Distribution and Conservation Status of European Pond Turtles Emys orbicularis (L., 1758) in Algeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Climate
2.2. Data Collection
2.3. Geographical Analysis
3. Results
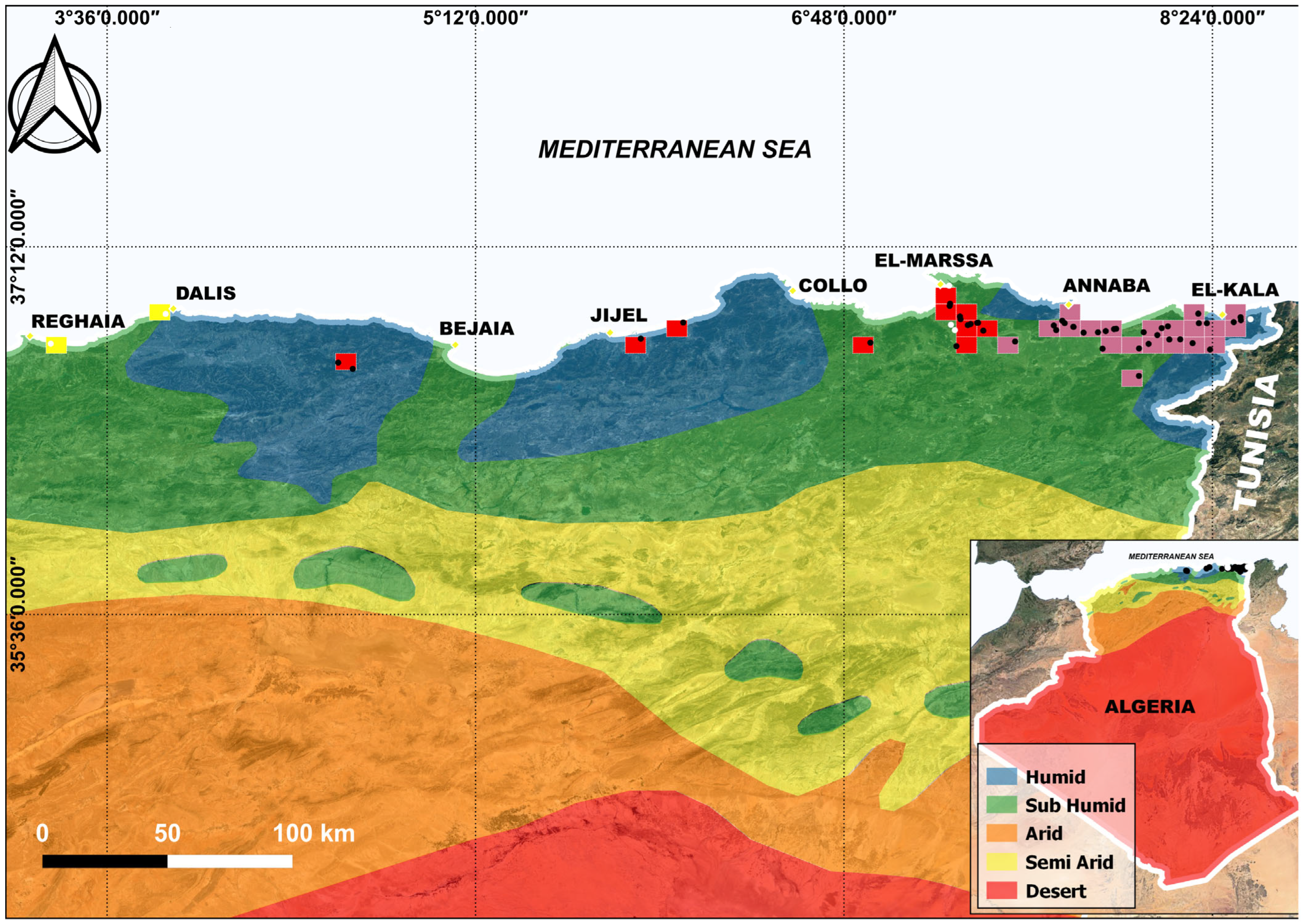
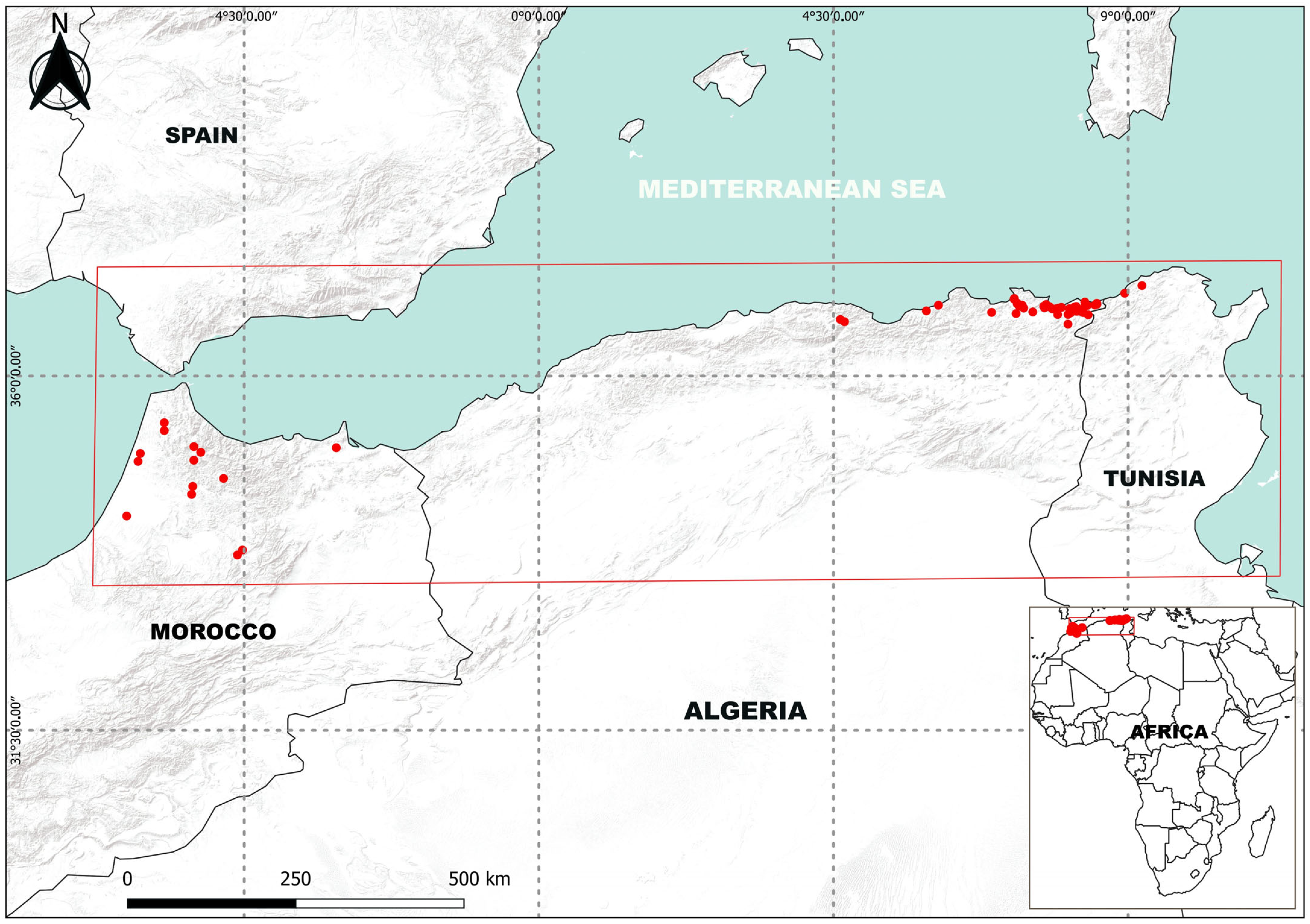
3.1. Distribution of Emys orbicularis in Algeria
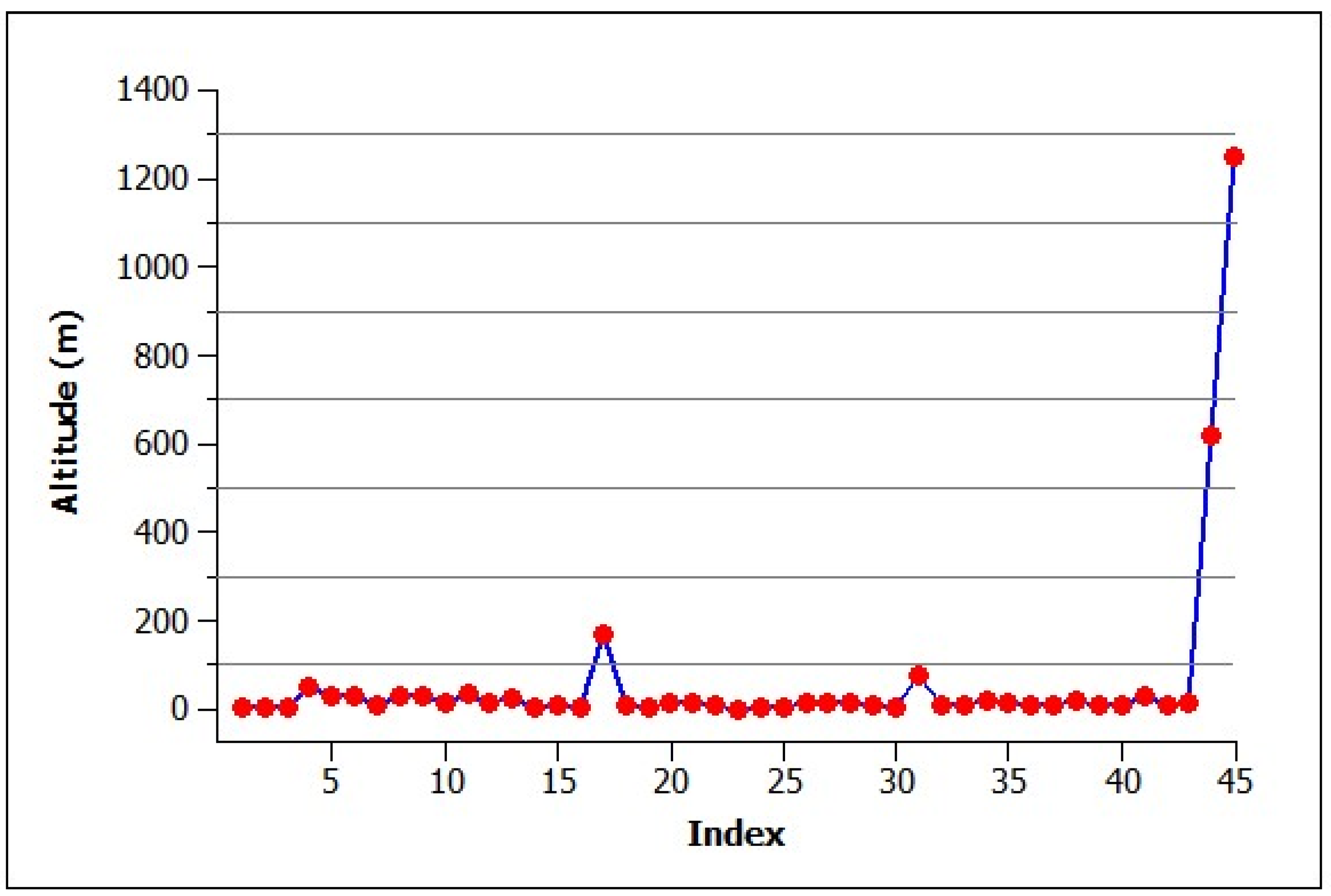
3.2. Climate and Elevation of Habitats
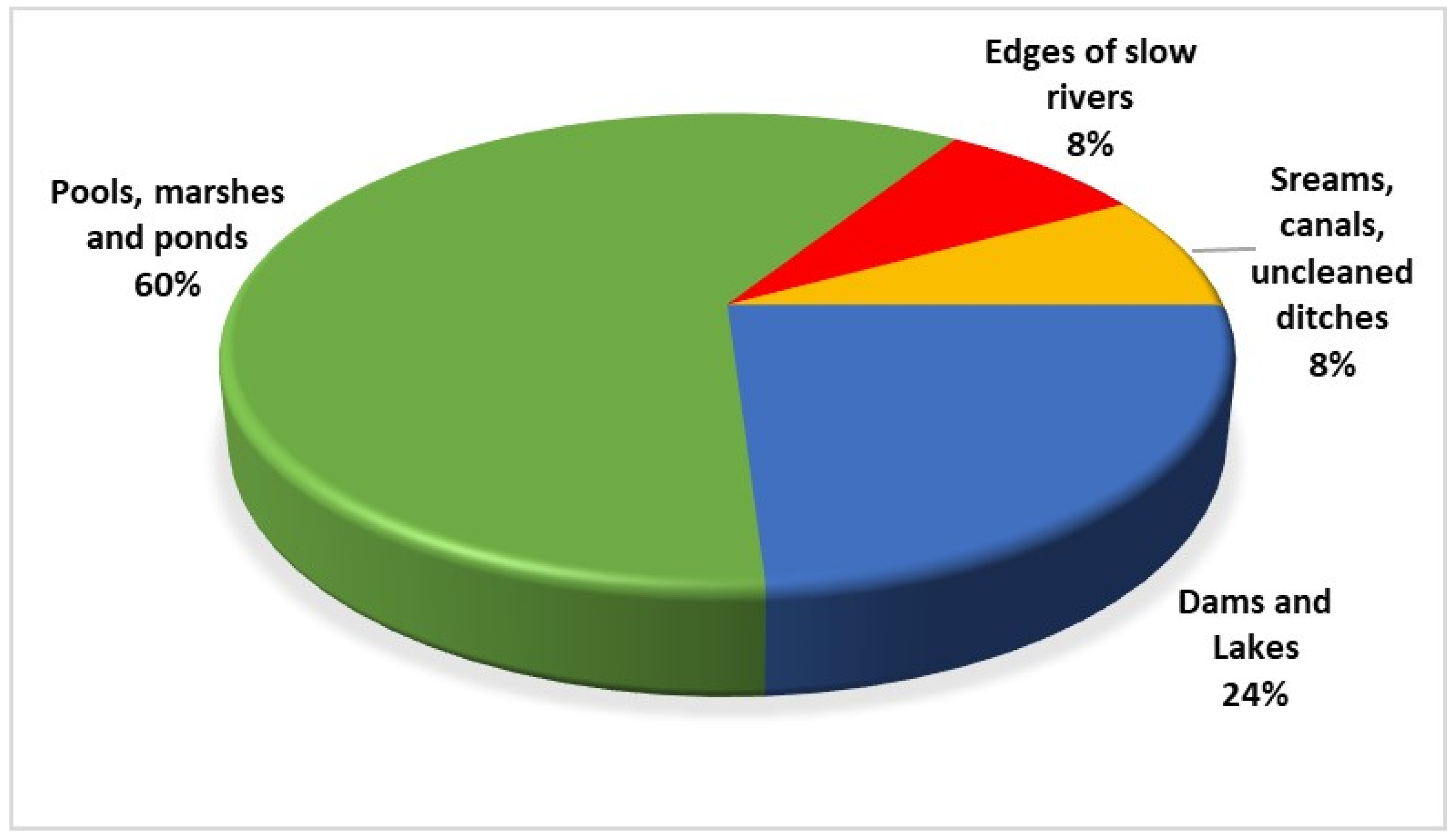
3.3. Diversity of Inhabited Biotopes
3.4. Conservation Status of Algerian Habitats of Emys orbicularis
4. Discussion
4.1. Distribution of E. orbicularis in Algeria
4.2. Climate and Elevation of Occupied Habitats
4.3. Diversity of Occupied Habitats
4.4. Conservation Status of Occupied Habitats
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiar-Saadi, M. Bioécologie des Tortues D’eau en Algérie. Ph.D. Thesis, Département de Biologie, Université Badji Mokhtar, Annaba, Algérie, 2018. [Google Scholar]
- Tiar, G.; Boudebza, R.; Souallem, I.; Tiar-Saadi, M. Enquête sur l’ampleur du ramassage illégal des tortues terrestres sauvages: Pratique non suffisamment contrôlée en Algérie (cas de la Wilaya d’El Tarf, nord-est algérien). Rev. Algérienne Sci. A 2019, 2, 71–75. [Google Scholar]
- Gahmous, S.A.; Tiar, G.; Tiar-Saadi, M.; Bouslama, Z.; Širokỳ, P. Reproductive traits demonstrate how well the Mediterranean stripe-necked turtle Mauremys leprosa can flourish under highly degraded–polluted conditions. Biology 2022, 11, 1562. [Google Scholar] [CrossRef] [PubMed]
- Tiar-Saadi, M.; Tiar, G.; Bouslama, Z.; Širokỳ, P. Mechanisms determining body size and shape difference in Algerian spur-thighed tortoises (Testudo graeca). Animals 2022, 12, 1330. [Google Scholar] [CrossRef] [PubMed]
- Fritz, U. Die Europäische Sumpfschildkröte Laurenti Verlag. Suppl. Z. Feldherpetol. 2003, 1, 224. [Google Scholar]
- Ernst, C.H.; Barbour, R.W.; Altenburg, R.G.M. Turtles of the World; Springer: Berlin/Heidelberg, Germany, 1989; Volume 272. [Google Scholar]
- Fritz, U. Emys orbicularis (Linnaeus, 1758)—Europäische Sumpfschildkröte. Aula Verlag. Handb. Reptil. Amphib. Eur. 2001, 3, 343–515. [Google Scholar]
