Abstract
Uropodina mites are organisms regularly found in the breeding sites of vertebrates. However, studies devoted to the nest dwellers of hole-nesting birds have been performed nearly exclusively in artificial places, i.e., nest boxes. Here, we describe an assemblage of mites from the Uropodina group living in excavated tree holes. We performed this study in western Poland, sampling material from 49 tree holes excavated by great spotted and black woodpeckers. We divided the material extracted from the tree holes into three categories: wood debris, remnants of bird nests, and remnants of insects. In total, we found 12 species from the Uropodina group. The two most numerous species, Leiodinychus orbicularis and Chiropturopoda nidiphila, constituted ca. 93% of the assemblage. Two other species, Apionoseius infirmus and Uroobovella obovata, were also relatively frequent. Among the assessed factors (woodpecker species, tree hole characteristics, and type of material), only the presence of insect remains, predominantly bat guano, affected species diversity and mite abundance the most. Our study is the first to describe an assemblage of Uropodina species in excavated tree holes and discover two extremely rare mite species, Ch. nidiphila and Nanteria banatica, related to the presence of bat guano in these cavities.
1. Introduction
Forests, as one of the most globally widespread ecosystems, cover ca. 30% of land [1]. One of the most distinctive and visible features of this ecosystem composed of trees are tree holes. Tree holes are globally distributed microenvironments in all types of forest but are characteristic for natural, old-growth forests [2,3]. They can be non-excavated, formed as a result of decaying processes or physical damage, or excavated, formed as a result of the destructive activity of excavators [3,4], among which the most important, worldwide, are woodpeckers [5]. The role of woodpeckers (primary excavators) as hole providers in forest ecosystems (except for Australia and New Zealand) cannot be overestimated. They are commonly considered to be keystone species in forests [6,7].
Tree holes excavated by woodpeckers are unique and constitute their specialised breeding sites, but, as long-lasting structures [8,9], they are regularly inhabited in the following years by other species of vertebrates, especially birds and mammals which rely upon existing holes for breeding and roosting [3,5,10,11]. To a much lesser extent, the role of excavated tree holes has been studied with respect to invertebrates [12,13,14].
The microclimate of natural tree holes that favours rapid decomposition, contrary to that of nest boxes [15,16], potentially makes them less attractive for nest-dwellers. The presence of old nest material in tree holes should not be neglected in ecological studies, as it may affect the nesting biology of hole-nesting birds with respect to at least four of the important aspects reviewed in Ref. [17]: increasing pressure of ectoparasites; increasing pressure of predators; serving informative cues for breeding birds; and enabling time and energy saving when building new nests.
Bird nests in tree holes constitute an attractive microenvironment for invertebrates from different ecological and systematic groups. In terms of foraging strategies alone, one may find the following organisms: saprophagous species feeding on decomposing nest materials and the prey and dropping remains of birds and mammal [18,19], scavengers and carnivores feeding on all the developmental stages of other invertebrates living commonly in the nest [20,21,22] or vertebrate ectoparasites [23,24].
It seems that, among the invertebrates, one of the most frequent arthropods found in bird nests are mites, especially from the Mesostigmata group. Surprisingly, apart from the relatively well-known European mites from the suborder Uropodina inhabiting bird nests, especially in the studies of [20,25,26,27,28,29], materials from tree holes excavated by woodpeckers have never been studied so far. The reason for this may be a problem with access to the nest sites (excavated holes are situated, on average, much higher than nest boxes) and sampling (tree hole content accessible only through a small opening), in contrast to data concerning mites in the nests of hole-nesting birds but extracted from nest boxes, which are well described, see [20,26,29,30]. Interestingly, the first intensive work using natural tree holes has lately brought about the very interesting discovery of Chiropturopoda nidiphila (Acari: Uropodina), which was previously recorded worldwide only once [31].
The diversity of mites from the suborder Uropodina is indisputably well studied in some regions of Europe, especially in Poland and Slovakia. The characteristic feature of Uropodina assemblages is a clear preference for specific microhabitats, dominated by single species [32,33]. Having collected materials from ca. 50 tree holes from Poland, we decided to present for the first time the species composition and structure of the assemblage of Uropodina mites from natural tree holes excavated by woodpeckers, thus providing novel data about species diversity of Uropodina mites in a previously unexplored microenvironment. We explored whether (i) the described assemblage is microhabitat-specific and whether (ii) the unique nature of the acari assemblage is shaped by tree hole characteristics or the presence of different types of nest materials. In accordance with our previous findings [31], we also predicted that the holes that contained bat guano would have the most unique and richest acari fauna.
