Abstract
The invasive pest, Spodoptera frugiperda, commonly known as the fall armyworm (FAW), is a serious threat to food security in multiple countries worldwide. Insects’ antennal sensilla play a crucial role in perceiving plant odors and communication between male and female insects. This study aimed to examine the antennal morphology and sensilla variations on the antennae of FAW larvae and adults through scanning electron microscope analysis. The results revealed that third and fifth instar larval antennae possessed smell pores, sensilla pegs, and five types of antennal sensilla, namely sensilla trichodea, sensilla basiconica, sensilla chaetica, sensilla campaniform, and sensilla styloconicum, and the smell pores were first observed in Lepidoptera larvae. Furthermore, the size of sensilla in fifth instar larvae was significantly greater than those in third instar. On the adult antennae, there were smell pores and 12 types of sensilla identified: sensilla trichodea, sensilla basicaonica, sensilla auricillica, sensilla cavity, sensilla placodea, sensilla ligulate, Böhm’s bristles, sensilla chaetica, sensilla squamous, sensilla coeloconica, sensilla styloconicum, and sensilla uniporous peg. Notably, the sensilla cavity, sensilla placodea, sensilla ligulate, sensilla uniporous peg, and smell pores were first discovered in FAW adults. Compared with larvae, FAW adults have more types and amounts of sensilla. Additionally, we also discussed the possible functions of these antennal sensilla. This study provides valuable information for a comprehensive understanding of the type and function of antennal sensilla in FAW and assists in the development of novel pest control strategies, such as pest behavior control technology, for the prevention of this invasive pest.
1. Introduction
The fall armyworm (FAW), Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae), is a migratory pest with strong fecundity, high-migration ability, and a wide range of hosts, making it difficult to control [1]. FAW comprises rice- and corn-preferring strains, with the latter favoring corn or sorghum as its host [2]. In January 2019, FAW was first detected in Yunnan Province in China, and has since spread to 27 provinces [3]. It was reported that the invasive FAW strain in China belongs to the corn strain, and adults possibly disperse northward via seasonal monsoons during spring and summer to reach major crop production areas along the Yangtze River Basin, the Yellow River Basin, and northeast China [4]. Moreover, prior studies show that FAW poses significant threats of economic loss to other crops such as cotton, wheat, soybean, cabbage, etc. [5,6]. Due to varying levels of resistance among FAW populations towards different pesticides and genetically modified corn strains, there is an urgent need for studying new sustainable prevention and control technologies against this pest insect [7,8,9].
The role of the olfactory system in insects for host location is widely recognized, with the antenna being the most crucial organ for detecting odors. Insect antennae are equipped with various sensilla that serve important functions in different behaviors such as foraging, courtship, and oviposition [10,11]. Numerous studies describe the antennal sensilla of multiple insect species, including Coleoptera [12,13], Hymenoptera [14], and Hemiptera [15]. However, research on the antennal sensilla of Lepidoptera larvae is limited compared to that of adults. This may be due to the fact that population reproduction is mainly carried out by adults [16,17,18,19,20]. Nevertheless, studying the antennal sensilla of insect larvae is also of great significance for us to understand their feeding behavior and escape from of natural enemies.
As the FAW is an invasive pest, different researchers have reported varying numbers of antennal sensilla types for FAW. Malo et al. [21] identified seven types of sensilla on FAW antennae, while Tian et al. [22] reported eleven types of sensilla on adult FAW antennae. More recently, Gargi et al. [23] found that male and female FAW antennae possess eight types of sensilla. These conflicting results could confound understanding of FAW sensilla and impede subsequent studies on sensilla function. To address this issue, we used optical and scanning electron microscopy to observe the antennal sensilla present in third and fifth instar larvae as well as adults in detail. We discussed the potential roles these sensilla may play in host location and host acceptance behavior. This work provides valuable information for fully understanding the types and functions of antennal sensilla in FAW larvae and adults. It could also aid development of new pest control strategies, such as pest behavior control technology for preventing this invasive pest.
2. Materials and Methods
In July 2019, we collected approximately 10 FAW egg masses from a maize field located in Yangling, Shaanxi Province, China. The egg masses were then incubated at a temperature of 25 °C. We raised around 100 neonate larvae individually in plastic boxes (4 × 3 × 3 cm) containing maize leaves in an artificial climate greenhouse with controlled conditions of 26 ± 1 °C, 60 ± 5% relative humidity, and a photoperiod of 14 L:10 D. We purchased maize seeds (Shaandan 636) from Yangling Nongcheng Seed Supplement Company in Yangling, China and grew them in plastic pots (10 × 15 cm) using a mixture of commercial peat moss (Pindstrup Mosebrug A/S, Ryomgaard, Denmark), perlite, and vermiculite within the same climate room. After the maize seedlings reached an age of 14 days old, we used them to rear FAW larvae.
We collected third instar (L3) and fifth instar (L5) larvae, as well as female and male adult FAW specimens that were within two days old. These specimens were anesthetized by freezing for 5–10 s at −20 °C using a cryogenic refrigerator (BCD-601WDPR, Qingdao Haier Co., Ltd., Qingdao, China). Next, we removed the antennae from their heads under a stereoscope (SZM45, Ningbo Sunny Instruments Co., Ltd., Ningbo, China) and cleaned them in an ultrasonic bath for 30 s. We then immersed the antennae in a fixative containing 2.5% glutaraldehyde (Beijing Leagene Biotechnology Co., Ltd., Beijing, China) for 24 h at 4 °C. After rinsing three times with 0.1 M PBS (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) we dehydrated them over a graded ethanol series of 10%, 30%, 50%, 70%, 80%, 90%, and finally twice with 100% ethanol (20–30 min each). Next, we treated them with tert-butanol (25%, 50%, and 75% for15 min each), followed by twice with 100% tert-butanol (30–40 min each) before freeze-drying the antennae for three hours. Using double-sided adhesive tape, we anchored the treated antennae on the platform dorsally before coating them with gold to observe them under a scanning electron microscope (S-3400N, Hitachi, Japan) with an accelerating voltage of 5–15 KV. We recorded images onto a computer and used SEM particle size statistics software to measure the length and basal width of each sensillum. We also measured the length and basal width of the entire antenna using an optical electron microscope (JT-H3, Shenzhen Jingchengtuoyou Technology Co., Ltd., Shenzhen, China). The antennae and sensillum samples were observed and measured in a frontal view angle to ensure no curling or folding. The length referred to the measurement from base to tip, the height of pegs was measured similarly, and the width referred to the diameter across the outer edges. A total of 15 antennae of larvae or adults were used for measurement. The identification of sensilla was based on available morphological characteristics and scientific reports published [18,21,22,23,24,25,26].