- Sillero, N.; Campos, J.; Bonardi, A.; Corti, C.; Creemers, R.; Crochet, P.-A.; Isailović, J.C.; Denoël, M.; Ficetola, G.F.; Gonçalves, J. Updated distribution and biogeography of amphibians and reptiles of Europe. Amphib. Reptil. 2014, 35, 1–31. [Google Scholar] [CrossRef]
- Seidel, M.; Ernst, C. A systematic review of the turtle family Emydidae. Vertebr. Zool. 2017, 67, 1–122. [Google Scholar] [CrossRef]
- Tortoise and Freshwater Turtle Specialist Group Nature (IUCN), International Union for Conservation of Nature. IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 1996. [Google Scholar]
- Ficetola, G.F.; Padoa-Schioppa, E.; Monti, A.; Massa, R.; Bernardi, F.D.; Bottoni, L. The importance of aquatic and terrestrial habitat for the European pond turtle (Emys orbicularis): Implications for conservation planning and management. Can. J. Zool. 2004, 82, 1704–1712. [Google Scholar] [CrossRef]
- Mollov, I.A. Influence of urbanization on the populations of the European pond turtle Emys orbicularis (L., 1758) in the city of Plovdiv (Bulgaria). Ecol. Balk. 2019, 11, 269–278. [Google Scholar]
- Stanford, C.B.; Iverson, J.B.; Rhodin, A.G.; Van Dijk, P.P.; Mittermeier, R.A.; Kuchling, G.; Berry, K.H.; Bertolero, A.; Bjorndal, K.A.; Blanck, T.E. Turtles and tortoises are in trouble. Curr. Biol. 2020, 30, R721–R735. [Google Scholar] [CrossRef]
- Nekrasova, O.; Marushchak, O.; Pupins, M.; Skute, A.; Tytar, V.; Čeirāns, A. Distribution and potential limiting factors of the European pond turtle (Emys orbicularis) in eastern Europe. Diversity 2021, 13, 280. [Google Scholar] [CrossRef]
- Ayaz, D.; Bayrakci, Y.; Çiçek, K.; Ihlow, F.; Tok, C.V.; Fritz, U. On the brink of extinction: Results of a 20-year quest for Eiselt’s pond turtle (Emys orbicularis eiselti) in southeastern Turkey. Chelonian Conserv. Biol. 2021, 20, 222–230. [Google Scholar] [CrossRef]
- Mediani, M.; Amezian, M.; Tattou, M.I.; Benhoussa, A.; Idrissi, H.R.; El Agbani, M.A.; Qninba, A. Nouvelles Citations de Deux Espèces Reliques Paléarctiques, Emys orbicularis Linnaeus, 1758 et Vipera latastei Boscá, 1878 Dans La Péninsule Tingitane (Rif Occidental, Maroc). Bull. Inst. Sci. Rabat. 2009, 31, 99–102. [Google Scholar]
- Velo-Antón, G.; el Marnisi, B.; Fritz, U.; Fahd, S. Distribution and conservation status of Emys orbicularis in Morocco. Vertebr. Zool. 2015, 65, 131–134. [Google Scholar] [CrossRef]