2. Materials and Methods
2.1. Study Area
This study was conducted in the Opole voivodeship, in south-western Poland. We sampled tree holes excavated by woodpeckers (for the tree hole characteristics, see Table 1) in different lowland forest complexes in the central and northern part of the voivodeship, between N 50.5955–50.9635 and S 17.7365–18.1398. Except for four sites, the rest came from tree stands dominated by the pine Pinus sylvestris, with an admixture of other tree species.

Table 1.
Characteristics of the tree holes excavated by the great spotted woodpecker and black woodpecker.
2.2. Data Collection
We checked tree holes excavated by great spotted woodpecker Dendrocopos major and black woodpecker Dryocopus martius that may have been inhabited by birds or bats in the subsequent years.
The holes were situated in five tree species, predominantly in pines (36). The mean tree hole height above the ground depending on the woodpecker species amounted to 5.5–8.6 m, and the mean depth of the hole chamber for both species amounted to 25 cm (Table 1).
The contents of the tree holes were collected using a handheld vacuum cleaner and long tweezers and placed into a sealed plastic bag with a label describing the hole’s location and date of collection. Each time, we attempted to extract all the contents of the tree holes at hand. The material was then transferred to a Berlese-Tullgren funnel for mite extraction. This process lasted ca. 72 h for each sample. The extracted specimens were collected in Eppendorf tubes filled with ca. 70–80% ethanol and labelled. The mite specimens were sorted and identified using a stereoscopic microscope (Olympus BX51) with the identification of the extracted species being conducted by the second author (JB). The hole contents were then placed back into their original bags until the examination of the nesting material composition.
Mites were determined using keys and papers comprising original descriptions of the species [32,34,35].
2.3. Tree Hole Contents’ Analysis
We divided the hole contents into three categories:
- -
- wood debris—loose or soft wood debris in different stages of decay;
- -
- bird nest remains—parts of bird nests consisting of different materials, such as moss, grass, leaves, feathers, and wool;
- -
- insect remains—predominantly bat guano and remains of woodpecker food. In two cases, this last category included also the remains of bumblebee and wasp nests with dead specimens. For the analysis of the presence of Chiropturopoda nidiphila only, we subdivided this category into holes containing only bat guano and holes containing only insect remains (other than bat guano).
2.4. Data Analysis
To describe the Uropodina assemblage, we used basic indices of dominance and frequency, as used in previous studies [27,32], with the following classes: dominance, i.e., eudominants (>30%), dominants (15.1–30.0%), subdominants (7.1–15.0%), residents (3.0–7.0%), and subresidents (<3%); and frequency, i.e., euconstants (>50%), constants (30.1–50%), subconstants (15.1–30.0%), accessory species (5.0–15.0%), and accidents (<5%).
2.5. Statistical Analysis
To test whether the number of acari species depended on tree hole origin (woodpecker species), tree hole characteristics, and the amount and type of the sampled material extracted from the holes, we used a generalised linear model (GLM) with a Poisson error distribution and log link function. The model contained the number of acari species as a response variable and the main effects of the following fixed predictors: excavator species (great spotted vs. black woodpecker), hole depth (vertical distance (cm) from the entrance to the cavity floor), material volume (cm3), and the type of sampled material (insect remains vs. bird nest or wood debris, set as a contrast). The overdispersion of the above model was 0.997.
To test the differences in the number of acari individuals, we used a negative binomial model with a log link function package MASS [36], which helped us to reduce overdispersion to the acceptable level of 1.3. The model contained the number of individuals as a response variable and the main effects of the following fixed predictors: excavator species (great spotted vs. black woodpecker), hole depth (cm), material volume (cm3), and the type of nest material (insect remains vs. bird nest or wood debris, set as a contrast).
To test whether the presence and the number of Chiropturopoda nidiphila depended on the type of material extracted from the tree holes, we used generalized linear models (GLMs) with binomial (for presence) and negative binomial (for abundance) error distributions with logit and log link functions, respectively. The models contained the occurrence (Boolean) variable (0—not found, 1—recorded) and the number of Ch. nidiphila individuals as the response variables, respectively, and the type of sampled material (four categories: bird nest, bat guano, insect remains, or wood debris). Additionally, the amount of material extracted was included as an offset variable in the model for abundance.
Model fitting was carried out with the TMBglmm [37], and model diagnostics were performed with the DHARMa [38] packages. Both models had satisfactory diagnostics: there were no issues with the dispersion (DHARMa-simulated residuals’ test), the normality of the residuals (K-S test), or the homogeneity of variances (Levene’s test).
Statistical analyses were performed in R version 4.1.0 [39].
3. Results
3.1. Species Composition and the Structure of Assemblage
Mites were present in 40 of the 49 inspected woodpecker tree holes. In total, we found 2186 specimens of Uropodina mites, including at least 12 species, 11 of which were identified to the species level (Table 2).

