Butterfly Assemblages Differ among Vegetation Types in Southern Amazonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Butterfly Sampling and Identification
2.3. Data Analyses
3. Results
4. Discussion
Conservation Remarks
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snyder, R.E.; Chesson, P. How the Spatial Scales of Dispersal, Competition, and Environmental Heterogeneity Interact to Affect Coexistence. Am. Nat. 2004, 164, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Tews, J.; Brose, U.; Grimm, V.; Tielbörger, K.; Wichmann, M.C.; Schwager, M.; Jeltsch, F. Animal species diversity driven by habitat heterogeneity/diversity: The importance of keystone structures. J. Biogeogr. 2004, 31, 79–92. [Google Scholar] [CrossRef]
- Lundholm, J.T. Plant species diversity and environmental heterogeneity: Spatial scale and competing hypotheses. J. Veg. Sci. 2009, 20, 377–391. [Google Scholar] [CrossRef]
- Tamme, R.; Hiiesalu, I.; Laanisto, L.; Szava-Kovats, R.; Pärtel, M. Environmental heterogeneity, species diversity and co-existence at different spatial scales. J. Veg. Sci. 2010, 21, 796–801. [Google Scholar] [CrossRef]
- Stein, A.; Kreft, H. Terminology and quantification of environmental heterogeneity in species-richness research. Biol. Rev. 2014, 90, 815–836. [Google Scholar] [CrossRef]
- MacArthur, R.H.; MacArthur, J.W. On bird species diversity. Ecology 1961, 42, 594–598. [Google Scholar] [CrossRef]
- Siemann, E.; Tilman, D.; Haarstad, J.; Ritchie, M. Experimental Tests of the Dependence of Arthropod Diversity on Plant Diversity. Am. Nat. 1998, 152, 738–750. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Rasmann, S.; Castagneyrol, B.; Mooney, K.A. Plant diversity effects on insect herbivores and their natural enemies: Current thinking, recent findings, and future directions. Curr. Opin. Insect Sci. 2016, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Connell, J.H. Diversity in Tropical Rain Forests and Coral Reefs: High diversity of trees and corals is maintained only in a nonequilibrium state. Science 1978, 199, 1302–1310. [Google Scholar] [CrossRef]
- Hartshorn, G.S. Neotropical Forest Dynamics. Biotropica 1980, 12, 23–30. [Google Scholar] [CrossRef]
- Uhl, C.; Clark, K.; Dezzeo, N.; Maquirino, P. Vegetation Dynamics in Amazonian Treefall Gaps. Ecology 1988, 69, 751–763. [Google Scholar] [CrossRef]
- McCarthy, J. Gap dynamics of forest trees: A review with particular attention to boreal forests. Environ. Rev. 2001, 9, 1–59. [Google Scholar] [CrossRef]
- Oliveira-Filho, A.T.; Ratter, J.A. Vegetation Physiognomies and Woody Flora of the Cerrado Biome. In The Cerrados of Brazil; Oliveira, P.S., Marquis, R.J., Eds.; Columbia University Press: New York, NY, USA, 2002; pp. 91–120. [Google Scholar] [CrossRef]
- Jenkins, C.N.; Pimm, S.L.; Joppa, L.N. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, E2602–E2610. [Google Scholar] [CrossRef]
- Antonelli, A.; Zizka, A.; Carvalho, F.A.; Scharn, R.; Bacon, C.D.; Silvestro, D.; Condamine, F.L. Amazonia is the primary source of Neotropical biodiversity. Proc. Natl. Acad. Sci. USA 2018, 115, 6034–6039. [Google Scholar] [CrossRef]
- Prance, G.T. Islands in Amazonia. Philos. Trans. R. Soc. Lond. B 1996, 351, 823–833. [Google Scholar] [CrossRef]
- Castello, L.; McGrath, D.G.; Hess, L.L.; Coe, M.T.; Lefebvre, P.A.; Petry, P.; Macedo, M.N.; Renó, V.F.; Arantes, C.C. The vulnerability of Amazon freshwater ecosystems. Conserv. lett. 2013, 6, 217–229. [Google Scholar] [CrossRef]
- Adeney, J.M.; Christensen, N.L.; Vicentini, A.; Cohn-Haft, M. White-sand Ecosystems in Amazonia. Biotropica 2016, 48, 7–23. [Google Scholar] [CrossRef]
- Melack, J.M.; Hess, L.L. Remote Sensing of the Distribution and Extent of Wetlands in the Amazon basin. In Amazonian Floodplain Forests: Ecophysiology, Biodiversity and Sustainable Management; Junk, W.J., Piedade, M.T.F., Wittman, F., Schöngart, J., Parolin, P., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 43–59. [Google Scholar] [CrossRef]
- Hess, L.L.; Melack, J.M.; Affonso, A.G.; Barbosa, C.; Gastil-Buhl, M.; Novo, E.M.L.M. Wetlands of the Lowland Amazon Basin: Extent, Vegetative Cover, and Dual-season Inundated Area as Mapped with JERS-1 Synthetic Aperture Radar. Wetlands 2015, 35, 745–756. [Google Scholar] [CrossRef]
- Wittmann, F.; Householder, J.E.; Piedade, M.T.F.; Schöngart, J.; Demarchi, L.O.; Quaresma, A.C.; Junk, W.J. A Review of the Ecological and Biogeographic Differences of Amazonian Floodplain Forests. Water 2022, 14, 3660. [Google Scholar] [CrossRef]
- INPE. Available online: http://www.inpe.br (accessed on 10 July 2022).
