Invasibility of Common Plant Community Types of the Middle Urals
Abstract
:1. Introduction
2. Materials and Methods
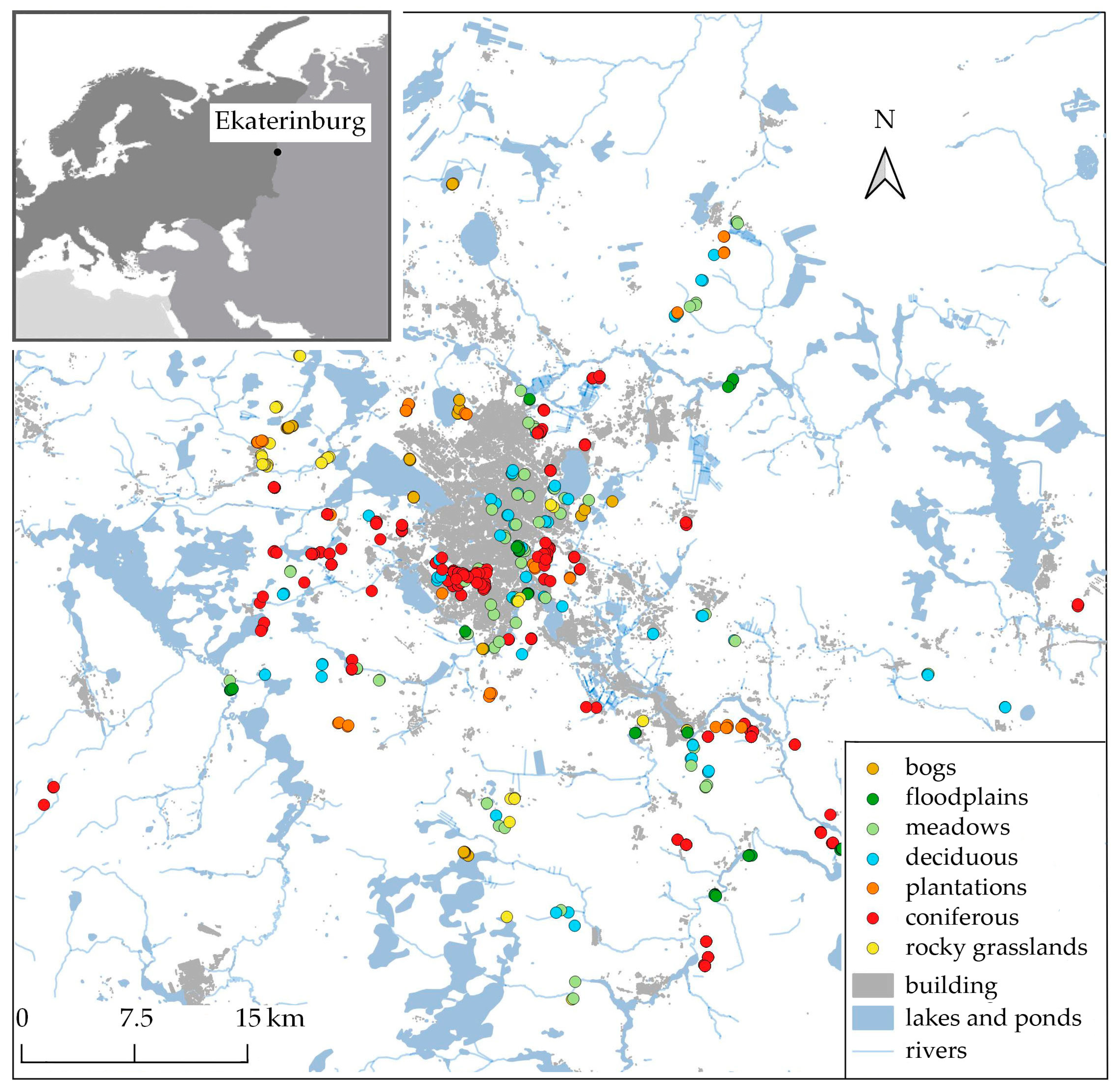
2.1. Study Area
2.2. Plant Communities
- Bog communities without trees—sedge and reedbeds fens and bogs without trees (EUNIS Q1, Q5);
- Bog communities with trees—swamps and boreal bog woodlands, shrub fens (EUNIS T16, T3J, S92);
- Floodplain communities without trees—floodplain meadows (EUNIS R35);
- Floodplain communities with trees—riparian forests formed by species of the genus Salix, Padus, Alnus (EUNIS T11);
- Meadows—mesic and wet meadows (EUNIS R2, R3);
- Birch forests—forests formed by species of the genus Betula (EUNIS T1);
- Coniferous plantations, generally Scots pine (Pinus sylvestris) (EUNIS T359);
- Coniferous forests (Pinus sylvestris) (EUNIS T3);
- Rocky grassland—rocky steppes of Festuco-Brometea class (EUNIS R1A) and anthropogenic herbaceous vegetation near the rocks (EUNIS V3);
- Forests dominated by Scots pine (Pinus sylvestris) on rocky outcrops (EUNIS T35).
