A New Species and Two New Records of the Lichen Genus Fissurina from China
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological and Chemical Analyses
2.2. DNA Extraction, Amplification and Sequencing
2.3. Phylogenetic Analysis
3. Results and Discussion
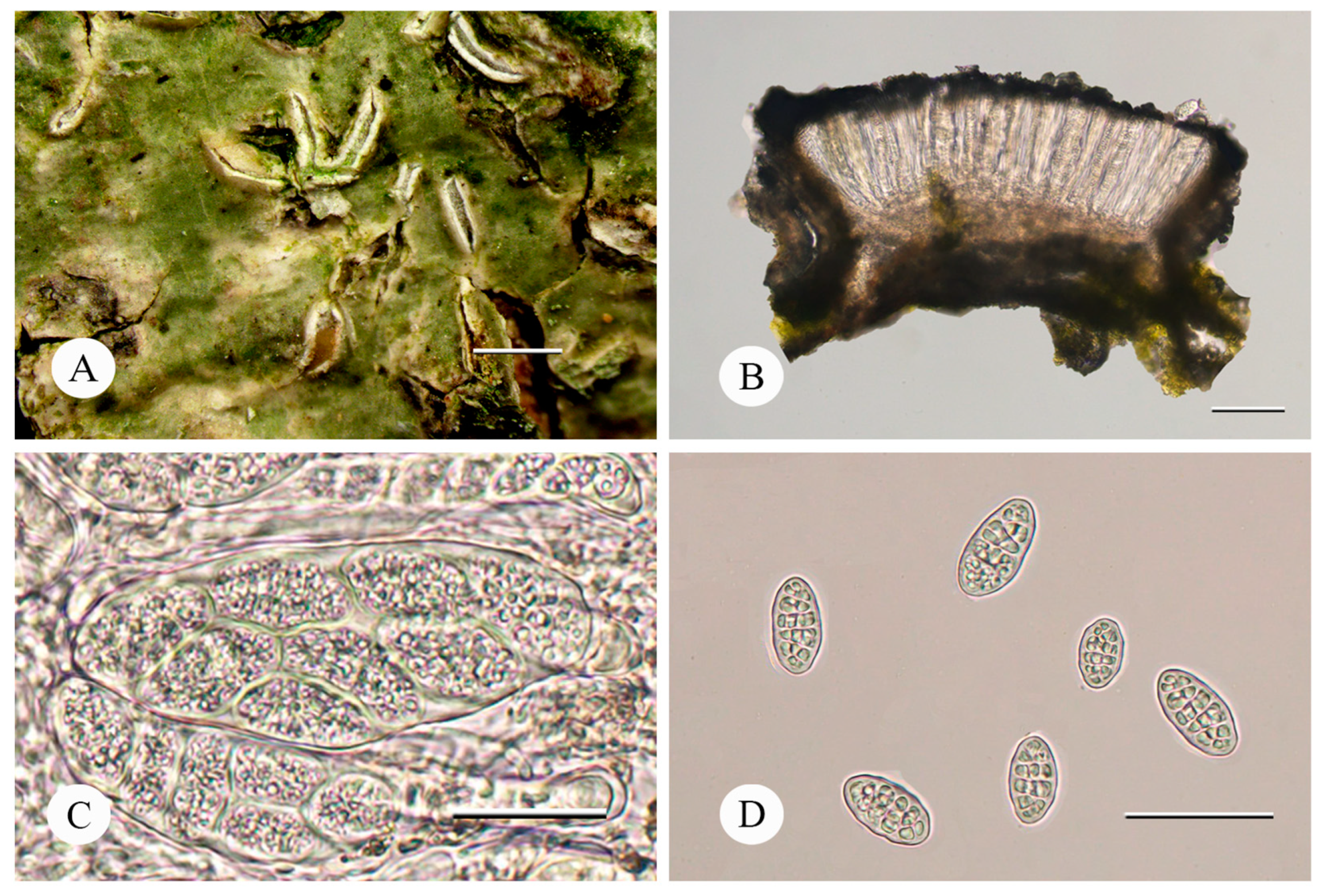
4. Taxonomy
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rivas Plata, E.; Lücking, R.; Lumbsch, H.T. A new classification for the family Graphidaceae (Ascomycota: Lecanoromycetes: Ostropales). Fungal Divers. 2012, 52, 107–121. [Google Scholar] [CrossRef]
- Rivas Plata, E.; Lücking, R. High diversity of Graphidaceae (lichenized Ascomycota: Ostropales) in Amazonian Perú. Fungal Divers. 2013, 58, 13–32. [Google Scholar] [CrossRef]
- Lücking, R.; Tehler, A.; Bungartz, F.; Rivas Plata, E.; Lumbsch, H.T. Journey from the West: Did tropical Graphidaceae (lichenized Ascomycota: Ostropales) evolve from a saxicolous ancestor along the American Pacific coast? Am. J. Bot. 2013, 100, 844–856. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Parnmen, S.; Kraichak, E.; Papong, K.B.; Lücking, R. High frequency of character transformations is phylogenetically structured within the lichenized fungal family Graphidaceae (Ascomycota: Ostropales). Syst. Biodivers. 2014, 12, 271–291. [Google Scholar] [CrossRef]
- Miadlikowska, J.; Kauff, F.; Högnabba, F.; Oliver, J.C.; Molnár, K.; Fraker, E.; Gaya, E.; Hafellner, J.; Hofstetter, V.; Gueidan, C. A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 317 genera and 66 families. Mol. Phylogenet. Evol. 2014, 79, 132–168. [Google Scholar] [CrossRef]
- Sharma, B.O.; Khadilkar, P.; Makhija, U. New species and new combinations in the lichen genera Fissurina and Hemithecium from India. Lichenologist 2012, 44, 339–362. [Google Scholar] [CrossRef]
- Jia, Z.F.; Li, J.; Meng, Q.F. A preliminary study of the lichen genus Fissurina (Graphidaceae) in China. Mycosystema 2018, 37, 881–895. Available online: https://manu40.magtech.com.cn/Jwxb/EN/Y2018/V37/I7/881 (accessed on 7 July 2023).
- Fée, A.L.A. Méthode Lichénographique et Genera: Ornée de Quatre Planches, Dont Trois Coloriées Donnant les Caractères des Genres qui Composent la Famille des Lichens avec Leurs Détails Grossis; Firmin Didot: Paris, France, 1824; pp. 1–124. [Google Scholar]
- Vainio, E.A. Lichenes Insularum Philippinarum III. Acta Soc. Sci. Fenn. 1921, 15, 1–368. [Google Scholar]
- Zahlbruckner, A. Catalogus Lichenum Universalis; Gebrüder Borntraeger: Stuttgart, Germany, 1923; pp. 145–452. [Google Scholar]
- Redinger, K. Die Graphidineen der ersten Regnell’schen Expedition nach Brasilien 1892–1894. III. Graphis, Phaeographis, nebst einem Nachtrage zu Graphina. Ark. För Bot. 1935, 27A, 1–103. [Google Scholar]