- El Hili, R.A.; Verneau, O.; Jrijer, J.; Achouri, M. Reassessment of distribution and conservation status of freshwater turtles (Testudines) in Tunisia. Salamandra 2020, 56, 362–372. [Google Scholar]
- Chaignon, V.H. Contributions à L’histoire Naturelle de la Tunisie. Bull. Soc. Hist. Nat. Autun 1904, 17, 1–280. [Google Scholar]
- Imprimerie et Librairie Dejussieu (Ed.) Société d’Histoire Naturelle d’Autun Dix-Septième Bulletin; La Société: Autun, France, 1905. [Google Scholar]
- Blanc, C.P. Notes Sur Les Reptiles de Tunisie. III. Distribution et Perspectives de Protection Des Tortues Terrestres et Dulçaquicoles. Arch. Inst. Pasteur Tunis 1978, 55, 55–66. [Google Scholar]
- Nouira, S. L’Herpétofaune. In Biodiversité Des Écosystèmes Côtiers et Des Zones Humides Du Cap-Bon, Tunisie; MedWetCoast, 2001; pp. 121–138. Available online: https://www.lacerta.de/AF/Bibliografie/BIB_13508.pdf (accessed on 3 August 2023).
- Eichwald, E. Naturhistorische Bemerkungen Iiber Algiers Und Den Atlas IX (Kept, and Batr.). Mem. Soc. Nat. Mosc. 1851, 2, 414–444. [Google Scholar]
- Strauch, A. Essai D’une Erpétologie de l’Algérie; Commissionnaires de l’Académie Impériale des Sciences: Bavaria, Germany, 1862. [Google Scholar]
- Lallemant, C. Erpétologie de l’Algérie ou Catalogue Synoptique et Analytique des Reptiles et Amphibies de la Colonie; Savy: Paris, France, 1867. [Google Scholar]
- Boulenger, G.A. Catalogue of the reptiles and batrachians of Barbary (Morocco, Algeria, Tunisia), based chiefly upon the notes and collections made in 1880-1884 by M. Fernand Lataste. Trans. Zool. Soc. Lond. 1891, 13, 93–164. [Google Scholar] [CrossRef]
- Service Algérien de la Carte Géologique. Bulletin: Stratigraphie de l’Algeria, Service de la Carte Géologique; Imp. de l’Université; Y. Cadoret: Paris, France, 1935. [Google Scholar]
- Guichenot, A. Histoire Naturelle Des Reptiles et Des Poissons de l’Algérie. [Section 2] Sciences Physiques. Zoologie. In Exploration Scientifique de l’Algérie Pendant les Annees 1840, 1841, 1842) par Ordre du Gouvernement et avec le Concours D’une Commission Académique; Arthus Bertrand, Imprimerie Nationale: Paris, France, 1850. [Google Scholar]
