Spatial Turnover and Functional Redundancy in the Ants of Urban Fragments of Tropical Dry Forest
Abstract
1. Introduction
2. Materials and Methods
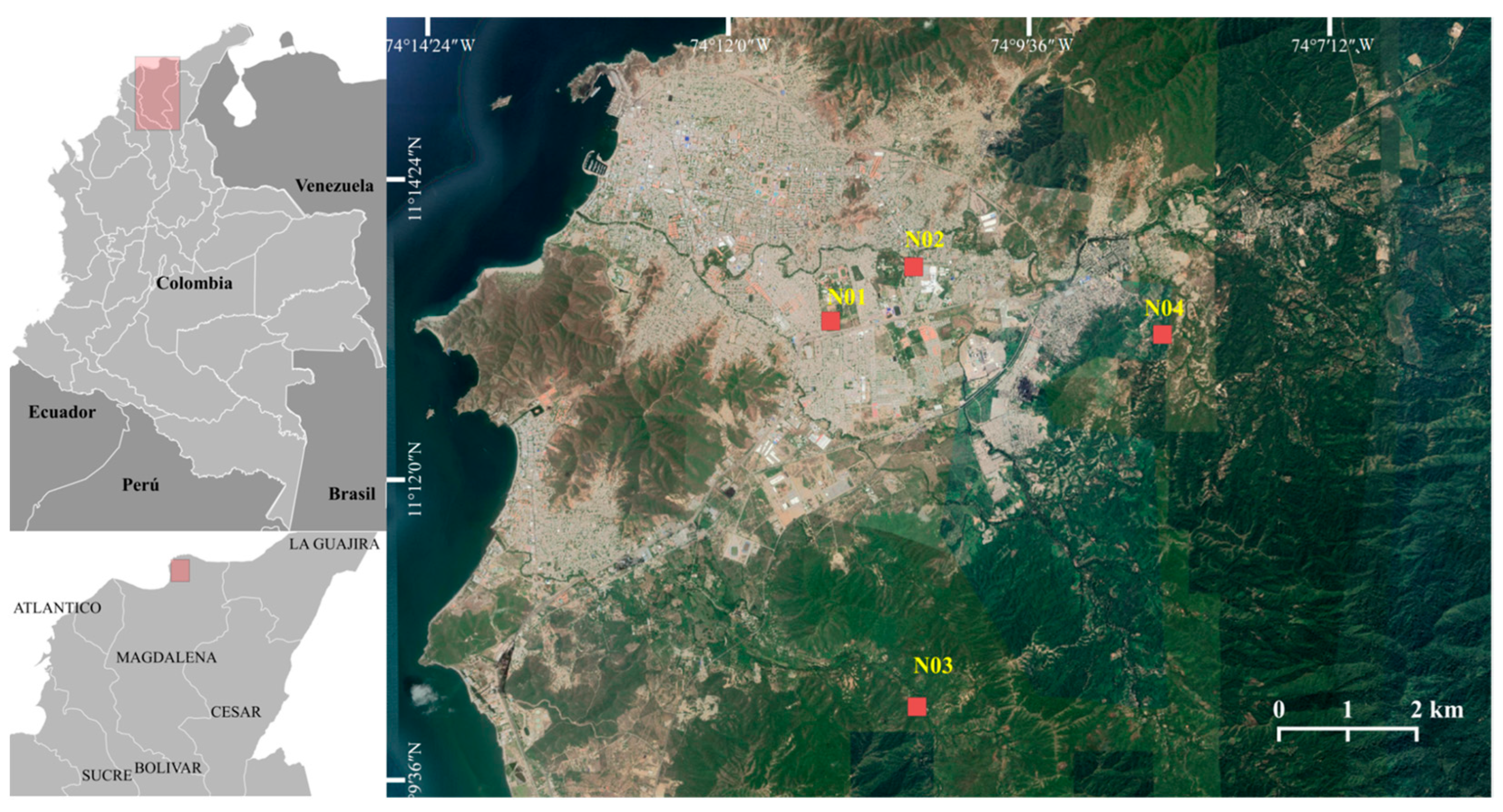
2.1. Study Area
2.2. Ants Sampling
2.3. Data Analysis
3. Results
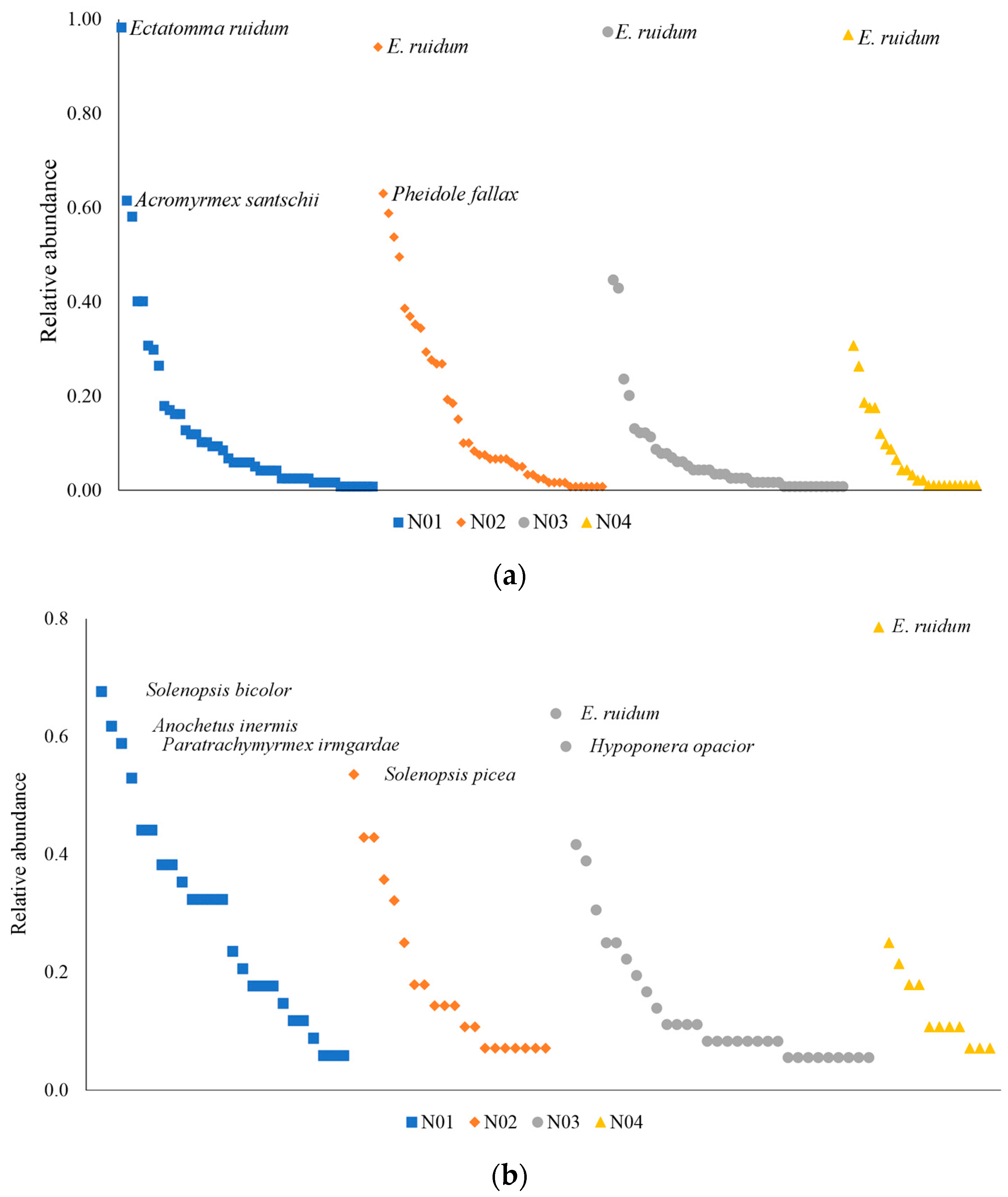
3.1. Composition and Completeness of Sampling
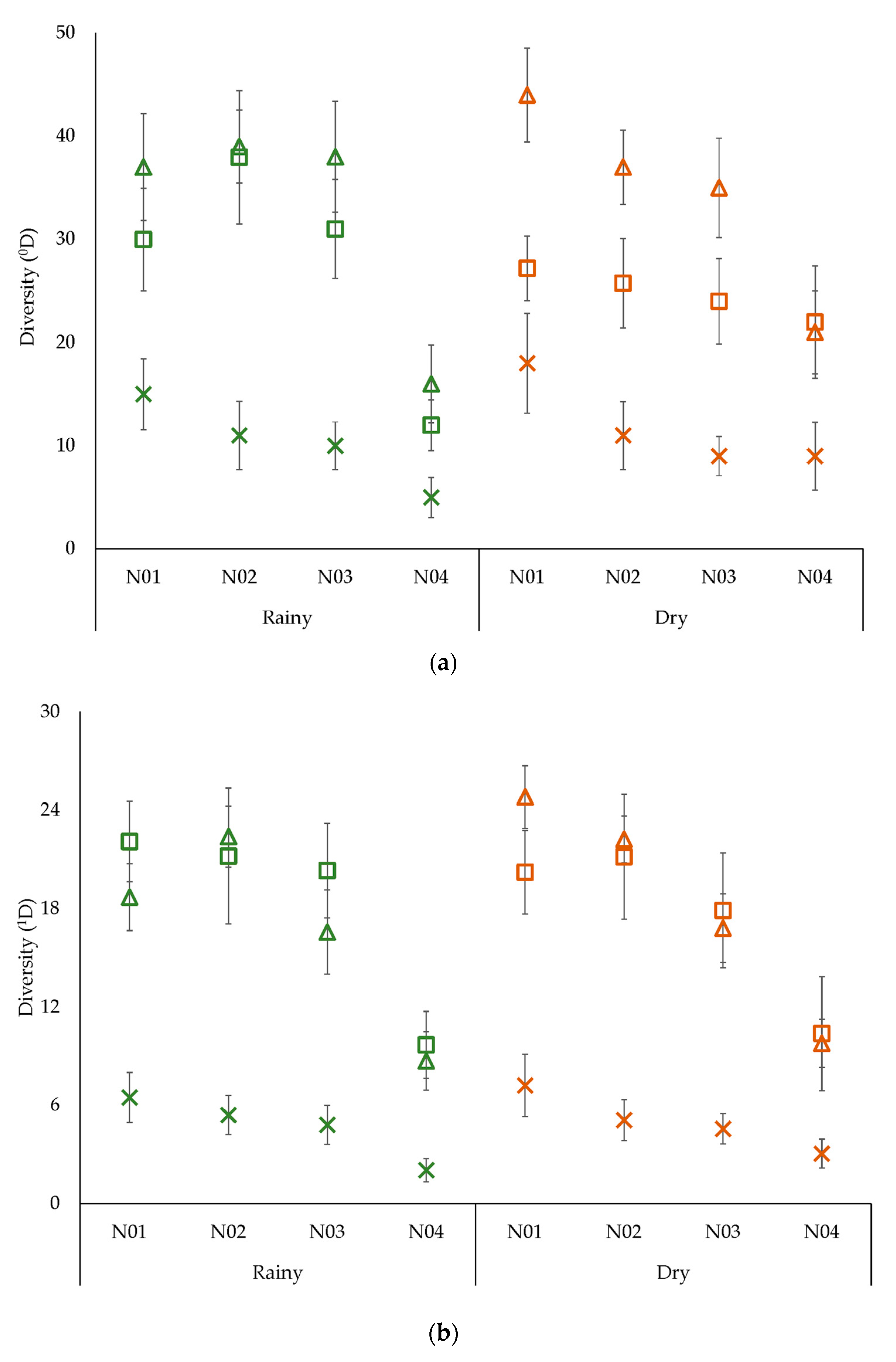
3.2. Alpha Diversity
3.3. Spatial and Temporal Variation
3.4. Functional Groups
4. Discussion
4.1. Composition and Completeness of Sampling
4.2. Alpha Diversity
4.3. Spatial and Temporal Variation
4.4. Functional Groups
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Subfamily | Species | Functional Group | N01 | N02 | N03 | N04 | Relative Abundance (%) |
|---|---|---|---|---|---|---|---|
| Myrmicinae | Acromyrmex octospinosus (Reich, 1793) | LCA | Hc, Pf | 0.7 | |||
| Acromyrmex santschii (Forel, 1912) | LCA | Ba, Hc, mW, Pf | Hc, Pf | Hc, mW, Pf | Pf | 19.2 | |
| Cephalotes aff. bimaculatus | AO | Hc | 0.1 | ||||
| Cephalotes femoralis (Smith, 1853) | AO | Hc, Pf | Hc, Pf | Hc, mW, Pf | Hc, Pf | 1.7 | |
| Cephalotes minutus (Fabricius, 1804) | AO | Ba, Hc, mW, Pf | Hc, Pf | mW, Pf | Hc, Pf | 3.9 | |
| Cephalotes pellans De Andrade, 1999 | AO | Hc, mW, Pf | mW, Pf | 0.7 | |||
| Cephalotes pusillus (Klug, 1824) | AO | Hc, mW, Pf | Hc, mW, Pf | Hc, mW, Pf | Hc, mW, Pf | 8.1 | |
| Cephalotes sp. | AO | mW | 0.1 | ||||
| Crematogaster crinosa Mayr, 1862 | AO | Hc, Pf | Ba, Hc, mW, Pf | Hc, Pf | Ba, Hc, Pf | 4.2 | |
| Crematogaster distans Mayr, 1870 | AO | Hc, mW | 0.2 | ||||
| Crematogaster limata Smith, 1858 | AO | Hc | 0.1 | ||||
| Crematogaster obscurata Emery, 1895 | OA | Hc, mW, Pf | Hc, mW, Pf | mW, Pf | 3.8 | ||
| Crematogaster rochai Forel, 1903 | AO | Hc, Pf | 0.4 | ||||
| Crematogaster torosa Mayr, 1870 | AO | Hc | Hc, Pf | Ba, Hc, Pf | 2.2 | ||
| Cyphomyrmex flavidus Pergande, 1896 | FYA | mW, Pf | 0.4 | ||||
| Cyphomyrmex rimosus (Spinola, 1851) | FYA | Hc, mW, Pf | Pf | mW, Pf | Pf | 5.9 | |
| Megalomyrmex silvestrii Wheeler, 1909 | MLO | mW | 0.1 | ||||
| Mycetomoellerius urichii (Forel, 1893) | FGHA | Ba, Hc, mW, Pf | mW, Pf | Hc, mW, Pf | Pf | 11.1 | |
| Mycetomoellerius zeteki (Weber, 1940) | FGHA | Hc, mW, Pf | 0.3 | ||||
| Myrmicocrypta buenzlii Borgmeier, 1934 | CHI | mW, Pf | mW, Pf | Pf | 4.7 | ||
| Nesomyrmex sp. n. | AO | Pf | 0.1 | ||||
| Paratrachymyrmex cornetzi (Forel, 1912) | FGHA | mW, Pf | mW, Pf | Pf | 4.1 | ||