Table 2.
Species composition and assemblage structure of Uropodina mites (with developmental stages) in woodpecker tree holes. N—sum of specimens of all development stages; F—female; M—male; D—deutonymph; P—protonymph; L—larva; D%—dominancy; Nps—number of positive samples; and F%—frequency.
The most common were Leiodinychus orbicularis and Chiropturopoda nidiphila, both classified as eudominants, which together constituted ca. 93% of the assemblage (Table 2). The analysis of frequency revealed only one euconstant (L. orbicularis), one constant (Ch. nidiphila), and two other relatively more frequent species (Apionoseius infirmus and Uroobovella obovata). All four species occurred in 12–55% of all the examined woodpecker tree holes (Table 2). The remaining species were much less numerous and occurred in fewer holes.
3.2. Effect of Tree Hole Characteristics and Type of Nest Material on the Structure of the Assemblage
The number of acari species recorded in the tree holes was unrelated to the woodpecker species (hole producer), the hole’s depth, or the material’s volume, but it differed between holes containing insect remains and wood debris (Table 3 and Table 4). The number of acari species was highest in the samples containing insect remains, slightly lower in bird nests, and the lowest in wood debris (Figure 1a).

Table 3.
Species composition of Uropodina mites (sum of all developmental stages) in different types of material sampled from woodpecker-excavated tree holes.

Table 4.
Results of a generalized linear model with Poisson error distribution and a log link function testing the difference in the number of acari species (response variable) recorded in tree holes excavated by the great spotted woodpecker vs. black woodpecker (n = 49) in relation to the depth of hole (cm), the volume (cm3), and the type of material (insect remains vs. bird nest or wood debris) extracted from the tree holes. Residual deviance = 42.9; df = 43; and AIC = 153.5.
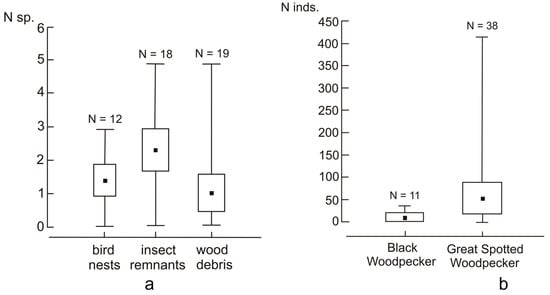
Figure 1.
(a) Number of acari species per tree hole in relation to the type of material sampled. Black squares—mean; box—95% confidence intervals; and whiskers—min–max ranges. (b) Number of acari individuals per tree hole in relation to the hole producer (black or great spotted woodpecker). Black squares—mean; box—95% confidence intervals; and whiskers—min–max ranges.
The number of acari individuals was usually higher in the holes excavated by great spotted woodpeckers than in those excavated by black woodpeckers (Table 5, Figure 1b). However, this difference was due to the exceptionally high abundance of acari in four holes of great spotted woodpeckers, as, when removing these four cases from the analysis, the abundance of acari was comparable between holes excavated by both species of woodpeckers (estimate = −0.86, CI 2.5–97.5% = −1.97–0.37). The number of acari individuals was unrelated to the hole’s depth or the material’s volume, but it was the highest in holes containing insect remains. Compared to the holes with insect remains, the abundance was slightly lower in the holes with bird nests and much lower in the holes with wood debris (Table 3 and Table 5, Figure 2).

Table 5.
Results of a negative binomial model with a log link function testing the difference in the number of acari individuals (response variable) in tree holes excavated by the great spotted woodpecker vs. black woodpecker (n = 49) in relation to the depth of the hole (cm), the volume (cm3), and the type of sampled material (insect remains vs. bird nest or wood debris) extracted from tree holes. Residual deviance = 56.7; df = 43; AIC = 392.5; and theta = 0.447.
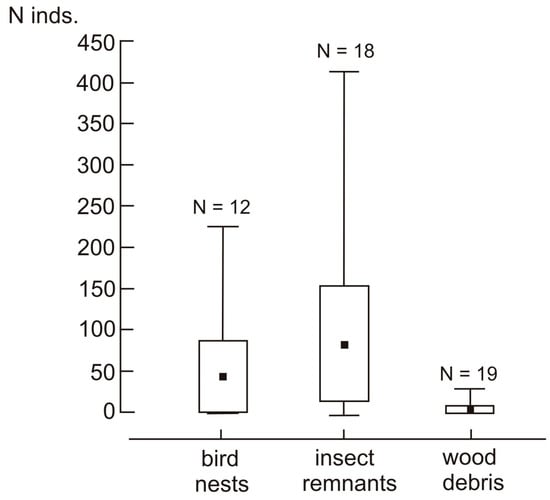
Figure 2.
