Presence of Endangered Red-Crowned Parrots (Amazona viridigenalis) Depends on Urban Landscapes
Abstract
1. Introduction
- (1)
- What is the current geographical range of Red-crowned Parrots in the USA, and what is the potential for range expansion?
- (2)
- Does a combined model that includes all populations predict presence similarly to individual population models?
- (3)
- What are the important climatic and environmental (anthropogenic) drivers for Red-crowned Parrot presence in the USA?
- (4)
- Are all populations of Red-crowned Parrots in the contiguous USA responding to climate and environmental (anthropogenic) variables similarly?
2. Methods
2.1. Occurrence Data
2.2. Environmental Data
2.2.1. Climatic Variables
2.2.2. Anthropogenic Variables
2.3. Species Distribution Modeling
2.3.1. Model Settings
2.3.2. Model Evaluation
2.3.3. Distribution
3. Results
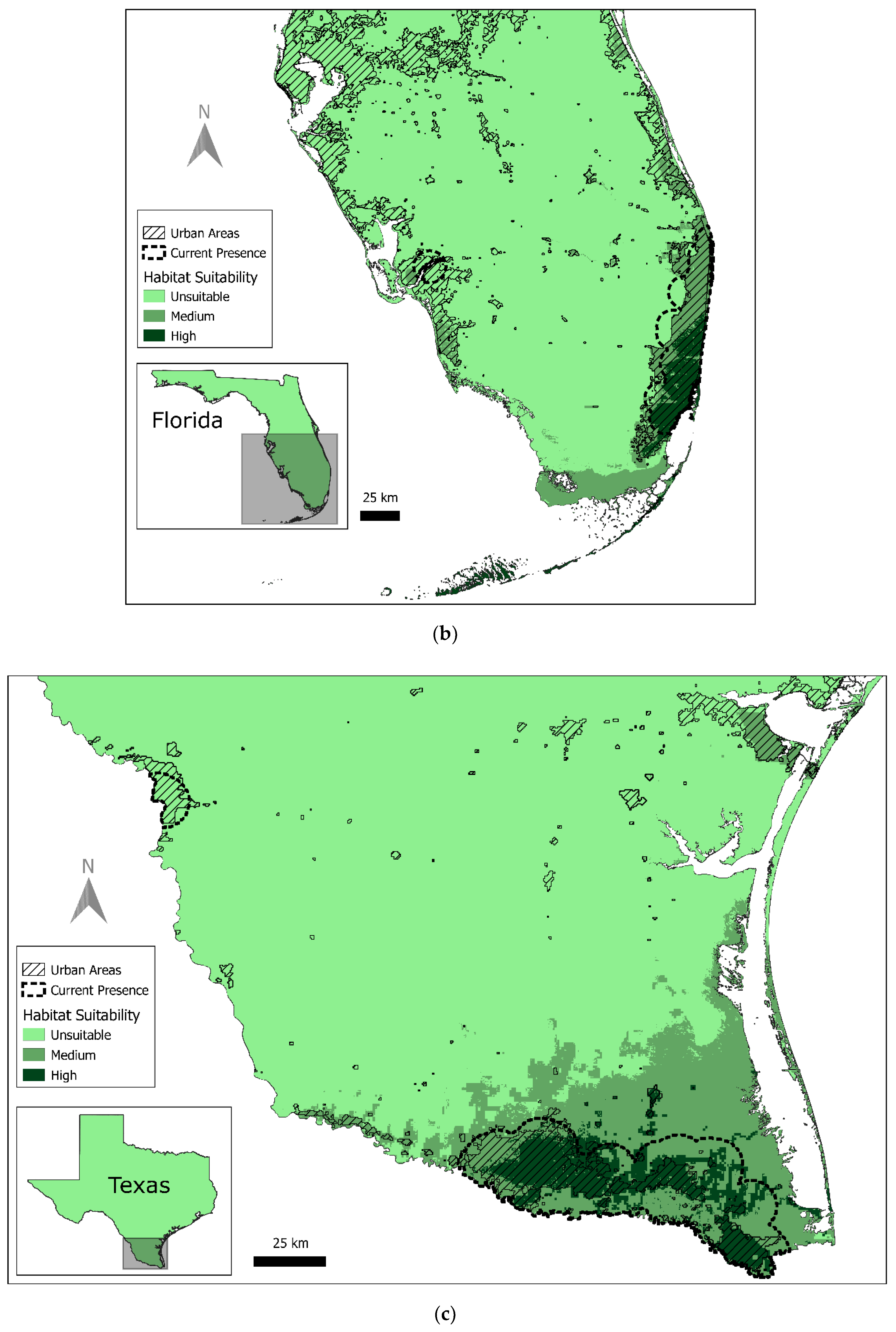
3.1. Current Red-Crowned Parrot Distribution
3.2. Predicted Habitat Distribution
3.3. Variable Importance
Response Curves
4. Discussion
4.1. Current Geographical Range and Potential for Expansion
4.2. Variation among Models
4.3. Important Climatic and Environmental Drivers of Presence
4.4. Temperature
4.5. Precipitation
4.6. Urbanization and Population Density
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ceballos, G.; Ehrlich, P.R.; Barnosky, A.D.; García, A.; Pringle, R.M.; Palmer, T.M. Accelerated modern human–induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef]
- United Nations; Department of Economic and Social Affairs; Population Division. World Urbanization Prospects: The 2018 Revision (ST/ESA/SER.A/420); United Nations: Geneva, Switzerland, 2019. [Google Scholar]
- Schmidt, C.; Domaratzki, M.; Kinnunen, R.; Bowman, J.; Garroway, C.J. Continent-wide effects of urbanization on bird and mammal genetic diversity. Proc. R. Soc. B 2020, 287, 20192497. [Google Scholar] [CrossRef] [PubMed]
- Kowarik, I. Novel urban ecosystems, biodiversity, and conservation. Environ. Pollut. 2011, 159, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Thompson, K.; McCarthy, M.A. Traits of British alien and native urban plants. J. Ecol. 2008, 96, 853–859. [Google Scholar] [CrossRef]
- Uehling, J.J.; Tallant, J.; Pruett-Jones, S. Introduced and naturalized parrots in the United States. In Naturalized Parrots of the World: Distribution, Ecology, and Impacts of the World’s Most Colorful Colonizers; Pruett-Jones, S., Ed.; Princeton University Press: Princeton, NJ, USA, 2021. [Google Scholar]
- Veech, J.A.; Small, M.F.; Baccus, J.T. The effect of habitat on the range expansion of a native and an introduced bird species. J. Biogeogr. 2011, 38, 69–77. [Google Scholar] [CrossRef]
- Brightsmith, D.J.; Kiacz, S. Are Parrots Priority Invasive Species? In Naturalized Parrots of the World: Distribution, Ecology, and Impacts of the World’s Most Colorful Colonizers; Pruett-Jones, S., Ed.; Princeton University Press: Princeton, NJ, USA, 2021. [Google Scholar]