| Paratrachymyrmex irmgardae (Forel, 1912) | FGHA | Ba, Hc, mW, Pf | mW | mW | 3.1 | ||
| Pheidole distorta Forel, 1899 | SO | mW, Pf | Ba, Pf | Pf | 1.8 | ||
| Pheidole fallax Mayr, 1870 | SO | Ba, Hc, mW, Pf | Ba, Pf | Ba, mW, Pf | Ba, Hc, Pf | 24.0 | |
| Pheidole guajirana Wilson, 2003 | SO | Ba, Hc, mW, Pf | Ba, mW, Pf | Ba, mW, Pf | mW, Pf | 16.2 | |
| Pheidole impressa Mayr, 1870 | SO | Pf | 0.2 | ||||
| Pheidole inversa Forel, 1901 | SO | Ba, mW, Pf | Ba, mW, Pf | Ba, mW, Pf | Ba, Hc, Pf | 9.4 | |
| Pheidole leptina Wilson, 2003 | SO | Ba, Hc | 0.2 | ||||
| Pheidole praeusta Roger, 1863 | SO | Ba | 0.1 | ||||
| Pheidole subarmata Mayr, 1884 | SO | Pf | Ba, Pf | 1.5 | |||
| Pheidole urbana Camargo y Guerrero, 2020 | SO | Pf | Ba, Pf | Ba, Pf | mW | 7.3 | |
| Pheidole sp. 10. | SO | Hc, mW, Pf | 0.4 | ||||
| Pogonomyrmex mayri Forel, 1899 | LEO | Ba, Pf | Ba, mW, Pf | Ba, mW, Pf | Ba, mW, Pf | 17.4 | |
| Rogeria curvipubens Emery, 1894 | SO | mW | 0.1 | ||||
| Rogeria foreli Emery, 1894 | SO | mW, Pf | mW | mW | 2.2 | ||
| Sericomyrmex bondari Borgmeier, 1937 | FGHA | mW, Pf | 0.7 | ||||
| Solenopsis altinodis Forel, 1912 | SO | Ba, Hc, mW, Pf | Ba, Hc, mW, Pf | mW | 8.4 | ||
| Solenopsis bicolor (Emery, 1906) | SO | Ba, Hc, mW, Pf | mW, Pf | mW, Pf | mW | 10.0 | |
| Solenopsis geminata (Fabricius, 1804) | LEO | Ba | mW, Pf | Hc, Pf | mW | 1.4 | |
| Solenopsis picea Emery, 1896 | SO | mW, Pf | mW, Pf | mW, Pf | mW, Pf | 7.4 | |
| Solenopsis whitfordi Mackay et al., 2013 | SO | mW | mW | mW, Pf | mW, Pf | 1.3 | |
| Strumigenys dyseides Bolton, 2000 | DP | Pf | 0.1 | ||||
| Strumigenys eggersi Emery, 1890 | DP | mW | mW | 0.2 | |||
| Strumigenys elongata Roger, 1863 | DP | mW | mW | mW | 1.3 | ||
| Strumigenys spathula Lattke & Goitía, 1997 | DP | Pf | mW | 0.5 | |||
| Strumigenys tanymastax (Brown, 1964) | DP | mW | mW | mW | 1.7 | ||
| Temnothorax subditivus (Wheeler, 1903) | SO | Ba, Hc, mW, Pf | Ba, mW, Pf | mW, Pf | mW, Pf | 9.2 | |
| Trichomyrmex destructor (Jerdon, 1851) | ASO | Hc, Pf | 0.3 | ||||
| Wasmannia auropunctata (Roger, 1863) | SO | Ba, Pf | Ba, mW, Pf | 1.5 | |||
| Ponerinae | Anochetus inermis André, 1889 | MLP | mW, Pf | Ba, mW, Pf | mW | 7.6 | |
| Hypoponera clavatula (Emery, 1906) | SLP | mW | 0.6 | ||||
| Hypoponera opacior (Forel, 1893) | SLP | mW, Pf | mW | mW | 3.8 | ||
| Leptogenys pubiceps Emery, 1890 | SSI | Hc, Pf | mW, Pf | Pf | 2.2 | ||
