Spatial Patterns in the Distribution and Diversity of Diploneis Genus-Level Diatoms in the Podlasie Springs (North-Eastern Poland)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Measurements
2.3. Physical and Chemical Water Analyses
2.4. Diatom Analyses
3. Results
3.1. Physical and Chemical Water Parameters
3.2. Diatoms
3.2.1. Diploneis burgitensis Prudent 1905, Figure 4a and Figure 5
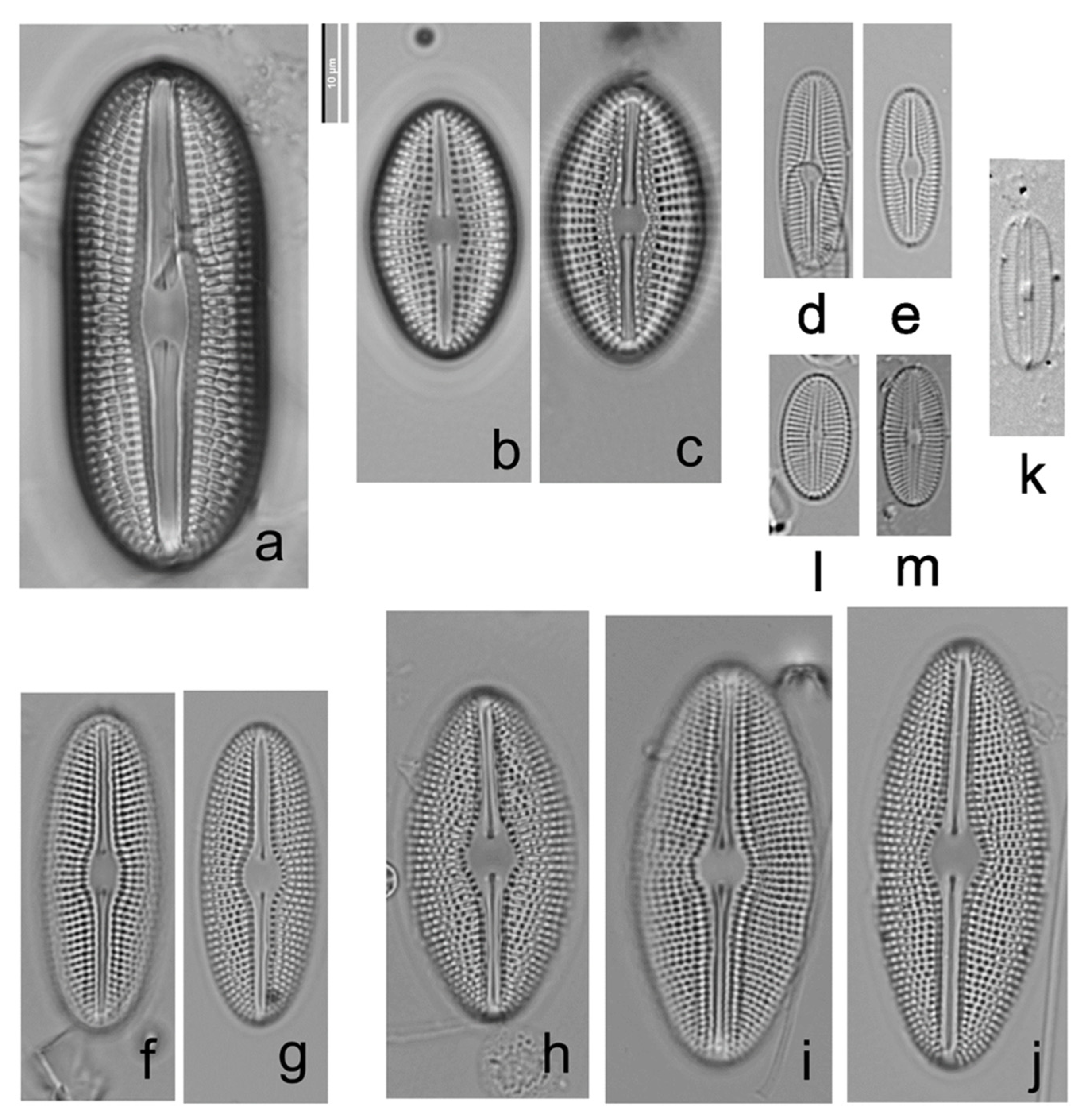
3.2.2. Diploneis elliptica (Kützing) Cleve 1891, Figure 4b,c
3.2.3. Diploneis fontanella, Lange-Bertalot in Werum and Lange-Bertalot 2004, Figure 4d,e
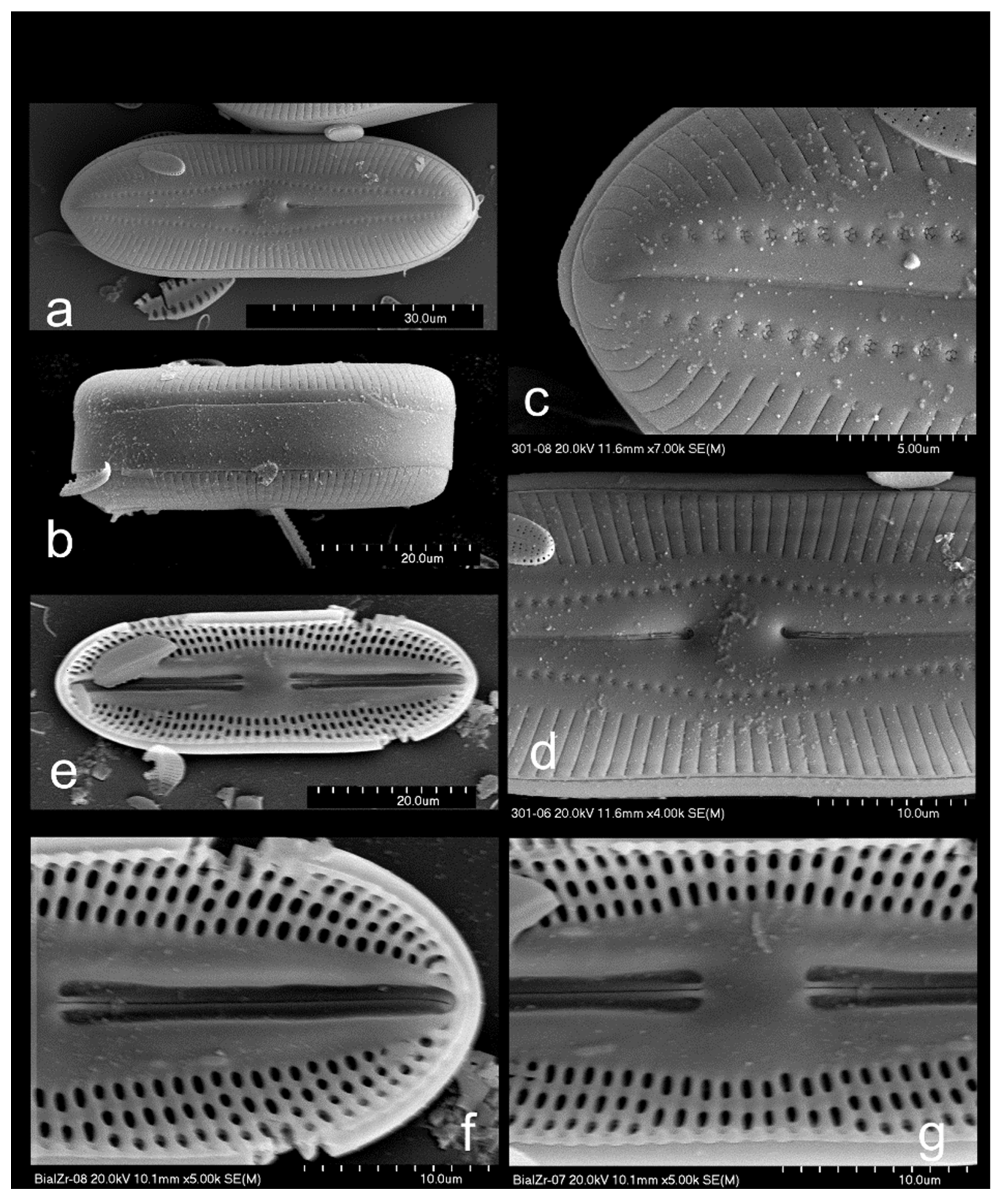
3.2.4. Diploneis fontium, Reichardt and Lange-Bertalot 2004, Figure 4f,g and Figure 6a–f

3.2.5. Diploneis krammeri, Lange-Bertalot and Reichardt 2004, Figure 4h–j
3.2.6. Diploneis parapetersenii Lange-Bertalot and Fuhrmann in Lange-Bertalot 2021, Figure 4k and Figure 6g,h
3.2.7. Diploneis separanda, Lange-Bertalot in Werum and Lange-Bertalot 2004, Figure 4l,m
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Żelazna-Wieczorek, J.; Bik, A. Dynamics of diatom communities in springs of different hydrobiological types. Fragm. Flor. Geobot. Pol. 2009, 16, 155–167, (In Polish with English Summary). [Google Scholar]
- Cantonati, M.; Komárek, J.; Montejano, G. Cyanobacteria in ambient springs. Biodivers. Conserv. 2015, 24, 865–888. [Google Scholar] [CrossRef]
- Wojtal, A.; Sobczyk, Ł. The influence of substrates and physicochemical factors on the composition of diatom assemblages in karst springs and their applicability in water quality assessment. Hydrobiologia 2012, 695, 97–108. [Google Scholar] [CrossRef][Green Version]
- Cantonati, M.; Gerecke, R.; Bertuzzi, E. Springs of the Alps, sensitive ecosystems to environmental change: From biodiversity assessments to long–term studies. Hydrobiologia 2006, 562, 59–96. [Google Scholar] [CrossRef]
- Angeli, N.; Cantonati, M.; Spitale, D.; Lange-Bertalot, H. A comparison between diatom assemblages in two groups of carbonate, low–altitude springs with different levels of anthropogenic disturbances. Fottea 2010, 10, 115–128. [Google Scholar] [CrossRef]