2.3. Vegetation Relevés
2.4. Alien Species
2.5. Invasibility Degree Estimation
2.6. Data Analysis
3. Results
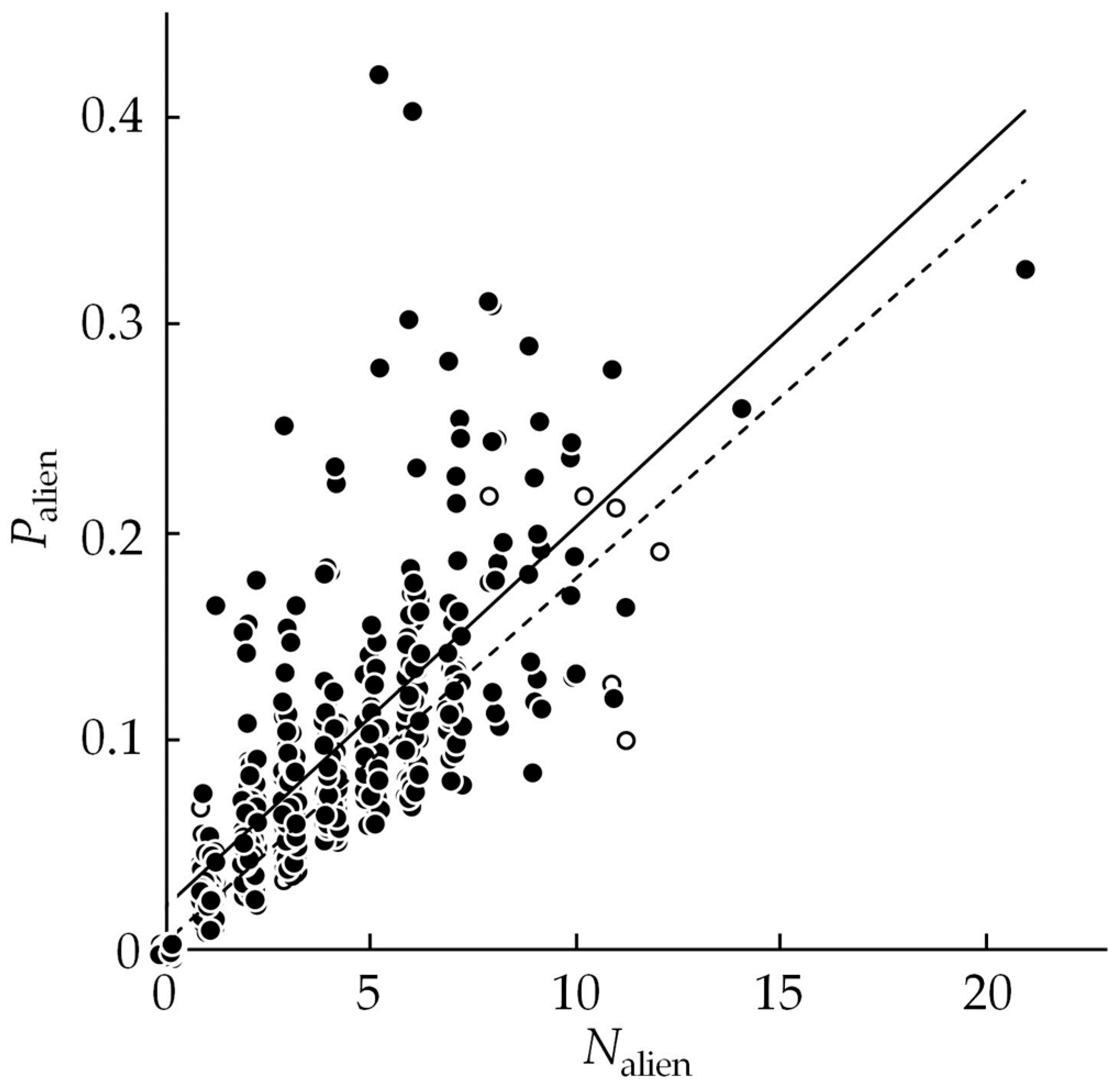
3.1. Alien Species Number and Proportion
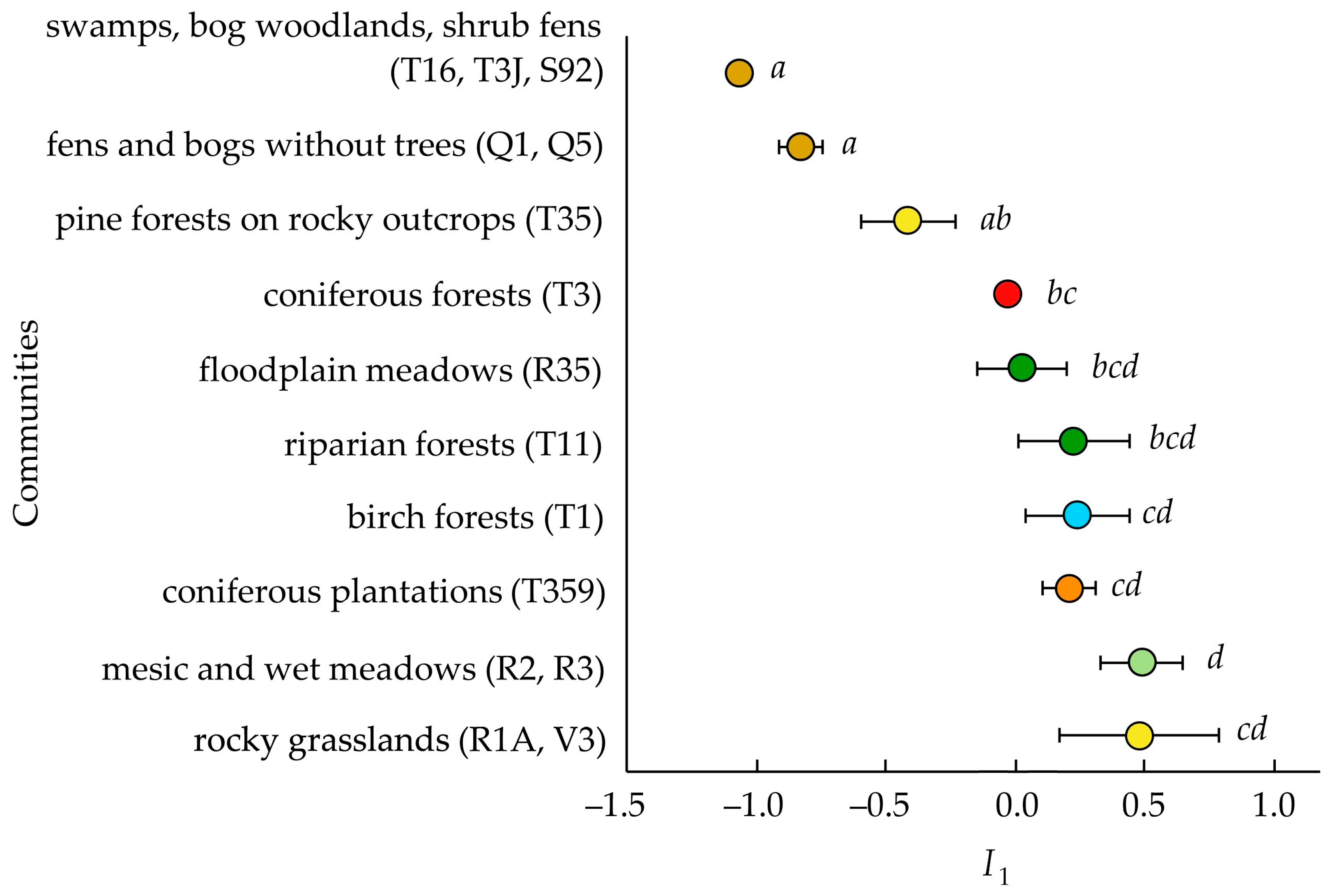
3.2. Invasibility Parameter I1
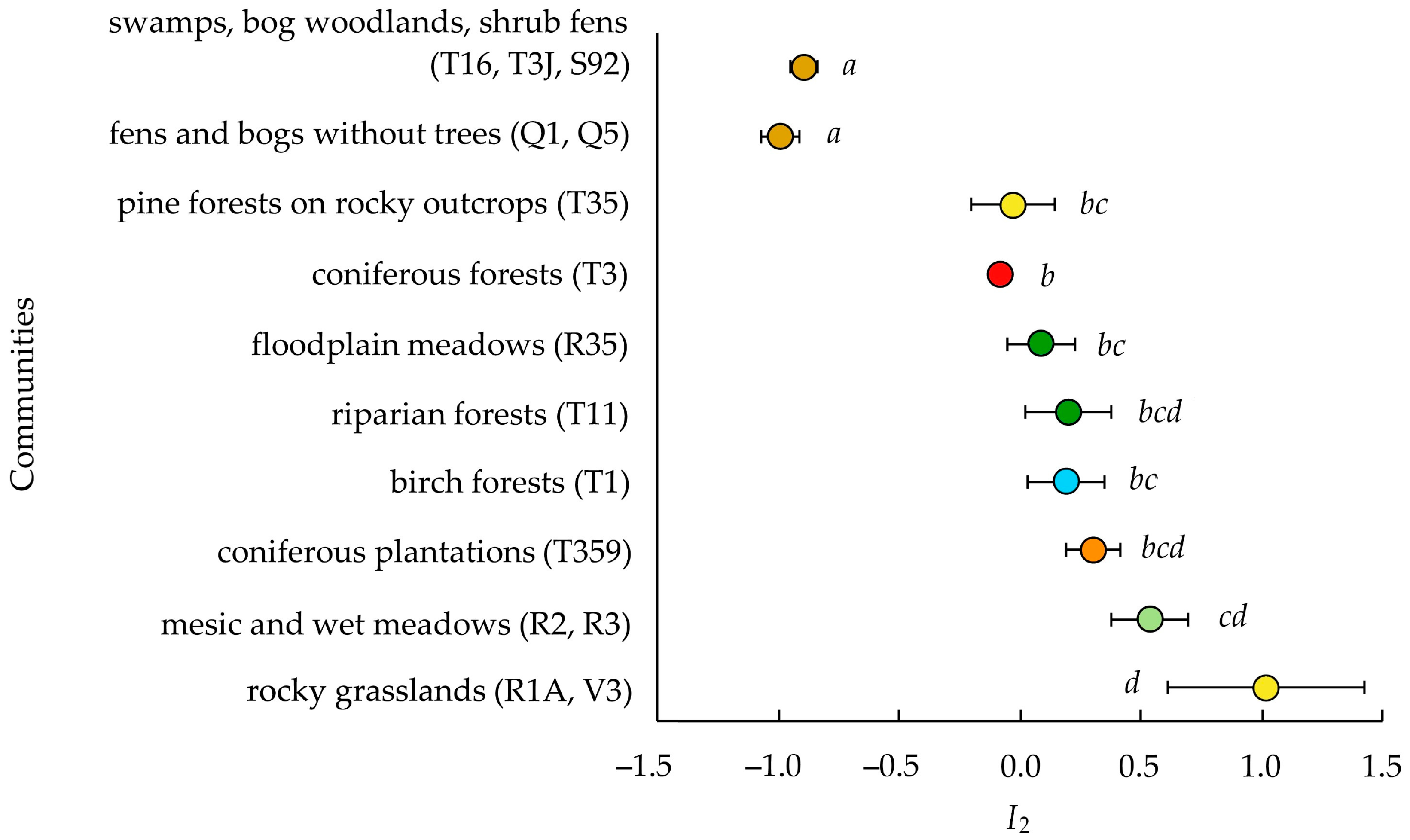
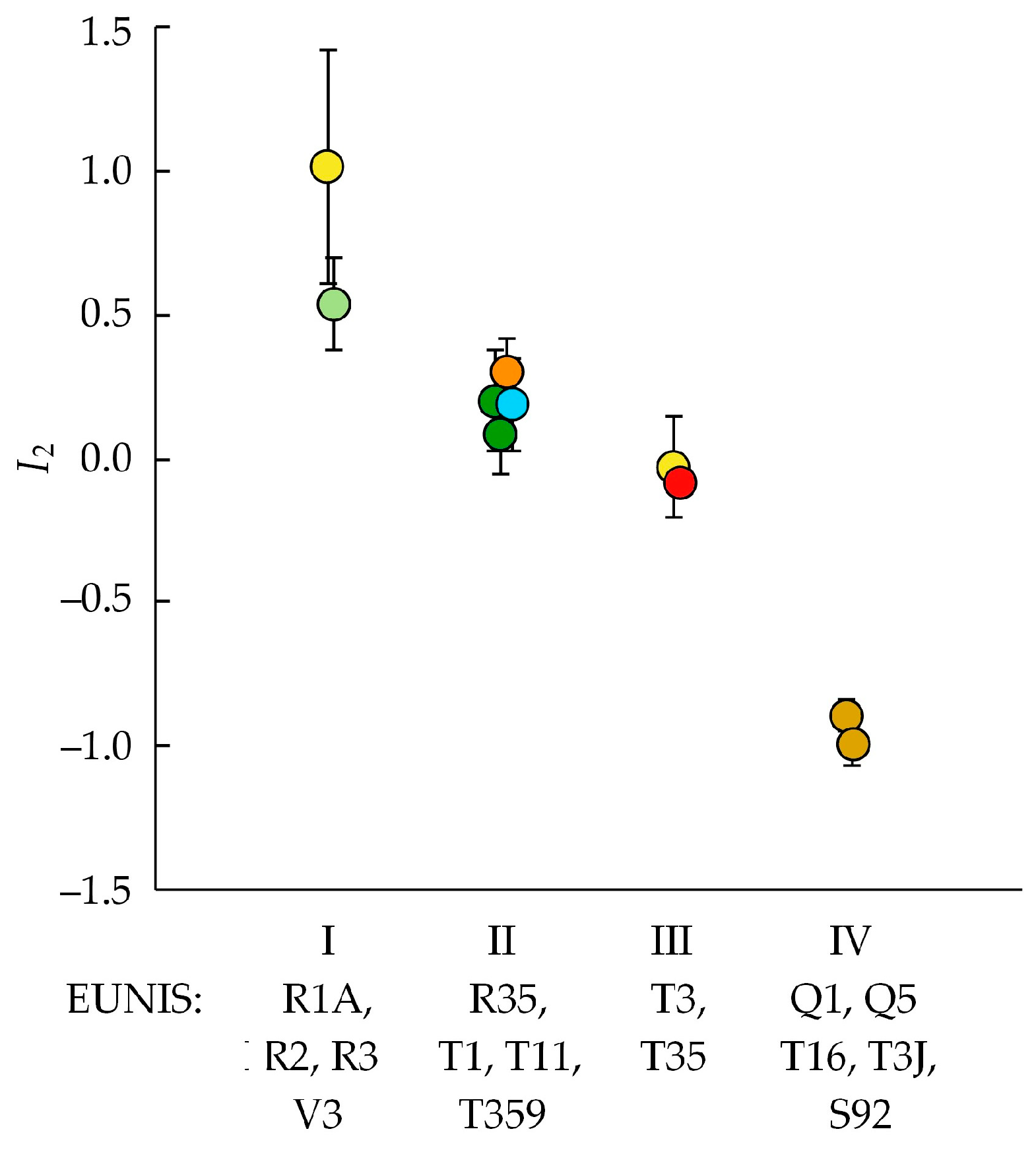
3.3. Invasibility Parameter I2
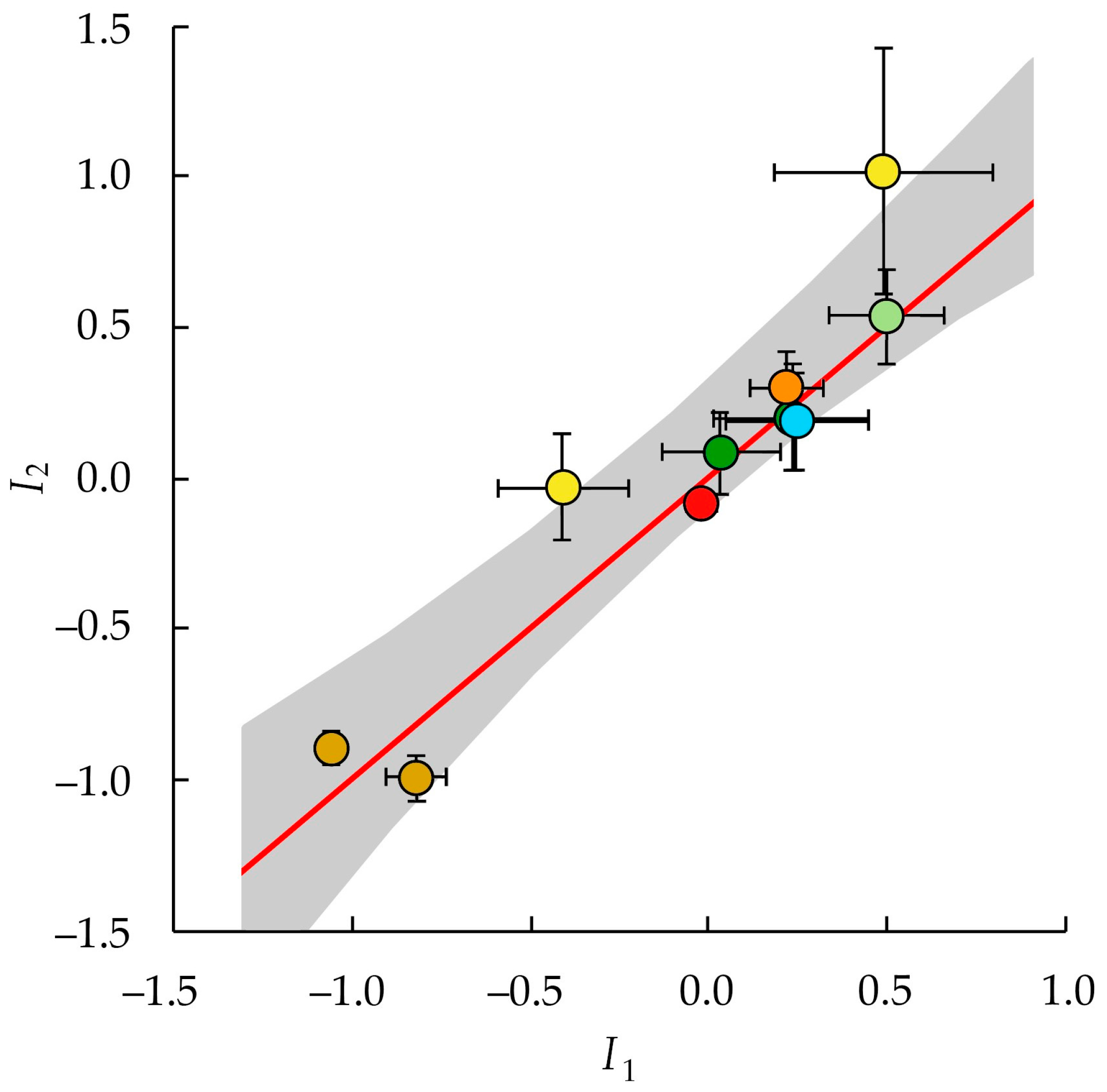
3.4. Relationship between I1 and I2
4. Discussion
4.1. Invasion Levels
4.2. Invasibility Estimation Methods
4.3. Invasibility of Different Community Types
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, M.A.; Grime, J.P.; Thompson, K. Fluctuating resources in plant communities: A general theory of invasibility. J. Ecol. 2000, 88, 528–534. [Google Scholar] [CrossRef]
- Catford, J.A.; Vesk, P.A.; Richardson, D.M.; Pyšek, P. Quantifying levels of biological invasion: Towards the objective classification of invaded and invasible ecosystems. Global Chang. Biol. 2012, 18, 44–62. [Google Scholar] [CrossRef]