- Doumergue, F. Essai sur la Faune Erpétologique de l’Oranie: Avec des Tableaux Analytiques et des Notions Pour la Détermination de Tous Les Reptiles & Batraciens du Maroc, de l’Algérie et de la Tunisie. Extrait du Bulletin de la Société de Géographie et d’Archéologie d’Oran; T. XIX à XXI; Imprimerie Typographique et Lithographique; L. Fouque: Oran, Algeria, 1901. [Google Scholar]
- Société Centrale d’Aquiculture et de Pèche. Bulletin de la Société Centrale d’Aquiculture et de Pèche; La Société: Urbana, IL, USA, 1912; Volume 24—25. [Google Scholar]
- Société d’Histoire Naturelle de l’Afrique du Nord. Bulletin de la Société D’histoire Naturelle de L’Afrique du Nord; La Société: Urbana, IL, USA, 1913. [Google Scholar]
- Samraoui, B.; De Belair, G. The Guerbes-Senhadja wetlands (NE Algeria). Part I: An overview. Ecologie 1997, 28, 233–250. [Google Scholar]
- De Belair, G.; Samraoui, B. L’écocomplexe Des Zones Humides de Beni-Belaid: Un Projet de Réserve Naturelle. Sci. Technol. A Exactes Sci. 2000, 14, 115–124. [Google Scholar]
- Tiar-Saadi, M.; Tiar, G.; Bouslama, Z.; Širokỳ, P. First data on the population of the European pond turtle Emys orbicularis at Lake Tonga, El Kala National Park, Algeria. Biologia 2017, 72, 819–824. [Google Scholar] [CrossRef]
- Escoriza, D.; Ben Hassine, J. Niche separation among North-West African semi-aquatic reptiles. Hydrobiologia 2017, 797, 47–56. [Google Scholar] [CrossRef]
- Segurado, P.; Araújo, A.P.R. Coexistence of Emys orbicularis and Mauremys leprosa in Portugal at two spatial scales: Is there evidence of spatial segregation. Biologia 2004, 59, 61–72. [Google Scholar]
- Mascort, R. Distribution and status of the European pond turtle, Emys orbicularis. Catalonia. Mertensiella 1998, 10, 177–186. [Google Scholar]
- Andueza, I.L.; Alcayde, V.S. Advances in the action plan for Emys orbicularis in the Valencia region, Spain. Biol. Bratisl. 2004, 59, 173–176. [Google Scholar]
- Cadi, A.; Nemoz, M.; Thienpont, S.; Joly, P. Home range, movements, and habitat use of the European pond turtle (Emys orbicularis) in the Rhône-Alpes region, France. Biologia 2004, 59, 89–94. [Google Scholar]