- Kiacz, S.; Brightsmith, D.J. Naturalized Parrots: Conservation and Research Opportunities. In Naturalized Parrots of the World: Distribution, Ecology, and Impacts of the World’s Most Colorful Colonizers; Pruett-Jones, S., Ed.; Princeton University Press: Princeton, NJ, USA, 2021. [Google Scholar]
- Shaffer, H.B. Urban biodiversity arks. Nat. Sustain. 2018, 1, 725–727. [Google Scholar] [CrossRef]
- Enkerlin-Hoeflich, E.C.; Hogan, K.M. Red-crowned Parrot (Amazona viridigenalis), version 1.0. Birds World 2020. [Google Scholar] [CrossRef]
- Kiacz, S.; Shackelford, C.E.; Henehan, A.K.; Brightsmith, D.J. History, status, and productivity of the Red-crowned Amazon Amazona viridigenalis in the Lower Rio Grande Valley of Texas. Bird Conserv. Internatn. 2020, 31, 519–533. [Google Scholar] [CrossRef]
- Enkerlin-Hoeflich, E.C. Las Aves de México en Peligro de Extinción; 970900011X; UNAM, Instituto de Ecologia Comision Nacional para el Conocimiento y Uso de la Biodiversidad Fondo de Cultura Economica: Tlalpan, Mexico, 2000; pp. 222–230. [Google Scholar]
- Uehling, J.J.; Tallant, J.; Pruett-Jones, S. Status of naturalized parrots in the United States. J. Ornithol. 2019, 160, 1–15. [Google Scholar] [CrossRef]
- Garrett, K. Twenty-five years of persistence and change in the naturalized parrots of Southern California. In Proceedings of the North American Ornithological Conference VII, Virtual, 15 August 2020. [Google Scholar]
- Garcia-Gonzalez, C.; Garcia-Vazquez, E. Urban ponds, neglected Noah’s ark for amphibians. J. Herpetol. 2012, 46, 507–514. [Google Scholar] [CrossRef]
- Jokimäki, J.; Suhonen, J.; Kaisanlahti-Jokimäki, M.-L. Urban core areas are important for species conservation: A European-level analysis of breeding bird species. Landsc. Urban Plann. 2018, 178, 73–81. [Google Scholar] [CrossRef]
- Faeth, S.H.; Bang, C.; Saari, S. Urban biodiversity: Patterns and mechanisms. Ann. N. Y. Acad. Sci. 2011, 1223, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Beninde, J.; Veith, M.; Hochkirch, A. Biodiversity in cities needs space: A meta-analysis of factors determining intra-urban biodiversity variation. Ecol. Lett. 2015, 18, 581–592. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Pacifici, M.; Foden, W.B.; Visconti, P.; Watson, J.E.; Butchart, S.H.; Kovacs, K.M.; Scheffers, B.R.; Hole, D.G.; Martin, T.G.; Akçakaya, H.R. Assessing species vulnerability to climate change. Nat. Clim. Change 2015, 5, 215–224. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species; Version 2020–2; IUCN: Cambridge, UK, 2020. [Google Scholar]
- Austin, M.P.; Van Niel, K.P. Improving species distribution models for climate change studies: Variable selection and scale. J. Biogeogr. 2011, 38, 1–8. [Google Scholar] [CrossRef]
- Pearson, R.G. Species’ distribution modeling for conservation educators and practitioners. Synth. Am. Mus. Nat. Hist. 2007, 50, 54–89. [Google Scholar]