We conducted a t-test to analyze the differences in antennae length or width between female and male adults, as well as the size of antennal sensilla between third and fifth instar larvae of FAW. The experimental data were analyzed using SPSS Statistics 28.0 package (IBM, Endicott, NY, USA).
3. Results
3.1. The Morphological Characteristics of Antennae in Spodoptera frugiperda Larvae
The antennae of L3 and L5 were found to be short and situated in sunken antennal fossae, which consisted of the scape, pedicel, and flagellum (Figure 1). The scape was located at the base of the antenna and had a thick, short shape (Figure 1A) with some scapes being covered by the antennal fossa (Figure 1B). Our analysis revealed that both the scape length (t = 12.609, df = 28, p < 0.0001) and basal diameter (t = 28.888, df = 28, p < 0.0001) of L5 were significantly greater than those of L3 (Table 1). The cylindrical pedicel was connected to the scape (Figure 1A,B), with our results showing that both the pedicel length (t = 27.467, df = 28, p < 0.0001) and basal diameter (t = 14.468, df = 28, p < 0.0001) of L5 were significantly greater than those of L3 (Table 1). Finally, we observed that the shorter flagellum was connected to the pedicel (Figure 1B), with our analysis indicating that the flagellum length of L5 was significantly greater than that of L3 (t = 8.475, df = 28, p < 0.0001) (Table 1).
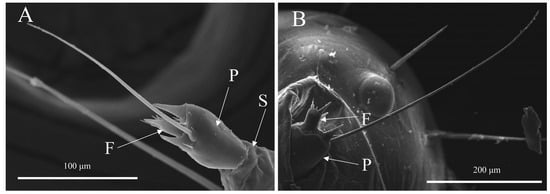
Figure 1.
The antennal morphological characteristics of Spodoptera frugiperda larvae. (A) Scape (S), pedicel (P), and flagellum (F) of 3rd-instar larvae; (B) Pedicel (P) and flagellum (F) of 5th-instar larvae.

Table 1.
The size of antennae and sensilla of Spodoptera frugiperda larvae.
3.2. The Antennal Sensilla Types and Distribution of Spodoptera frugiperda Larvae
The antennae of FAW larvae were observed to have five distinct types of sensilla, namely sensilla trichodea (with two subtypes), sensilla basiconica (with two subtypes), sensilla styloconicum, sensilla chaetica, and sensilla campaniform. Notably, we found no presence of sensilla on the scape (Figure 2A). Moving further along the antenna structure, the top of pedicel was observed to contain one each of the following: sensilla trichodea I, sensilla trichodea II, sensilla campaniform, sensilla chaetica, and two sensilla basiconica I (Figure 2C). Additionally, our investigation revealed that L3 and L5 had sensilla pegs distributed on their respective pedicels (Figure 2A). Lastly, the flagellum was observed to possess one each of the following: a single sensilla styloconicum, a single sensilla basiconica I and II each, one single sensilla chaetica, as well as at least one smell pore feature (Figure 2B).
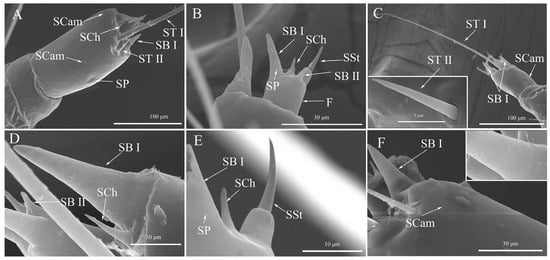
Figure 2.
The sensilla, smell pores, and sensilla pegs of Spodoptera frugiperda larvae. (A) Sensilla on the pedicel; (B) Sensilla on the flagellum; (C) Sensilla trichodea, sensilla basiconica I, and sensilla campaniform; (D) Sensilla chaetica, sensilla basiconica I and II; (E) Sensilla chaetica, sensilla styloconicum, sensilla basiconica I, and smell pores; (F) Sensilla basiconica I and sensilla campaniform. ST I: Sensilla trichodea I; ST II: Sensilla trichodea II; SB I: Sensilla basiconica I; SB II: Sensilla basiconica II; SCam: Sensilla campaniform; SSt: Sensilla styloconicum; SCh: Sensilla chaetica; SP: Smell pores; F: Flagellum.
3.2.1. Sensilla Trichodea (ST)
The structure of sensilla trichodea was observed to resemble a slender hair. These sensory units were found to be inserted into the antennal socket with their diameter gradually reducing towards the tip, possessing a smooth surface throughout (Figure 2C). We also identified two subtypes of ST: sensilla trichodea I (ST I) and sensilla trichodea II (ST II). Comparing these subtypes, we noted that ST I appeared blunter and more curved than ST II. In terms of length, our measurements revealed that both ST I (t = 29.421, df = 28, p < 0.0001) and ST II (t = 11.915, df = 28, p < 0.0001) units were significantly longer in L5 as compared to L3 (Table 1).