- Chytrý, M.; Wild, J.; Pyšek, P.; Jarošík, V.; Dendoncker, N.; Reginster, I.; Pino, J.; Maskell, L.; Vilà, M.; Pergl, J.; et al. Projecting trends in plant invasions in Europe under different scenarios of future land-use change. Global Ecol. Biogeogr. 2012, 21, 75–87. [Google Scholar] [CrossRef]
- Gioria, M.; Hulme, P.E.; Richardson, D.M.; Pyšek, P. Why Are Invasive Plants Successful? Annu. Rev. Plant Biol. 2023, 74, 635–670. [Google Scholar] [CrossRef] [PubMed]
- Chytrý, M.; Jarošík, V.; Pyšek, P.; Hájek, O.; Knollová, I.; Tichý, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Chytrý, M.; Pyšek, P.; Wild, J.; Pino, J.; Maskell, L.C.; Vilà, M. European map of alien plant invasions based on the quantitative assessment across habitats. Divers. Distrib. 2009, 15, 98–107. [Google Scholar] [CrossRef]
- Daehler, C.C. Invasibility of tropical islands by introduced plants: Partitioning the influence of isolation and propagule pressure. Preslia 2006, 78, 389–404. [Google Scholar]
- Richardson, D.M.; Pyšek, P. Plant invasions: Merging the concepts of species invasiveness and community invasibility. Prog. Phys. Geogr. 2006, 30, 409–431. [Google Scholar] [CrossRef]