- Cadi, A.; Nemoz, M.; Thienpont, S.; Joly, P. Annual home range and movement in freshwater turtles: Management of the endangered European pond turtle (Emys orbicularis). Rev. Esp. Herp 2008, 22, 71–86. [Google Scholar]
- Zuffi, M.A.L. Conservation biology of the European pond turtle Emys orbicularis (L.) in Italy. Stapfia 2000, 69, 219–228. [Google Scholar]
- Govedič, M.; Vogrin, M.; Bordjan, D.; Bombek, D.; Denac, D.; Gregorc, T.; Janžekovič, F.; Kirbiš, N.; Vamberger, M. New data on distribution of the European pond turtle Emys orbicularis (Linnaeus, 1758) in the Podravje region (NE Slovenia). Nat. Slov. 2016, 18, 77–82. [Google Scholar]
- Krizmanić, I.; Urošević, A.; Simović, A.; Krstić, M.; Jović, D.; Ajtić, R.; Anđelković, M.; Slijepčević, M.; Đorđević, S.; Golubović, A. Updated distribution of the European pond turtle Emys orbicularis (Linnaeus, 1758) and its conservation issues in Serbia. Arch. Biol. Sci. 2015, 67, 1043–1053. [Google Scholar] [CrossRef]
- Sacdanaku, E.; Haxhiu, I. Distribution, habitats and preliminary data on the population structure of the European pond turtle, Emys orbicularis (Linnaeus, 1758), in Vlora Bay, Albania. Acta Zool. Bulg. 2017, 10, 9–14. [Google Scholar]
- Puky, M.; Gémesi, D.; Schád, P. Distribution of Emys orbicularis in Hungary with notes on related conservational and environmental education activities. Biol. Bratisl. 2004, 59, 55–60. [Google Scholar]
- Horváth, E.; Martvoňová, M.; Danko, S.; Havaš, P.; Kaňuch, P.; Uhrin, M. Distribution range and population viability of Emys orbicularis in Slovakia: A review with conservation implications. Nat. Conserv. 2021, 44, 141. [Google Scholar] [CrossRef]
- Rybacki, M.; Maciantowicz, M. Status, Distribution and protection of the European pond turtle (Emys orbicularis, L.) in western Poland. Rev. Esp. Herp 2008, 22, 131–137. [Google Scholar]
- Meeske, A.C.M.; Pupins, M.; Rybczynski, K. Erste Ergebnisse zur Verbreitung und zum Status der Europäischen Sumpfschildkröte (Emys orbicularis) am nördlichen Rand ihrer Verbraitung in Litauen und Lettland. Z. Feldenherpetologie 2006, 13, 71–99. [Google Scholar]
- Kotenko, T. Distribution, habitats, abundance and problems of conservation of the European pond turtle (Emys orbicularis) in the Crimea (Ukraine): First results. Biol. Bratisl. 2004, 59, 33–46. [Google Scholar]