3.2.2. Sensilla Basiconica (SB)
Sensilla basiconica were observed to be positioned on the top surface of the antennae. Its bases were broader, and apices blunter. The SB outer walls exhibited longitudinal lines (Figure 2F) and smell pores (Figure 2B,E). We identified two subtypes of SB, namely sensilla basiconica I (SB I) and sensilla basiconica II (SB II). Our observations revealed that pedicel had two SB I (Figure 2C), whereas the flagellum possessed one SB I and one SB II (Figure 2B). In terms of length comparison, we noted that the length of SB I in L5 was significantly greater than that in L3 (t = 3.976, df = 28, p = 0.0004), while no significant difference was found in length for SB II between L5 and L3 (t = 1.643, df = 28, p = 0.1114) (Table 1).
3.2.3. Sensilla Chaetica (SCh)
Two sensilla chaetica, SCh, were found on the upper surface of the antenna. Unlike SBs, SCh had a thicker base without a basal socket. The tip of SCh was sharper than that of SB II (Figure 2B,D). Our analysis revealed that the length of SCh in L5 was significantly greater than that in L3 (t = 2.859, df = 28, p = 0.0079) (Table 1).
3.2.4. Sensilla Styloconicum (SSt)
The FAW larvae had a single SSt on their antennae, which had a smooth surface. The SSt was thicker at the base and erected on the top surface of the antenna with a sharper and slightly curved tip (Figure 2B,E). Our measurements indicated that both the basal column height (t = 2.062, df = 28, p = 0.0486) and peg height (t = 3.009, df = 28, p = 0.0055) of SSt were significantly longer in L5 than in L3 (Table 1).
3.2.5. Sensilla Campaniform (SCam)
3.3. General Description of Antennae of FAW Adults
Both male and female adults had thread-like antennae, which consisted of the scape, pedicel, and flagellum (Figure 3A). The scape was short and thick, while the pedicel was thinner and shorter than the scape (Figure 3B). The flagellum, on the other hand, was long and thin, comprised of 69–73 subsegments (Figure 3B,C). Although males had slightly longer antennae than females, this difference was not significant (t = 1.069, df = 28, p = 0.2940) (Table 2). The dorsal surface of the antennae was covered with imbricate scales, while two rows of scales could be observed covering the subsegments of the flagellum (Figure 3C,D).
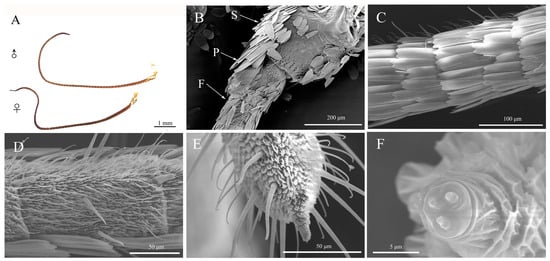
Figure 3.
The antennae morphological characteristics of Spodoptera frugiperda adults. (A) Overall appearance of adult antennae; (B) Scape, pedicel, and flagellum; (C) Dorsal side of the flagellum; (D) Ventral side of the flagellum; (E) The end of the flagellum; (F) The tip of the flagellum. ♀: Female; ♂: Male; S: Scape; P: Pedicel; F: Flagellum.

Table 2.
The antennal size of Spodoptera frugiperda adults.
3.4. The Antennal Sensilla Type and Distribution of Spodoptera frugiperda Adults
The antennae of FAW adults were observed to have smell pores and 12 types of sensilla, including sensilla trichodea, sensilla basicaonica, sensilla auricillica, sensilla cavity, sensilla placodea, sensilla ligulate, Böhm bristles, sensilla chaetica, sensilla squamous, sensilla coeloconica, sensilla styloconicum, and a single type of uniporous peg. The majority of these sensilla were found on the ventral surface of the antenna with only SCh and sensilla squamous present on the dorsal scale (Figure 3D). Furthermore, it was observed that the end of the flagellum had a greater amount of SCh while there was one specialized SSt present at the tip (Figure 3E,F).
3.4.1. Böhm’s Bristles (BB)
Böhm’s bristles occurred bilaterally on the scape and pedicel base (Figure 4A). These BBs had a spiny texture with a smooth, non-porous surface, and a thicker base that tapered towards the tip. Two subtypes of BB were distinguished based on their length and basal socket: BB I and BB II (Figure 4B). In females, the long and thick BB I inserted slightly above the antennal cuticle had significantly greater length compared to male adults (t = −2.762, df = 28, p < 0.0001). No significant difference in basal diameter was observed between female and male adults (t = −1.611, df = 28, p = 0.1183). Numerous BB II clustered around BB I, comprising short and thin bristles without a socket at the base. There was no significant difference in length of BB II between female and male adults (t = 0.076, df = 28, p = 0.9399) (Table 3).
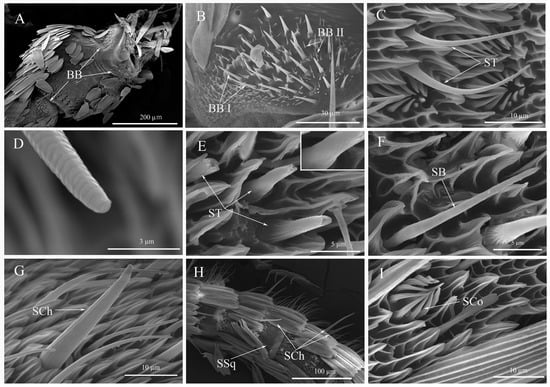
Figure 4.
The sensilla morphology of Spodoptera frugiperda adults. (A) The distribution of Böhm bristles; (B) Böhm’s bristles; (C) Sensilla trichodea; (D) The tip of sensilla trichodea; (E) The thick wall of sensilla trichodea; (F) Sensilla basiconica; (G) Sensilla chaetica; (H) Sensilla chaetica and sensilla squamous on the dorsal flagellum; (I) Sensilla coeloconica. BB: Böhm’s bristles; BB I: Böhm’s bristles I; BB II: Böhm’s bristles II; ST: Sensilla trichodea; SB: Sensilla basiconica; SCh: Sensilla chaetica; SCo: Sensilla coeloconica; SSq: Sensilla squamous.

Table 3.
The size of antennae and sensilla of Spodoptera frugiperda adult.