Number of acari individuals per tree hole in relation to the type of material sampled. Black squares—mean; box—95% confidence intervals; and whiskers—min–max ranges.
3.3. Effect of Bat Guano Presence on the Occurrence of Ch. nidiphila
Chiropturopoda nidiphila was recorded, in total, in 18 tree holes, including holes of both woodpecker species and holes containing each of the material types. Ch. nidiphila was more frequent in the holes with bat guano (seven out of nine, estimated at 0.77, and 95% CI: 0.42–0.94, compared to 0.08, 0.33, and 0.37 in the holes with bird nests, insect remains, and wood debris, respectively, Table 6). Likewise, its abundance was the highest in the tree holes with bat guano (on average 22.7 ind., 95% CI: 8.7–59.3) than in the ones containing bird nests (1.4 ind., CI: 0.18–11.9), insect remains (7.9 ind., CI: 2.0–31.0), or wood debris (14 ind., CI: 4.8–41.5) (all these values were estimated for a fixed volume of extracted material of 200 cm3) (Table 7).

Table 6.
Results of a generalised binomial model for the presence of Chiropturopoda nidiphila in tree holes in relation to material type. Intercept stands for presence in tree holes with bird nests, remaining effects given as contrasts.

Table 7.
Results of a generalized negative binomial model for the abundance of Chiropturopoda nidiphila in tree holes excavated by the great spotted woodpecker vs. the black woodpecker (n = 49) in relation to material type. Intercept stands for presence in tree holes with bird nests, remaining effects given as contrasts.
4. Discussion
The Uropodina assemblage inhabiting tree holes excavated by woodpeckers is rich in species, with 12 species observed over the course of this study. Equally as rich or richer assemblages as the ones observed in the excavated tree holes in our study have, to date, only exhibited merocenoses of the nests of two analysed bird species, i.e., the nests of the white stork Ciconia ciconia (13) and the wood warbler Phylloscopus sibilatrix (12) [27]. The assemblages of mites inhabiting tree holes excavated by woodpeckers may be also compared to the wood-mould microhabitats in forest, which various publications also refer to as ‘tree holes’ but are different in origin. Wood-mould microhabitats emerge as a result of the natural decay of wood, are open, and rarely host the nests of birds or mammals. A total of 34 species have been found in such merocenoses, including (in addition to Ch. nidiphila and N. banatica) all those observed in woodpecker holes [33]. Apart from the two species mentioned above, all Uropodina found in the excavated holes in our study also appear in other merocenoses. Because Uroobovella obovata, Pulchellaobovella pyriformis, and Pulchellaobovella pulchella inhabit dead wood [33], their presence inside tree holes excavated by woodpeckers is unsurprising. In turn, Leiodinychus orbicularis and Apionoseius infirmus are typical nidicoles that settle in the nests of various bird species but also in bat boxes and in animal boxes inhabited by Gliridae [40]. Uropolyaspis hamulifera and Oplitis paradoxa are relatively rare species in Poland. They have been observed in the soil and in various merocenoses, such as in dead wood or in bird nesting boxes. Urodiaspis tecta is one of the most common species found in the litter of different types of forests. It also occasionally appears in the merocenoses of dead wood [32]. The biology and ecology of mites from the genus Pseudoropoda (Trichouropoda sensu Hirschmann et Zirgiebl-Nicol) are mostly unknown. These mites inhabit both the soil and the merocenoses of dead wood.
The assemblage of Uropodina mites in the excavated tree holes in our study are characterised by the presence of species that have, to date, not been observed in other microenvironments in Poland, including Chiropturopoda nidiphila and Nenteria banatica. Both species have been observed exclusively in holes excavated by woodpeckers. Ch. nidiphila was described by Wiśniewski and Hirschmann (1983) based on a deutonymph found in dead pine wood in the Greater Poland province in western Poland. The material collected recently from woodpecker tree holes allowed for a description of adult specimens and a full set of juvenile stages [31]. Conversely, N. banatica was for the first time described following its observation in bat guano in caves in southern Romania [41]; this constituted only the second observation of this species in Europe and the first one in Poland. According to previous observations, both Ch. nidiphila and N. banatica seem to be associated with the tree holes excavated by woodpeckers that are visited by bats. Ch. nidiphila reaches high abundance in tree holes, while N. banatica is very rare and forms relatively small population sizes.