- Kalb, K.; Josef, H. Bemerkenswerte Flechten und lichenicole Pilze von der Insel Madeira. Herzogia 1992, 9, 45–102. [Google Scholar] [CrossRef]
- Staiger, B.; Kalb, K. Acanthothecis and other graphidioid lichens with warty periphysoids or paraphysis-tips. Mycotaxon 1999, 73, 69–134. [Google Scholar]
- Staiger, B. Die Flechtenfamilie Graphidaceae; Bibliotheca Lichenologica: Berlin, Germany, 2002; Volume 85, pp. 1–526. [Google Scholar]
- Kalb, K.; Staiger, B.; Elix, J.A. A monograph of the lichen genus Diorygma—A first attempt. Symb. Bot. Ups. 2004, 34, 133–181. [Google Scholar]
- Archer, A.W. The lichen genera Cyclographina, Diplogramma, Glyphis, Gymnographa, Medusulina, Sarcographa and Sarcographina (Graphidaceae) in Australia. Telopea 2004, 10, 589–605. [Google Scholar]
- Lücking, R.; Chaves, J.L.; Sipman, H.J.; Umaña, L.; Aptroot, A. A first assessment of the Ticolichen biodiversity inventory in Costa Rica: The genus Graphis, with notes on the genus Hemithecium (Ascomycota: Ostropales: Graphidaceae). Fieldiana Bot. 2008, 46, 1–126. [Google Scholar] [CrossRef]
- Lücking, R.; Kalb, K.; Staiger, B.; McNeill, J. (1792) Proposal to conserve the name Phaeographis, with a conserved type, against Creographa, Ectographis, Flegographa, Hymenodecton, Platygramma, and Pyrographa (Ascomycota: Ostropales: Graphidaceae), along with notes on the names Graphina and Phaeographina. Taxon 2007, 56, 1296–1299. [Google Scholar] [CrossRef]
- Mangold, A.; Martín, M.P.; Lücking, R.; Lumbsch, H.T. Molecular phylogeny suggests synonymy of Thelotremataceae within Graphidaceae (Ascomycota: Ostropales). Taxon 2008, 57, 476–486. [Google Scholar]
- Staiger, B.; Kalb, K.; Grube, M. Phylogeny and phenotypic variation in the lichen family Graphidaceae (Ostropomycetidae, Ascomycota). Mycol. Res. 2006, 110, 765–772. [Google Scholar] [CrossRef]
- Rivas Plata, E.; Parnmen, S.; Staiger, B.; Mangold, A.; Frisch, A.; Weerakoon, G.; Hernandez, J.; Caceres, M.; Kalb, K.; Sipman, H. A molecular phylogeny of Graphidaceae (Ascomycota, Lecanoromycetes, Ostropales) including 428 species. MycoKeys 2013, 6, 55–94. [Google Scholar] [CrossRef]
- Kraichak, E.; Huang, J.-P.; Nelsen, M.; Leavitt, S.D.; Lumbsch, H.T. A revised classification of orders and families in the two major subclasses of Lecanoromycetes (Ascomycota) based on a temporal approach. Bot. J. Linn. Soc. 2018, 188, 233–249. [Google Scholar] [CrossRef]
- Lücking, R. Stop the abuse of time! Strict temporal banding is not the future of rank-based classifications in fungi (including lichens) and other organisms. Crit. Rev. Plant Sci. 2019, 38, 199–253. [Google Scholar] [CrossRef]
- Wijayawardene, N.; Hyde, K.; Dai, D.; Sánchez-García, M.; Goto, B.T.; Magurno, F. Outline of Fungi and fungus-like taxa–2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Archer, A.W. Key and checklist for the lichen family Graphidaceae (lichenised Ascomycota) in the Solomon Islands. Syst. Biodivers. 2007, 5, 9–22. [Google Scholar] [CrossRef]