- Barbosa, R.I.; Nascimento, S.P.; Amorim, P.A.F.; Silva, R.F. Notas sobre a composição arbóreo-arbustiva de uma fisionomia das savanas de Roraima, Amazônia Brasileira. Acta Bot. Bras. 2005, 19, 323–329. [Google Scholar] [CrossRef]
- Anderson, A.B. White-sand vegetation of Brazilian Amazonia. Biotropica 1981, 13, 199–210. [Google Scholar] [CrossRef]
- Griscom, B.W.; Daly, D.C.; Ashton, M.S. Floristics of bamboo-dominated stands in lowland terra-firma forests of southwestern Amazonia. J. Torrey Bot. Soc. 2007, 134, 108–125. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Nelson, B.W.; Bianchini, M.C.; Plagnol, D.; Kuplich, T.M.; Daly, D.C. Bamboo-Dominated Forests of the Southwest Amazon: Detection, Spatial Extent, Life Cycle Length and Flowering Waves. PLoS ONE 2013, 8, e54852. [Google Scholar] [CrossRef]
- Ratter, J.A.; Richards, P.W.; Argent, G.; Gifford, D.R. Observations on the vegetation of northeastern Mato Grosso: I. The woody vegetation types of the Xavantina-Cachimbo Expedition area. Philos. Trans. R. Soc. Lond. B 1973, 266, 449–492. [Google Scholar] [CrossRef]
- Ackerly, D.D.; Thomas, W.; Ferreira, C.A.; Pirani, J.R. The forest-cerrado transition zone in southern Amazonia: Results of the 1985 Projeto Flora Amazônica expedition to Mato Grosso. Brittonia 1989, 41, 113–128. [Google Scholar] [CrossRef]
- Zappi, D.C.; Sasaki, D.; Milliken, W.; Iva, J.; Henicka, G.S.; Biggs, N.; Frisby, S. Plantas vasculares da região do Parque Estadual Cristalino, norte de Mato Grosso, Brasil. Acta Amazon. 2011, 41, 29–38. [Google Scholar] [CrossRef]
- May, R.; Jacobs, J.M.; Santa-Cruz, R.; Valdivia, J.; Huamán, J.M.; Donnelly, M.A. Amphibian community structure as a function of forest type in Amazonian Peru. J. Trop. Ecol. 2010, 26, 509–519. [Google Scholar] [CrossRef]
- Bobrowiec, P.E.D.; Rosa, L.S.; Gazarini, J.; Haugaasen, T. Phyllostomid Bat Assemblage Structure in Amazonian Flooded and Unflooded Forests. Biotropica 2014, 46, 312–321. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, C.A. Primate assemblage structure in amazonian flooded and unflooded forests. Am. J. Primatol. 2005, 67, 243–258. [Google Scholar] [CrossRef]
- Beja, P.; Santos, C.D.; Santana, J.; Pereira, M.J.; Marques, J.T.; Queiroz, H.L.; Palmeirim, J.M. Seasonal patterns of spatial variation in understory bird assemblages across a mosaic of flooded and unflooded Amazonian forests. Biodivers. Conserv. 2010, 19, 129–152. [Google Scholar] [CrossRef]
- Alonso, J.Á.; Metz, M.R.; Fine, P.V.A. Habitat Specialization by Birds in Western Amazonian White-sand Forests. Biotropica 2013, 45, 365–372. [Google Scholar] [CrossRef]
- Oliveira, I.F.; Baccaro, F.B.; Werneck, F.P.; Zacca, T.; Haugaasen, T. Marked Differences in Butterfly Assemblage Composition between Forest Types in Central Amazonia, Brazil. Forests 2021, 12, 942. [Google Scholar] [CrossRef]
- Oliveira, I.F.; Baccaro, F.B.; Werneck, F.P.; Haugaasen, T. Seasonal flooding decreases fruit-feeding butterfly species dominance and increases spatial turnover in floodplain forests of central Amazonia. Ecol. Evol. 2023, 13, e9718. [Google Scholar] [CrossRef]
- Rabelo, R.M.; Pereira, G.C.N.; Valsecchi, J.; Magnusson, W.E. The Role of River Flooding as an Environmental Filter for Amazonian Butterfly Assemblages. Front. Ecol. Evol. 2021, 9, 693178. [Google Scholar] [CrossRef]