Despite the different characteristics of the tree holes from which we collected the materials (Table 1), the species of the excavators, and, as a result, the dimensions of the holes (depth and diameter) and the volume of the material did not affect the species diversity or abundance of mites (Table 4 and Table 5). The type of material present in the tree holes had the strongest effect on the results, with the highest species diversity and abundance being observed in the holes containing insect remains (Table 4 and Table 5) and, especially in the case of presence and abundance of Ch. nidiphila, bat guano (Table 6 and Table 7). It should also be noted that the material present in the tree holes cannot by itself determine the resulting assemblage: this has been researched in previous studies, yet species diversity and specificity are different. Consequently, the rotting wood present in a tree hole can be compared to the extensively studied wood-mould and dead wood environments [32,33], whereas the remains of bird nests present in tree holes can be compared to those researched extensively in nest boxes such as those of the great tit Parus major, the blue tit Cyanistes caeruleus, the nuthatch Sitta europaea, the pied flycatcher Ficedula hypoleuca, and the European starling Sturnus vulgaris [27]. A study also researched the bat guano of as many as 10 bat species from their forest refuges in the vicinity (ca. 40 km) of Stobrawski Natural Landscape Park (southern Poland) but only found one species of mites, L. orbicularis. However, the study was conducted in artificial refuges (wooden boxes) [42]. The conditions for the occurrence of such a diverse and specific Uropodina fauna are, thus, determined by both the type of material present in a tree hole and the conditions of the hole itself. In contrast to boxes made for hole-nesting birds, natural tree holes have a different climate, both in terms of the range of temperatures and humidity and their dynamics [16].
In the assemblage of the Uropodina analysed in this study, Ch. nidiphila was the most frequently observed species after L. orbicularis, with a frequency of 37%. Interestingly, Ch. nidiphila more frequently inhabited the tree holes with a presence of bat feces, which may suggest that this species feeds on them. Bats were present in five of the nine tree holes containing bat guano in our study, and, in each case, the bat species found was the common noctule, Nyctalus noctula. In another study, Ch. nidiphila was also considerably more common and more numerous in the bird boxes in northern Poland that were visited by bats than in the boxes not visited by them [43]. Surprisingly, however, the species was not found in the guano collected from wooden bat boxes in western Poland, including the guano of the common noctule [42]. Furthermore, Ch. nidiphila was not observed in natural tree holes or in dead wood in two other studies [27,33].
The rarity of Ch. nidiphila and N. banatica gives rise to the question about how these two species travel from tree hole to tree hole. Their deutonymphs do not exhibit any adaptations to phoresis, nor have they ever been observed on insects or myriapods. They are also unlikely to be carried by bats, as research on the mites they carry has been fairly extensive, including research on the common noctule analysed in this study, as, so far, their presence has not been observed [44,45,46,47,48]. Consequently, deutonymphs are most likely carried by birds, especially since the specific structure of the barbs allows the deutonymphs to ‘hook’ onto feathers. Moss mites (Oribatida) are carried in a similar manner by birds while also having no special adaptations to phoresis, e.g., [49,50,51]. Unfortunately, we are unable to present the accurate age, detailed history, or specific visitors of the tree holes we surveyed. We were usually able to determine only their most recent inhabitants over the year of hole sampling. Because excavated cavities in Europe can persist for 31 years or more [8], they may be used by a wide variety of birds and mammals after being excavated [52].
Napierała and Błoszyk [33] showed that each merocenose has its characteristic acari species, which dominate in this microhabitat. Our study supports these findings; among the twelve recorded species, only two constituted as much as 93% of the total number of collected individuals: L. orbicularis, a typical nidicole species, and Ch. nidiphila, a mite clearly characteristic for excavated tree holes which are often visited by bats. Taking into account the rich species composition of the mites from the Uropodina group in our study, especially when comparing them with the merocenoses of bird nests and the dominant presence of characteristic and merocenose-specific mite species, tree holes excavated by woodpeckers appear to be important and exceptional microhabitats for mites in the forest ecosystem.
Author Contributions
Conceptualization, G.H. and J.B.; methodology, G.H. and J.B.; software, G.H. and J.B.; validation, G.H. and J.B.; formal analysis, G.H. and J.B.; investigation, G.H.; resources, J.B.; data curation, J.B.; writing—original draft preparation, G.H. and J.B.; writing—review and editing, G.H.; visualization, G.H.; supervision, J.B. and G.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are stored in an Invertebrate Fauna Bank (Natural History Collections, Faculty of Biology, Adam Mickiewicz University, Poznań, Poland).
