Diversity of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata) Parasitizing Starlings of the Genus Lamprotornis (Passeriformes: Sturnidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Host Sampling
2.2. Mite Preparation and Morphological Analyses
3. Results
3.1. Descriptions
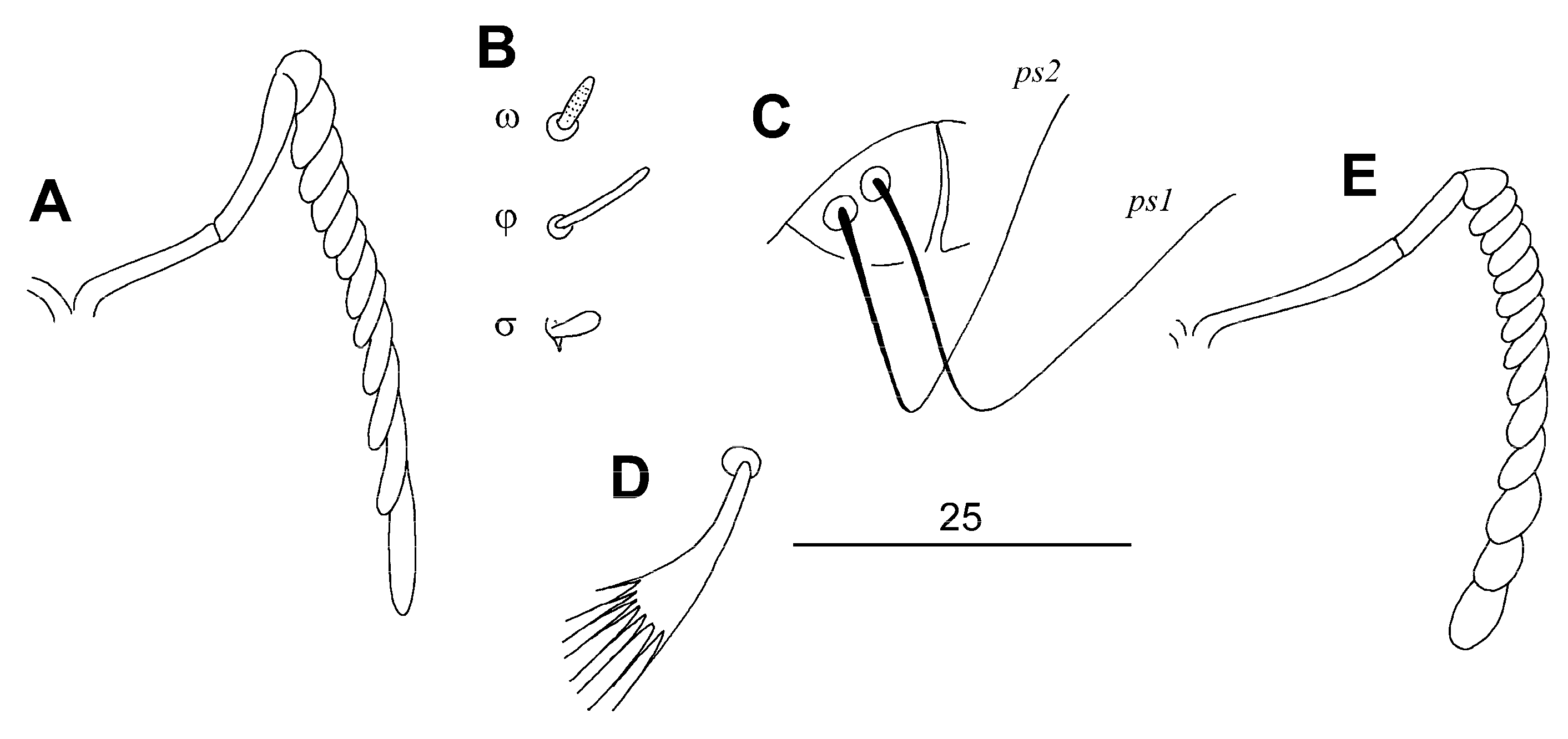
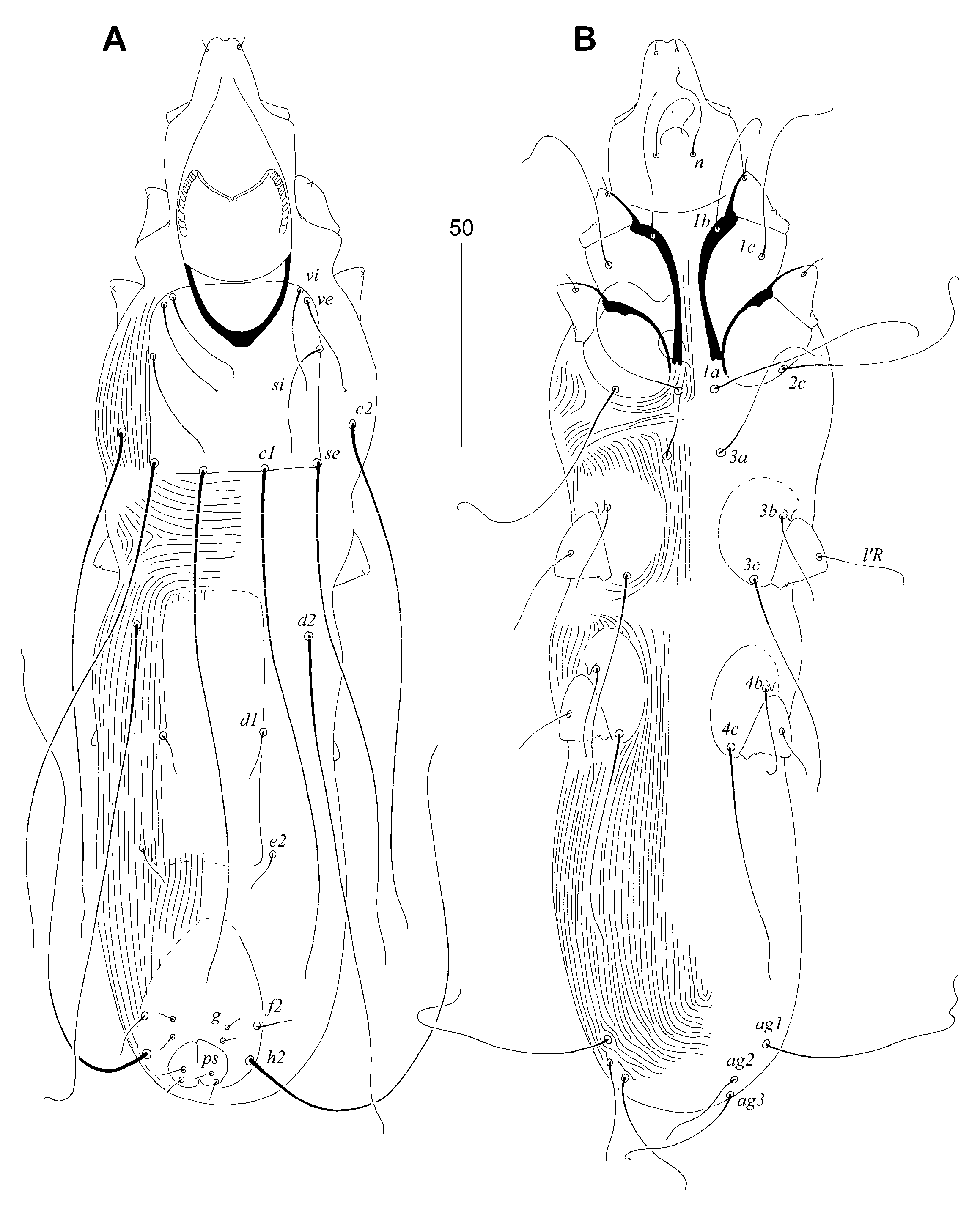
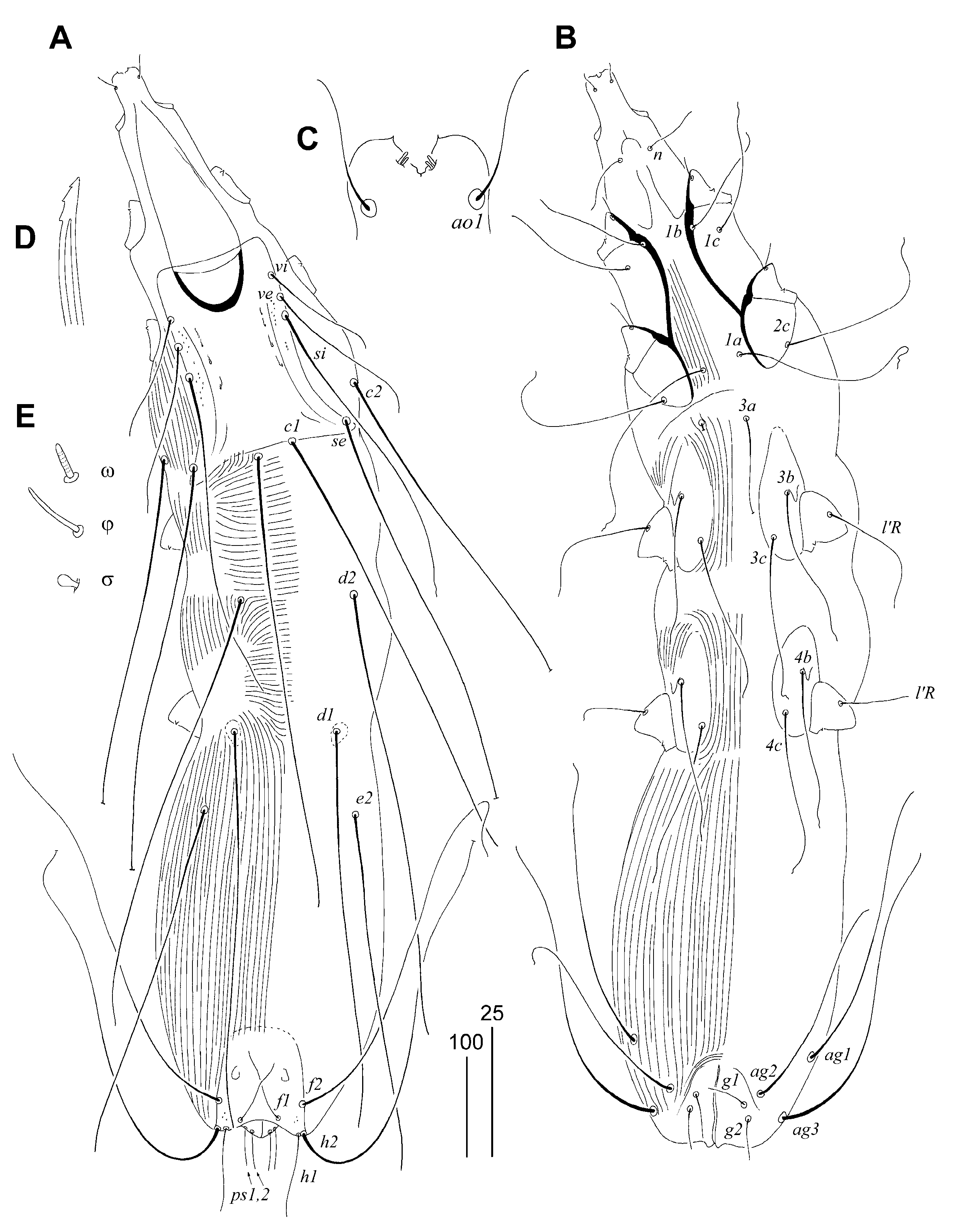
3.1.1. Syringophiloidus saponai Skoracki, Patan and Unsoeld sp. n.
Type Material
Type Material Deposition
Additional Material
Differential Diagnosis
Etymology
3.1.2. Syringophilopsis parasturni Skoracki, Patan and Unsoeld sp. n.
Type Material
Type Material Deposition
Additional Material
Differential Diagnosis
Etymology
3.1.3. Picobia lamprotornis Klimovičová, Skoracki, Wamiti and Hromada, 2014
Material Examined
3.2. Prevalence
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zmudzinski, M.; Skoracki, M.; Sikora, B. An Updated Checklist of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata). 2023. Available online: https://figshare.com/articles/dataset/An_updated_checklist_of_quill_mites_of_the_family_Syringophilidae_Acariformes_Prostigmata_/16529574 (accessed on 23 November 2023).