- Graça, M.B.; Pequeno, P.A.C.L.; Franklin, E.; Souza, J.L.P.; Morais, J.W. Taxonomic, functional, and phylogenetic perspectives on butterfly spatial assembly in northern Amazonia. Ecol. Entomol. 2017, 42, 816–826. [Google Scholar] [CrossRef]
- Brown, K.S., Jr.; Hutchings, R.W. Disturbance, Fragmentation, and the Dynamics of Diversity in Amazonian Forest Butterflies. In Tropical Forest Remnants-Ecology, Management, and Conservation of Fragmented Communities; Laurance, W.F., Bierregaard, R.O., Jr., Eds.; The University of Chicago Press: Chicago, IL, USA, 1997; pp. 91–110. [Google Scholar]
- Brown, K.S., Jr.; Freitas, A.V.L. Atlantic Forest Butterflies: Indicators for Landscape Conservation 1. Biotropica 2000, 32, 934–956. [Google Scholar] [CrossRef]
- DeVries, P.J.; Walla, T.R. Species diversity and community structure in neotropical fruit-feeding butterflies. Biol. J. Linn. Soc. 2001, 74, 1–15. [Google Scholar] [CrossRef]
- Ferrer-Paris, J.R.; Sánchez-Mercado, A.; Viloria, Á.L.; Donaldson, J. Congruence and Diversity of Butterfly-Host Plant Associations at Higher Taxonomic Levels. PLoS ONE 2013, 8, e63570. [Google Scholar] [CrossRef]
- Nimer, E. Clima. In Geografia do Brasil; Duarte, A.C., Ed.; Região Centro-Oeste IBGE: Rio de Janeiro, Brazil, 1989; Volume 1, pp. 23–34. [Google Scholar]
- Dubreuil, V.; Debortoli, N.; Funatsu, B.; Nédélec, V.; Durieux, L. Impacto f land-cover change in Southern Amazonia climate: A case study for the region of Alta Floresta, Mato Grosso, Brazil. Environ. Monit. Assess. 2012, 184, 877–891. [Google Scholar] [CrossRef]
- Sazaki, D.; Farias, R.A. Plano de Manejo das Reservas Particulares do Patrimônio Natural Cristalino I, II e III, (Novo Mundo, Mato Grosso–Brasil); Fundação Ecológica Cristalino: Alta Floresta, Brazil, 2008; pp. 1–193. [Google Scholar]
- Müller, M.V.Y.; Farias, R. Reserva Particular do Patrimônio Natural Lote Cristalino–Plano de Manejo; Fundação Ecológica Cristalino: Alta Floresta, Brazil, 2010; pp. 1–181. [Google Scholar]
- Mota, L.L.; Boddington, S.J.; Brown, K.S., Jr.; Callaghan, C.J.; Carter, G.; Carter, W.; Dantas, S.M.; Dolibaina, D.R.; Garwood, K.; Hoyer, R.C.; et al. The butterflies of Cristalino Lodge, in the Brazilian southern Amazonia: An updated species list with a significant contribution from citizen science. Biota Neotrop. 2022, 22, 1–25. [Google Scholar] [CrossRef]
- Espeland, M.; Nakahara, S.; Zacca, T.; Barbosa, E.P.; Huertas, B.; Marín, M.A.; Lamas, G.; Benmesbah, M.; Brévignon, C.; Casagrande, M.M.; et al. Combining target enrichment and Sanger sequencing data to clarify the systematics of the diverse Neotropical butterfly subtribe Euptychiina (Nymphalidae, Satyrinae). Syst. Entomol. 2023. early view. [Google Scholar] [CrossRef]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 1 February 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Lamas, G.; Mielke, O.H.H.; Robbins, R.K. The Ahrenholtz technique for attracting tropical skippers (Hesperiidae). J. Lepid. Soc. 1993, 47, 80–82. [Google Scholar]