- Bîrsan, C.-C.; Iosif, R.; Székely, P.; Cogălniceanu, D. Spatio-temporal bias in the perceived distribution of the European pond turtle, Emys orbicularis (Linnaeus, 1758), in Romania. Acta Zool. Bulg. 2017, 10, 37–41. [Google Scholar]
- Khabibullin, V. Distribution of Emys orbicularis in the South Urals, Russia. Biol. Bratisl. 2004, 59, 27–32. [Google Scholar]
- Mazanaeva, L.; Orlova, V. Distribution and ecology of Emys orbicularis in Daghestan, Russia. Biol. Bratisl. 2004, 59, 47–53. [Google Scholar]
- Loveridge, A.; Williams, E.E. Revision of the African tortoises and turtles of the suborder Cryptodira. Bull. Mus. Comp. Zool. 1957, 115, 163–557. [Google Scholar]
- Bons, J.; Geniez, P. Amphibiens et Reptiles du Maroc, Atlas Biogéographique; AHE: Barcelona, Spain, 1996. [Google Scholar]
- Real, R.; Pleguezuelos, J.M.; Fahd, S. The distribution patterns of reptiles in the Riff region, northern Morocco. Afr. J. Ecol. 1997, 35, 312–325. [Google Scholar] [CrossRef][Green Version]
- Fahd, S.; El Marnisi, B.; Mediani, M.; Fritz, U. Zur Verbreitung Und Zum Bedrohungsstatus Der Europäischen Sumpfschildkröte (Emys orbicularis) in Marokko. Elaphe 2009, 17, 30–33. [Google Scholar]
- Fritz, U.; Guicking, D.; Kami, H.; Arakelyan, M.; Auer, M.; Ayaz, D.; Fernández, C.A.; Bakiev, A.; Celani, A.; Džukić, G.; et al. Mitochondrial phylogeography of European pond turtles (Emys orbicularis, Emys irinacris)—An update. Amphib. Reptil. 2007, 28, 418–426. [Google Scholar] [CrossRef]
- Stuckas, H.; Velo-Antón, G.; Fahd, S.; Kalboussi, M.; Rouag, R.; Arculeo, M.; Marrone, F.; Sacco, F.; Vamberger, M.; Fritz, U. Where are you from, stranger? The enigmatic biogeography of North African pond turtles (Emys orbicularis). Org. Divers. Evol. 2014, 14, 295–306. [Google Scholar] [CrossRef]
- Messaoudene, M.; Tessier, L. Relations Cerne-Climat Dans des Peuplements de Quercus Afares Willd et Quercus Canariensis Pomel en Algérie. Ann. Sci. For. 1997, 54, 347–358. [Google Scholar] [CrossRef]
- Bouldjedri, M.; de Bélair, G.; Mayache, B.; Muller, S.D. Menaces et Conservation Des Zones Humides d’Afrique Du Nord: Le Cas Du Site Ramsar de Beni-Belaid (NE Algérien). Comptes Rendus Biol. 2011, 334, 757–772. [Google Scholar] [CrossRef]