Acknowledgments
Grzegorz Hebda is very grateful to Magdalena Cielniak (Institute of Biology, University of Opole) for her participation in the fieldwork, including the finding of the tree holes and sample collection. We are also grateful to Marta Maziarz and Grzegorz Neubauer for their help with the statistical analysis. Two anonymous reviewers significantly improved the first version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pan, Y.; Birdsey, R.A.; Phillips, O.L.; Jackson, R.B. The structure, distribution, and biomass of the World’s forests. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 593–622. [Google Scholar] [CrossRef]
- Rehm, J.; Lohmus, A. Tree cavities in forests—The broad distribution pattern of a keystone structure for biodiversity. For. Ecol. Manag. 2011, 262, 579–585. [Google Scholar] [CrossRef]
- Cockle, K.L.; Martin, K.; Wesołowski, T. Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Front. Ecol. Environ. 2011, 9, 377–382. [Google Scholar] [CrossRef]
- Cockle, K.L.; Martin, K.; Robledo, G. Linking fungi, trees, and hole-using birds in a Neotropical tree-cavity network: Pathways of cavity production and implications for conservation. For. Ecol. Manag. 2012, 264, 210–219. [Google Scholar] [CrossRef]
- Van der Hoek, Y.; Gaona, G.V.; Martin, K. The diversity, distribution and conservation status of the tree-cavity-nesting birds of the world. Divers. Distrib. 2017, 23, 1120–1131. [Google Scholar] [CrossRef]
- Blanc, L.A.; Walters, J.R. Cavity-nest webs in a longleaf pine ecosystem. Condor 2008, 110, 80–92. [Google Scholar] [CrossRef]
- Trzcinski, M.K.; Cockle, K.L.; Norris, A.R.; Edworthy, M.; Wiebe, K.L.; Martin, K. Woodpeckers and other excavators maintain the diversity of cavity-nesting vertebrates. J. Anim. Ecol. 2022, 91, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Wesołowski, T. “Lifespan” of woodpecker-made holes in a primeval temperate forest: A thirty year study. For. Ecol. Manag. 2011, 262, 1846–1852. [Google Scholar] [CrossRef]
- Cockle, K.L.; Trzcinski, M.K.; Wiebe, K.L.; Edworthy, A.B.; Martin, K. Lifetime productivity of tree cavities used by cavity-nesting animals in temperate and subtropical forests. Ecol. Appl. 2019, 29, e01916. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, P.; Lindenmayer, D. Tree Hollows and Wildlife Conservation in Australia; CSIRO Publishing: Collingwood, Australia, 2002. [Google Scholar]
- Czeszczewik, D.; Walankiewicz, W.; Stańska, M. Small mammals in nests of cavity-nesting birds: Why should ornithologists study rodents? Can. J. Zool. 2008, 86, 286–293. [Google Scholar] [CrossRef]
- McComb, W.C.; Noble, R.E. Invertebrate use of natural tree cavities and vertebrate nest boxes. Am. Midl. Nat. 1982, 107, 163–172. [Google Scholar] [CrossRef]
- Nilsson, S.G.; Baranowski, R. Habitat predictability and the occurrence of wood beetles in old-growth beech forests. Ecography 1997, 20, 491–498. [Google Scholar] [CrossRef]
- Hanzelka, J.; Baroni, D.; Martikainen, P.; Eeva, T.; Laaksonen, T. Cavity-breeding birds create specific microhabitats for diverse arthropod communities in boreal forests. Biodivers. Conserv. 2023, 32, 3845–3874. [Google Scholar] [CrossRef]
- Hebda, G.; Kandziora, A.; Mitrus, S. Decomposition of nest material in tree holes and nest-boxes occupied by European Starlings Sturnus vulgaris: An experimental study. Acta Ornithol. 2017, 52, 119–125. [Google Scholar] [CrossRef]
- Maziarz, M.A.; Broughton, R.K.; Wesołowski, T. Microclimate in tree cavities and nest-boxes: Implications for hole-nesting birds. For. Ecol. Manag. 2017, 389, 306–313. [Google Scholar] [CrossRef]
- Mazgajski, T.D. Effect of old nest material on nest site selection and breeding parameters in secondary hole nesters—Review. Acta Ornithol. 2007, 42, 1–14. [Google Scholar] [CrossRef]
- Roy, L.; Bouvier, J.-C.; Lavigne, C.; Galès, M.; Buronfosse, T. Impact of pest control strategies on the arthropodofauna living in bird nests built in nestboxes in pear and apple orchards. Bull. Entomol. Res. 2013, 103, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Boyes, D.H.; Lewis, O.T. Ecology of Lepidoptera associated with bird nests in mid-Wales, UK: Ecology of Lepidoptera associated with bird nests. Ecol. Entomol. 2019, 44, 1–10. [Google Scholar] [CrossRef]