- Von der Lippe, M.; Kowarik, I. Do cities export biodiversity? Traffic as dispersal vector across urban–rural gradients. Divers. Distrib. 2008, 14, 18–25. [Google Scholar] [CrossRef]
- Aronson, M.F.J.; Handel, S.N.; La Puma, I.P.; Clemants, S.E. Urbanization promotes alien woody species and diverse plant assemblages in the New York metropolitan region. Urban Ecosyst. 2015, 18, 31–45. [Google Scholar] [CrossRef]
- Chytrý, M.; Pyšek, P.; Tichý, L.; Danihelka, J. Invasions by alien plants in the Czech Republic: A quantitative assessment across habitats. Preslia 2005, 77, 339–354. [Google Scholar]
- Hierro, J.L.; Maron, J.L.; Callaway, R.M. A biogeographical approach to plant invasions: The importance of studying exotics in their introduced and native range. J. Ecol. 2005, 93, 5–15. [Google Scholar] [CrossRef]
- González-Moreno, P.; Pino, J.; Carreras, D.; Basnou, C.; Fernández-Rebollar, I.; Vila, M. Quantifying the landscape influence on plant invasions in Mediterranean coastal habitats. Landsc. Ecol. 2013, 28, 891–903. [Google Scholar] [CrossRef]
