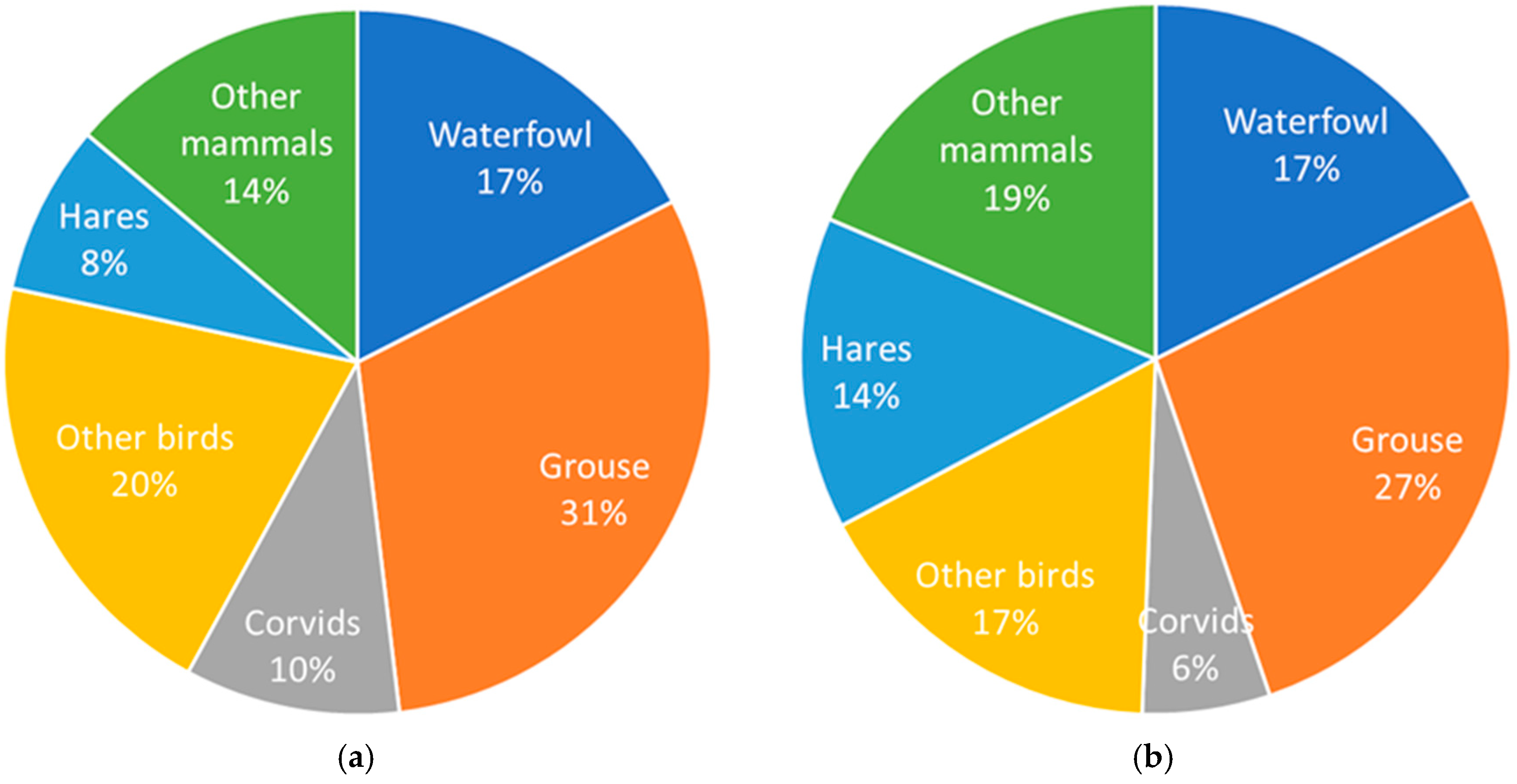


| Numbers (N) | Biomass (B) | Mean (N) | Mean (B) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | ||||
| Black Grouse | Tetrao tetrix | 24.3 | 33.5 | 32.0 | 31.0 | 22.5 | 19.1 | 20.7 | 13.2 | 18.9 | 18.3 | 24.8 | 21.6 | 22.7 | 16.8 | 13.5 | 13.8 | 8.0 | 11.8 | 23.9 | 16.8 |
| Hares | Lepus sp. | 6.5 | 4.3 | 6.7 | 7.5 | 4.8 | 10.7 | 10.1 | 9.7 | 9.8 | 13.0 | 8.5 | 12.1 | 14.7 | 9.6 | 20.2 | 18.0 | 15.7 | 16.3 | 7.8 | 14.2 |
| Common Crane | Grus grus | 5.9 | 2.1 | 3.6 | 3.3 | 5.9 | 4.6 | 3.8 | 4.7 | 6.4 | 18.0 | 6.1 | 9.9 | 9.8 | 17.7 | 13.1 | 10.2 | 11.5 | 16.2 | 4.5 | 12.5 |
| Capercaillie | Tetrao urogallus | 3.2 | 7.2 | 5.2 | 5.1 | 6.4 | 4.6 | 7.6 | 7.0 | 4.2 | 6.4 | 14.0 | 9.2 | 9.9 | 12.6 | 8.5 | 13.3 | 11.2 | 6.8 | 5.6 | 10.2 |
| Mallard | Anas platyrchynchos | 12.4 | 10.5 | 6.2 | 8.7 | 12.8 | 12.2 | 8.9 | 6.2 | 9.5 | 8.7 | 7.2 | 3.9 | 6.0 | 8.9 | 8.1 | 5.5 | 3.5 | 5.5 | 9.8 | 6.4 |
| White-fronted goose | Anser albifrons | 0.5 | 3.9 | 2.6 | 3.0 | 3.7 | 4.6 | 5.1 | 7.9 | 6.8 | 0.9 | 6.3 | 3.8 | 4.8 | 6.1 | 7.1 | 7.3 | 10.5 | 9.2 | 4.2 | 6.3 |
| Racoon Dog | Nyctereutes procyonoides | 1.1 | 6.2 | 5.2 | 5.7 | 2.1 | 3.1 | 4.6 | 4.4 | 3.0 | 1.8 | 10.1 | 7.7 | 9.3 | 3.5 | 4.8 | 6.9 | 5.9 | 4.2 | 3.9 | 6.0 |
| Roe Deer | Capreolus capreolus | 1.1 | 1.2 | 1.5 | 1.2 | 1.1 | 2.3 | 1.7 | 3.8 | 4.2 | 3.6 | 4.1 | 4.6 | 3.9 | 3.5 | 7.2 | 5.0 | 10.3 | 11.5 | 2.0 | 6.0 |
| Raven | Corvus corax | 7.0 | 7.4 | 4.1 | 7.8 | 7.5 | 11.5 | 5.9 | 7.0 | 6.4 | 5.5 | 5.7 | 2.9 | 6.0 | 5.8 | 8.5 | 4.1 | 4.5 | 4.2 | 7.2 | 5.2 |