- Lendemer, J.C. Lichens of Eastern North America Exsiccati, Fascicle V, nos. 201–250. Opusc. Philolichenum 2007, 4, 69–80. [Google Scholar]
- Lücking, R.; Johnston, M.K.; Aptroot, A.; Kraichak, E.; Lendemer, J.C.; Boonpragob, K.; Caceres, M.E.; Ertz, D.; Ferraro, L.I.; Jia, Z.F.; et al. One hundred and seventy-five new species of Graphidaceae: Closing the gap or a drop in the bucket? Phytotaxa 2014, 189, 7–38. [Google Scholar] [CrossRef]
- Diederich, P.; Lücking, R.; Aptroot, A.; Sipman, H.J.; Braun, U.; Ahti, T.; Ertz, D. New species and new records of lichens and lichenicolous fungi from the Seychelles. Herzogia 2017, 30, 182–236. [Google Scholar] [CrossRef]
- Aptroot, A.; Feuerstein, S. New Graphidaceae from South and Central Brazil. Universitätsbibliothek Johann Christian Senckenberg. Arch. Lichenol. 2020, 16, 1–10. [Google Scholar]
- Lücking, R.; Álvaro-Alba, W.R.; Moncada, B.; Marín-Canchala, N.L.; Tunjano, S.S.; Cárdenas-López, D. Lichens from the Colombian Amazon: 666 Taxa Including 28 new Species and 157 New Country Records Document an Extraordinary Diversity. Bryologist 2023, 126, 242–303. [Google Scholar] [CrossRef]
- Van den Boom, P.P.; Lücking, R.; Sipman, H.J. Notes on Graphidaceae in Macaronesia, with Descriptions of Four New Species. Diversity 2023, 15, 817. [Google Scholar] [CrossRef]
- Fang, J.Y.; Song, Y.C.; Liu, H.Y.; Piao, S.L. Vegetation-climate relationship and its application in the division of vegetation zone in China. Acta Bot. Sin. 2002, 44, 1105–1122. [Google Scholar]
- Xu, Q.; Dong, Y.; Wang, Y.; Yang, R.; Xu, C. Determinants and identification of the northern boundary of China’s tropical zone. J. Geogr. Sci. 2018, 28, 31–45. [Google Scholar] [CrossRef]
- Jia, Z.F.; Lücking, R. Resolving the species of the lichen genus Graphina Müll. Arg. in China, with some new combinations. MycoKeys 2017, 25, 13–29. [Google Scholar] [CrossRef][Green Version]
- Nakanishi, M.; Kashiwadani, H.; Moon, K.H. Notes on the genera Graphina and Graphis (Graphidaceae) in Thailand. Bull. Natl. Sci. Mus. Ser. B 2001, 27, 47–55. [Google Scholar]
- Wei, J.C.; Jia, Z.F. Flora Lichenum Sinicorum Vol. 13 Ostropales(I) Graphidaceae (1); Science Press: Beijing, China, 2016; pp. 1–210. [Google Scholar]
- Culberson, C.F. Improved conditions and new data for identification of lichen products by standardized thin-layer chromatographic method. J. Chromatogr. A 1972, 72, 113–125. [Google Scholar] [CrossRef]