- Knysak, P.J. Human Impact on Crenic Ecosystems Based on the Diversity of Diatoms and Their Autecology. Ph.D. Thesis, University of Lodz, Lodz, Poland, 2019. (In Polish with English Abstract). [Google Scholar]
- Wojtal, A.Z. Species composition and distribution of diatom assemblages in spring waters from various geological formations in southern Poland. Bibl. Diatomol. 2013, 59, 1–436. [Google Scholar]
- Żelazna-Wieczorek, J. Diatom Flora in Springs of Łódź Hills (Central Poland): Biodiversity, Taxonomy, and Temporal Changes of Epipsammic Diatom Assemblages in Springs Affected by Human Impact; A. R. G. Gantner: Liechtenstein, Germany, 2011; pp. 1–419. [Google Scholar]
- Gutwiński, R. Materyjały do flory wodorostów Galicyi. Spraw. Komis. Fizjograf. 1884, 18, 127–138. (In Polish) [Google Scholar]
- Schumann, J. Die Diatomeen der Hohen Tatra; Wien, W. Braumüller: Wien, Austria, 1867; 102p. [Google Scholar]
- Grabowska, M. The role of springs in maintaining the biodiversity of freshwater algae. In Proceedings of the 1st International Electronic Conference on Biological Diversity, Ecology and Evolution, Online, 15–31 March 2021. [Google Scholar]
- Grabowska, M.; Danilczyk, M.; Jekatierynczuk-Rudczyk, E.; Fieducik, J.; Jankowska, L. A preliminary study of benthic diatoms in Knyszyńska Forest springs. In Proceedings of the Book of Abstracts, XXXVth International Conference of the Polish Phycological Society “Algae in Anthropogenically Transformed Ecosystems”, Łódź—Stryków, Poland, 1–4 June 2016; p. 45. [Google Scholar]
- Jekatierynczuk-Rudczyk, E.; Zieliński, P.; Puczko, K.; Micun, K.; Puczyłowska, E. The Role of the Catchment Area in Shaping Water Quality in the Lowland Springs of the Knyszyn Forest (NE Poland). Water 2022, 14, 3202. [Google Scholar] [CrossRef]