| Leptogenys ritae | SSI | mW, Pf | 0.2 | ||||
| Odontomachus bauri Emery, 1892 | LEP | Ba, mW, Pf | Ba, mW, Pf | Hc, mW, Pf | Pf | 7.9 | |
| Odontomachus ruginodis Wheeler, 1908 | LEP | mW, Pf | Hc, mW, Pf | mW, Pf | 3.5 | ||
| Pachycondyla harpax (Fabricius, 1804) | LEP | Ba, mW, Pf | mW | mW, Pf | Pf | 2.5 | |
| Platythyrea pilosula (Smith, 1858) | LEP | Pf | Pf | 0.3 | |||
| Thaumatomyrmex sp. mutilatus group | SSM | Hc | 0.1 | ||||
| Formicinae | Brachymyrmex cordemoyi Forel, 1895 | ASO | mW | 0.2 | |||
| Brachymyrmex minutus Forel, 1893 | ASO | Ba, Hc, mW, Pf | Hc, mW, Pf | mW, Pf | Ba, mW, Pf | 6.6 | |
| Camponotus blandus pronotalis Santschi, 1936 | ASO | Ba, Pf | Ba, Hc, mW, Pf | mW | Ba | 2.1 | |
| Camponotus zonatus Emery, 1894 | ASO | Pf | Hc, Pf | mW, Pf | Pf | 12.5 | |
| Camponotus coruscus (Smith, 1862) | ASO | Pf | Ba, mW, Pf | 1.5 | |||
| Camponotus lindigi Mayr, 1870 | ASO | Ba, Hc, Pf | Ba, Hc, mW, Pf | Ba, Hc, mW, Pf | Cm, mW, Pf | 11.6 | |
| Camponotus sp. 5 | ASO | Pf | 0.1 | ||||
| Nylanderia nodifera (Mayr, 1870) | MLO | mW, Pf | Ba, mW, Pf | 0.7 | |||
| Paratrechina longicornis (Latreille, 1802) | MLO | Pf | Ba, Cm, Pf | Ba, Hc, Pf | 2.0 | ||
| Pseudomyrmecinae | Pseudomyrmex boopis (Roger, 1863) | LAP | mW, Pf | mW, Pf | Ba, Hc, mW, Pf | Ba, Pf | 3.8 |
| Pseudomyrmex elongatus (Mayr, 1870) | LAP | Hc, Pf | Hc | 0.8 | |||
| Pseudomyrmex gracilis (Fabricius, 1804) | LAP | Hc | 0.1 | ||||
| Pseudomyrmex simplex (Smith, 1877) | LAP | Hc, Pf | Hc, Pf | Hc | 1.0 | ||
| Pseudomyrmex urbanus (Smith, 1877) | LAP | Pf | Hc, mW, Pf | Hc | Hc | 0.8 | |
| Pseudomyrmex venustus (Smith, 1858) | LAP | Hc | Pf | Hc, Pf | Hc | 0.7 | |
| Dolichoderinae | Dolichoderus diversus Emery, 1894 | AO | mW | 0.1 | |||
| Dorymyrmex biconis Forel, 1912 | SO | mW | Hc, Pf | 0.9 | |||
| Forelius damiani Guerrero y Fernández, 2008 | SO | Ba, Pf | mW, Pf | 1.6 | |||
| Tapinoma melanocephalum (Fabricius, 1793) | ASO | Ba, Hc, Pf | Hc | 1.2 | |||
| Ectatomminae | Ectatomma aff. ruidum | LEP | Pf | 0.1 | |||
| Ectatomma ruidum (Roger, 1860) | LEP | Ba, Hc, mW, Pf | Ba, Hc, mW, Pf | Ba, Hc, mW, Pf | Ba, Hc, mW, Pf | 81.2 | |
| Ectatomma tuberculatum (Olivier, 1792) | LAP | Hc | 0.1 | ||||
| Dorylinae | Labidus coecus (Latreille, 1802) | AA | Pf | mW, Pf | mW, Pf | 2.0 | |
| Neivamyrmex iridescens Borgmeier, 1950 | AA | Pf | 0.2 |
References