- Culberson, C.F.; Kristinsson, H.D. A standardized method for the identification of lichen products. J. Chromatogr. A 1970, 46, 85–93. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Zoller, S.; Scheidegger, C.; Sperisen, C. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist 1999, 31, 511–516. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Kraichak, E.; Parnmen, S.; Plata, E.R.; Aptroot, A.; Cáceres, M.E.S.; Ertz, D.; Feuerstein, S.C.; Mercado-Díaz, J.A.; Staiger, B.; et al. New higher taxa in the lichen family Graphidaceae (lichenized Ascomycota: Ostropales) based on a three-gene skeleton phylogeny. Phytotaxa 2014, 189, 39–51. [Google Scholar] [CrossRef][Green Version]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v. 1.4.2. Institute of Evolutionary Biology, University of Edinburgh. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 15 July 2023).
- Fée, A.L.A. Essai sur les Cryptogames des Ecorces Exotiques Officinales; Firmin Didot: Paris, France, 1825; pp. 1–167. [Google Scholar]
- Chesnokov, S.V.; Pan’kova, V.V.; Konoreva, L.A. Fissurina inabensis (Graphidaceae, Ascomycota), a new record to Russia from Shikotan Island. Turczaninowia 2023, 26, 116–123. [Google Scholar] [CrossRef]
- Lücking, R.; Seavey, F.; Common, R.S.; Beeching, S.Q.; Breuss, O.; Buck, W.R.; Crane, L.; Hodges, M.; Hodkinson, B.P.; Lay, E. The lichens of Fakahatchee Strand Preserve State Park, Florida: Proceedings from the 18th tuckerman workshop. Bull. Fla. Mus. Nat. Hist. 2011, 49, 127–186. [Google Scholar]
- Herrera-Campos, M.A.; Barcenas-Pena, A.; Miranda-González, R.; Mejía, M.A.; González, J.A.B.; Colín, P.M.; Téllez, N.S.; Lücking, R. New lichenized Arthoniales and Ostropales from Mexican seasonally dry tropical forest. Bryologist 2019, 122, 62–83. [Google Scholar] [CrossRef]
- Lendemer, J.C.; Harris, R.C. Seven new species of Graphidaceae (lichenized Ascomycetes) from the Coastal Plain of southeastern North America. Phytotaxa 2014, 189, 153–175. [Google Scholar] [CrossRef]
- Cáceres, M.E.; Aptroot, A.; Parnmen, S.; Lücking, R. Remarkable diversity of the lichen family Graphidaceae in the Amazon rain forest of Rondônia, Brazil. Phytotaxa 2014, 189, 87–136. [Google Scholar] [CrossRef]
- Papong, K.B.; Luecking, R.; Kraichak, E.; Parnmen, S.; Konrat, M.; Lumbsch, H.T. Twenty-three new species in the lichen family Graphidaceae from New Caledonia Ostropales, Ascomycota. Phytotaxa 2014, 189, 204–231. [Google Scholar] [CrossRef]
- Lumbsch, H.T.; Ahti, T.; Altermann, S.; De Paz, G.A.; Aptroot, A.; Arup, U.; Peña, A.B.; Bawingan, P.A.; Benatti, M.N.; Betancourt, L.; et al. One hundred new species of lichenized fungi: A signature of undiscovered global diversity. Phytotaxa 2011, 18, 1–127. [Google Scholar] [CrossRef]
- Joshi, S.; Upreti, D.K.; Nguyen, T.T.; Nguyen, A.D.; Oh, S.O.; Hur, J.S. A new species of Fissurina and new records of Graphidaceae from Vietnam. Cryptogam. Mycol. 2015, 36, 383–397. [Google Scholar] [CrossRef]