- Brown, K.S., Jr.; Freitas, A.V.L. Diversidade Biológica no Alto Juruá: Avaliação, Causas e Manutenção. In Enciclopedia da Floresta. O Alto Juruá: Práticas e Conhecimento das Populações; Cunha, M.M.C., Almeida, M.B., Eds.; Companhia das Letras: São Paulo, Brazil, 2002; pp. 33–42. [Google Scholar]
- Francini, R.B.; Duarte, M.; Mielke, O.H.H.; Caldas, A.; Freitas, A.V.L. Butterflies (Lepidoptera, Papilionoidea and Hesperioidea) of the “Baixada Santista” region, coastal São Paulo, southeastern Brazil. Rev. Bras. Entomol. 2011, 55, 55–68. [Google Scholar] [CrossRef]
- Robbins, R.K.; Lamas, G.; Mielke, O.H.H.; Harvey, D.J.; Casagrande, M.M. Taxonomic Composition and Ecological Structure of the Species-Rich Butterfly Community at Pakitza, Parque Nacional del Manu, Perú. In Manu: The Biodiversity of Southeastern Peru; Wilson, D.E., Sandoval, A., Eds.; Smithsonian Institution Press: Washington, DC, USA, 1996; pp. 217–252. [Google Scholar]
- Brown, K.S., Jr. Geological, evolutionary, and ecological bases of the diversification of Neotropical butterflies: Implications for conservation. In Tropical Rainforests: Past, Present and Future; Bermingham, E., Dick, C.W., Moritz, C., Eds.; University of Chicago Press: Chicago, IL, USA, 2005; pp. 166–201. [Google Scholar]
- Espeland, M.; Hall, J.P.W.; DeVries, P.J.; Lees, D.C.; Cornwall, M.; Hsu, Y.-F.; Wu, L.-W.; Campbell, D.L.; Talavera, G.; Vila, R.; et al. Ancient Neotropical origin and recent recolonisation: Phylogeny, biogeography and diversification of the Riodinidae (Lepidoptera: Papilionoidea). Mol. Phylogenet. Evol. 2015, 93, 296–306. [Google Scholar] [CrossRef]
- DeVries, P.J. Stratification of fruit-feeding nymphalid butterflies in a Costa Rican rainforest. J. Res. Lepid. 1988, 26, 98–108. [Google Scholar]
- Hall, J.P.W.; Willmott, K.R. Patterns of feeding behaviour in adult male riodinid butterflies and their relationship to morphology and ecology. Biol. J. Linn. Soc. 2000, 69, 1–23. [Google Scholar] [CrossRef]
- Orlandin, E.; Piovesan, M.; D’Agostini, F.M.; Carneiro, E. Use of microhabitats affects butterfly assemblages in a rural landscape. Pap. Avulsos Zool. 2019, 59, e20195949. [Google Scholar] [CrossRef]
- Scheffers, B.R.; Phillips, B.L.; Laurance, W.F.; Sodhi, N.S.; Diesmos, A.; Williams, S.E. Increasing arboreality with altitude: A novel biogeographic dimension. Proc. R. Soc. B 2013, 280, 20131581. [Google Scholar] [CrossRef] [PubMed]
- Mena, S.; Kozak, K.M.; Cárdenas, R.E.; Checa, M.F. Forest stratification shapes allometry and flight morphology of tropical butterflies. Proc. R. Soc. B 2020, 287, 20201071. [Google Scholar] [CrossRef] [PubMed]
- Papageorgis, C. Mimicry in Neotropical butterflies. Am. Sci. 1975, 63, 522–532. [Google Scholar]
- Beccaloni, G.W. Vertical stratification of ithomiine butterfly (Nymphalidae: Ithomiinae) mimicry complexes: The relationship between adult flight height and larval host-plant height. Biol. J. Linn. Soc. 1997, 62, 313–341. [Google Scholar] [CrossRef]
- DeVries, P.J.; Alexander, L.G.; Chacon, I.A.; Fordyce, J.A. Similarity and difference among rainforest fruit-feeding butterfly communities in Central and South America. J. Anim. Ecol. 2012, 81, 472–482. [Google Scholar] [CrossRef]
- Fordyce, J.A.; DeVries, P.J. A tale of two communities: Neotropical butterfly assemblages show higher beta diversity in the canopy compared to the understory. Oecologia 2016, 181, 235–243. [Google Scholar] [CrossRef]