- Kethley, J.B. A revision of the family Syringophilidae (Prostigmata: Acarina). Contrib. Am. Entomol. Inst. 1970, 5, 1–76. [Google Scholar]
- Skoracki, M.; Fajfer, M.; Hromada, M.; Hušek, J.; Sikora, B. Tinamiphilopsis temmincki sp. n., a New Quill Mite Species from Tataupa Tinamou, and the Early History of Syringophilid Mites. Animals 2023, 13, 2728. [Google Scholar] [CrossRef] [PubMed]
- Clements, J.F.; Rasmussen, P.C.; Schulenberg, T.S.; Iliff, M.J.; Fredericks, T.A.; Gerbracht, J.A.; Lepage, D.; Spencer, A.; Billerman, S.M.; Sullivan, B.L.; et al. The eBird/Clements Checklist of Birds of the World. 2023. Available online: https://www.birds.cornell.edu/clementschecklist/download/ (accessed on 23 November 2023).
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Starlings (Sturnidae), version 1.0. In Birds of the World; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Klimovičová, M.; Skoracki, M.; Wamiti, W.; Hromada, M. Quill mites of the subfamily Picobiinae (Acari: Syringophilidae) parasitising African birds, with description of two new species. Folia Parasitol. 2014, 61, 394–400. [Google Scholar] [CrossRef]
- Skoracki, M. Quill mites (Acari: Syringophilidae) of the Palaearctic region. Zootaxa 2011, 2840, 1–414. [Google Scholar] [CrossRef]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology, 3rd ed.; Texas Tech University Press: Lubbock, TX, USA, 2009. [Google Scholar]
- Grandjean, F. Les segments post-larvaires de l’hystérosoma chez les Oribates (Acariens). Bull. Soc. Zool. Fr. 1939, 64, 273–284. [Google Scholar]
- Kethley, J.B. Acarina: Prostigmata (Actinedida). In Soil Biology Guide; Dindal, D.L., Ed.; John Wiley & Sons: New York, NY, USA, 1990; pp. 667–756. [Google Scholar]
- Grandjean, F. Observations Sur Les Acariens de La Famille Des Stigmaeidae. Arch. Sci. Phys. Nat. 1944, 26, 103–131. [Google Scholar]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for Parasitologists—A Primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Fain, A.; Bochkov, A.V.; Mironov, S.V. New genera and species of quill mites of the family Syringophilidae (Acari: Prostigmata). Bull. Inst. R. Sci. Nat. Belg. 2000, 70, 33–70. [Google Scholar]
- Bochkov, A.V.; Galloway, T.D. New species and records of cheyletoid mites (Acari: Cheyletoidea) from birds in Canada. J. Kans. Entomol. 2004, 77, 26–44. [Google Scholar] [CrossRef]
- Chirov, P.A.; Kravtsova, N.T. A new genus and new species of mites of the family Syringophilidae. Parazitologiya 1995, 29, 370–379. [Google Scholar]
- Skoracki, M.; Glowska, E. Two new species of the genus Picobia Haller (Acari: Syringophilidae) from Australian and Indonesian passeriform birds. N. Z. J. Zool. 2008, 35, 281–286. [Google Scholar] [CrossRef]
- Skoracki, M.; Bochkov, A.V.; Wauthy, G. Revision of the quill mites of the genus Picobia Haller, 1878 (Acari: Syringophilidae) with notes on their host-parasites relationships. Insect Syst. Evol. 2004, 35, 155–176. [Google Scholar] [CrossRef]
- Skoracki, M.; Sikora, B.; Spicer, G.S. A review of the subfamily Picobiinae Johnston and Kethley, 1973 (Acariformes: Prostigmata: Syringophilidae). Zootaxa 2016, 4113, 1–95. [Google Scholar] [CrossRef] [PubMed]