- Wang, H.-H.; Wonkka, C.L.; Treglia, M.L.; Grant, W.E.; Smeins, F.E.; Rogers, W.E. Species distribution modelling for conservation of an endangered endemic orchid. AoB Plants 2015, 7, plv039. [Google Scholar] [CrossRef]
- Randklev, C.R.; Wang, H.-H.; Groce, J.E.; Grant, W.E.; Robertson, S.; Wilkins, N. Land use relationships for a rare freshwater mussel species endemic to central Texas. J. Fish Wildl. Manag. 2015, 6, 327–337. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Sohl, T.L. The relative impacts of climate and land-use change on conterminous United States bird species from 2001 to 2075. PLoS ONE 2014, 9, e112251. [Google Scholar] [CrossRef]
- Wang, H.-H.; Wonkka, C.L.; Treglia, M.L.; Grant, W.E.; Smeins, F.E.; Rogers, W.E. Incorporating local-scale variables into distribution models enhances predictability for rare plant species with biological dependencies. Biodivers. Conserv. 2019, 28, 171–182. [Google Scholar] [CrossRef]
- Illán, J.G.; Gutiérrez, D.; Wilson, R.J. The contributions of topoclimate and land cover to species distributions and abundance: Fine-resolution tests for a mountain butterfly fauna. Glob. Ecol. Biogeogr. 2010, 19, 159–173. [Google Scholar] [CrossRef]
- Martin, Y.; Van Dyck, H.; Dendoncker, N.; Titeux, N. Testing instead of assuming the importance of land use change scenarios to model species distributions under climate change. Glob. Ecol. Biogeogr. 2013, 22, 1204–1216. [Google Scholar] [CrossRef]
- Mainali, K.; Hefley, T.; Ries, L.; Fagan, W. Matching expert range maps with species distribution model predictions. Conserv. Biol. 2020, 34, 1292–1304. [Google Scholar] [CrossRef]
- Sullivan, B.L.; Wood, C.L.; Iliff, M.J.; Bonney, R.E.; Fink, D.; Kelling, S. eBird: A citizen-based bird observation network in the biological sciences. Biol. Conserv. 2009, 142, 2282–2292. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Zuckerberg, B.; Bonter, D.N. Citizen science as an ecological research tool: Challenges and benefits. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 149–172. [Google Scholar] [CrossRef]
- Abellán, P.; Tella, J.L.; Carrete, M.; Cardador, L.; Anadón, J.D. Climate matching drives spread rate but not establishment success in recent unintentional bird introductions. Proc. Natl. Acad. Sci. USA 2017, 114, 9385–9390. [Google Scholar] [CrossRef]
- Hayes, K.R.; Barry, S.C. Are there any consistent predictors of invasion success? Biol. Invasions 2008, 10, 483–506. [Google Scholar] [CrossRef]
- Blair, R.B.; Launer, A.E. Butterfly diversity and human land use: Species assemblages along an urban grandient. Biol. Conserv. 1997, 80, 113–125. [Google Scholar] [CrossRef]
- Lemoine, N.; Bauer, H.G.; Peintinger, M.; Böhning-Gaese, K. Effects of climate and land-use change on species abundance in a central European bird community. Conserv. Biol. 2007, 21, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Monterrubio-Rico, T.C.; Renton, K.; Ortega-Rodríguez, J.M.; Pérez-Arteaga, A.; Cancino-Murillo, R. The Endangered yellow-headed parrot Amazona oratrix along the Pacific coast of Mexico. Oryx 2010, 44, 602–609. [Google Scholar] [CrossRef]