- Lange-Bertalot, H.; Fuhrmann, A. Contribution to the genus Diploneis (Bacillariophyta): Twelve species from Holarctic freshwater habitats proposed as new to science. Fottea 2016, 16, 157–183. [Google Scholar] [CrossRef]
- Jovanovska, E.; Levkov, Z. The genus Diploneis in the Republic of North Macedonia. In Diatoms of Europe; Lange-Bertalot, H., Ed.; Koeltz Botanical Books: Glashütten, Germany, 2020; Volume 9, pp. 527–653. [Google Scholar]
- Lange-Bertalot, H.; Fuhrmann, A.; Werum, M. Species Diversity in the Holarctic and Spot Checks from Elsewhere. In Diatoms of Europe; Lange-Bertalot, H., Ed.; Koeltz Botanical Books: Glashütten, Germany, 2020; Volume 9, pp. 1–526. [Google Scholar]
- Cantonati, M.; Kelly, M.G.; Lange-Bertalot, H. Freshwater Benthic Diatoms of Central Europe: Over 800 Common Species Used in Ecological Assessmen; Koeltz Botanical Books: Glashütten, Germany, 2017; pp. 1–942. [Google Scholar]
- Wojtal, A.Z. The diatoms of Kobylanka stream near Kraków (Wyżyna Krakowsko- Częstochowska, S Poland). Pol. Bot. J. 2009, 54, 129–330. [Google Scholar]
- Pociecha, A.; Wojtal, A.Z.; Szarek-Gwiazda, E.; Cieplok, A.; Ciszewski, D.; Cichoń, S. Neo- and paleo-limnological studies on diatom and cladoceran communities of subsidence ponds affected by mine waters (S. Poland). Water 2020, 12, 1581. [Google Scholar] [CrossRef]
- Puczko, K.; Zieliński, P.; Jusik, S.; Kołakowska, A.; Jekatierynczuk-Rudczyk, E. Vascular plant and bryophyte species richness in response to water quality in lowland spring niches with different anthropogenic impacts. Environ. Monit. Assess 2018, 190, 338. [Google Scholar] [CrossRef]
- Jekatierynczuk-Rudczyk, E.; Zieliński, P.; Puczko, K. Is the protection of springs in Knyszyn Forest effective and satisfactory? Prot. Nativ. Nat. 2017, 73, 135–147, (In Polish with English Summary). [Google Scholar]