- Dirzo, R.; Young, H.S.; Mooney, H.A.; Ceballos, G. (Eds.) Seasonally Dry Tropical Forests: Ecology and Conservation; Island Press: Washington, DC, USA, 2011; pp. xi–xiii. [Google Scholar]
- Pizano, C.; Cabrera, M.; García, H. Bosque seco tropical en Colombia; generalidades y contexto. In El Bosque Seco Tropical en Colombia; Pizano, C., García, H., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH): Bogotá, Colombia, 2014; pp. 36–47. [Google Scholar]
- García, H.; González-M, R. Bosque Seco Colombia: Biodiversidad y Gestión; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2019; p. 8. [Google Scholar]
- Andersen, A.N. Functional groups in ant community ecology. In Ant Ecology; Lach, L., Parr, C.L., Abbott, K.L., Eds.; Oxford University Press: New York, NY, USA, 2010; pp. 142–144. [Google Scholar]
- Neves, F.S.; Braga, R.F.; do Espírito-Santo, M.M.; Delabie, J.H.C.; Fernandea, D.W.; Sánchez-Azofeifa, G. Diversity of Arboreal Ants In a Brazilian Tropical Dry Forest: Effects Of Seasonality and Successional Stage. Sociobiology 2010, 56, 177–194. [Google Scholar]
- Marques, T.; Espírito-Santo, M.M.; Schoereder, J.H. Ant Assemblage Structure in a Secondary Tropical Dry Forest: The Role of Ecological Succession and Seasonality. Sociobiology 2017, 64, 261–275. [Google Scholar] [CrossRef][Green Version]
- Silva, L.; Souza, M.; Solar, R.R.C.; Neves, F.S. Ant diversity in Brazilian tropical dry forests across multiple vegetation domains. Environ. Res. Lett. 2017, 12, 035002. [Google Scholar] [CrossRef]
- Rocha-Ortega, M.; Castaño-Meneses, G. Effects of urbanization on the diversity of ant assemblages in tropical dry forests, Mexico. Urban Ecosyst. 2015, 18, 1373–1388. [Google Scholar] [CrossRef]
- Hernández-Flores, J.; Flores-Palacio, A.; Vásquez-Bolaños, M.; Toledo-Hernández, V.H.; Sotelo-Caro, O.; Ramos-Robles, M. Effect of forest disturbance on ant (Hymenoptera: Formicidae) diversity in a Mexican tropical dry forest canopy. Insect Conserv. Divers. 2021, 14, 393–402. [Google Scholar] [CrossRef]
- Armbrecht, I.; Chacón, P. Rareza y Diversidad de Hormigas en Fragmentos de Bosque Seco Colombianos y sus Matrices. Biotropica 1999, 31, 646–653. [Google Scholar] [CrossRef]
- Armbrecht, I.; Tischer, I.; Chacón, P. Nested subsets and partition patterns in ant assemblages (Hymenoptera, Formicidae) of Colombian dry forest fragments. Pan-Pac. Entomol. 2001, 77, 196–209. [Google Scholar]
- Chacón de Ulloa, P.; Osorio-García, A.M.; Achury, R.; Bermúdez-Rivas, C. Hormigas (Hymenoptera: Formicidae) del Bosque seco Tropical (Bs-T) de la cuenca alta del río Cauca, Colombia. Biota Colomb. 2012, 13, 165–181. [Google Scholar]