- Molleman, F.; Kop, A.; Brakefield, P.M.; DeVries, P.J.; Zwaan, B.J. Vertical and temporal patterns of biodiversity of fruit-feeding butterflies in a tropical forest in Uganda. Biodivers. Conserv. 2006, 15, 107–121. [Google Scholar] [CrossRef]
- Fermon, H.; Waltert, M.; Mühlenberg, M. Movement and vertical stratification of fruit-feeding butterflies in a managed West African rainforest. J. Insect Conserv. 2003, 7, 7–19. [Google Scholar] [CrossRef]
- Fermon, H.; Waltert, M.; Vane-Wright, R.I.; Mühlenberg, M. Forest use and vertical stratification in fruit-feeding butterflies of Sulawesi, Indonesia: Impacts for conservation. Biodivers. Conserv. 2005, 14, 333–350. [Google Scholar] [CrossRef]
- Schulze, C.H.; Linsenmair, K.E.; Fiedler, K. Understorey versus canopy: Patterns of vertical stratification and diversity among Lepidoptera in a Bornean rain forest. In Tropical Forest Canopies: Ecology and Management; Linsenmair, K.E., Davis, A.J., Fiala, B., Speight, M.R., Eds.; Springer: Dordrecht, The Netherlands, 2001; pp. 133–152. [Google Scholar] [CrossRef]
- Ribeiro, D.B.; Freitas, A.V.L. The effect of reduced-impact logging on fruit-feeding butterflies in Central Amazon, Brazil. J. Insect Conserv. 2012, 16, 733–744. [Google Scholar] [CrossRef]
- Santos, J.P.; Iserhard, C.A.; Carreira, J.Y.O.; Freitas, A.V.L. Monitoring fruit-feeding butterfly assemblages in two vertical strata in seasonal Atlantic Forest: Temporal species turnover is lower in the canopy. J. Trop. Ecol. 2017, 33, 345–355. [Google Scholar] [CrossRef]
- Brown, K.S., Jr. Borboletas da Serra do Japi: Diversidade, hábitats, recursos alimentares e variação temporal. In História natural da Serra do Japi: Ecologia e preservação de uma área florestal no Sudeste do Brasil; Morellato, L.P.C., Ed.; Editora da Unicamp: Campinas, SP, Brazil, 1992; pp. 142–187. [Google Scholar]
- Pardonnet, S.; Beck, H.; Milberg, P.; Bergman, K.-O. Effect of tree-fall gaps on fruit-feeding nymphalid butterfly assemblages in a Peruvian rain forest. Biotropica 2013, 45, 612–619. [Google Scholar] [CrossRef]
- Sparrow, H.R.; Sisk, T.D.; Ehrlich, P.R.; Murphy, D.D. Techniques and Guidelines for Monitoring Neotropical Butterflies. Conserv. Biol. 1994, 8, 800–809. [Google Scholar] [CrossRef]
- Spitzer, K.; Jaroš, J.; Havelka, J.; Lepš, J. Effect of small-scale disturbance on butterfly communities of an Indochinese montane rainforest. Biol. Conserv. 1997, 80, 9–15. [Google Scholar] [CrossRef]
- Hill, J.; Hamer, K.; Tangah, J.; Dawood, M. Ecology of tropical butterflies in rainforest gaps. Oecologia 2001, 128, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Hamer, K.C.; Hill, J.K.; Benedick, S.; Mustaffa, N.; Sherratt, T.N.; Maryati, M.; Chey, V.K. Ecology of butterflies in natural and selectively logged forests of northern Borneo: The importance of habitat heterogeneity. J. Appl. Ecol. 2003, 40, 150–162. [Google Scholar] [CrossRef]
- Pryke, J.S.; Vrdoljak, S.M.; Grant, P.B.; Samways, M.J. Butterfly behavioural responses to natural Bornean tropical rain-forest canopy gaps. J. Trop. Ecol. 2012, 28, 45–54. [Google Scholar] [CrossRef]