- Puczko, K.; Jekatierynczuk-Rudczyk, E. Analysis of urban land cover influence to organic carbon and nutrients in surface water via impacted groundwater. Environ. Monit. Assess 2020, 192, 145. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, P.; Jekatierynczuk-Rudczyk, E.; Puczko, K. Factors affecting the abundance and activity of the bacterioplankton in lowland forest springs in northeastern Poland. Ecohydrol. Hydrobiol. 2020, 20, 675–686. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Waste Water; APHA, AWWA and WPCF: Washington, DC, USA, 1992. [Google Scholar]
- Neal, C.; Neal, M.; Wickham, H. Phosphate measurement in natural waters: Two examples of analytical problems associated with silica interference using phosphomolybdic acid methodologies. Sci. Total Environ. 2000, 251–252, 513–542. [Google Scholar] [CrossRef]
- Hartley, B.; Ross, R.; Williams, D.M. A check-list of the freshwater, brackish and marine diatoms of the British Isles and adjoining coastal waters. J. Mar. Biol. Assoc. UK 1986, 66, 531–610. [Google Scholar] [CrossRef]
- Ludwig, G.; Schnittler, M. Rote Liste gefährdeter Phlanzen Deutschlands. Schriftenreihe Veg. 1996, 28, 1–744. [Google Scholar]
- Hindák, F.; Hindáková, A. Cyanobacteria and diatoms of cold mineral springs in the National Natural Landmark of Mičiná (Central Slovakia). Bull. Slov. Bot. Spoločn. 2016, 38, 13–19, (In Slovak with English Abstract). [Google Scholar]
- Levkov, Z.; Williams, D.M. Checklist of diatoms (Bacillariophyta) from Lake Ohrid and Lake Prespa (Macedonia), and their watersheds. Phytotaxa 2012, 45, 1–76. [Google Scholar] [CrossRef][Green Version]
- Bąk, M.; Witkowski, A.; Żelazna-Wieczorek, J.; Wojtal, A.Z.; Szczepocka, E.; Szulc, K.; Szulc, B. The Key to the Determination of Diatoms in Phytobenthos for the Assessment of the Ecological Status of Surface Waters in Poland; Biblioteka Monitoringu Środowiska: Warszawa, Poland, 2012; pp. 1–452. (In Polish) [Google Scholar]
- Pliński, M.; Witkowski, A. Diatoms from the Gulf of Gdańsk and Surrounding Waters (the Southern Baltic Sea); Gdańsk University Press: Gdańsk, Poland, 2020; pp. 1–442. (In Polish) [Google Scholar]
- Noga, T.; Stanek-Tarkowska, J.; Pajaczek, A.; Kochman, N.; Peszek, L. Ecological assessment of the San River water quality on the area of the San Valley Landscape Park. J. Ecol. Eng. 2014, 15, 1222. [Google Scholar]