3.4.2. Sensilla Trichodea (ST)
The morphological characteristics of sensilla trichodea of adults were similar to those of larvae, but ST of adults had spiral lines and shallow pores on the surface (Figure 4C–E). No significant difference was observed in either length (t = −0.399, df = 28, p = 0.6931) or basal diameter (t = −1.576, df = 28, p = 0.1262) of ST between female and male adults (Table 3).
3.4.3. Sensilla Basiconica (SB)
The morphological characteristics of sensilla basiconica of adults were similar to those of larvae (Figure 4F). No significant difference in length was observed between female and male adults for SB (t = −0.503, df = 28, p = 0.619). However, the diameter of the circular socket in males was significantly larger than that of females (t = 2.643, df = 28, p = 0.0133) (Table 3).
3.4.4. Sensilla Chaetica (SCh)
The SCh was characterized by a straight shape with a spine-like tip, longitudinal lines on the surface, and a socket at the base. Both males and females had six evenly distributed SCh surrounding each flagellum subsegment (Figure 4H and Figure 5A), with the end subsegment of the flagellum having twelve SCh (possibly due to two fused subsegments) (Figure 3E). The angle between SCh and the antennal surface was greater than that of other sensilla (Figure 4G). Males had significantly longer SCh compared to females (t = −4.103, df = 28, p < 0.0003) (Table 3).
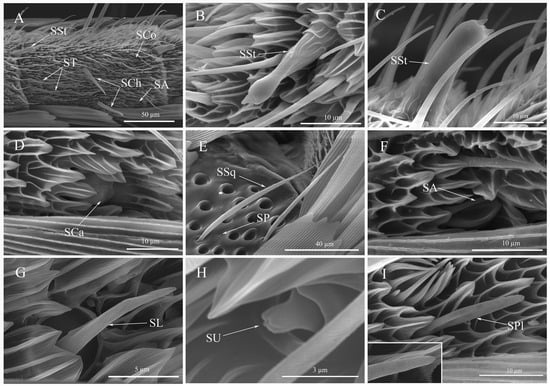
Figure 5.
The sensilla and smell pores morphology of Spodoptera frugiperda adults. (A) Sensilla on the dorsal flagellum; (B,C) Sensilla styloconicum; (D) Sensilla cavity; (E) Sensilla squamous and smell pores; (F) Sensilla auricillica; (G) Sensilla ligulate; (H) Sensilla uniporous peg; (I) Sensilla placodea. SSt: Sensilla styloconicum; SSq: Sensilla squamous; SA: Sensilla auricillica; SL: Sensilla ligulate; SU: Sensilla uniporous peg; SPl: Sensilla placodea; SP: Smell pores; SCo: Sensilla coeloconica; ST: Sensilla trichodea; SCh: Sensilla chaetica; SCa: Sensilla cavity.
3.4.5. Sensilla Coeloconica (SCo)
The SCo had a daisy-like shape, and each subsegment of the flagellum contained 2–6 SCo (Figure 5A). It was a shallow circular cavity created by the antennal surface’s inward depression. The center of the cavity featured a vertical sensory cone with a blunt round tip that protruded out of the epidermal depression. Around the cavity, there were 11–13 petal-shaped marginal pegs that formed a semilunar shape by bending toward the central pegs. Both marginal pegs and sensory cones had longitudinal lines on their surfaces (Figure 4I). There was no significant difference in the sensilla cavity diameters between females and males (t = −0.817, df = 28, p = 0.4208) (Table 3).
3.4.6. Sensilla Styloconicum (SSt)
Sensilla styloconicum, also known as the cylinder sensilla, were distributed on the edge of the end subsegment of the flagellum, extending from the former to the latter (Figure 5A). The tip of the end subsegment had a single SSt (Figure 3E,F), which resembled a thumb in shape. The base was cylindrical and featured raised pleated mesh-like structures, while the tip was thick with a small cavity at the top and one to three small papillary bulges within it. A constriction existed on the middle surface of SSt (Figure 5B,C). Males had significantly larger sensilla heights than females (t = −4.619, df = 28, p < 0.0001) (Table 3).
3.4.7. Sensilla Cavity (SCa)
The distribution of Sensilla cavity on the flagellum was less frequent, and its surface was sunken to create a cavity. The base of SCa was mound-shaped, and the middle surface had lines (Figure 5D). There was no significant difference in sensilla diameter between females and males (t = −0.188, df = 28, p = 0.8522) (Table 3).
3.4.8. Sensilla Squamous (SSq)
Sensilla squamous was present throughout the antennae, resembling scales but thinner and longer with a socket at the base (Figure 5E). The surface of SSq had five to seven parallel longitudinal ridges, with evenly distributed discontinuities on each ridge. The length (t = −1.417, df = 28, p = 0.1675) and basal diameter (t = 1.388, df = 28, p = 0.1760) of SSq did not significantly differ between females and males (Table 3). Additionally, smell pores were distributed around the SSq (Figure 5E).
3.4.9. Sensilla Auricillica (SA)
Sensilla auricillica was found dispersed throughout the middle and posterior subsegments of the flagellum, near the inner scale site, with an average of 1–2 SA per subsegment (Figure 5A). The shape of SA resembled an outward curling scale with a base inserted in a socket-shaped nest and an end shaped like a bell mouth (Figure 5F). There was no significant difference in sensilla length between females and males (t = −1.840, df = 28, p = 0.0763) (Table 3).
3.4.10. Sensilla Ligulate (SL)
Sensilla ligulate were found on the ventral surface of the antennae, curving towards the antenna surface (Figure 5G). The SL had small pores and grooves on their surfaces. The SL were embedded in a socket, tapering apically to a blunt end. Females had significantly larger sensilla length than males (t = 2.319, df = 28, p = 0.0279), while there was no significant difference in basal diameters between females and males (t = 1.495, df = 28, p = 0.1461) (Table 3).