- Akatov, V.V.; Akatova, T.V.; Chefranov, S.G.; Shadzhe, A.E. Species saturation and invasibility of the plant communities: A hypothesis of species pools correlation. Zhurnal Obs. Biol. 2009, 70, 328–340. (In Russian) [Google Scholar]
- Akatov, V.V.; Akatova, T.V.; Zagurnaya, J.S.; Shadzhe, A.E. Invasiveness of plant communities: Prognosis based on the analysis of cenotic parameters. New Technol. 2009, 3, 112–119. (In Russian) [Google Scholar]
- Guo, H.-D.; Zhang, L.; Zhu, L.-W. Earth observation big data for climate change research. Adv. Clim. Chang. Res. 2015, 6, 108–117. [Google Scholar] [CrossRef]
- Chadaeva, V.A.; Pshegusov, R.H. Factors of adventivization of roadside plant com-munities in the south of the Russian Black Sea Region. Uchenye Zap. Kazan. Univ. Seriya Estestv. Nauk. 2021, 163, 115–136. (in Russian) [Google Scholar] [CrossRef]
- Smith, M.D.; Wilcox, J.C.; Kelly, T.; Knapp, A.K. Dominance not richness determines invasibility of tallgrass prairie. Oikos 2004, 106, 253–262. [Google Scholar] [CrossRef]
- Holle, B.V.; Simberloff, D. Ecological resistance to biological invasion overwhelmed by propagule pressure. Ecology 2005, 86, 3212–3218. [Google Scholar] [CrossRef]
- Waddell, E.H.; Chapman, D.S.; Hill, J.K.; Hughes, M.; Bin Sailim, A.; Tangah, J.; Banin, L.F. Trait filtering during exotic plant invasion of tropical rainforest remnants along a disturbance gradient. Funct. Ecol. 2020, 34, 2584–2597. [Google Scholar] [CrossRef]
- Vilà, M.; Pino, J.; Font, X. Regional assessment of plant invasions across different habitat types. J. Veg. Sci. 2007, 18, 35–42. [Google Scholar] [CrossRef]
- Kowarik, I.; von der Lippe, M.; Cierjacks, A. Prevalence of alien versus native species of woody plants in Berlin differs between habitats and at different scales. Preslia 2013, 85, 113–132. [Google Scholar]
- The Official Internet Portal of Legal Information of the Sverdlovsk Region. Available online: http://www.pravo.gov66.ru/33322/ (accessed on 29 April 2023).
- Federal State Statistics Service. Available online: https://rosstat.gov.ru/vpn_popul (accessed on 29 April 2023).