- Joshi, S.; Nguyen, T.T.; Dzung, N.A.; Jayalal, U.; Oh, S.O.; Hur, J.S. The lichen genus Fissurina (Graphidaceae) from Vietnam. Mycotaxon 2013, 124, 309–321. [Google Scholar] [CrossRef]



| Species | Country | Voucher Specimens | ITS | mtSSU | nuLSU |
|---|---|---|---|---|---|
| Clandestinotrema clandestinum | Costa Rica | Sipman 44327 | — | JX421014 | — |
| C. leucomelaenum | Ecuador | Luecking 26216 | — | JX421016 | — |
| C. leucomelaenum | Costa Rica | Luecking LCB6b | — | JX421015 | — |
| C. stylothecium | Nicaragua | Luecking 28636 | — | HQ639597 | HQ639662 |
| Cruentotrema cruentatum | Brazil | Luecking 263 | — | HQ639587 | HQ639660 |
| Cr. thailandicum | Myanmar | TNS:YO12327 | LC573996 | — | — |
| Cr. thailandicum | Thailand | Lumbsch 19955d1 | — | JF828960 | JF828975 |
| Cr. thailandicum | Thailand | Lumbsch 19955d2 | — | JX421020 | — |
| Cr. thailandicum | Thailand | Lumbsch 19955d3 | — | JX421021 | — |
| Dyplolabia afzelii | Australia | MEL:2382721 | KP012902 | — | — |
| D. afzelii | / | RMG246 | MK503256 | — | — |
| D. afzelii | USA | Luecking 26509 | — | HQ639594 | HQ639628 |
| D. afzelii | Australia | Kalb 33915 | — | DQ431950 | AY640013 |
| Fissurina adscribens | China | HNX18044 | OR264094 | OR264108 | OR264070 |
| F. adscribens | China | FJ220250 | OR264085 | OR264107 | OR264068 |
| F. adscribens | China | FJ211195 | OR264084 | — | — |
| F. adscribens | Thailand | Lumbsch 20200c | — | JX421032 | — |
| F. aff. dumastii | Thailand | Kalb 38899 | — | JX421034 | JX421487 |
| F. aff. humilis | Tanzania | A. Frisch No. 99/Tz2002 | — | DQ431948 | DQ431921 |
| F. aggregatula | Peru | RivasPlata 107C | — | JX421036 | JX421490 |
| F. aggregatula | Thailand | Luecking 24019 | — | JX421038 | — |
| F. aggregatula | Thailand | Kalb 38890 | — | JX421037 | — |
| F. amazonica | Brazil | Caceres 11030 | — | KJ608636 | KJ608633 |
| F. astroisidiata | Mexico | Luecking RLD057 | — | JX421040 | JX421491 |
| F. bullata | Australia | Mangold 6f | — | JX421041 | KF875537 |
| F. comparimuralis | El Salvador | Luecking 28103 | — | JX421042 | JX421492 |
| F. crassilabra | China | FJ211425 | OR264081 | OR264114 | OR264065 |
| F. crystallifera | USA | Mercado 4462 | — | — | KF875538 |
| F. dumastii | Cameroon | Frisch & Tamnjong Idi 99/Ka3855 | — | DQ384926 | — |
| F. illiterata | USA | Common 9115B | — | JX421045 | — |
| F. inabensis | China | GZ18113 | OR264087 | OR264105 | OR264071 |
| F. inabensis | China | YN221499 | OR264086 | OR264109 | OR264067 |
| F. inabensis | China | FJ211545 | OR264088 | OR264104 | OR264066 |
| F. inabensis | Russia | LE L-18645 | OP901516 | — | — |
| F. insidiosa | China | HN19093 | OR264080 | OR264111 | — |
| F. insidiosa | / | AFTOL-ID 1662 | — | DQ972995 | DQ973045 |