- Skoracki, M.; Spicer, G.S.; OConnor, B.M. A systematic review of the subfamily Syringophilinae (Acari: Syringophilidae) of the Nearctic region. Part 1: Quill mites associated with passerines (Aves: Passeriformes). Zootaxa 2016, 4084, 451–494. [Google Scholar] [CrossRef]
- Skoracki, M. Three new species of the ectoparasitic mites of the genus Syringophiloidus Kethley, 1970 (Acari: Syringophilidae) from passeriform birds from Slovakia. Folia Parasitol. 2002, 49, 305–313. [Google Scholar] [CrossRef] [PubMed]
- del Hoyo, J.; Collar, N.J.; Christie, D.A.; Elliott, A.; Fishpool, L.D.C.; Boesman, P.; Kirwan, G.M. HBW and BirdLife International Illustrated Checklist of the Birds of the World. Volume 2: Passerines; Lynx Edicions and BirdLife International: Barcelona, Spain; Cambridge, UK, 2016. [Google Scholar]
- BirdLife International: Species Factsheet: Sturnus vulgaris. Available online: http://datazone.birdlife.org/species/factsheet/common-starling-sturnus-vulgaris (accessed on 23 November 2023).
- Casto, S.D. Quill wall thickness and feeding of Syringophiloidus minor (Berlese) (Acarina: Syringophilidae). Ann. Entomol. Soc. Am. 1974, 67, 824. [Google Scholar] [CrossRef]
- Kethley, J.B. Population regulation in quill mites (Acari: Syringophilidae). Ecology 1971, 52, 1113–1118. [Google Scholar] [CrossRef]
- Johnston, D.E.; Kethley, J.B. A numerical phenetic study of the quill mites of the family Syringophilidae (Acari). J. Parasitol. 1973, 59, 520–530. [Google Scholar] [CrossRef]
- Skoracki, M.; Zabludovskaya, S.A.; Bochkov, A.V. A review of Prostigmata (Acariformes: Trombidiformes) permanently associated with birds. Acarina 2012, 20, 67–107. [Google Scholar]
- Skoracki, M.; Glowska, E.; Bochkov, A.V. Phylogeny of quill mites of the family Syringophilidae (Acari: Prostigmata) based on their external morphology. Eur. J. Entomol. 2013, 110, 663–675. [Google Scholar] [CrossRef]
- Skoracki, M.; Michalik, J.; Sikora, B. Prevalence and habitat preference of quill mites (Acari, Syringophilidae) parasitizing forest passerine birds in Poland. Acta Parasitol. 2010, 55, 188–193. [Google Scholar] [CrossRef]
- Kaszewska-Gilas, K.; Kosicki, J.Z.; Hromada, M.; Skoracki, M. Global Studies of the Host-Parasite Relationships between Ectoparasitic Mites of the Family Syringophilidae and Birds of the Order Columbiformes. Animals 2021, 11, 3392. [Google Scholar] [CrossRef] [PubMed]
- Marciniak-Musial, N.; Skoracki, M.; Kosicki, J.Z.; Unsöld, M.; Sikora, B. Host-Parasite Relationships of Quill Mites (Syringophilidae) and Parrots (Psittaciformes). Diversity 2023, 15, 1. [Google Scholar] [CrossRef]
- Kethley, J.B.; Johnston, D.E. Resource tracking patterns in bird and mammal ectoparasites. Misc. Publ. Entomol. Soc. Am. 1975, 9, 227–236. [Google Scholar]