- Horn, H.S. Markovian properties of forest succession. In Ecology and Evolution of Communities; Cody, M.L., Diamond, J.M., Eds.; Harvard University Press: Cambridge, MA, USA, 1975; pp. 196–211. [Google Scholar]
- Gueratto, P.E.; Carreira, J.Y.O.; Santos, J.P.; Tacioli, A.; Freitas, A.V.L. Effects of forest trails on the community structure of tropical butterflies. J. Insect Conserv. 2020, 24, 309–319. [Google Scholar] [CrossRef]
- Lourenço, G.M.; Soares, G.R.; Santos, T.P.; Dáttilo, W.; Freitas, A.V.L.; Ribeiro, S.P. Equal but different: Natural ecotones are dissimilar to anthropic edges. PLoS ONE 2019, 14, e0213008. [Google Scholar] [CrossRef]
- DeVries, P.J.; Penz, C.M.; Hill, R.I. Vertical distribution, flight behaviour and evolution of wing morphology in Morpho butterflies. J. Anim. Ecol. 2010, 79, 1077–1085. [Google Scholar] [CrossRef]
- DeVries, P.J.; Murray, D.; Lande, R. Species diversity in vertical, horizontal, and temporal dimensions of a fruit-feeding butterfly community in an Ecuadorian rainforest. Biol. J. Linn. Soc. 1997, 62, 343–364. [Google Scholar] [CrossRef]
- Uehara-Prado, M.; Freitas, A.V.L. The effect of rainforest fragmentation on species diversity and mimicry ring composition of ithomiine butterflies. Insect Conserv. Diver. 2009, 2, 23–28. [Google Scholar] [CrossRef]
- Willmott, K.R.; Mallet, J. Correlations between adult mimicry and larval host plants in ithomiines butterflies. Proc. R. Soc. Lond. B 2004, 271, S266–S269. [Google Scholar] [CrossRef]
- Murray, D.L. Systematics of Neotropical Satyrine Butterflies (Nymphalidae: Satyrinae: Euptychiina) Based on Larval Morphology and DNA Sequence Data and the Evolution of Life History Traits. Ph.D. thesis, Louisiana State University and Agricultural & Mechanical College, Baton Rouge, LA, USA, 2001. [Google Scholar]
- Freitas, A.V.L.; Mota, L.L.; Zacca, T.; Barbosa, E.P. Description of a new and highly distinctive genus and species of Euptychiina (Lepidoptera: Nymphalidae: Satyrinae) from the Brazilian southern Amazon. Rev. Bras. Entomol. 2019, 63, 254–261. [Google Scholar] [CrossRef]
- Vu, L.V.; Bonebrake, T.C.; Vu, M.Q.; Nguyen, N.T. Butterfly diversity and habitat variation in a disturbed forest in northern Vietnam. Pan-Pac. Entomol. 2015, 91, 29–38. [Google Scholar] [CrossRef]
- Prance, G.T. Notes on the vegetation of amazonia III. The terminology of amazonian forest types subject to inundation. Brittonia 1979, 31, 26–38. [Google Scholar] [CrossRef]
- Haugaasen, T.; Peres, C.A. Floristic, edaphic and structural characteristics of flooded and unflooded forests in the lower Rio Purús region of central Amazonia, Brazil. Acta Amazon. 2006, 36, 25–35. [Google Scholar] [CrossRef]
- Carim, M.J.V.; Wittmann, F.K.; Piedade, M.T.F.; Guimarães, J.R.S.; Tostes, L.C.L. Composition, diversity, and structure of tidal “Várzea” and “Igapó” floodplain forests in eastern Amazonia, Brazil. Braz. J. Bot. 2017, 40, 115–124. [Google Scholar] [CrossRef]
- Freitas, A.V.L.; Brown, K.S., Jr. Immature stages of Vila emilia (Lepidoptera: Nymphalidae, Biblidinae). Trop. Lepid. Res. 2008, 18, 74–77. [Google Scholar]
- Duarte, M.; Robbins, R.K.; Mielke, O.H.H. Immature stages of Calycopis caulonia (Hewitson, 1877) (Lepidoptera, Lycaenidae, Theclinae, Eumaeini), with notes on rearing detritivorous hairstreaks on artificial diet. Zootaxa 2005, 1063, 1–31. [Google Scholar] [CrossRef]