3.4.11. Sensilla Uniporous Peg (SU)
The sensilla uniporous peg was found exclusively on the subsegments of the female flagellum, with two to six SU present on each subsegment. These papillate SU were embedded in deep sockets and had an enlarged base with a small hole at the end, along with longitudinal notches surrounding the hole (Figure 5H). This smallest type of sensilla in FAW is listed as the SU peg in Table 3.
3.4.12. Sensilla Placodea (SPl)
Sensilla placodea was situated within sensilla fossae, which was an elongated oval shape with longitudinal lines and small holes. The width of SPl remained consistent from base to end, ending in a blunt round tip (Figure 5I). The length (t = −1.948, df = 28, p = 0.0615), basal diameter (t = 0.503, df = 28, p = 0.6186), and width (t = −1.226, df = 28, p = 0.2304) of SPl showed no significant differences between males and females (Table 3).
4. Discussion
Antennal sensilla play a crucial role in insect behavior and perception of environmental changes [27]. In comparison to previous studies by Malo et al. [21], Tian et al. [22], and Gargi et al. [23], our study revealed that FAW larvae possess sensilla pegs, smell pores, and five types of sensilla, including the first reported instances of smell pores in Lepidoptera larvae. FAW adults had smell pores and 12 types of sensilla, with sensilla cavity, sensilla uniporous peg, sensilla ligulate, sensilla placodea, and smell pores being discovered for the first time in this species. These findings suggest that FAW possesses a diverse array of antennal sensilla, which may contribute to its rapid dispersal ability and adaptation to various environments.
Lepidopteran larvae primarily use their head sensilla to identify host plants and facilitate feeding activities [28]. Our study showed that the sensilla of FAW larvae were primarily located on the pedicel and flagellum of their antennae, with the most commonly observed types being sensilla trichodea, sensilla chaetica, and sensilla basiconica. These findings are consistent with studies conducted on Spodoptera litura larvae by Zhang et al. [29] and Ectropis grisescens larvae by Qin et al. [30]. Additionally, we discovered smell pores on FAW larval antennae, marking the first report of these structures in Lepidoptera larvae. While chemosensory functions were observed in Hymenoptera [31], the role of smell pores in Lepidoptera insects remains unclear. Lepidopteran larvae rely on their mandibles and maxillae’s sensilla trichodea to detect mechanical stimuli and determine food type and nature [16]. Sensilla styloconicum found on maxillary palps of Cossidae larvae function as gustatory receptors [28]. Albert [32] documented that sensilla basiconica on Choristoneura fumiferana larvae’s maxillary palp could be used to detect odor substances. In Antheraea assamensis larvae, multiporous sensilla chaetica located on their mandibles can perceive chemical stimuli [33]. Sensilla campaniform of Antheraea proylei larvae probably govern the preferred feeding posture of the silk moth larvae [34]. Sensilla campaniform on the integument of the first instar larvae of Dermatobia hominis, suggesting that it has importance in establishing the parasitic phase of the life cycle of this insect [35]. These studies suggest that the function of sensilla of insect larvae may vary depending on the species of insect. Therefore, further research is necessary to explore these specific functions of sensilla in FAW larvae. In addition, our results found the types and numbers of antennal sensilla of FAW larvae did not change, but the size of sensilla of fifth instar larvae was significantly larger than that of third instar larvae. It is well known that the elderly larvae of FAW (over third instar) have the characteristic of cannibalism, and their food intake accounts for over 98% of the entire larval stage [36]. Therefore, we speculate that the larger sensilla of older larvae of FAW might help them perceive complex environmental information more quickly and make behavioral responses that are beneficial to themselves.
Lepidoptera adults rely on olfactory and tactile sensilla to sense external stimuli, which play a crucial role in host plant selection and insect reproduction [26,37]. Insect sensilla can be classified into chemoreceptors, mechanoreceptors, thermo- and hygroreceptors based on their physiological functions. Chemoreceptors, such as olfactory and tactile sensilla, are essential for locating habitats and mates [38], with sensilla trichodea and sensilla basiconica potentially serving an olfactory function [39,40]. Sensilla auricillica were found to play a vital role in the host localization of female Scoliopteryx libatrix adults [26,41], while gustatory function was identified in Sensilla placodea located on the antennae of Coleophora obducta [42]. In our study, we observed the distribution of these sensilla types on FAW antennae. However, further research is needed to determine whether they play a similar role.
Mechanoreceptors play a critical role in providing accurate information to insects about host movement and body size [19,43]. Previous studies have identified Böhm’s bristles, sensilla chaetica, and sensilla squamous as structures that sense mechanical stimuli [44]. For instance, Böhm’s bristles are associated with the mechanical rotation of antennae in fir longhorn beetles [13], while Holcocerus hippophaecolus relies on these structures for flight control [45]. In FAW, we found that Böhm’s bristles were distributed at the base of the scape and pedicel, suggesting they may be involved in sensing position, velocity, and acceleration. Sensilla chaetica respond to mechanical shocks and play a crucial role in selecting suitable sites, behavioral environments, and courtship microenvironments [44]. However, it is worth noting that Jiang et al. [46] reported that sensilla chaetica is sensitive to D-fructose and has a gustatory function. In our study, we observed that sensilla chaetica of FAW were prominently located on both ventral and dorsal surfaces of antennae, suggesting they may be the first structures to encounter objects and could serve functions related to perceiving mechanical stimuli. Additionally, we found that the sensilla chaetica of male FAW were longer than those of females, which could aid in better perceiving female movement and status. Previous studies suggest that sensilla squamous may have mechanoreceptive functions [17,47]. Our investigation of FAW indicates that these structures are distributed on the scape, pedicel, and dorsal surface of the flagellum. This distribution suggests they may play a role in sensing mechanical stimulation and potentially reducing mechanical damage to the antenna.