- Skoracki, M.; Hromada, M.; Zmudzinski, M.; Unsoeld, M.; Sikora, B. Parasitic quill mites of the family Syringophilidae (Acariformes: Prostigmata) associated with Sub-Saharan Sunbirds (Passeriformes: Nectariniidae): Species composition and host-parasite relationships. J. Med. Entomol. 2018, 55, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Skoracki, M.; Hromada, M.; Prevuznakova, P.; Wamiti, W. Mites of the family Syringophilidae (Acariformes: Cheyletoidea) parasitizing waxbills of the genus Estrilda (Passeriformes: Estrildidae). Syst. Appl. Acarol. 2019, 24, 1799–1808. [Google Scholar]
- Skoracki, M.; Sikora, B.; Unsoeld, M.; Hromada, M. Mite Fauna of the Family Syringophilidae (Acariformes: Prostigmata) Parasitizing Darwin’s Finches in Galápagos Archipelago. Diversity 2022, 14, 585. [Google Scholar] [CrossRef]
- Skoracki, M.; Unsöld, M.; Sikora, B. Redescription of Syringophilioidus glandarii (Fritsch, 1958) with new records of hosts and localities (Acariformes, Syringophilidae). Spixiana 2022, 45, 39–44. [Google Scholar]
- Craig, A.J.F.; Feare, C.J. Greater Blue-eared Starling (Lamprotornis chalybaeus), version 1.0. In Birds of the World; del Hoyo, J., Elliott, J.A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Craig, A.J.F.; Feare, C.J.; de Juana, E. Lesser Blue-eared Starling (Lamprotornis chloropterus), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Grossi, A.; Proctor, H. The distribution of quill mites (Betasyringophiloidus seiuri) among flight feathers of the ovenbird (Seiurus aurocapilla). J. Parasitol. 2020, 106, 82–89. [Google Scholar] [CrossRef]
- Skirnisson, K.; Nielsen, Ó.K. Quill mite infestation of rock ptarmigan Lagopus muta (Aves: Phasianidae) in relation to year and host age, sex, body condition, and density. Parasitol. Res. 2019, 118, 2643–2650. [Google Scholar] [CrossRef]
- Skoracki, M.; Kosicki, J.Z.; Kwieciński, Z. Distribution of the parasitic mite Bubophilus aegolius sp. n. (Acariformes: Syringophilidae) on the Boreal Owl Aegolius funereus (L) (Strigiformes: Strigidae) and the low effectiveness of infestation. Eur. Zool. J. 2021, 88, 352–362. [Google Scholar] [CrossRef]



| Host Species, No. of Examined Specimens | No. of Infested Specimens | Prevalence (CI) | Habitat | Quill Mite Species |
|---|---|---|---|---|
| L.chalybaeus, n = 14 | 2 | 14.3% (3.0–41.4) | cov | S. saponai sp. n. |
| “ | 1 | 7.1% (0.1–31.7) | con | P. lamprotornis |
| “ | 1 | 7.1% (0.1–31.7) | cov + con | S. saponai sp. n. + P. lamprotornis |
| Total | 4 | 28.6% (10.4–57.4) | con, cov | S. saponai sp. n., P. lamprotornis |
| L. superbus, n = 22 | 2 | 9.1% (1.6–29.1) | cov | S. saponai sp. n. |
| “ | 3 | 13.6% (3.8–33.8) | con | P. lamprotornis |
| Total | 5 | 22.7% (9.4–45.3) | con, cov | S. saponai sp. n., P. lamprotornis |
| L. chloropterus, n = 17 | 1 | 5.9% (0.3–28.7) | cov | S. saponai sp. n. |
| “ | 2 | 11.8% (2.1–35.0) | con | P. lamprotornis |
| “ | 2 | 11.8% (2.1–35.0) | con + cov | S. saponai sp. n. + P. lamprotornis |
| Total | 5 | 29.4% (12.4–54.4) | con, cov | S. saponai sp. n., P. lamprotornis |
| L. unicolor, n = 8 | 1 | 12.5% (0.6–50.0) | con | S. saponai sp. n. |
| L. pulcher, n = 2 | 1 | 50% (2.5–97.5) | cov | S. parasturni sp. n. |
| L. chalcurus, n = 1 | 1 | 100% (50–100) | cov | S. parasturni sp. n. |
| Quill Mite Species | Host Species | Locality | References |
|---|---|---|---|
| Subfamily Picobiinae Johnston and Kethley, 1973 | |||