- Eliasz-Kowalska, M.; Wojtal, A.Z.; Barinova, S. Influence of Selected Environmental Factors on Diatom β Diversity (Bacillariophyta) and the Value of Diatom Indices and Sampling Issues. Water 2022, 14, 2315. [Google Scholar] [CrossRef]
- Jekatierynczuk-Rudczyk, E.; Ejsmont-Karabin, J. Rotifers of Inter-Forest Springs. Diversity 2023, 15, 153. [Google Scholar] [CrossRef]
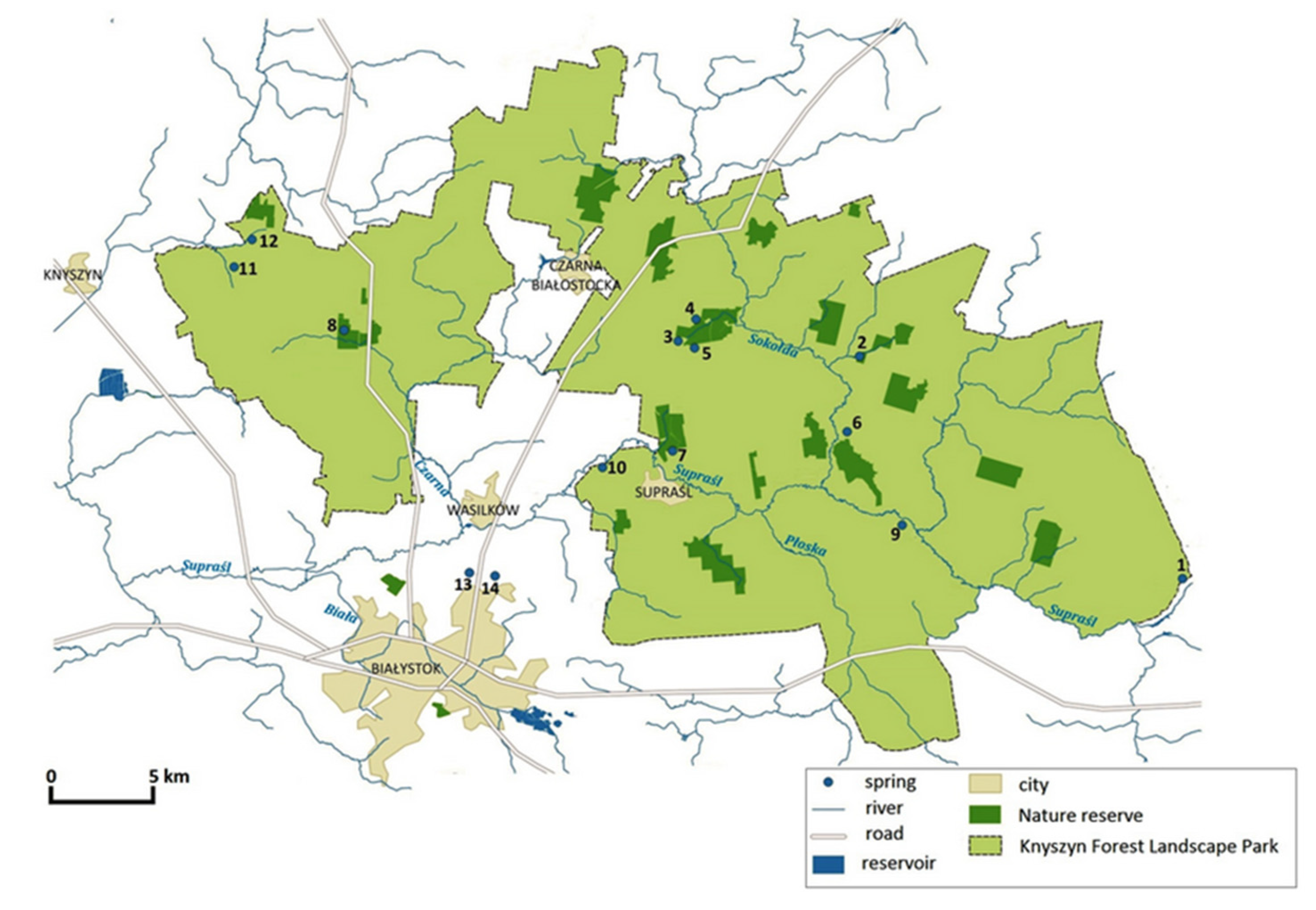


| Spring No. | Spring Name | Geographical Coordinates | Hydrological Location | Qav. [L s−1] | Geomorphological Type of Spring | Substrate | Land Use |
|---|---|---|---|---|---|---|---|
| KNYSZYN FOREST | |||||||
| 1. | Piłatowszczyzna | N: 53°8′58.19″ E: 23°42′56.39″ | right-side direct tributary of the Supraśl river → Breszczeczka catchment | 1.5 | hillslope | epiliton | forest |
| 2. | Woronicze | N: 53°16′5.05″ E: 23°31′1.89″ | Sokołda catchment → Łanga catchment | 2.3 | hillslope | epiliton | rural area |
| 3. | Budzisk reserve I | N: 53°16′53.67″ E: 23°22′15.75″ | Sokołda catchment → Migówka catchment | 6.8 | hillslope | mud | forest |
| 4. | Budzisk reserve II | N: 53°16′54.62″ E: 23°22′27.36″ | Sokołda catchment → Migówka catchment | 4.5 | hillslope | mud | forest |
| 5. | Pstrągownia | N: 53°16′26.7″ E: 23°21′24.73″ | Sokołda catchment → Migówka catchment | 5.4 | valley | epiliton | forest |
| 6. | Łaźnie | N: 53°14′53.01″ E: 23°28′59.46″ | direct tributary of the Sokołda river → Sokołda catchment | 4.8 | hillslope | epiliton | forest |
| 7. | Jałówka reserve | N: 53°14′4.58″ E: 23°20′41.57″ | Sokołda catchment → Jałówka catchment | 2.3 | valley | epiliton | forest |
| 8. | Krzemianka reserve | N: 53°16′55.79″ E: 23°7′5.98″ | Czarna catchment → Krzemianka catchment | 8.6 | valley | epiliton/moss | forest |
| 9. | Nowosiółki | N: 53°10′21.9″ E: 23°31′2.85″ | left-side direct tributary of the Supraśl river → Supraśl catchment | 0.5 | hillslope | mud | forest |