The temperature and humidity sensing abilities of insects are linked to sensilla styloconicum, sensilla uniporous peg, and sensilla coeloconica. Sensilla styloconicum, typically non-porous, is known to sense temperature and humidity [48] and is widely distributed on the antennae of Lepidopteran like Copitarsia consueta [49] and Plutella xylostella [18]. Our research revealed that the sensilla styloconicum of FAW lacked pores, suggesting its function might be in sensing environmental changes. Sensilla uniporous peg is present on the antennae of Earias vittella and S. littoralis females, potentially sensing humidity or CO2 sensitivity [50]. While sensilla cavity was observed in Hymenoptera, its function remains unknown [51]. In our study, we found that sensilla uniporous peg were only located on the ventral surface of the flagellum in FAW females, which might be play role in detecting temperature and humidity changes within the host habitat. Sensilla coeloconica are frequently found on Lepidoptera antennae [40,52] and can be classified into subtypes in Sitotroga cerealella [53]. Shanbhag et al. [54] reported that sensilla coeloconica of Drosophila melanogaster lacks pore-like structures on its walls but senses temperature and humidity in the environment through its inner central sense cone. Our research shows that FAW has only one type of sensilla coeloconica with no pores on its walls. However, the surrounding annular microtrichia on the surface of the sensilla may protect the inner central sense cone from physical damage caused by external environmental factors.
5. Conclusions
Insects use their sensilla to detect a wide range of substances, including plant secondary compounds, salts, sugars, and amino acids. These abilities help them select suitable host plants. The FAW is a global agricultural pest that feeds on more than 350 host plant species. Our study comprehensively documented the antennal sensilla of FAW larvae, which include smell pores, sensilla pegs, and five types of sensilla. We also identified 12 types of sensilla on the antennae of adult FAW. While these antennal sensilla share similarities in morphology with other Lepidoptera species, there are differences in size and surface microstructure. However, the physiological function and role in host localization and receptive behavior of each sensillum in FAW have not been fully explored. Therefore, further research into these functions is crucial for developing new pest control strategies, such as behavioral control technology, to more effectively manage this invasive pest.
Author Contributions
Conceptualization, S.Z., X.J. and W.W.; Methodology, S.Z., W.W., P.H., T.L. and X.J.; Software, W.W. and P.H.; Validation, S.Z., W.W. and X.J.; Data Curation, S.Z. and W.W.; Writing—Original Draft Preparation, S.Z., X.J., W.W. and P.H.; Visualization, S.Z., W.W. and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research and Development Program of Shaanxi (No. 2021NY038) and National Key R&D Program of China (2022YFD1401200).
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the fact that no human subjects were involved.
Data Availability Statement
The data that support the findings of this study are included within the article.
Acknowledgments
We are grateful for the assistance of all staff members and students in the Key Laboratory of Applied Entomology, Northwest A&F University at Yangling, Shaanxi, China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Montezano, D.G.; Specht, A.; Sosa-Gómez, D.R.; Roque-Specht, V.F.; Sousa-Silva, J.C.; Paula-Moraes, S.V.; Peterson, J.A.; Hunt, T.E. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef]
- Murúa, M.G.; Nagoshi, R.N.; Dos Santos, D.A.; Hay-Roe, M.M.; Meagher, R.L.; Vilardi, J.C. Demonstration using field collections that argentina fall armyworm populations exhibit strain-specific host plant preferences. J. Econ. Entomol. 2015, 108, 2305–2315. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.L.; Jiang, C.X.; Guo, X.; Chen, D.D.; You, C.; Zhang, Y.; Wang, M.T.; Li, Q. Potential distribution of Spodoptera frugiperda (J. E. Smith) in China and the major factors influencing distribution. Glob. Ecol. Conserv. 2020, 21, e00865. [Google Scholar] [CrossRef]
- Wu, K.M. Management strategies of fall armyworm (Spodoptera frugiperda) in China. Plant Prot. 2020, 46, 1–5. [Google Scholar] [CrossRef]
- Hardke, J.T.; Jackson, R.E.; Leonard, B.R.; Temple, J.H. Fall armyworm (Lepidoptera: Noctuidae) development, survivorship, and damage on cotton plants expressing insecticidal plant-incorporated protectants. J. Econ. Entomol. 2015, 108, 1086–1093. [Google Scholar] [CrossRef]
- Wang, W.W.; He, P.Y.; Zhang, Y.Y.; Liu, T.X.; Jing, X.F.; Zhang, S.Z. The population growth of Spodoptera frugiperda on six cash crop species and implications for its occurrence and damage potential in China. Insects 2020, 11, 639. [Google Scholar] [CrossRef]
- Liang, P.; Gu, S.; Zhang, L.; Gao, X. Research status and prospects of Spodoptera frugiperda (Lepidoptera: Noctuidae) in China. Acta Entomol. Sin. 2020, 63, 624–638. [Google Scholar] [CrossRef]
- Sisay, B.; Sevgan, S.; Weldon, C.W.; Krüger, K.; Torto, B.; Tamiru, A. Responses of the fall armyworm (Spodoptera frugiperda) to different host plants: Implications for its management strategy. Pest Manag. Sci. 2023, 79, 845–856. [Google Scholar] [CrossRef]