- Lemdani, S.; Ouaked, L. Etude D’une Zone Humide Montagneuse en Kabylie Cas Du: Lac Noir d’Akfadou. Master’s Thesis, Université Mouloud Mammeri, Tizi Ouzou, Algeria, 2020. [Google Scholar]
- Laribi, M.; Derridj, A.; Acherar, M. Phytosociologie de La Forêt Caducifoliée à Chêne Zéen (Quercus Canariensis Willd.) Dans Le Massif d’Ath Ghobri-Akfadou (Grande Kabylie, Algérie). Fitosociologia 2008, 45, 77–92. [Google Scholar]
- Clarté, P.; Pinet, F.; D’amico, F. Importance Des Habitats Terrestres Dans La Dynamique d’occupation d’Emys orbicularis (Linnaeus, 1758) Sur Le Site Natura 2000 «Grande Brenne». Naturae 2020, 4, 71–84. [Google Scholar] [CrossRef]
- Roe, J.H.; Brinton, A.C.; Georges, A. Temporal and spatial variation in landscape connectivity for a freshwater turtle in a temporally dynamic wetland system. Ecol. Appl. 2009, 19, 1288–1299. [Google Scholar] [CrossRef]
- Roe, J.H.; Georges, A. Maintenance of variable responses for coping with wetland drying in freshwater turtles. Ecology 2008, 89, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Cushman, S.A. Effects of habitat loss and fragmentation on amphibians: A review and prospectus. Biol. Conserv. 2006, 128, 231–240. [Google Scholar] [CrossRef]
- Gilpin, M.E.; Soulé, M.E. Minimum Viable Populations: Processes of Species Extinction; Soulé, M.E., Ed.; Conservation Biology: The Science of Scarcity and Diversity; Sinauer Associates: Sunderland, MA, USA, 1986; pp. 19–34. [Google Scholar]
- Harrison, S. Local extinction in a metapopulation context: An empirical evaluation. Biol. J. Linn. Soc. 1991, 42, 73–88. [Google Scholar] [CrossRef]
- Medail, F.; Quezel, P. Hot-spots analysis for conservation of plant biodiversity in the Mediterranean basin. Ann. Mo. Bot. Gard. 1997, 84, 112–127. [Google Scholar] [CrossRef]
- Véla, E.; Benhouhou, S. Évaluation d’un nouveau point chaud de biodiversité végétale dans le Bassin méditerranéen (Afrique du Nord). Comptes Rendus Biol. 2007, 330, 589–605. [Google Scholar] [CrossRef]
- General Directorate of Forests Stratégie Nationale de Gestion Écosystémique des Zones Humides d’Algérie. FAOLEX. Available online: https://www.fao.org/faolex/results/details/fr/c/LEX-FAOC210820/ (accessed on 3 August 2023).