| 10. | Pólko | N: 53°13′13.9″ E: 23°18′5.54″ | left-side direct tributary of the Supraśl river → Supraśl catchment | 1.5 | valley | mud | forest/grassland |
| 11. | Krynice | N: 53°18′19.94″ E: 23°1′55.69″ | Narew catchment → Jaskranka catchment | 3.2 | hillslope | psammon | rural area |
| 12. | Hatka | N: 53°19′12.49″ E: 23°3′23.81″ | Narew catchment → Jaskranka catchment | 1.1 | hillslope | mud | rural area |
| BIAŁYSTOK | |||||||
| 13. | Jaroszówka | N: 53°10′38.80″ E: 23°11′59.10″ | Supraśl catchment → Jaroszówka catchment | 5.2 | hillslope | sand/mud | forest |
| 14. | Pietrasze | N: 53°09′15.41″ E: 23°04′23.61″ | Supraśl catchment | 4.9 | hillslope | sand | forest |
| Spring No. | Spring Name | Temperature [°C] | Reaction [pH] | Electrolytic Conductivity [µS L−1] | Oxygen [mg L−1] | Oxygen Saturation [%] | Hydrochemistry Type of Water |
|---|---|---|---|---|---|---|---|
| KNYSZYN FOREST | |||||||
| 1 * | Piłatowszczyzna | 11.1 | 7.27 | 354 | 7.63 | 63.3 | HCO3-Ca |
| 2 * | Woronicze | 15.4 | 8.16 | 321 | 8.02 | 80.7 | HCO3-Ca-Mg |
| 3 ***** | Budzisk reserve I | 13.3 | 7.67 | 386 | 9.97 | 97.1 | HCO3-Ca |
| 4 ***** | Budzisk reserve II | 12.6 | 7.74 | 379 | 9.83 | 94.1 | HCO3-Ca-Mg |
| 5 ** | Pstrągownia | 8.30 | 7.77 | 395 | 10.95 | 95.1 | |
| 5 ***** | Pstrągownia | 13.2 | 8.07 | 401 | 9.36 | 91.0 | HCO3-Ca-Mg |
| 6 * | Łaźnie | 12.0 | 7.46 | 395 | 9.12 | 88.2 | |
| 6 ***** | Łaźnie | 11.9 | 8.30 | n.a. | 6.45 | 60.8 | HCO3-Ca-Mg |
| 7 ** | Jałówka reserve | 15.1 | 7.99 | 424 | 8.48 | 86.9 | |
| 7 ***** | Jałówka reserve | 15.1 | 7.99 | 424 | 8.48 | 86.9 | HCO3-Ca-Mg |
| 8 *** | Krzemianka reserve | 8.30 | 7.85 | 590 | 10.14 | 86.7 | HCO3-Ca-Mg |
| 9 * | Nowosiółki | 11.5 | 8.21 | 261 | 9.74 | 89.4 | HCO3-Ca-Mg |
| 10 ***** | Pólko | 11.0 | 8.03 | 425 | 8.3 | 76.6 | HCO3-Ca-Mg |
| 11 ** | Krynice | 19.7 | 8.13 | 465 | 7.05 | 78.6 | HCO3-Ca-Mg |
| 12 ** | Hatka | 17.9 | 7.81 | 484 | 6.03 | 64.9 | HCO3-Ca |
| BIAŁYSTOK | |||||||
| 13 ***** | Jaroszówka | 14.9 | 8.08 | 412 | 8.9 | 90.3 | HCO3-Ca |