- Kenis, M. Prospects for classical biological control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in invaded areas using parasitoids from the Americas. J. Econ. Entomol. 2023, 116, 331–341. [Google Scholar] [CrossRef]
- Cardoso, V.; Linardi, P.M. Scanning electron microscopy studies of sensilla and other structures of the head of Polygenis (Polygenis) tripus (Siphonapera: Rhopalopsyllidae). Micron 2006, 37, 557–565. [Google Scholar] [CrossRef]
- Rebora, M.; Piersanti, S.; Gaino, E. The antennal sensilla of the adult of Libellula depressa (Odonata: Libellulidae). Arthropod Struct. Dev. 2008, 37, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Diakite, M.M.; Ali, S.; Wang, M.Q. Morphology and ultrastructure of the antennal sensilla of Sitophilus granarius (Coleoptera: Curculionidae). Bull. Entomol. Res. 2016, 106, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.S.; Dou, F.G.; Yang, Y.B.; Wickham, J.D.; Tang, R.; Zhang, Y.J.; Huang, Z.Y.; Zheng, X.L.; Wang, X.Y.; Lu, W. First description and comparison of the morphological and ultramicro characteristics of the antennal sensilla of two fir longhorn beetles. PLoS ONE 2020, 15, e0241115. [Google Scholar] [CrossRef]
- Ribeiro Júnior, C.; Serrão, J.E. Antennal sensilla in Vespidae: A comparison between a diurnal and a nocturnal polistinae wasp. Microsc. Microanal. 2022, 28, 880–893. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.X.; Wu, W.J.; Liang, G.W.; Fu, Y.G. Nymphal antennae and antennal sensilla in Aleurodicus dispersus (Hemiptera: Aleyrodidae). Bull. Entomol. Res. 2014, 104, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Singh, S.; Chakraborty, R. Surface ultrastructure of larval mouthpart sensilla of the muga silkmoth, Antheraea assamensis, an endemic species of North-East India. Microsc. Res. Tech. 2011, 74, 292–300. [Google Scholar] [CrossRef]
- Ndomo-Moualeu, A.F.; Ulrichs, C.; Radek, R.; Adler, C. Structure and distribution of antennal sensilla in the Indianmeal moth, Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae). J. Stored Prod. Res. 2014, 59, 66–75. [Google Scholar] [CrossRef]
- Wee, S.L.; Oh, H.W.; Park, K.C. Antennal sensillum morphology and electrophysiological responses of olfactory receptor neurons in trichoid sensilla of the diamondback moth (Lepidoptera: Plutellidae). Fla. Entomol. 2016, 99, 146–158. [Google Scholar] [CrossRef]
- Ma, L.Y.; Hu, K.; Li, P.D.; Liu, J.Q.; Yuan, X.Q. Ultrastructure of the proboscis sensilla of ten species of butterflies (Insecta: Lepidoptera). PLoS ONE 2019, 14, e0214658. [Google Scholar] [CrossRef]
- Rani, A.T.; Shashank, P.R.; Meshram, N.M.; Sagar, D.; Srivastava, C.; Pandey, K.K.; Singh, J. Morphological characterization of antennal sensilla of Earias vittella (Fabricius) (Lepidoptera: Nolidae). Micron 2021, 140, 102957. [Google Scholar] [CrossRef]
- Malo, E.A.; Castrejongomez, V.R.; Cruzlopez, L.; Rojas, J.C. Antennal sensilla and electrophysiological response of male and female Spodoptera frugiperda (Lepidoptera: Noctuidae) to conspecific sex pheromone and plant pdors. Ann. Entomol. Soc. Am. 2004, 97, 1273–1284. [Google Scholar] [CrossRef]
- Tian, C.H.; Huang, J.R.; Wang, Y.N.; Zhang, S.G.; Li, G.P. Ultrastructure and morphology of antennal sensilla of the adult Spodoptera frugiperda (J. E. Smith). Plant Prot. 2021, 47, 216–221. [Google Scholar] [CrossRef]
- Gargi, C.; Kennedy, J.S.; Jayabal, T.D. Morphometrics and distribution of antennal sensillae of both sexes of Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). J. Appl. Nat. Sci. 2022, 14, 41–48. [Google Scholar] [CrossRef]
- Schneider, D. Insect Antennae. Annu. Rev. Entomol. 1964, 9, 103–122. [Google Scholar] [CrossRef]
- Zacharuk, R.Y.; Shields, V.D. Sensilla of immature insects. Annu. Rev. Entomol. 1991, 36, 331–354. [Google Scholar] [CrossRef]
- Ansebo, L.; Ignell, R.; Lofqvist, J.; Hansson, B.S. Responses to sex pheromone and plant odours by olfactory receptor neurons housed in sensilla auricillica of the codling moth, Cydia pomonella (Lepidoptera: Tortricidae). J. Insect Physiol. 2005, 51, 1066–1074. [Google Scholar] [CrossRef]
- Ronderos, D.; Smith, D. Diverse signaling mechanisms mediate volatile odorant detection in Drosophila. Fly 2009, 3, 290–297. [Google Scholar] [CrossRef]
- Xu, L.L.; Pei, J.H.; Wang, T.; Ren, L.L.; Zong, S.X. The larval sensilla on the antennae and mouthparts of five species of Cossidae (Lepidoptera). Can. J. Zool. 2017, 95, 611–622. [Google Scholar] [CrossRef]
- Zhang, F.M.; Jin, Y.L.; Zhang, L.L.; Yin, J.; Chen, J.H.; Zhao, Q.; Pan, P.L. Ultrastructure of the sensilla on adult antenna and larval head of Ectropis grisescens (Lepidoptera: Geometridae). Acta Entomol. Sin. 2019, 62, 743–755. [Google Scholar] [CrossRef]
- Qin, D.Q.; Zhang, P.W.; Zhou, Y.; Liu, B.J.; Xiao, C.X.; Chen, W.B.; Zhang, Z.X. Antifeeding effects of azadirachtin on the fifth instar Spodoptera litura larvae and the analysis of azadirachtin on target sensilla around mouthparts. Arch. Insect Biochem. Physiol. 2020, 103, e21646. [Google Scholar] [CrossRef]
- Ahmed, T.; Zhang, T.T.; Wang, Z.Y.; He, K.L.; Bai, S.X. Morphology and ultrastructure of antennal sensilla of Macrocentrus cingulum Brischke (Hymenoptera: Braconidae) and their probable functions. Micron 2013, 50, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.J. Electrophysiological responses to sucrose from a gustatory sensillum on the larval maxillary palp of the spruce budworm, Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae). J. Insect Physiol. 2003, 49, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.G.; Dey, S.; Kalita, J. Fine structural studies on major larval mouth part sensilla of Antheraea assamensis, an endemic silk moth species of North East India in regard to sensory physiology. J. Appl. Fundam. Sci. 2016, 2, 6–16. [Google Scholar]