- Crnobrnja-Isailović, J.; Mesaroš, G. Conservation activities for the European pond turtle (Emys orbicularis) in Serbia. Herpetol. Notes 2013, 6, 119–123. [Google Scholar]
- Ficetola, G.F.; Salvidio, S.; D’Angelo, S.; Bonardi, A.; Bottoni, L.; Canalis, L.; Crosetto, S.; Di Martino, S.; Ferri, V.; Filetto, P. Conservation activities for European and Sicilian pond turtles (Emys orbicularis and Emys trinacris, respectively) in Italy. Herpetol. Notes 2013, 6, 127–133. [Google Scholar]
- Thienpont, S.; Cadi, A.; Quesada, R.; Cheylan, M. Overwintering habits of the European pond turtle (Emys orbicularis) in the Isère department (France). Biol. Bratisl. 2004, 59, 143–147. [Google Scholar]
- Cadi, A.; Faverot, P. La Cistude d’Europe–Gestion et Restauration des Populations et de Leur Habitat; Guide Technique; Conservatoire Rhône-Alpes des Espaces Naturels: Vourles, France, 2004; 108p. [Google Scholar]
- Gagno, S.; Jardé, N.; Marchis, N.; Ballouard, J.-M. Pressions Anthropiques Subies Par Les Chéloniens Dans Le Var-Testudo hermanni (Gmelin, 1789) et Emys orbicularis (Linnaeus, 1758): Premier Retour d’un Centre de Soins de La Faune Sauvage. Bull. Soc. Herp. Fr. 2013, 145, 157–168. [Google Scholar]
| Period, Year | 1 April–31 July 2020 | 1 April–31 July 2021 | 1 April–31 July 2022 | 1 April–31 July 2023 |
|---|---|---|---|---|
| Field Monitoring Effort | ||||
| Positive to one up to four field observations | 2–4,11,20,22,26,29–31,34–35 | 2,11,22,26,29–31,34–35,37–44,46–47,49,52 | 2,10–11,14,17–18,20–22,26,29–31,34–35,37–44,46–47,49,52 | 2,11,14,17–18,21,26,29–31,34–35,37–44,46–47,49–52 |
| Positive to five up to eight field observations | - | 3,8,10,12,14,16–23,27–28,32–33,48,50–51 | 3–4,8,12,16,19,23,27–28,32–33,48,50–51 | 7–8,10,12,16,19–20,22–25,27–28,32,36 |
| Negative at eight field observations | 1,5,6,9,13,15,45,53–54 | 1,5–7,9,13,15,36,45,53–54 | 1,5–6,9,13,15,33,45,48,53–54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherbi, N.; Tiar-Saadi, M.; Boucheker, A.; Široký, P.; Mezghiche, C.; Draidi, K.; Bouslama, Z.; Tiar, G. Distribution and Conservation Status of European Pond Turtles Emys orbicularis (L., 1758) in Algeria. Diversity 2023, 15, 993. https://doi.org/10.3390/d15090993
Gherbi N, Tiar-Saadi M, Boucheker A, Široký P, Mezghiche C, Draidi K, Bouslama Z, Tiar G. Distribution and Conservation Status of European Pond Turtles Emys orbicularis (L., 1758) in Algeria. Diversity. 2023; 15(9):993. https://doi.org/10.3390/d15090993
Chicago/Turabian StyleGherbi, Nourhane, Manel Tiar-Saadi, Abdennour Boucheker, Pavel Široký, Chahinez Mezghiche, Khalil Draidi, Zihad Bouslama, and Ghoulem Tiar. 2023. "Distribution and Conservation Status of European Pond Turtles Emys orbicularis (L., 1758) in Algeria" Diversity 15, no. 9: 993. https://doi.org/10.3390/d15090993
APA StyleGherbi, N., Tiar-Saadi, M., Boucheker, A., Široký, P., Mezghiche, C., Draidi, K., Bouslama, Z., & Tiar, G. (2023). Distribution and Conservation Status of European Pond Turtles Emys orbicularis (L., 1758) in Algeria. Diversity, 15(9), 993. https://doi.org/10.3390/d15090993