| 14 **** | Pietrasze | 12.5 | 8.14 | 853 | 7.7 | 97.3 | HCO3-Ca |
| Spring No. | Ca 2+ | Mg 2+ | Na + | K+ | HCO3− | SO42− | Cl− | TFe | DOC | TN | N-NH4+ | N-NO3− | N-NO2− | TP | SRP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [mg L−1] | |||||||||||||||
| KNYSZYN FOREST | |||||||||||||||
| 1 * | 70.6 | 9.1 | 2.95 | 1.38 | 251 | 35.6 | 8.00 | 0.614 | 4.01 | 2.068 | 0.246 | 0.352 | 0.002 | 0.071 | 0.070 |
| 2 * | 62.5 | 16.0 | 4.37 | 0.20 | 238 | 20.6 | 6.00 | 2.207 | 1.66 | 0.789 | 0.493 | 0.188 | 0.001 | 0.305 | 0.060 |
| 3 ***** | 65.1 | 8.33 | 2.56 | 0.40 | 275 | 21.3 | 2.70 | 0.051 | 2.88 | 0.788 | 0.006 | 0.327 | 0.001 | 0.309 | 0.044 |
| 4 ***** | 62.7 | 8.45 | 2.38 | 0.57 | 269 | 20.3 | 2.74 | 0.088 | 2.59 | 0.682 | 0.00 | 0.323 | 0.001 | 0.349 | 0.049 |
| 5 ** | 69.9 | 17.5 | 3.66 | 0.64 | 277 | 29.9 | 5.50 | 1.60 | 1.28 | 0.588 | 0.201 | 0.618 | 0.001 | 0.304 | 0.077 |
| 5 ***** | 62.8 | 9.40 | 2.45 | 0.46 | 268 | 20.4 | 2.41 | 0.044 | 2.94 | 1.254 | 0.006 | 0.618 | 0.0002 | 0.422 | 0.030 |
| 6 * | 74.7 | 13.5 | 4.33 | 1.39 | n.a. | 24.0 | 11.30 | 0.809 | 3.21 | 1.667 | 0.210 | 0.066 | 0.001 | 0.028 | 0.013 |
| 6 ***** | 58.4 | 10.1 | 4.18 | 0.75 | 259 | 21.0 | 4.09 | 0.037 | 2.35 | 0.723 | 0.004 | 0.314 | 0.001 | 0.433 | 0.025 |
| 7 ** | 68.5 | 21.6 | 5.84 | 1.30 | 281 | 26.2 | 10.57 | 1.841 | 2.81 | 1.890 | 0.327 | 1.330 | 0.003 | 0.154 | 0.062 |
| 7 ***** | 67.6 | 9.40 | 2.86 | 0.55 | 278 | 32.4 | 4.68 | 0.037 | 4.17 | 1.166 | 0.010 | 0.487 | 0.001 | 0.390 | 0.036 |
| 8 *** | 91.4 | 17.9 | 14.98 | 1.16 | 319 | 38.9 | 41.50 | 0.726 | 4.05 | 1.442 | 0.314 | 0.310 | 0.001 | 0.112 | 0.024 |
| 9 * | 60.5 | 10.1 | 2.19 | 0.27 | 162 | 25.7 | 8.40 | 0.979 | 3.43 | 1.223 | 0.197 | 0.186 | 0.002 | 0.156 | 0.077 |
| 10 ***** | 71.1 | 10.2 | 4.24 | 0.61 | 275 | 39.4 | 4.79 | 0.029 | 2.79 | 3.276 | 0.012 | 1.891 | 0.003 | 0.321 | 0.027 |
| 11 ** | 59.5 | 16.8 | 4.00 | 0.42 | 290 | 42.3 | 8.02 | 1.580 | 2.70 | 0.203 | 0.242 | 0.242 | 0.001 | 0.178 | 0.097 |