- Molloy, S.W.; Burbidge, A.H.; Comer, S.; Davis, R.A. Using climate change models to inform the recovery of the western ground parrot Pezoporus flaviventris. Oryx 2020, 54, 52–61. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- CIESIN. Gridded Population of the World, Version 4 (GPWv4): Population Density, Revision 11; CIESIN: Palisades, NY, USA, 2018. [Google Scholar]
- Homer, C.; Dewitz, J.; Jin, S.; Xian, G.; Costello, C.; Danielson, P.; Gass, L.; Funk, M.; Wickham, J.; Stehman, S. Conterminous United States land cover change patterns 2001–2016 from the 2016 National Land Cover Database. ISPRS J. Photogramm. Remote Sens. 2020, 162, 184–199. [Google Scholar] [CrossRef]
- U.S. Geological Survey Gap Analysis Program. GAP/LANDFIRE National Terrestrial Ecosystems; U.S. Geological Survey: Reston, VA, USA, 2016. [Google Scholar] [CrossRef]
- Comer, P.; Faber-Langendoen, D.; Evans, R.; Gawler, S.; Josse, C.; Kittel, G.; Menard, S.; Pyne, M.; Reid, M.; Schulz, K. Ecological Systems of the United States: A Working Classification of US Terrestrial Systems; NatureServe: Arlington, VA, USA, 2003; p. 75. [Google Scholar]
- Homer, C.; Dewitz, J.; Yang, L.; Jin, S.; Danielson, P.; Xian, G.; Coulston, J.; Herold, N.; Wickham, J.; Megown, K. Completion of the 2011 National Land Cover Database for the conterminous United States–representing a decade of land cover change information. Photogramm. Eng. Remote Sens. 2015, 81, 345–354. [Google Scholar]
- Coulston, J.W.; Moisen, G.G.; Wilson, B.T.; Finco, M.V.; Cohen, W.B.; Brewer, C.K. Modeling percent tree canopy cover: A pilot study. Photogramm. Eng. Remote Sens. 2012, 78, 715–727. [Google Scholar] [CrossRef]
- Strubbe, D.; Matthysen, E. Establishment success of invasive ring-necked and monk parakeets in Europe. J. Biogeogr. 2009, 36, 2264–2278. [Google Scholar] [CrossRef]
- Davis, A.Y.; Malas, N.; Minor, E.S. Substitutable habitats? The biophysical and anthropogenic drivers of an exotic bird’s distribution. Biol. Invasions 2014, 16, 415–427. [Google Scholar] [CrossRef]
- Liu, X.; de Sherbinin, A.; Zhan, Y. Mapping Urban Extent at Large Spatial Scales Using Machine Learning Methods with VIIRS Nighttime Light and MODIS Daytime NDVI. Remote Sens. 2019, 11, 1274. [Google Scholar] [CrossRef]
- CIESIN. 2015 Urban Extents from VIIRS and MODIS for the Continental U.S. Using Machine Learning Methods; CIESIN: Palisades, NY, USA, 2019. [Google Scholar]
- Baldwin, R.A. Use of maximum entropy modeling in wildlife research. Entropy 2009, 11, 854–866. [Google Scholar] [CrossRef]
- Elith, J.; Kearney, M.; Phillips, S. The art of modelling range-shifting species. Methods Ecol. Evol. 2010, 1, 330–342. [Google Scholar] [CrossRef]
- West, A.M.; Kumar, S.; Brown, C.S.; Stohlgren, T.J.; Bromberg, J. Field validation of an invasive species Maxent model. Ecol. Inform. 2016, 36, 126–134. [Google Scholar] [CrossRef]