- Dey, S.; Choudhury, S. Physiological significance of gravity receptors on larval cephalic cuticle in the silk moth, Antheraea proylei Jolly. Microsc. Res. Tech. 2018, 81, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Filippis, T.D.; Leite, A.C.R. Scanning electron microscopy studies on the first-instar larva of Dermatobia hominis. Med. Vet. Entomol. 1997, 11, 165–171. [Google Scholar] [CrossRef]
- Wang, W.W.; He, P.Y.; Liu, T.X.; Jing, X.F.; Zhang, S.Z. Comparative studies of ovipositional preference, larval feeding selectivity, and nutritional indices of Spodoptera frugiperda (Lepidoptera: Noctuidae) on 6 crops. J. Econ. Entomol. 2023, 116, 790–797. [Google Scholar] [CrossRef]
- Isidoro, N.; Bartlet, E.; Ziesmann, J.; Williams, I.H. Antennal contact chemosensilla in Psylliodes chrysocephala responding to cruciferous allelochemicals. Physiol. Entomol. 1998, 23, 131–138. [Google Scholar] [CrossRef]
- Ruschioni, S.; Riolo, P.; Verdolini, E.; Peri, E.; Guarino, S.; Colazza, S.; Romani, R.; Isidoro, N. Fine structure of antennal sensilla of Paysandisia archon and electrophysiological responses to volatile compounds associated with host palms. PLoS ONE 2015, 10, e0124607. [Google Scholar] [CrossRef]
- Binyameen, M.; Anderson, P.; Ignell, R.; Seada, M.A.; Hansson, B.S.; Schlyter, F. Spatial organization of antennal olfactory sensory neurons in the female Spodoptera littoralis moth: Differences in sensitivity and temporal characteristics. Chem. Senses 2012, 37, 613–629. [Google Scholar] [CrossRef]
- Roh, H.S.; Park, K.C.; Oh, H.-W.; Park, C.G. Morphology and distribution of antennal sensilla of two tortricid moths, Cydia pomonella and C. succedana (Lepidoptera). Microsc. Res. Tech. 2016, 79, 1069–1081. [Google Scholar] [CrossRef]
- Anderson, P.; Hallberg, E.; Subchev, M. Morphology of antennal sensilla auricillica and their detection of plant volatiles in the Herald moth, Scoliopteryx libatrix L. (Lepidoptera: Noctuidae). Arthropod Struct. Dev. 2000, 29, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yan, S.C.; Liu, D. Ultrastructural observations on antennal sensilla of Coleophora obducta (Meyrick) (Lepidoptera: Coleophoridae). Micron 2009, 40, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Nacro, S.; Nénon, J.P. Comparative study of the morphology of the ovipositor of Platygaster diplosisae (Hymenoptera: Platygasteridae) and Aprostocetus procerae (Hymenoptera: Eulophidae) two parasitoids associated with the African rice gall midge, Orseolia oryzivora (Diptera: Cecidomyiidae). Psyche A J. Entomol. 2009, 2009, 675242. [Google Scholar] [CrossRef]
- Dong, Z.S.; Yang, Y.B.; Dou, F.G.; Zhang, Y.J.; Huang, H.X.; Zheng, X.L.; Wang, X.Y.; Lu, W. Observations on the ultrastructure of antennal sensilla of adult Glenea cantor (Cerambycidae: Lamiinae). J. Insect Sci. 2020, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, L.; Xu, L.L.; Zong, S.X.; Luo, Y.Q. Sensilla on the antennae and ovipositor of the sea buckthorn Carpenter moth, Holcocerus hippophaecolus Hua et al. (Lepidoptera: Cossidae). Neotrop. Entomol. 2015, 44, 68–76. [Google Scholar] [CrossRef]
- Jiang, X.J.; Ning, C.; Guo, H.; Jia, Y.Y.; Huang, L.Q.; Qu, M.J.; Wang, C.Z. A gustatory receptor tuned to D-fructose in antennal sensilla chaetica of Helicoverpa armigera. Insect Biochem. 2015, 60, 39–46. [Google Scholar] [CrossRef]
- Chang, X.Q.; Zhang, S.; Lv, L.; Wang, M.Q. Insight Into the ultrastructure of antennal sensilla of Mythimna separata (Lepidoptera: Noctuidae). J. Insect Sci. 2015, 15, 124. [Google Scholar] [CrossRef]
- Altner, H.; Loftus, R. Ultrastructure and function of insect thermo- and hygroreceptors. Annu. Rev. Entomol. 1985, 30, 273–295. [Google Scholar] [CrossRef]
- Castrejón-Gómez, V.R.; Valdez-Carrasco, J.; Cibrian-Tovar, J.; Camino-Lavin, M.; Rodolfo Osorio, O. Morphology and distribution of the sense organs on the antennae of Copitarsia consueta (Lepidoptera: Noctuidae). Fla. Entomol. 1999, 82, 546–555. [Google Scholar] [CrossRef]
- Seada, M.A. Antennal morphology and sensillum distribution of female cotton leaf worm Spodoptera littoralis (Lepidoptera: Noctuidae). JOBAZ. 2015, 68, 10–18. [Google Scholar] [CrossRef]
- Bai, J.C.; Chen, K.W.; Chen, L.; Liang, G.W.; Zeng, L. Antennal sensilla of Diachasmimorpha longcicaudata (Ashmead) observed with scanning electron microscopy. J. Environ. Entomol. 2012, 34, 339–344. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.Q.; Zhang, G. Ultrastructural observations on antennal sensilla of Cnaphalocrocis medinalis (Lepidoptera: Pyralidae). Microsc. Res. Tech. 2011, 74, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Chang, M.M.; Lu, Y.; Lei, C.L.; Yang, F.L. Ultrastructure of sensilla of antennae and ovipositor of Sitotroga cerealella (Lepidoptera: Gelechiidae), and location of female sex pheromone gland. Sci. Rep. 2017, 7, 40637. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.R.; Singh, K.; Singh, R.N. Fine structure and primary sensory projections of sensilla located in the sacculus of the antenna of Drosophila melanogaster. Cell Tissue Res. 1995, 282, 237–249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).