- Gallego-Ropero, M.C.; Salguero, B. Ensamblaje de hormigas del bosque secotropical, jardín botánico de Cali. Colomb. For. 2015, 18, 139–150. [Google Scholar] [CrossRef]
- Domínguez, Y.; Fontalvo, L.; Gutiérrez, L.C. Composición y distribución espacio-temporal de las hormigas cazadoras (Formicidae: Grupos Poneroide y Ectatomminoide) en tres fragmentos de bosque seco tropical del departamento del Atlántico, Colombia. In Sistemática, Biogeografía Y Conservación de las Hormigas Cazadoras de Colombia; Jiménez, E., Fernández, F., Arias, T., Lozano-Zambrano, F., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2008; pp. 497–511. [Google Scholar]
- Díaz, P.J.; Molano, P.C.; Gaviria, J.C. Diversidad genérica de hormigas (Hymenoptera: Formicidae) en ambientes de bosque seco de los Montes de María, Sucre, Colombia. Rev. Colomb. Cienc. Anim. 2009, 1, 279–285. [Google Scholar] [CrossRef]
- Simanca-Fontalvo, R.; Fajardo-Herrera, R.J.; Hernandez, N.J. Fauna de hormigas (Hymenoptera: Formicidae) en dos remanentes de bosque seco tropical (Bs-T) en corrales de San Luis, Atlántico, Colombia. Boletín Mus. Entomol. Univ. Val. 2013, 14, 1–15. [Google Scholar]
- Fontalvo-Rodríguez, L.; Domínguez-Haydar, Y. Ectatomma ruidum (Roger) como indicadora de diversidad de hormigas cazadoras (Hymenoptera: Formicidae) y relación con estructura vegetal en parches de bosque seco del Caribe colombiano. Intropica 2009, 4, 29–39. [Google Scholar]
- Domínguez-Haydar, Y.; Armbrecht, I. Response of Ants and Their Seed Removal in Rehabilitation Areas and Forests at El Cerrejón Coal Mine in Colombia. Restor. Ecol. 2011, 19, 178–184. [Google Scholar] [CrossRef]
- Guerrero, R.; Fernández, F. A new species of the ant genus Forelius (Formicidae: Dolichoderinae) from the dry forest of Colombia. Zootaxa 2008, 1958, 51–60. [Google Scholar] [CrossRef]
- Camargo-Vanegas, J.J.; Guerrero, R.J. Las hormigas Pheidole (Formicidae: Myrmicinae) en el bosque seco tropical de Santa Marta, Colombia. Rev. Colomb. Entomol. 2020, 46, e8433. [Google Scholar] [CrossRef]
- Kattan, G.H.; Sánchez, C.E.; Vélez, C.; Ramírez, L.; Celis, M. Beta diversity and knowledge gaps of Colombia’s dry forests: Implications for their conservation. Caldasia 2019, 41, 5–11. [Google Scholar] [CrossRef]