- Duarte, M.; Robbins, R.K. Immature stages of Calycopis bellera (Hewitson) and C. janeirica (Felder) (Lepidoptera, Lycaenidae, Theclinae, Eumaeini): Taxonomic significance and new evidence for detritivory. Zootaxa 2009, 2325, 39–61. [Google Scholar] [CrossRef]
- Duarte, M.; Robbins, R.K. Description and phylogenetic analysis of the Calycopidina (Lepidoptera, Lycaenidae, Theclinae, Eumaeini): A subtribe of detritivores. Rev. Bras. Entomol. 2010, 54, 45–65. [Google Scholar] [CrossRef]
- Schöngart, J.; Wittmann, F.; Resende, A.F.; Assahira, C.; Lobo, G.S.; Neves, J.R.D.; Rocha, M.; Mori, G.B.; Quaresma, A.C.; Demarchi, L.O.; et al. The shadow of the Balbina dam: A synthesis of over 35 years of downstream impacts on floodplain forests in Central Amazonia. Aquat. Conserv. 2021, 31, 1117–1135. [Google Scholar] [CrossRef]
- Correa, S.B.; van der Sleen, P.; Siddiqui, S.F.; Bogotá-Gregory, J.D.; Arantes, C.C.; Barnett, A.A.; Couto, T.B.A.; Goulding, M.; Anderson, E.P. Biotic Indicators for Ecological State Change in Amazonian Floodplains. Bioscience 2022, 72, 753–768. [Google Scholar] [CrossRef]
- Santos, A.C.; Carmo, D.L.R.; Plaza, T.G.D.; Arrua, B.A.; Nacagawa, V.A.F.; Fernades, R.A.M.; Pontes, F.T.N.; Ribeiro, D.B. Active Sampling and Understory Traps Can Cost-Effectively Detect Changes in Butterfly Communities after Hydroelectric Dam Construction. Diversity 2022, 14, 873. [Google Scholar] [CrossRef]
- Lees, A.C.; Peres, C.A.; Fearnside, P.M.; Schneider, M.; Zuanon, J.A.S. Hydropower and the future of Amazonian biodiversity. Biodivers. Conserv. 2016, 25, 451–466. [Google Scholar] [CrossRef]
- Nobre, C.A.; Sampaio, G.; Borma, L.S.; Castilla-Rubio, J.C.; Silva, J.S.; Cardoso, M. Land-use and climate change risks in the Amazon and the need of a novel sustainable development paradigm. Proc. Natl. Acad. Sci. USA 2016, 113, 10759–10768. [Google Scholar] [CrossRef]
- Lovejoy, T.E.; Nobre, C. Amazon tipping point: Last chance for action. Sci. Adv. 2019, 5, eaba2949. [Google Scholar] [CrossRef]
- Sales, L.P.; Galetti, M.; Pires, M.M. Climate and land-use change will lead to a faunal ‘savannization’ on tropical rainforests. Glob. Chang. Biol. 2020, 26, 7036–7044. [Google Scholar] [CrossRef]
- Reis, S.M.; Marimon, B.S.; Esquivel-Muelbert, A.; Marimon, B.H., Jr.; Morandi, P.S.; Elias, F.; Oliveira, E.A.; Galbraith, D.; Feldpausch, T.R.; Menor, I.O.; et al. Climate and crown damage drive tree mortality in southern Amazonian edge forests. J. Ecol. 2022, 110, 876–888. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X.; Riley, W.J.; Xue, Y.; Nobre, C.A.; Lovejoy, T.E.; Jia, G. Deforestation triggering irreversible transition in Amazon hydrological cycle. Environ. Res. Lett. 2022, 17, 034037. [Google Scholar] [CrossRef]
- Wunderling, N.; Staal, A.; Sakschewski, B.; Hirota, M.; Tuinenburg, O.A.; Donges, J.F.; Barbosa, H.M.J.; Winkelmann, R. Recurrent droughts increase risk of cascading tipping events by outpacing adaptive capacities in the Amazon rainforest. Proc. Natl. Acad. Sci. USA 2022, 119, e2120777119. [Google Scholar] [CrossRef]
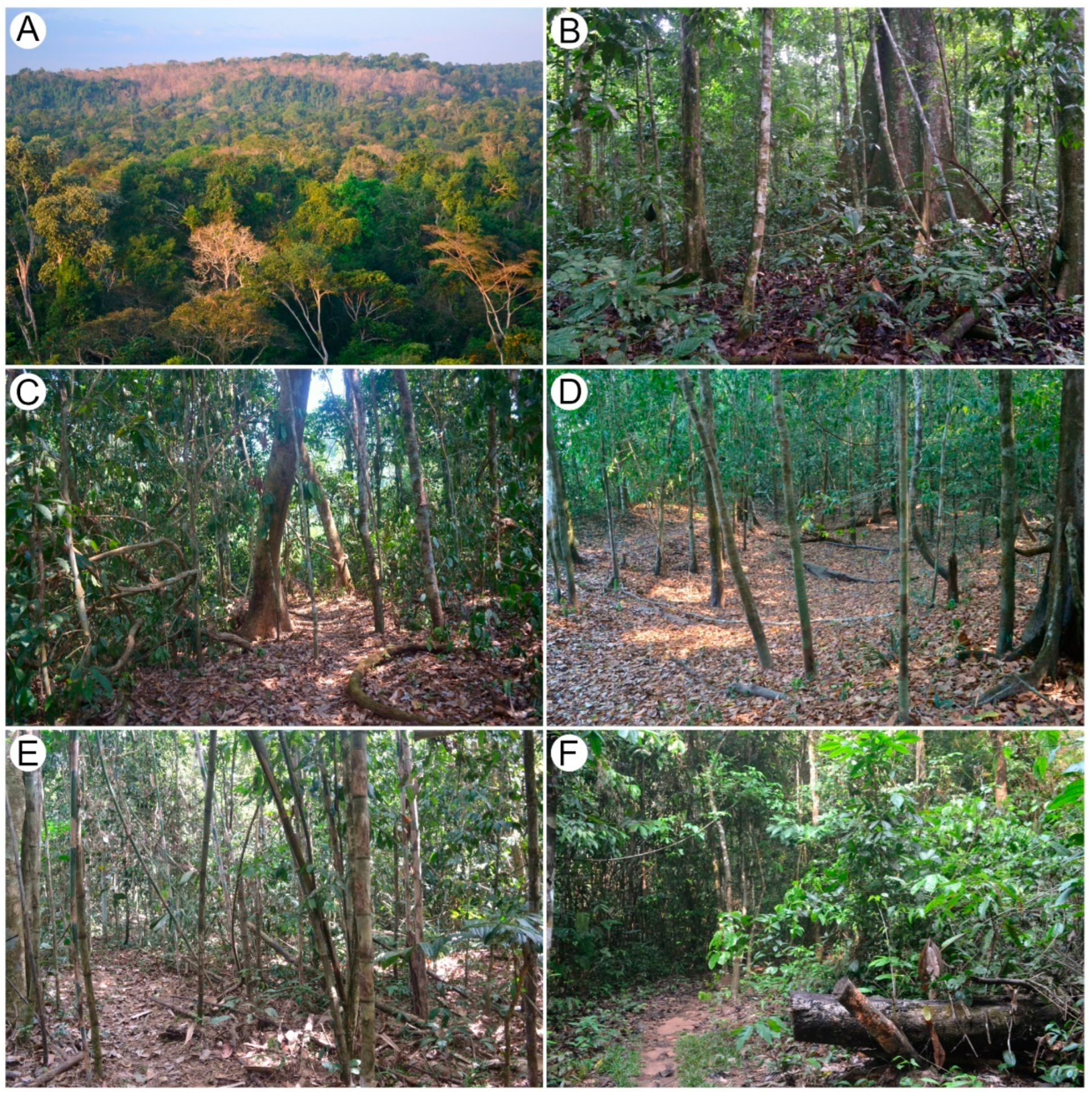
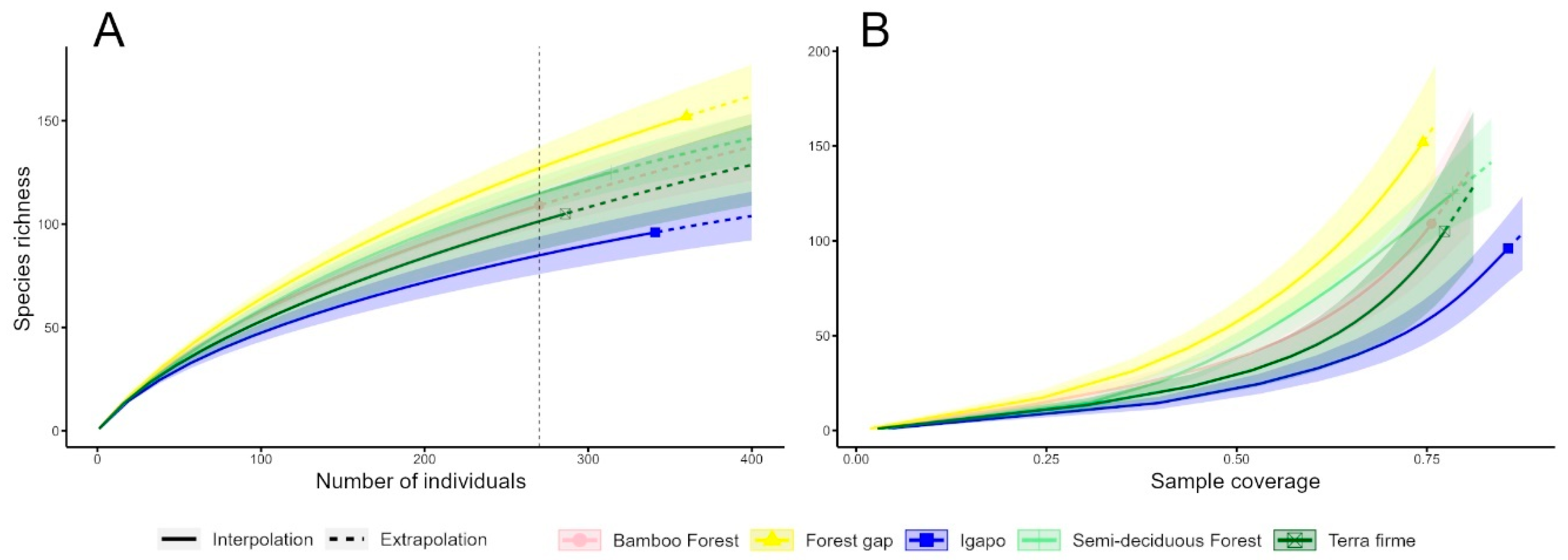
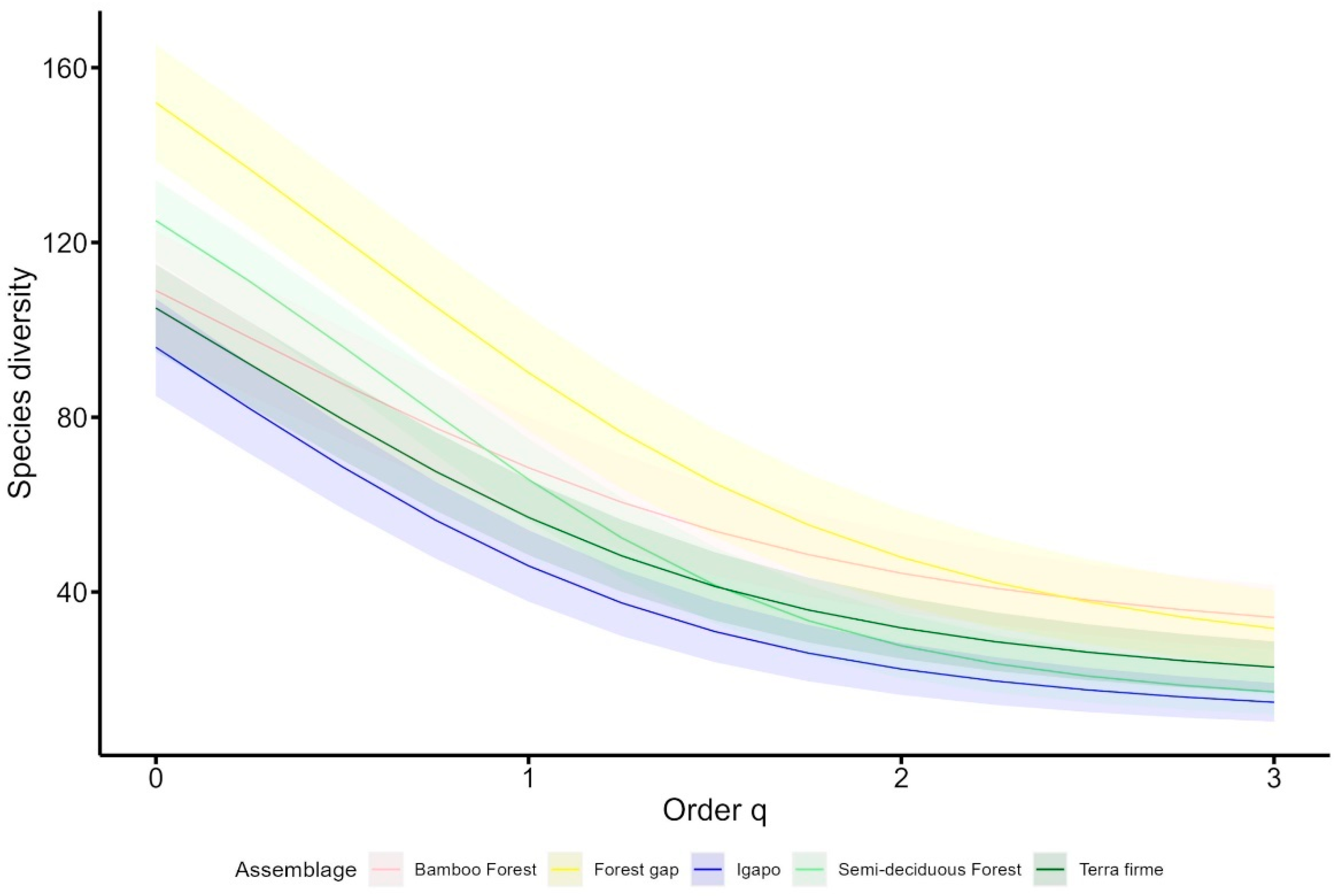