- Smith, A.B.; Godsoe, W.; Rodríguez-Sánchez, F.; Wang, H.-H.; Warren, D. Niche estimation above and below the species level. Trends Ecol. Evol. 2019, 34, 260–273. [Google Scholar] [CrossRef]
- Koralewski, T.E.; Wang, H.-H.; Grant, W.E.; Byram, T.D. Plants on the move: Assisted migration of forest trees in the face of climate change. For. Ecol. Manag. 2015, 344, 30–37. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.; Li, J.; Peterson, A.T.; Graham, C.; Guisan, A.; NCEAS Predicting Species Distributions Working Group. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 398. [Google Scholar]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Phillips, S.J. A brief tutorial on Maxent. ATT Res. 2005, 190, 231–259. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- O’Donnell, M.S.; Ignizio, D.A. Bioclimatic Predictors for Supporting Ecological Applications in the Conterminous United States; U.S. Geological Survey: Reston, VA, USA, 2012; p. 691. [Google Scholar]
- Feng, X.; Park, D.S.; Liang, Y.; Pandey, R.; Papeş, M. Collinearity in ecological niche modeling: Confusions and challenges. Ecol. Evol. 2019, 9, 10365–10376. [Google Scholar] [CrossRef] [PubMed]
- Monterrubio-Rico, T.C.; Charre-Medellín, J.F.; Pacheco-Figueroa, C.; Arriaga-Weiss, S.; de Dios Valdez-Leal, J.; Cancino-Murillo, R.; Escalona-Segura, G.; Bonilla-Ruz, C.; Rubio-Rocha, Y. Distribución potencial histórica y contemporánea de la familia Psittacidae en México. Rev. Mex. Biodivers. 2016, 87, 1103–1117. [Google Scholar] [CrossRef]
- Kamp, J.; Sheldon, R.D.; Koshkin, M.A.; Donald, P.F.; Biedermann, R. Post-Soviet steppe management causes pronounced synanthropy in the globally threatened Sociable Lapwing Vanellus gregarius. Ibis 2009, 151, 452–463. [Google Scholar] [CrossRef]
- Winter, R.; Mantilla-Contreras, J.; Schmidt, S. Usage of buildings in the life cycle of two endangered Rhinolophus species in the Mediterranean region: Implications for roost protection. Eur. J. Wildl. Res. 2020, 66, 38. [Google Scholar] [CrossRef]
- Renton, K.; Salinas-Melgoza, A.; Rueda-Hernández, R.; Vázquez-Reyes, L.D. Differential resilience to extreme climate events of tree phenology and cavity resources in tropical dry forest: Cascading effects on a threatened species. For. Ecol. Manag. 2018, 426, 164–175. [Google Scholar] [CrossRef]
- White, T.H.; Abreu, W.; Benitez, G.; Jhonson, A.; Lopez, M.; Ramirez, L.; Rodriguez, I.; Toledo, M.; Torres, P.; Velez, J. Minimizing potential allee effects in psittacine reintroductions: An example from Puerto Rico. Diversity 2021, 13, 13. [Google Scholar] [CrossRef]
- Kowarik, I. Time lags in biological invasions with regard to the success and failure of alien species. In Plant Invasions—General Aspects and Special Problems; SPB Academic Publishing: Amsterdam, The Netherlands, 1995; pp. 15–38. [Google Scholar]