- Geografía Urbana. Plan de Ordenamiento Territorial Santa Marta Documento de Formulación. Alcaldía de Santa Marta. Available online: https://www.santamarta.gov.co/plan-de-ordenamiento-territorial (accessed on 4 February 2021).
- Rangel, J.O.; Carvajal-Cogollo, J.E. Clima de la región Caribe colombiana. In Diversidad Biótica XII: La Región Caribe de Colombia; Rangel, J.O., Ed.; Universidad Nacional de Colombia-Instituto de Ciencias Naturales: Bogotá, Colombia, 2012; pp. 67–129. [Google Scholar]
- Hernández-Camacho, J.; Sánchez, H. Biomas terrestres de Colombia. In La Diversidad Biológica de Iberoamérica; Halffter, G., Ed.; Volumen Especial, Acta Zoológica Mexicana (n.s.): Xalapa, México, 1992; pp. 153–173. [Google Scholar]
- Carbonó-Delahoz, E.; Barros-Barraza, A.; Jiménez-Vergara, J. Cactaceae de Santa Marta, Magdalena, Colombia. Rev. de la Acad. Colomb. de Cienc. Exactas Físicas y Nat. 2013, 37, 177–187. [Google Scholar] [CrossRef]
- Guerrero, R.; Delsinne, T.; Dekoninck, W. Métodos de recolección y curadurías. In Hormigas de Colombia; Fernández, F., Guerrero, R., Delsinne, T., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2019; pp. 319–369. [Google Scholar]
- Fernández, F.; Guerrero, R.; Delsinne, T. (Eds.) Hormigas de Colombia; Universidad Nacional de Colombia: Bogotá, Colombia, 2019; pp. 387–1113. [Google Scholar]
- Jost, L. Entropy and diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-based rarefaction and extrapolation: Standardizing samples by completeness rather than size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Cultid, C.; Escobar, F. Pautas para la estimación y comparación estadística de la diversidad biológica (qD). In La Biodiversidad en un Mundo Cambiante: Fundamentos Teóricos y Metodológicos Para su Estudio; Moreno, C., Ed.; Universidad Autónoma del Estado de Hidalgo: Pachuca de Soto, México, 2019; pp. 175–202. [Google Scholar]
- Pineda-López, R. Estimadores de la riqueza de especies. In La Biodiversidad en un Mundo Cambiante: Fundamentos Teóricos y Metodológicos Para su Estudio; Moreno, C., Ed.; Universidad Autónoma del Estado de Hidalgo: Pachuca de Soto, México, 2019; pp. 59–174. [Google Scholar]
- Cumming, G.; Fidler, F.; Vaux, D.L. Error bars in experimental biology. J. Cell Biol. 2007, 177, 7–11. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (H ill numbers). Methods Ecol. Evol 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Clarke, R.; Gorley, R.; Somerfield, P.J.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2014; pp. 6–11. [Google Scholar]