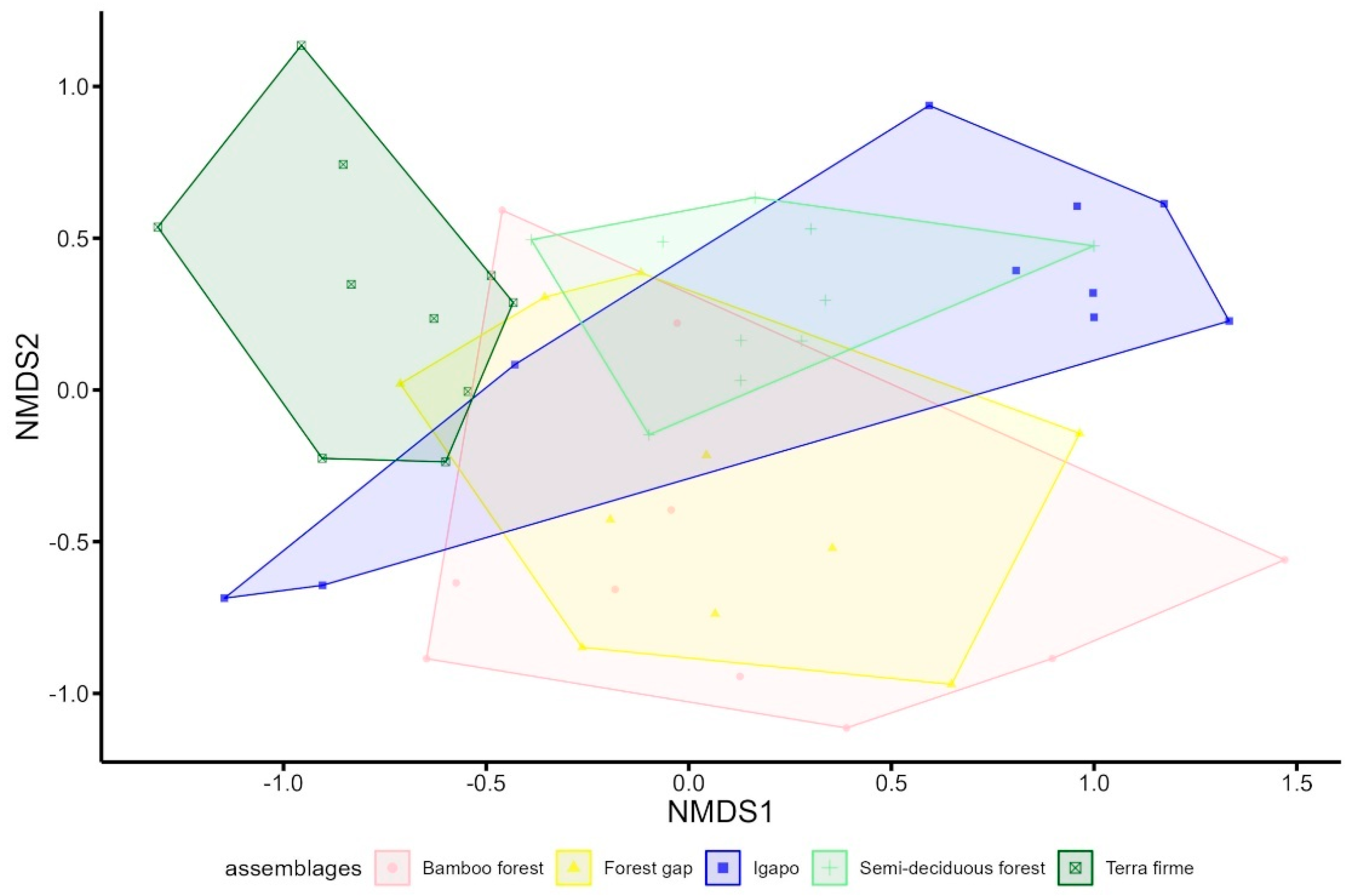
| Terra Firme | Semi-Deciduous Forest | Igapó | Bamboo Forest | Forest Gap | Total | |
|---|---|---|---|---|---|---|
| Nymphalidae | 46 (173) | 49 (203) | 36 (204) | 60 (197) | 57 (195) | 112 (972) |
| Hesperiidae | 23 (30) | 29 (36) | 23 (30) | 22 (23) | 41 (62) | 90 (181) |
| Riodinidae | 28 (72) | 38 (60) | 26 (81) | 22 (43) | 36 (81) | 84 (337) |
| Lycaenidae | 6 (6) | 5 (9) | 2 (11) | 0 | 16 (20) | 23 (46) |
| Pieridae | 1 (4) | 2 (2) | 5 (6) | 2 (4) | 1 (1) | 7 (17) |
| Papilionidae | 0 | 2 (4) | 4 (9) | 2 (2) | 1 (1) | 5 (16) |
| Hedylidae | 1 (1) | 0 | 0 | 1 (1) | 0 | 1 (2) |
| All butterflies | 105 (286) | 125 (314) | 96 (341) | 109 (270) | 152 (360) | 322 (1571) |
| Family | Indicator Species | Vegetation Type | N | IndVal (%) | p (Raw) |
|---|---|---|---|---|---|
| Nymphalidae | Amphidecta calliomma (C. Felder & R. Felder, 1862) | Bamboo forest | 12 (4) | 55 | 0.0001 |
| Nymphalidae | Splendeuptychia sp. | Bamboo forest | 10 (4) | 40 | 0.0014 |
| Nymphalidae | Morpho sp.* | Forest gap | 56 (23) | 40 | 0.0021 |
| Riodinidae | Mesosemia lacernata Stichel, 1909 | Forest gap | 5 (4) | 40 | 0.001 |
| Lycaenidae | Calycopis msp.1 * | Igapó | 6 (5) | 50 | 0.0002 |
| Lycaenidae | Calycopis msp.2 * | Igapó | 9 (8) | 27.78 | 0.0043 |
| Nymphalidae | Pseudeuptychia herseis (Godart, 1824) * | Igapó | 32 (21) | 35 | 0.0031 |
| Nymphalidae | Heliconius erato (Linnaeus, 1758) | Igapó | 18 (9) | 46.67 | 0.0002 |
| Nymphalidae | Hermeuptychia sp. | Igapó | 109 (25) | 33.39 | 0.0077 |
| Nymphalidae | Deltaya ocypete (Fabricius, 1776) | Igapó | 5 (4) | 40 | 0.0007 |
| Nymphalidae | Pierella hyalinus (Gmelin, 1790) | Igapó | 11 (7) | 29.09 | 0.0063 |
| Nymphalidae | Vila emilia (Cramer, 1779) | Igapó | 15 (6) | 60 | 0.0001 |
| Riodinidae | Nymphidium caricae (Linnaeus, 1758) | Igapó | 32 (11) | 72.5 | 0.0001 |
| Nymphalidae | Euptychia westwoodi A. Butler, 1867 | Semi-deciduous forest | 104 (35) | 38.46 | 0.0008 |
| Nymphalidae | Heliconius aoede (Hübner, 1813) | Semi-deciduous forest | 5 (5) | 32 | 0.004 |
| Nymphalidae | Pierella astyoche (Erichson, 1849) | Terra firme | 19 (12) | 34.74 | 0.0025 |
| Riodinidae | Mesosemia marisa (Hewitson, 1858) | Terra firme | 26 (5) | 38.46 | 0.0015 |
| Riodinidae | Stalachtis calliope (Linnaeus, 1758) | Terra firme | 24 (15) | 33.33 | 0.0053 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, L.L.; Santos, J.P.; Willmott, K.R.; Freitas, A.V.L. Butterfly Assemblages Differ among Vegetation Types in Southern Amazonia. Diversity 2023, 15, 624. https://doi.org/10.3390/d15050624
Mota LL, Santos JP, Willmott KR, Freitas AVL. Butterfly Assemblages Differ among Vegetation Types in Southern Amazonia. Diversity. 2023; 15(5):624. https://doi.org/10.3390/d15050624
Chicago/Turabian StyleMota, Luísa L., Jessie P. Santos, Keith R. Willmott, and André V. L. Freitas. 2023. "Butterfly Assemblages Differ among Vegetation Types in Southern Amazonia" Diversity 15, no. 5: 624. https://doi.org/10.3390/d15050624
APA StyleMota, L. L., Santos, J. P., Willmott, K. R., & Freitas, A. V. L. (2023). Butterfly Assemblages Differ among Vegetation Types in Southern Amazonia. Diversity, 15(5), 624. https://doi.org/10.3390/d15050624