| Picobia indonesiana Skoracki and Glowska, 2008 | Aplonis panayensis (Scopoli) * | Orie: Indonesia | [16] |
| “ | Enodes erythrophris (Temminck) | Orie: Indonesia (Sulawesi) | [16] |
| “ | Mino dumontii (Lesson) | Ocea: Indonesia (New Guinea) | [16] |
| Picobia sturni Skoracki, Bochkov and Wauthy, 2004 | Sturnus vulgaris (Linnaeus) * | Pala: Poland, Slovakia, Moldova | [7,17] |
| “ | Sturnus unicolor (Temminck) | Pala: Morocco | [18] |
| “ | Spodiopsar cineraceus (Temminck) | Pala: Japan | [7] |
| Picobia lamprotornis Klimovičová, Skoracki, Wamiti and Hromada, 2014 | Lamprotornis superbus (Rüppell) * | Afro: Kenya | [6], c.s. |
| “ | Lamprotornis chalybaeus (Hemprich & Ehrenberg) | Afro: Tanzania, Kenya | c.s. |
| “ | Lamprotornis chloropterus (Swainson) | Afro: Tanzania | c.s. |
| Subfamily Syringophilinae Lavoipierre, 1953 | |||
| Syringophiloidus graculae Fain, Bochkov and Mironov, 2000 | Gracula religiosa (Linnaeus) | Orie: SE Asia | [13] |
| Syringophiloidus presentalis Chirov and Kravtsova, 1995 | Sturnus vulgaris (Linnaeus) | Pala: France, Poland, Slovakia, Kyrgyzstan; Near: USA | [7,15,19] |
| Syringophiloidus saponai sp. n. | Lamprotornis chalybaeus (Hemprich & Ehrenberg) * | Afro: Kenya, Tanzania, Ethiopia | c.s. |
| “ | Lamprotornis superbus (Rüppell) | Afro: Tanzania, Kenya | c.s. |
| “ | Lamprotornis chloropterus (Swainson) | Afro: Tanzania | c.s. |
| “ | Lamprotornis unicolor (Shelley) | Afro: Tanzania | c.s. |
| Syringophilopsis sturni Chirov and Kravtsova, 1995 | Sturnus vulgaris (Linnaeus) | Pala: Poland, Kazakhstan, Kyrgyzstan, Ukraine | [7,15] |
| Syringophilopsis parasturni sp. n. | Lamprotornis pulcher (Müller) * | Afro: Senegal | c.s. |
| “ | Lamprotornis chalcurus (Nordmann) | Afro: Senegal | c.s. |
| Krantziaulonastus buczekae (Skoracki, 2002) | Sturnus vulgaris (Linnaeus) | Pala: Poland | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoracki, M.; Patan, M.; Unsoeld, M.; Hromada, M.; Kwieciński, Z.; Marcisova, I. Diversity of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata) Parasitizing Starlings of the Genus Lamprotornis (Passeriformes: Sturnidae). Diversity 2024, 16, 51. https://doi.org/10.3390/d16010051
Skoracki M, Patan M, Unsoeld M, Hromada M, Kwieciński Z, Marcisova I. Diversity of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata) Parasitizing Starlings of the Genus Lamprotornis (Passeriformes: Sturnidae). Diversity. 2024; 16(1):51. https://doi.org/10.3390/d16010051
Chicago/Turabian StyleSkoracki, Maciej, Milena Patan, Markus Unsoeld, Martin Hromada, Zbigniew Kwieciński, and Iva Marcisova. 2024. "Diversity of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata) Parasitizing Starlings of the Genus Lamprotornis (Passeriformes: Sturnidae)" Diversity 16, no. 1: 51. https://doi.org/10.3390/d16010051
APA StyleSkoracki, M., Patan, M., Unsoeld, M., Hromada, M., Kwieciński, Z., & Marcisova, I. (2024). Diversity of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata) Parasitizing Starlings of the Genus Lamprotornis (Passeriformes: Sturnidae). Diversity, 16(1), 51. https://doi.org/10.3390/d16010051