- Carvalho, J.C.; Cardoso, P.; Gomes, P. Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Glob. Ecol. Biogeogr. 2012, 21, 760–771. [Google Scholar] [CrossRef]
- Cardoso, P.; Rigal, R.; Carvalho, J.C. BAT—Biodiversity Assessment Tools, an R package for themeasurement and estimation of alpha and beta taxon, phylogenetic and functional diversity. Methods Ecol. Evol. 2015, 6, 232–236. [Google Scholar] [CrossRef]
- Brandão, R.F.B.; Silva, R.R.; Delabie, J.H.C. Neotropical Ants (Hymenoptera) Functional Groups: Nutritional and Applied Implications. In Insect Bioecology and Nutrition for Integrated Pest Management; Panizzi, J., Parra, J., Eds.; CRC Press Taylor and Francis Group: Boca Ratón, FL, USA, 2012; pp. 213–236. [Google Scholar]
- Branstetter, M.G.; Ješovnik, A.; Sosa-Calvo, J.; Lloyd, M.W.; Faircloth, B.C.; Brady, S.G.; Schultz, T.R. Dry habitats were crucibles of domestication in the evolution of agriculture in ants. Proc. Royal Soc. B 2017, 284, 20170095. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.B.A.; dos Santos, J.R.M.; Nascimento, I.C.; Delabie, J.H.C. Comparative evaluation of taxonomic and functional diversities of leaf-litter ants of the Brazilian Atlantic Forest. Turk. J. Zool. 2019, 43, 437–546. [Google Scholar] [CrossRef]
- Fernández, F.; Guerrero, R.; Delsinne, T. Filogenia y sistemática de las hormigas neotropicales. In Hormigas de Colombia; Fernández, F., Guerrero, R., Delsinne, T., Eds.; Universidad Nacional de Colombia: Bogotá, Colombia, 2019; pp. 57–89. [Google Scholar]
- García, E.I.; Tocora, M.C.; Fiorentino, G.; Escárraga, M.E.; Fernández, F.; Guerrero, R.J. New records of ants (Hymenoptera: Formicidae) for Colombia. Biota Neotrop. 2020, 20, e20201088. [Google Scholar] [CrossRef]
- Dix, O.; Martínez, J.; Fernández, C. Contribucion al conocimiento de la mirmecofauna en el municipio de San Antero, cordoba, Colombia. Rev. Colomb. Entomol. 2005, 31, 97–103. [Google Scholar] [CrossRef]
- Fontalvo-Rodríguez, L.; Solís-Medina, C. Ensamblaje de hormigas (Hymenoptera formicidae) en fragmentos de bosque seco en el complejo carbonífero el Cerrejón (La Guajira, Colombia). Intropica 2009, 4, 5–15. [Google Scholar]
- Ward, P.S.; Brady, S.G.; Fisher, B.L.; Schultz, T.R. The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae): Phylogeny and evolution of myrmicine ants. Syst. Entomol. 2015, 40, 61–81. [Google Scholar] [CrossRef]