| F. insidiosa | USA | Spribille 39035 | KR017123 | KR017325 | KR017185 |
| F. inspersa | USA | Common 9113G | — | JX421047 | — |
| F. marginata | Australia | Kalb 33944 | — | DQ431951 | AY640012 |
| F. marginata | Thailand | Luecking 24122 | — | HQ639613 | JX421493 |
| F. monilifera | Puerto Rico | Mercado 838 | — | KJ435167 | KJ440941 |
| F. nigrolabiata | Philippines | Rivas Plata 1198B | — | JF828961 | JF828976 |
| F. nigrolabiata | Philippines | Rivas Plata 1016E | — | — | JX421494 |
| F. nitidescens | USA | Common 9113C | — | JX421048 | — |
| F. pseudostromatica | China | YN222451 | OR264089 | OR264098 | OR264074 |
| F. pseudostromatica | China | YN222474 | OR264090 | OR264099 | OR264072 |
| F. pseudostromatica | USA | Common 9126B | — | JX421051 | — |
| F. pseudostromatica | USA | Common 9113A | — | JX421050 | — |
| F. pseudostromatica | Bolivia | Luecking 29014 | — | JX421049 | — |
| F. pseudostromatica | Thailand | Kalb 38827 | — | — | JX421495 |
| F. pseudostromatica | China | YN210691 | OR264091 | OR264100 | OR264075 |
| F. rufula | Fiji | Lumbsch 20521l | — | JX421053 | JX421497 |
| F. rufula | Fiji | Lumbsch 20500l | — | JX421052 | — |
| F. subcomparimuralis | China | GD19219 | OR264092 | OR264112 | OR264079 |
| F. subcomparimuralis | China | GD19359 | OR264093 | OR264113 | OR264078 |
| F. subfurfuracea | Brazil | Caceres 11981 | — | KJ608635 | KJ608632 |
| F. subundulata | China | FJ220370 | OR264082 | OR264110 | — |
| F. subundulata | China | GZ18148 | OR264083 | OR264106 | OR264069 |
| F. triticea | Tanzania | A. Frisch No. 99/Tz1855 | — | DQ431952 | AY640011 |
| F. wuyinensis | China | FJ230605 | OR264095 | OR264101 | OR264076 |
| F. wuyinensis | China | FJ230610 | OR264097 | OR264103 | OR264073 |
| F. wuyinensis | China | FJ230464 | OR264096 | OR264102 | OR264077 |
| Porina aenea | Czech Republic | PRA-Vondrak25464 | OQ718026 | OQ646398 | — |
| P. leptalea | Czech Republic | PRA-Vondrak24457 | OQ718029 | OQ646400 | — |
| Pycnotrema pycnoporellum | USA | Luecking 26546 | — | JX421295 | JX421615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, K.; Jia, Z.; Zhao, X. A New Species and Two New Records of the Lichen Genus Fissurina from China. Diversity 2023, 15, 959. https://doi.org/10.3390/d15090959
Shi K, Jia Z, Zhao X. A New Species and Two New Records of the Lichen Genus Fissurina from China. Diversity. 2023; 15(9):959. https://doi.org/10.3390/d15090959
Chicago/Turabian StyleShi, Kaijie, Zefeng Jia, and Xin Zhao. 2023. "A New Species and Two New Records of the Lichen Genus Fissurina from China" Diversity 15, no. 9: 959. https://doi.org/10.3390/d15090959
APA StyleShi, K., Jia, Z., & Zhao, X. (2023). A New Species and Two New Records of the Lichen Genus Fissurina from China. Diversity, 15(9), 959. https://doi.org/10.3390/d15090959