- Hitch, A.T.; Leberg, P.L. Breeding distributions of North American bird species moving north as a result of climate change. Conserv. Biol. 2007, 21, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Sol, D.; Duncan, R.P.; Blackburn, T.M.; Cassey, P.; Lefebvre, L. Big brains, enhanced cognition, and response of birds to novel environments. Proc. Natl. Acad. Sci. USA 2005, 102, 5460–5465. [Google Scholar] [CrossRef]
- Colautti, R.I.; Grigorovich, I.A.; MacIsaac, H.J. Propagule pressure: A null model for biological invasions. Biol. Invasions 2006, 8, 1023–1037. [Google Scholar] [CrossRef]
- Habibzadeh, N.; Storch, I.; Ludwig, T. Differential habitat associations in peripheral populations of threatened species: The case of the Caucasian grouse. Ecol. Res. 2019, 34, 309–319. [Google Scholar] [CrossRef]
- Peterson, M.L.; Doak, D.F.; Morris, W.F. Incorporating local adaptation into forecasts of species’ distribution and abundance under climate change. Glob. Chang. Biol. 2019, 25, 775–793. [Google Scholar]
- Olson, L.E.; Bjornlie, N.; Hanvey, G.; Holbrook, J.D.; Ivan, J.S.; Jackson, S.; Kertson, B.; King, T.; Lucid, M.; Murray, D. Improved prediction of Canada lynx distribution through regional model transferability and data efficiency. Ecol. Evol. 2021, 11, 1667–1690. [Google Scholar] [CrossRef] [PubMed]
- Boria, R.A.; Blois, J.L. The effect of large sample sizes on ecological niche models: Analysis using a North American rodent, Peromyscus maniculatus. Ecol. Model. 2018, 386, 83–88. [Google Scholar] [CrossRef]
- Ancillotto, L.; Strubbe, D.; Menchetti, M.; Mori, E. An overlooked invader? Ecological niche, invasion success and range dynamics of the Alexandrine parakeet in the invaded range. Biol. Invasions 2016, 18, 583–595. [Google Scholar] [CrossRef]
- Mori, E.; Cardador, L.; Reino, L.; White, R.L.; Hernández-Brito, D.; Le Louarn, M.; Mentil, L.; Edelaar, P.; Pârâu, L.G.; Nikolov, B.P. Lovebirds in the air: Trade patterns, establishment success and niche shifts of Agapornis parrots within their non-native range. Biol. Invasions 2020, 22, 421–435. [Google Scholar] [CrossRef]
- Pereira, P.F.; Barbosa, A.M.; Godinho, C.; Salgueiro, P.A.; Silva, R.R.; Lourenço, R. The spread of the red-billed leiothrix (Leiothrix lutea) in Europe: The conquest by an overlooked invader? Biol. Invasions 2020, 22, 709–722. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Barnett, D.; Flather, C.; Fuller, P.; Peterjohn, B.; Kartesz, J.; Master, L.L. Species richness and patterns of invasion in plants, birds, and fishes in the United States. Biol. Invasions 2006, 8, 427–447. [Google Scholar] [CrossRef]
- Strubbe, D.; Jackson, H.; Groombridge, J.; Matthysen, E. Invasion success of a global avian invader is explained by within-taxon niche structure and association with humans in the native range. Divers. Distrib. 2015, 21, 675–685. [Google Scholar] [CrossRef]
- Pigot, A.L.; Owens, I.P.; Orme, C.D.L. The environmental limits to geographic range expansion in birds. Ecol. Lett. 2010, 13, 705–715. [Google Scholar] [CrossRef]