- Ramírez, D. Diversidad de Hormigas en las Formaciones Vegetales Subxerofiticas de Santa Marta, Colombia. Tesis de Pregrado, Universidad del Magdalena, Santa Marta, Colombia, 2007. [Google Scholar]
- Arcila-Cardona, A.; Osorio, A.M.; Bermúdez, C.; Chacón de Ulloa, P. Diversidad de hormigas cazadoras asociadas a los elementos del paisaje del bosque seco. In Sistemática, Biogeografía y Conservación de las Hormigas Cazadoras de Colombia; Jiménez, E., Fernández, F., Arias, T., Lozano-Zambrano, F., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogotá, Colombia, 2008; pp. 531–552. [Google Scholar]
- Santamaría, C.; Domínguez Haydar, Y.; Armbrecht, I. Cambios en la distribución de nidos y abundancia de la hormiga Ectatomma ruidum (Roger 1861) en dos zonas de Colombia. Boletín Mus. De Entomol. Univ. Val. 2009, 10, 10–18. [Google Scholar]
- Torres, J.A. Diversity and distribution of ant Communities in Puerto Rico. Biotropica 1984, 16, 296–303. [Google Scholar] [CrossRef]


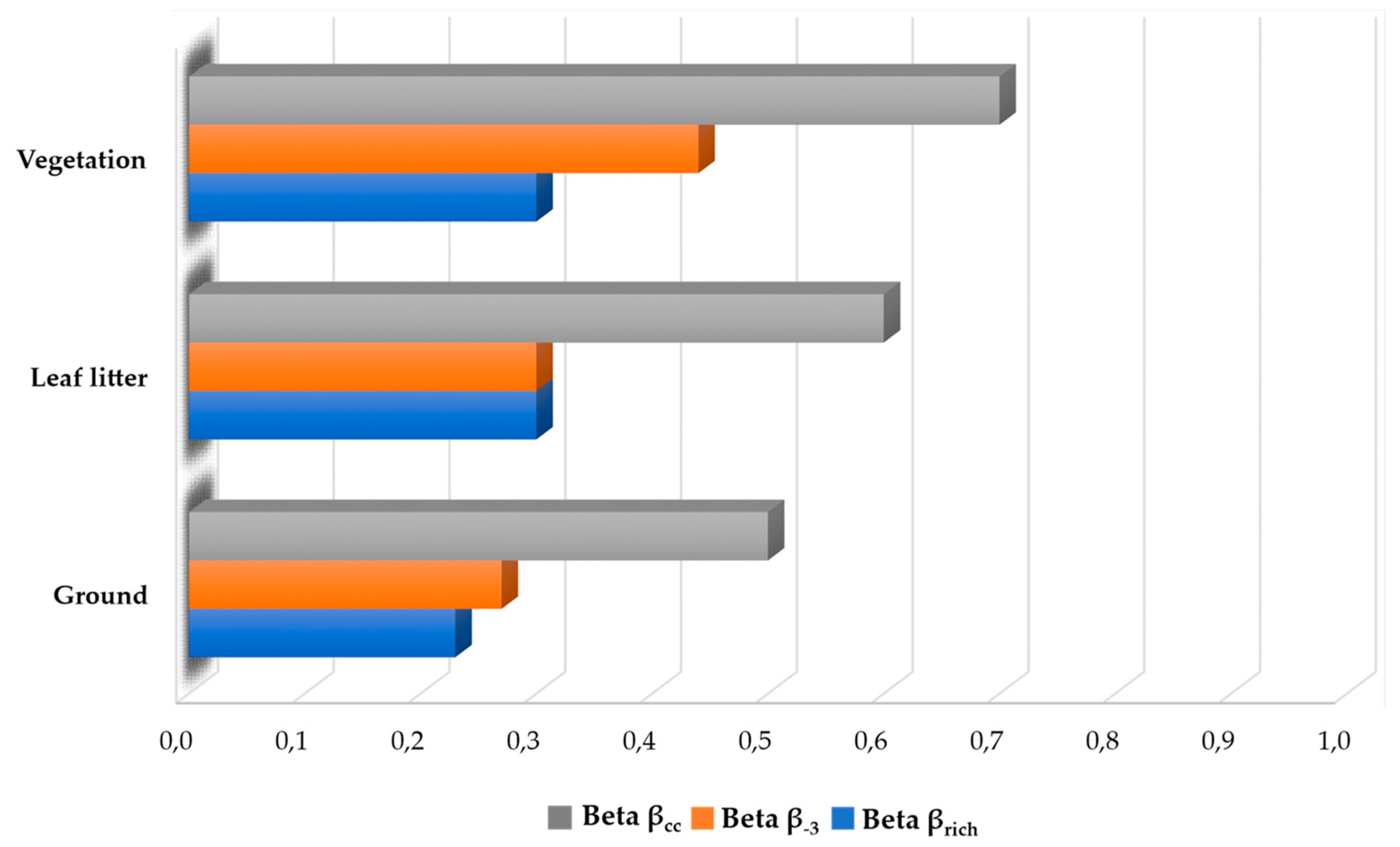

| Beta Diversity | N01 | N02 | N03 | N04 |
|---|---|---|---|---|
| Ground (pitfall) | ||||
| N01 | 50 | 0.10 | 0.08 | 0.44 |
| N02 | 0.36 | 44 | 0.02 | 0.36 |
| N03 | 0.36 | 0.45 | 45 | 0.40 |
| N04 | 0.15 | 0.24 | 0.09 | 26 |
| Leaf litter (Winkler) | ||||
| N01 | 38 | 0.06 | 0.06 | 0.52 |
| N02 | 0.48 | 35 | 0.12 | 0.51 |
| N03 | 0.45 | 0.36 | 41 | 0.39 |
| N04 | 0.27 | 0.21 | 0.14 | 15 |
| Vegetation (hand collection) | ||||
| N01 | 26 | 0.17 | 0.08 | 0.50 |
| N02 | 0.51 | 20 | 0.09 | 0.32 |
| N03 | 0.63 | 0.65 | 23 | 0.30 |
| N04 | 0.14 | 0.40 | 0.30 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Ortega, L.M.; Guerrero, R.J. Spatial Turnover and Functional Redundancy in the Ants of Urban Fragments of Tropical Dry Forest. Diversity 2023, 15, 880. https://doi.org/10.3390/d15070880
Ramos Ortega LM, Guerrero RJ. Spatial Turnover and Functional Redundancy in the Ants of Urban Fragments of Tropical Dry Forest. Diversity. 2023; 15(7):880. https://doi.org/10.3390/d15070880
Chicago/Turabian StyleRamos Ortega, Lina María, and Roberto J. Guerrero. 2023. "Spatial Turnover and Functional Redundancy in the Ants of Urban Fragments of Tropical Dry Forest" Diversity 15, no. 7: 880. https://doi.org/10.3390/d15070880
APA StyleRamos Ortega, L. M., & Guerrero, R. J. (2023). Spatial Turnover and Functional Redundancy in the Ants of Urban Fragments of Tropical Dry Forest. Diversity, 15(7), 880. https://doi.org/10.3390/d15070880