- Krištofík, J.; Mašán, P.; Šustek, Z.; Nuhličková, S. Arthropods (Acarina, Coleoptera, Siphonaptera) in nests of hoopoe (Upupa epops) in Central Europe. Biologia 2013, 68, 155–161. [Google Scholar] [CrossRef]
- Cosandey, V.; Séchaud, R.; Béziers, P.; Chittaro, Y.; Sanchez, A.; Roulin, A. Nidicolous beetle species richness is driven by Barn Owl’s nests occupancy and landscape structure. J. Ornithol. 2021, 162, 857–864. [Google Scholar] [CrossRef]
- Jaworski, T.; Gryz, J.; Krauze-Gryz, D.; Plewa, R.; Bystrowski, C.; Dobosz, R.; Horák, J. My home is your home: Nest boxes for birds and mammals provide habitats for diverse insect communities. Insect Conserv. Divers. 2022, 15, 461–469. [Google Scholar] [CrossRef]
- Rendell, W.B.; Verbeek, N.A.M. Are avian ectoparasites more numerous in nest boxes with old nest material? Can. J. Zool. 1996, 74, 1819–1825. [Google Scholar] [CrossRef]
- Eeva, T.; Andersson, T.; Berglund, Å.M.M.; Brommer, J.E.; Hyvönen, R.; Klemola, T.; Laaksonen, T.; Loukola, O.; Morosinotto, C.; Rainio, K.; et al. Species and abundance of ectoparasitic flies (Diptera) in pied flycatcher nests in Fennoscandia. Parasites Vectors 2015, 8, 648. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, S. Biologisch-ökologische Untersuchungen über die Vogelnidicolen. Acta Zool. Fenn. 1936, 21, 1–168. [Google Scholar]
- Błoszyk, J.; Olszanowski, J. Materiały do znajomości fauny roztoczy gniazd i budek lęgowych ptaków. II. Różnice w liczebności i składzie gatunkowym populacji Uropodina (Acari, Anactinotrichida) budek lęgowych na Mierzei Wiślanej na podstawie dwuletnich obserwacji. Przegl. Zool. 1986, 30, 63–66. [Google Scholar]
- Napierała, A.; Maziarz, M.; Hebda, G.; Broughton, R.K.; Rutkowski, T.; Zacharyasiewicz, M.; Błoszyk, J. Lack of specialist nidicoles as a characteristic of mite assemblages inhabiting nests of the ground-nesting wood warbler, Phylloscopus sibilatrix (Aves: Passeriformes). Exp. Appl. Acarol. 2021, 84, 149–170. [Google Scholar] [CrossRef]
- Mašan, P. Mites of the Cohort Uropodina (Acarina, Mesostigmata) in Slovakia, 1st ed.; Annotationes Zoologicae et Botanicae: Bratislava, Slovakia, 2001. [Google Scholar]
- Błoszyk, J.; Bajerlein, D.; Gwiazdowicz, D.J.; Halliday, R.B.; Dylewska, M. Uropodine mite communities (Acari: Mesostigmata) in birds’ nests in Poland. Belg. J. Zool. 2006, 136, 145–153. [Google Scholar]
- Melekhina, E.N.; Korolev, A.N.; Selivanova, N.P. Oribatid Mites (Oribatida) Associated with Nests of Hollow Nesting Birds, on the Example of a Model Species, the European Pied Flycatcher (Ficedula hypoleuca), in the Taiga Forests of the European North-East of Russia. Diversity 2023, 15, 765. [Google Scholar] [CrossRef]
- Błoszyk, J.; Hebda, G.; Adamski, Z.; Zacharyasiewicz, M. Redescription of Chiropturopoda nidiphila Wiśniewski & Hirschmann (Acari: Uropodina) from a woodpecker’s tree holes, including all development stages and first notes on its ecology. Syst. Appl. Acarol. 2021, 26, 1867–1899. [Google Scholar] [CrossRef]
- Błoszyk, J. Geograficzne i Ekologiczne Zróżnicowanie Zgrupowań Roztoczy z Kohorty Uropodina (Acari: Mesostigmata) w Polsce: Uropodina Lasów Grądowych (Carpinion betuli), 1st ed.; Kontekst: Poznań, Poland, 1999. [Google Scholar]
- Napierała, A.; Błoszyk, J. Unstable microhabitats (merocenoses) as specific habitats of Uropodina mites (Acari: Mesostigmata). Exp. Appl. Acarol. 2013, 60, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Bregetova, N.G.; Kaditje, B.A.; Petrova, A.D. Nagkogorta Mesostigmata, Kogorta Trchytina Tragardh, 1938, Kogorta Uropodina Kramer, 1881. In Opredelitel’ Obitajuscich v Pocve Klescej, Mesostigmata; Ghilarov, M.C., Bregetova, N.G., Eds.; Nauka: Leningrad, Russia, 1977; pp. 12–25, 621–691. [Google Scholar]
- Karg, W. Acari (Acarina), Milben Uunterordnung Parasitiformes (Anactinochaeta), Uropodina Kramer, Schildkotenmilben; Die Tirwelt Deutschlands; Gustav Fischer: Jena, Germany, 1989; Volume 67, 202p. [Google Scholar]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB Balances Speed and Flexibility among Packages for Zero-inflated Generalized Linear Mixed Modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.6. 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 10 November 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 November 2023).