- Socolar, J.B.; Epanchin, P.N.; Beissinger, S.R.; Tingley, M.W. Phenological shifts conserve thermal niches in North American birds and reshape expectations for climate-driven range shifts. Proc. Natl. Acad. Sci. USA 2017, 114, 12976–12981. [Google Scholar] [CrossRef] [PubMed]
- Bellard, C.; Thuiller, W.; Leroy, B.; Genovesi, P.; Bakkenes, M.; Courchamp, F. Will climate change promote future invasions? Glob. Change Biol. 2013, 19, 3740–3748. [Google Scholar] [CrossRef]
- Hogue, T.S.; Pincetl, S. Are you watering your lawn? Science 2015, 348, 1319–1320. [Google Scholar] [CrossRef]
- Mini, C.; Hogue, T.; Pincetl, S. Estimation of residential outdoor water use in Los Angeles, California. Landsc. Urban Plann. 2014, 127, 124–135. [Google Scholar] [CrossRef]
- Mayer, P.W.; Martien, L.; Hayden, M.; Funk, A.; Kramer-Duffield, M.; Davis, R.; Henderson, J.; Raucher, B.; Gleick, P.; Heberger, M. California Single Family Water Use Efficiency Study; Aquacraft, Inc. Water Engineering and Management: Boulder, CO, USA, 2011. [Google Scholar]
- Mini, C.; Hogue, T.S.; Pincetl, S. Patterns and controlling factors of residential water use in Los Angeles, California. Water Policy 2014, 16, 1054–1069. [Google Scholar] [CrossRef]
- Meseck, K.A. Habitat Distribution for Non-Native Amazona viridigenalis within San Diego County Using Maxent Predictive Model. Master’s Thesis, San Diego State University, San Diego, CA, USA, 2013. [Google Scholar]
- Neck, R.W. Expansion of Red-crowned Parrot, Amazona viridigenalis, into southern Texas and changes in agricultural practices in northern Mexico. Bull. Tex. Ornithol. Soc. 1986, 19, 6–12. [Google Scholar]
- Garrett, K.L. Introducing change: A current look at naturalized bird species in western North America. In Trends and Traditions: Avifaunal Change in Western North America. Studies of Western Birds 3; Shuford, W.D., Gill, R.E., Jr., Handel, C.M., Eds.; Western Field Ornithologists: Camarillo, CA, USA, 2018; pp. 116–130. [Google Scholar]
- Duncan, R.P.; Blackburn, T.M.; Sol, D. The Ecology of Bird Introductions. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 71–98. [Google Scholar] [CrossRef]
- Vall-Llosera, M.; Cassey, P. Leaky doors: Private captivity as a prominent source of bird introductions in Australia. PloS ONE 2017, 12, e0172851. [Google Scholar] [CrossRef]
- Olah, G.; Butchart, S.H.M.; Symes, A.; Guzmán, I.M.; Cunningham, R.; Brightsmith, D.J.; Heinsohn, R. Ecological and socio-economic factors affecting extinction risk in parrots. Biodivers. Conserv. 2016, 25, 205–223. [Google Scholar] [CrossRef]
- Meager, J.J.; Schlacher, T.A.; Nielsen, T. Humans alter habitat selection of birds on ocean-exposed sandy beaches. Divers. Distrib. 2012, 18, 294–306. [Google Scholar] [CrossRef]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Why garden for wildlife? Social and ecological drivers, motivations and barriers for biodiversity management in residential landscapes. Ecol. Econ. 2013, 86, 258–273. [Google Scholar] [CrossRef]
- Wood, E.M.; Esaian, S. The importance of street trees to urban avifauna. Ecol. Appl. 2020, 30, e02149. [Google Scholar] [CrossRef] [PubMed]