| Pine Marten | Martes martes | 5.9 | 3.7 | 6.2 | 3.6 | 7.5 | 2.3 | 4.2 | 8.5 | 6.1 | 4.8 | 2.9 | 4.5 | 2.8 | 6.0 | 1.7 | 3.0 | 5.5 | 4.0 | 5.3 | 3.9 |
| Bean Goose | Anser fabalis | 0.5 | 1.0 | 2.6 | 1.5 | 0.0 | 0.0 | 1.7 | 2.6 | 2.3 | 1.2 | 2.3 | 5.3 | 3.3 | 0.0 | 0.0 | 3.4 | 4.8 | 4.3 | 1.4 | 2.8 |
| Red Fox | Vulpes vulpes | 3.2 | 0.6 | 1.0 | 0.9 | 0.0 | 0.0 | 1.3 | 1.8 | 1.1 | 5.4 | 1.0 | 1.5 | 1.5 | 0.0 | 0.0 | 1.9 | 2.4 | 1.6 | 1.1 | 1.7 |
| Food niche breath (Levins’ index) | 6.6 | 4.6 | 5.3 | 5.5 | 6.7 | 7.7 | 6.5 | 8.8 | 7.5 | 6.6 | |||||||||||
| Sample size | 185 | 486 | 194 | 3 | 187 | 131 | 237 | 341 | 264 | 185 | 486 | 194 | 332 | 187 | 131 | 237 | 341 | 264 | |||
| Number of nests | 12 | 27 | 16 | 21 | 12 | 10 | 14 | 20 | 16 | 12 | 27 | 16 | 21 | 12 | 10 | 14 | 20 | 16 | |||
| Estimate ± SE | t | p | |
|---|---|---|---|
| Productivity | |||
| F2,6 = 6.53, R2adj = 0.58, p = 0.032 | |||
| Capercaillie Tetrao urogallus | 0.035 ± 0.011 | 3.238 | 0.017 |
| Roe Deer Capreolus capreolus | 0.055 ± 0.017 | 3.168 | 0.019 |
| Breeding frequency | |||
| F2,6 = 40.44, R2adj = 0.91, p < 0.001 | |||
| White-fronted Goose Anser albifrons | 0.026 ± 0.006 | 4.461 | 0.004 |
| Hares Lepus sp. | −0.012 ± 0.002 | −4.849 | 0.002 |
| Breeding success | |||
| F2,6 = 3.06, R2adj = 0.34, p = 0.121 | |||
| Capercaillie Tetrao urogallus | 0.029 ± 0.018 | 1.674 | 0.145 |
| Roe Deer Capreolus capreolus | 0.069 ± 0.028 | 2.454 | 0.049 |
| Estimate ± SE | t | p | |
|---|---|---|---|
| Productivity | |||
| F3,5 = 2.15, R2adj = 0.30, p = 0.212 | |||