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Sturman, V.I. Natural and Technogenic Factors of Air Pollution in Russian Cities. Bull. Udmurt Univ. Ser. Biol. Earth Sci. 2008, 2, 15–29. (In Russian) [Google Scholar]
- Antropov, K.M.; Varaksin, A.N. Assessing nitrogen dioxide air pollution in Ekaterinburg with land use regression model. Ecol. Syst. Devices 2011, 8, 47–54. (In Russian) [Google Scholar]
- Veselkin, D.V. Urbanization increases the range, but not the depth, of forest edge influences on Pinus sylvestris bark pH. Urban For. Urban Green. 2023, 79, 127819. [Google Scholar] [CrossRef]
- Shavnin, S.A.; Veselkin, D.V.; Vorobeichik, E.L.; Galako, V.A.; Vlasenko, V.E. Factors of pine-stand transformation in the city of Yekaterinburg. Contemp. Probl. Ecol. 2016, 9, 844–852. [Google Scholar] [CrossRef]
- Tretyakova, A.S.; Yakimov, B.N.; Kondratkov, P.V.; Grudanov, N.Y.; Cadotte, M.W. Phylogenetic Diversity of Urban Floras in the Central Urals. Front. Ecol. Evol. 2021, 9, 663244. [Google Scholar] [CrossRef]
- Tretyakova, A.S. Distribution of plant species composition in natural and anthropogenic habitats of Ekaterinburg city. Bot. Zhurnal 2014, 99, 1277–1282. (In Russian) [Google Scholar]
- Tretyakova, A.S. Regularities of distribution of alien plants in anthropogenous habitats of Sverdlovsk oblast. Rus. J. Biol. Invasions 2015, 8, 117–128. (In Russian) [Google Scholar]
- Veselkin, D.V.; Korzhinevskaya, A.A.; Podaevskaya, E.N. The edge effect on the grass and bush layer of urbanized southern taiga forests. Rus. J. Ecol. 2018, 49, 411–420. [Google Scholar] [CrossRef]
- Veselkin, D.V.; Korzhinevskaya, A.A. Spatial factors of the alien understory shrubs and trees distribution in urban forests of large city. Izv. Ross. Akad. Nauk. Seriya Geogr. 2018, 4, 55–65. (In Russian) [Google Scholar] [CrossRef]
- Veselkin, D.V.; Dubrovin, D.I. Diversity of the grass layer of urbanized communities dominated by invasive Acer negundo. Rus. J. Ecol. 2019, 50, 413–421. [Google Scholar] [CrossRef]
- EUNIS Habitat Classification. Available online: https://www.eea.europa.eu/data-and-maps/data/eunis-habitat-classification-1 (accessed on 29 April 2023).
- Chytrý, M.; Otýpková, Z. Plot Sizes Used for Phytosociological Sampling of European Vegetation. J. Veg. Sci. 2003, 4, 563–570. [Google Scholar] [CrossRef]
- Mueller-Dombois, D.; Jacobi, J.D.; Daehler, C.C. (Eds.) Biodiversity Assessment of Tropical Island Ecosystems: PABITRA Manual for Interactive Ecology and Management; Bishop Museum Press: Honolulu, HI, USA, 2008; pp. 17–47. [Google Scholar]
- Richardson, D.M.; Pyšek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirscher, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 200453, 131–143. [Google Scholar] [CrossRef]
- Knyazev, M.S.; Zolotareva, N.V.; Podgaevskaya, E.N.; Tretyakova, A.S.; Kulikov, P.V. An annotated check list of the flora of Sverdlovsk region. Part I: Spore and gymnosperms plants. Phytodivers. East. Eur. 2016, 10, 11–41. (In Russian) [Google Scholar]
- Knyazev, M.S.; Tretyakova, A.S.; Podgaevskaya, E.N.; Zolotareva, N.V.; Kulikov, P.V. An annotated checklist of the flora of Sverdlovsk Region. Part II: Monocotyledonous plants. Phytodivers. East. Eur. 2017, 11, 4–108. (In Russian) [Google Scholar]
- Knyazev, M.S.; Tretyakova, A.S.; Podgaevskaya, E.N.; Zolotareva, N.V.; Kulikov, P.V. An annotated checklist of the flora of Sverdlovsk Region. Part III: Dicotyledonous plants (Aristolochiaceae–Monotropaceae). Phytodivers. East. Eur. 2018, 12, 4–95. (In Russian) [Google Scholar] [CrossRef]
- Knyazev, M.S.; Tretyakova, A.S.; Podgaevskaya, E.N.; Zolotareva, N.V.; Kulikov, P.V. Annotated checklist of the flora of Sverdlovsk region. Part IV: Dicotyledonous plants (Empetraceae–Droseraceae). Phytodivers. East. Eur. 2019, 13, 130–196. (In Russian) [Google Scholar] [CrossRef]
- Knyazev, M.S.; Chkalov, A.V.; Tretyakova, A.S.; Zolotareva, N.V.; Podgaevskaya, E.N.; Pakina, D.V.; Kulikov, P.V. Annotated checklist of the flora of Sverdlovsk region. Part V: Dicotyledonous plants (Rosaceae). Phytodivers. East. Eur. 2019, 13, 305–352. (In Russian) [Google Scholar] [CrossRef]