- Kuruneri-Chitepo, C.; Shackleton, C.M. The distribution, abundance and composition of street trees in selected towns of the Eastern Cape, South Africa. Urban For. Urban Green. 2011, 10, 247–254. [Google Scholar] [CrossRef]
- Chalker-Scott, L. Nonnative, noninvasive woody species can enhance urban landscape biodiversity. Arboric. Urban For. 2015, 41, 173–185. [Google Scholar] [CrossRef]
- Pena, J.C.d.C.; Martello, F.; Ribeiro, M.C.; Armitage, R.A.; Young, R.J.; Rodrigues, M. Street trees reduce the negative effects of urbanization on birds. PLoS ONE 2017, 12, e0174484. [Google Scholar] [CrossRef] [PubMed]
- Schlaepfer, M.A.; Sax, D.F.; Olden, J.D. The potential conservation value of non-native species. Conserv. Biol. 2011, 25, 428–437. [Google Scholar] [CrossRef]
- Runde, D.; Pitt, W.; Foster, J. Population ecology and some potential impacts of emerging populations of exotic parrots. In Proceedings of the Managing Vertebrate Invasive Species 2007, Fort Collins, CO, USA, 7–9 August 2007; pp. 338–360. [Google Scholar]
- Nosil, P.; Egan, S.P.; Funk, D.J. Heterogeneous genomic differentiation between walking-stick ecotypes:“isolation by adaptation” and multiple roles for divergent selection. Evol. Int. J. Org. Evol. 2008, 62, 316–336. [Google Scholar] [CrossRef]
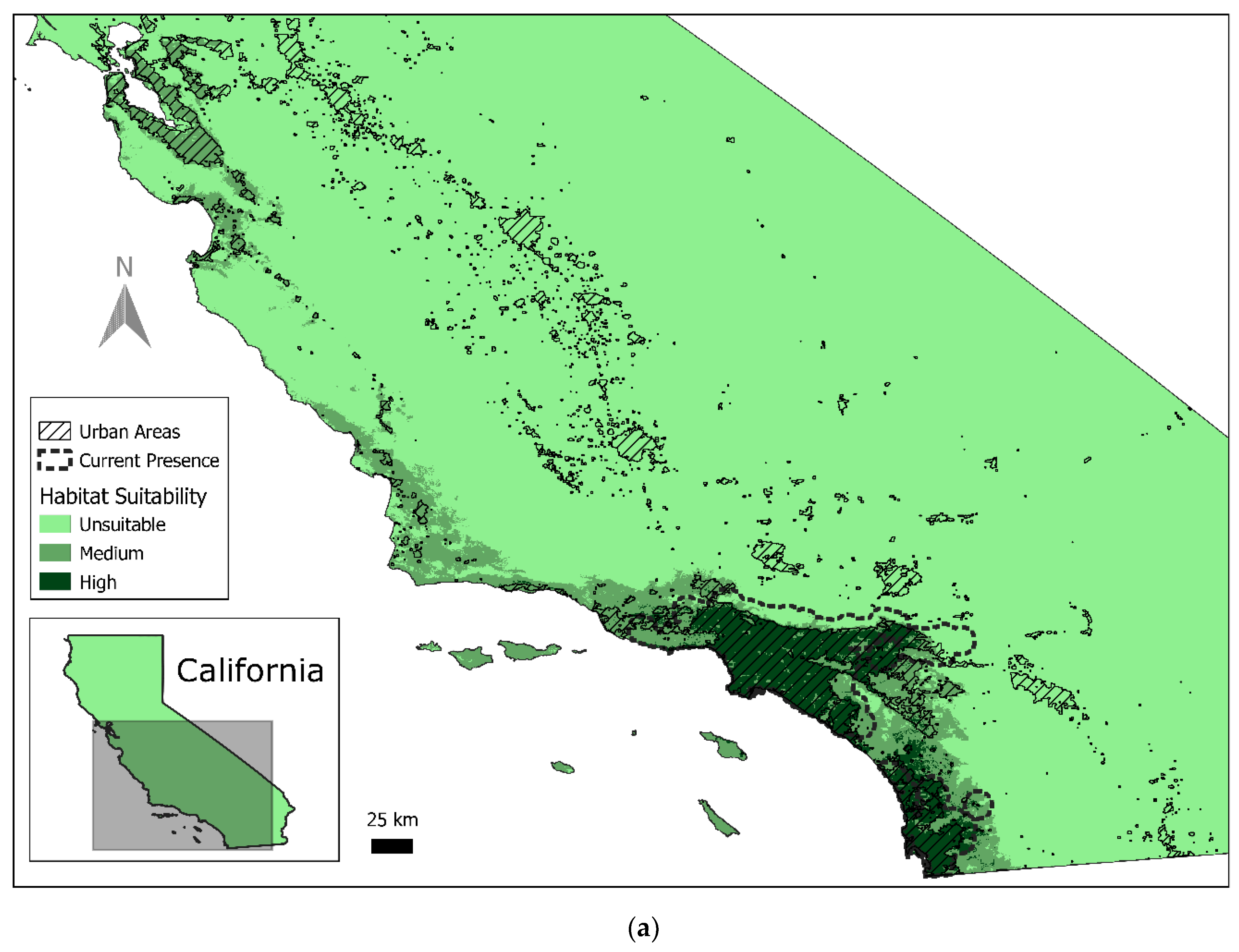


| Predicted High Quality (km2) | % of State | Predicted Medium Quality (km2) | % of State | Predicted Unsuitable (km2) | % of State | |
|---|---|---|---|---|---|---|
| California | 8250 | 2% | 17,489 | 4.30% | 383,661 | 94% |
| Texas | 1523 | 0.20% | 5623 | 0.80% | 677,208 | 99% |
| Florida | 1690 | 1.20% | 4854 | 3.30% | 139,117 | 96% |
| Total | 11,463 | 27,966 | 1,199,986 |
| Predicted High Quality (km2) | % of State | Predicted Medium Quality (km2) | % of State | Predicted Unsuitable (km2) | % of State | |
|---|---|---|---|---|---|---|
| California | 7999 | 2% | 14,577 | 3.60% | 386,824 | 94% |
| Texas | 1486 | 0.20% | 4546 | 0.70% | 678,322 | 99% |
| Florida | 1986 | 1.40% | 1722 | 1.20% | 141,953 | 97% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiacz, S.; Wang, H.-H.; Brightsmith, D.J. Presence of Endangered Red-Crowned Parrots (Amazona viridigenalis) Depends on Urban Landscapes. Diversity 2023, 15, 878. https://doi.org/10.3390/d15070878
Kiacz S, Wang H-H, Brightsmith DJ. Presence of Endangered Red-Crowned Parrots (Amazona viridigenalis) Depends on Urban Landscapes. Diversity. 2023; 15(7):878. https://doi.org/10.3390/d15070878
Chicago/Turabian StyleKiacz, Simon, Hsiao-Hsuan Wang, and Donald J. Brightsmith. 2023. "Presence of Endangered Red-Crowned Parrots (Amazona viridigenalis) Depends on Urban Landscapes" Diversity 15, no. 7: 878. https://doi.org/10.3390/d15070878
APA StyleKiacz, S., Wang, H.-H., & Brightsmith, D. J. (2023). Presence of Endangered Red-Crowned Parrots (Amazona viridigenalis) Depends on Urban Landscapes. Diversity, 15(7), 878. https://doi.org/10.3390/d15070878