| Capercaillie Tetrao urogallus | 0.093 ± 0.038 | 2.443 | 0.058 |
| White-fronted Goose Anser albifrons | −0.116 ± 0.055 | −2.086 | 0.091 |
| Roe Deer Capreolus capreolus | 0.115 ± 0.053 | 2.169 | 0.082 |
| Breeding frequency | |||
| F2,6 = 61.02, R2adj = 0.94, p < 0.001 | |||
| White-fronted Goose Anser albifrons | 0.032 ± 0.003 | 11.013 | <0.001 |
| Hares Lepus sp. | −0.011 ± 0.002 | −4.981 | 0.002 |
| Breeding success | |||
| F3,5 = 2.41, R2adj = 0.35, p = 0.182 | |||
| Capercaillie Tetrao urogallus | 0.140 ± 0.061 | 2.320 | 0.068 |
| White-fronted Goose Anser albifrons | −0.219 ± 0.088 | −2.485 | 0.055 |
| Roe Deer Capreolus capreolus | 0.186 ± 0.084 | 2.213 | 0.078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sein, G.; Väli, Ü. Diet Composition Explains Interannual Fluctuations in Reproductive Performance in a Lowland Golden Eagle Population. Diversity 2024, 16, 69. https://doi.org/10.3390/d16010069
Sein G, Väli Ü. Diet Composition Explains Interannual Fluctuations in Reproductive Performance in a Lowland Golden Eagle Population. Diversity. 2024; 16(1):69. https://doi.org/10.3390/d16010069
Chicago/Turabian StyleSein, Gunnar, and Ülo Väli. 2024. "Diet Composition Explains Interannual Fluctuations in Reproductive Performance in a Lowland Golden Eagle Population" Diversity 16, no. 1: 69. https://doi.org/10.3390/d16010069
APA StyleSein, G., & Väli, Ü. (2024). Diet Composition Explains Interannual Fluctuations in Reproductive Performance in a Lowland Golden Eagle Population. Diversity, 16(1), 69. https://doi.org/10.3390/d16010069