- Knyazev, M.S.; Podgaevskaya, E.N.; Tretyakova, A.S.; Zolotareva, N.V.; Kulikov, P.V. Annotated checklist of the flora of Sverdlovsk Region. Part VI: Dicotyledonous plants (Fabaceae–Lobeliaceae). Phytodivers. East. Eur. 2020, 14, 190–331. (In Russian) [Google Scholar] [CrossRef]
- Knyazev, M.S.; Podgaevskaya, E.N.; Zolotareva, N.V.; Tretyakova, A.S.; Kulikov, P.V. Annotated checklist of the flora of Sverdlovsk Region. Part VII: Dicotyledonous plants (Asteraceae, Cichorioideae). Divers. Plant World 2021, 11, 5–33. [Google Scholar] [CrossRef]
- Knyazev, M.S.; Podgaevskaya, E.N.; Zolotareva, N.V.; Tretyakova, A.S.; Kulikov, P.V. Annotated checklist of the flora of Sverdlovsk Region. Part VIII: Dicotyledonous plants (Asteraceae, Asteroideae). Divers. Plant World 2022, 12, 28–56. (In Russian) [Google Scholar] [CrossRef]
- Zolotareva, N.V.; Zolotarev, M.P. The phenomenon of forest invasion to steppe areas in the Middle Urals and its probable causes. Rus. J. Ecol. 2017, 48, 21–31. [Google Scholar] [CrossRef]
- Zaretskaya, N.E.; Panova, N.K.; Antipina, T.G.; Zhilin, M.G.; Uspenskaya, O.N.; Savchenko, S.N. Geochronology, stratigraphy, and evolution of Middle Uralian peatlands during the holocene (exemplified by the Shigir and Gorbunovo peat bogs). Stratigr. Geol. Correl. 2014, 22, 632–654. [Google Scholar] [CrossRef]
- Hobbs, R.J.; Huenneke, L. Disturbance, diversity, and invasion: Implications for conservation. Conserv. Biol. 1992, 6, 324–337. [Google Scholar] [CrossRef]
| Urbanization Levels | |||
|---|---|---|---|
| Community Types (EUNIS Habitat Types) | Non-Urban Habitats | Urban Habitats | Total |
| Fens and bogs without trees (Q1, Q5) | 14 | 25 | 39 |
| Swamps, bog woodlands, shrub fens (T16, T3J, S92) | 16 | 3 | 19 |
| Flood meadows (R35) | 20 | 15 | 35 |
| Riparian forests (T11) | 15 | 16 | 31 |
| Mesic and wet meadows (R2, R3) | 30 | 33 | 63 |
| Birch forests (T1) | 30 | 28 | 58 |
| Coniferous plantations (T359) | 26 | 26 | 52 |
| Coniferous forests (T3) | 180 | 230 | 410 |
| Rocky grasslands (R1A, V3) | 13 | 4 | 17 |
| Pine forests on rocky outcrops (T35) | 23 | 2 | 25 |
| Total | 367 | 382 | 749 |
| Community Types (EUNIS Habitat Types) | Nalien | Palien, % | ||||
|---|---|---|---|---|---|---|
| Non-Urban Habitats | Urban Habitats | Differences 1 | Non-Urban Habitats | Urban Habitats | Differences 1 | |
| Fens and bogs without trees (Q1, Q5) | 0 | 0.72 ± 0.23 | n.s. | 0 | 2.8 ± 1.00 | n.s. |
| Swamps, bog woodlands, shrub fens (T16, T3J, S92) | 0 | 0 | n.s. | 0 | 0 | n.s. |
| Flood meadows (R35) | 1.15 ± 0.29 | 3.40 ± 0.74 | n.s. | 3.2 ± 0.8 | 14.9 ± 1.7 | *** |
| Riparian forests (T11) | 1.33 ± 0.39 | 4.19 ± 0.66 | * | 3.4 ± 0.9 | 14.3 ± 2.6 | *** |
| Mesic and wet meadows (R2, R3) | 2.40 ± 0.45 | 4.97 ± 0.59 | ** | 5.6 ± 1.0 | 14.0 ± 1.4 | *** |
| Birch forests (T1) | 1.00 ± 0.22 | 5.50 ± 0.76 | *** | 1.8 ± 0.4 | 15.3 ± 2.1 | *** |
| Coniferous plantations (T359) | 2.77 ± 0.38 | 4.54 ± 0.39 | n.s. | 5.0 ± 0.7 | 8.7 ± 0.7 | n.s. |
| Coniferous forests (T3) | 1.58 ± 0.15 | 4.04 ± 0.15 | *** | 2.6 ± 0.2 | 7.9 ± 0.3 | *** |
| Rocky grasslands (R1A, V3) | 3.31 ± 0.94 | 5.75 ± 1.25 | n.s. | 8.2 ± 2.1 | 14.1 ± 2.8 | n.s. |
| Pine forests on rocky outcrops (T35) | 1.13 ± 0.31 | 7.00 ± 1.00 | * | 2.7 ± 0.6 | 20.5 ± 3.8 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veselkin, D.V.; Zolotareva, N.V.; Dubrovin, D.I.; Podgaevskaya, E.N.; Pustovalova, L.A.; Korzhinevskaya, A.A. Invasibility of Common Plant Community Types of the Middle Urals. Diversity 2023, 15, 955. https://doi.org/10.3390/d15090955
Veselkin DV, Zolotareva NV, Dubrovin DI, Podgaevskaya EN, Pustovalova LA, Korzhinevskaya AA. Invasibility of Common Plant Community Types of the Middle Urals. Diversity. 2023; 15(9):955. https://doi.org/10.3390/d15090955
Chicago/Turabian StyleVeselkin, Denis V., Natalya V. Zolotareva, Denis I. Dubrovin, Elena N. Podgaevskaya, Liliya A. Pustovalova, and Anastasia A. Korzhinevskaya. 2023. "Invasibility of Common Plant Community Types of the Middle Urals" Diversity 15, no. 9: 955. https://doi.org/10.3390/d15090955
APA StyleVeselkin, D. V., Zolotareva, N. V., Dubrovin, D. I., Podgaevskaya, E. N., Pustovalova, L. A., & Korzhinevskaya, A. A. (2023). Invasibility of Common Plant Community Types of the Middle Urals. Diversity, 15(9), 955. https://doi.org/10.3390/d15090955