- Błoszyk, J.; Hebda, G.; Kulczak, M.; Zacharyasiewicz, M.; Rutkowski, T.; Napierała, A. Communities of Uropodina (Acari: Mesostigmata) in Nest Boxes Inhabited by Dormice (Glis glis and Muscardinus avellanarius) and Differences in Percentages of Nidicoles in Nests of Various Hosts. Animals 2023, 13, 3567. [Google Scholar] [CrossRef]
- Palacios-Vargas, J.G.; Decu, V.; Iavorski, V.; Hutzu, M.; Juberthie, C. Acari Terestia. In Encyklopedia Biospeologicai; Juberthie, C., Decu, V., Eds.; Socieate de Biospeologie: Bucarest, Romania, 1998; Volume 2, pp. 929–952. [Google Scholar]
- Błoszyk, J.; Rutkowski, T.; Wojtaszyn, G.; Książkiewicz-Parulska, Z.; Zacharyasiewicz, M.; Napierała, A. Leiodinychus orbicularis (CL Koch, 1839) in bat boxes in Poland. Eur. J. Biol. Res. 2020, 10, 150–155. [Google Scholar] [CrossRef]
- Błoszyk, J.; Wendzonka, J.; Kulczak, M.; Lubińska, K.; Napierała, A. Bird nesting boxes as a specific artificial microenvironment increasing biodiversity of mites from the suborder Uropodina (Acari: Mesostigmata): A case study of Bory Tucholskie National Park. Exp. Appl. Acarol. 2023, submitted.
- Ferenc, H.; Skoracki, M. Stan zbadania roztoczy z rodziny Spinturnicidae (Acari: Mesostigmata) w Polsce. Wiad. Parazytol. 2000, 46, 433–438. [Google Scholar] [PubMed]
- Baker, A.S.; Craven, J.S. Checklist of the mites (Arachnida: Acari) associated with bats (Mammalia: Chiroptera) in the British Isles. Syst. Appl. Acarol. Spec. Publ. 2003, 14, 1–20. [Google Scholar] [CrossRef]
- Haitlinger, R.; Łupicki, D. Arthropods (Acari, Siphonaptera, Heteroptera, Psocoptera) associated with Nyctalus noctula (Schreber, 1774) (Chiroptera: Vespertilionidae) in Southern Poland. Wiad. Parazytol. 2008, 54, 123–130. [Google Scholar] [PubMed]
- Léger, C. Bat parasites (Acari, Anoplura, Cestoda, Diptera, Hemiptera, Nematoda, Siphonaptera, Trematoda) in France (1762–2018): A literature review and contribution to a checklist. Parasite 2020, 27, 61. [Google Scholar] [CrossRef]
- Orlova, M.V.; Stanyukovich, M.K.; Orlov, O.L. Gamasid Mites (Mesostigmata: Gamasina) Parasiting Bats (Chiroptera: Rhinolophida, Vespertilionidae, Molossidae) of Palearctic Boreal Zone (Russia and Adjacent Countries); Babenko, A.S., Ed.; National Research Tomsk Stae University, Biological Institute, Russian Academy of Science, Zoological Institute, Publishing House of Tomsk State Uniwersity: Tomsk, Russia, 2015; pp. 3–149. [Google Scholar]
- Krivolutsky, D.A.; Lebedeva, N.V. Oribatid Mites (Oribatei, Acariformes) in Bird Plumage; ABF Publ.: Moscow, Russia, 2003. (In Russian) [Google Scholar]
- Krivolutsky, D.A.; Lebedeva, N.V. Oribatid Mites (Oribatei, Acariformes) in Bird Feathers: Passerines. Acta Zool. Litu. 2004, 14, 19–38. [Google Scholar] [CrossRef]
- Lebedeva, N.V. Oribatid mites transported by birds to polar islands—A review. Berichte Polar Meeresforsch. Rep. Polar Mar. Res. 2012, 640, 152–161. [Google Scholar]
- Günther, E.; Hellmann, M. Development and new tenants of holes of spotted woodpeckers (Dendrocopos) in the “Swift-forest” in the Harz Mountains (Sachsen-Anhalt). Results of twenty years investigations of the use of natural tree holes. Orn. Jber. Mus. Heine. 2005, 23, 103–122, (In German with English Summary). [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).