| 12 ** | 70.4 | 11.6 | 4.46 | 1.96 | 299 | 30.3 | 12.18 | 1.561 | 2.22 | 1.975 | 0.266 | 1.572 | 0.003 | 0.216 | 0.127 |
| BIAŁYSTOK | |||||||||||||||
| 13 **** | 123.4 | 12.63 | 31.23 | 12.27 | 476 | 79.66 | 54.26 | 0.037 | 3.04 | 3.211 | 0.014 | 1.970 | 0.002 | 0.397 | 0.096 |
| 14 *** | 67.2 | 7.13 | 3.06 | 0.48 | 280 | 43.03 | 5.54 | 0.037 | 2.97 | 0.504 | 0.017 | 0.181 | 0.006 | 0.431 | 0.078 |
| Species/Locality | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diploneis burgitensis | + | + | + | + | + | + | + | + | + | + | + | + | ||
| Diploneis elliptica | + | + | + | + | ||||||||||
| Diploneis fontanella | + | + | + | |||||||||||
| Diploneis fontium | + | + | ||||||||||||
| Diploneis krammeri | + | + | + | + | + | + | ||||||||
| Diploneis parapetersenii | + | |||||||||||||
| Diploneis separanda | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabowska, M.; Wojtal, A.Z.; Jekatierynczuk-Rudczyk, E.; Kryvosheia-Zakharova, O. Spatial Patterns in the Distribution and Diversity of Diploneis Genus-Level Diatoms in the Podlasie Springs (North-Eastern Poland). Diversity 2023, 15, 897. https://doi.org/10.3390/d15080897
Grabowska M, Wojtal AZ, Jekatierynczuk-Rudczyk E, Kryvosheia-Zakharova O. Spatial Patterns in the Distribution and Diversity of Diploneis Genus-Level Diatoms in the Podlasie Springs (North-Eastern Poland). Diversity. 2023; 15(8):897. https://doi.org/10.3390/d15080897
Chicago/Turabian StyleGrabowska, Magdalena, Agata Z. Wojtal, Elżbieta Jekatierynczuk-Rudczyk, and Olha Kryvosheia-Zakharova. 2023. "Spatial Patterns in the Distribution and Diversity of Diploneis Genus-Level Diatoms in the Podlasie Springs (North-Eastern Poland)" Diversity 15, no. 8: 897. https://doi.org/10.3390/d15080897
APA StyleGrabowska, M., Wojtal, A. Z., Jekatierynczuk-Rudczyk, E., & Kryvosheia-Zakharova, O. (2023). Spatial Patterns in the Distribution and Diversity of Diploneis Genus-Level Diatoms in the Podlasie Springs (North-Eastern Poland). Diversity, 15(8), 897. https://doi.org/10.3390/d15080897






