Abstract
Reproductive isolating barriers are a crucial element in the speciation process. From these barriers, and among those acting early in the speciation process (premating), the ethological ones can play a pivotal role in isolating populations or closely related species. In fact, the high diversity of some taxa has been correlated with an elevated divergence in sexual signals, which facilitates isolation. The present study explored whether variation in chemical signals may contribute to the high diversity of Liolaemus lizards, a genus with more than 290 species. Specifically, population scent discrimination was investigated in two sympatric species, L. tenuis and L. lemniscatus, studying the response of closely located populations most likely from the same genetic lineages. Lizards of both species discriminated between scents of their own and different populations, and in some cases, scents from their own population were preferred. These results suggest a fast evolution of scents and/or of their discrimination, implying that the ethological barriers involving the chemical modality may evolve fast. The studied species, however, differed in the strength of the exhibited population scent discrimination, suggesting that the ethological barriers may evolve at different rates across species. It can be concluded that ethological barriers involving chemical modality may disrupt species’ cohesion, potentially contributing to Liolaemus diversification, in synergy or not with geographical barriers.
1. Introduction
A critical step in the speciation process is the occurrence of reproductive barriers that limit gene flow between populations or incipient species (e.g., [1,2,3]). Such barriers are classified based on when they restrict gene exchange, and those that preclude copulation, premating barriers [2], tend to evolve early in the speciation process [4]. Among the premating barriers, the behavioral ones, also called ethological or sexual isolating barriers [2], have been proposed to play a pivotal role in isolating populations or closely related species [5]. Thus, in the context of sexual selection, signals involved in mate attraction and male–male competition can play a significant role in speciation [6,7,8], supported by various studies involving different taxa and sensory modalities (e.g., [9,10,11,12]). Remarkably, the high speciation rates of some taxa have been associated with high divergence in sexual signals (e.g., [13,14]). For example, Laupala crickets, the genus with the highest speciation rate among arthropods, have a high divergence in song characteristics across taxa [15]. Similarly, the increased diversification of African cichlid fishes has been linked to variation in male colorations involved in mate selection [16,17].
Liolaemus, a genus with more than 290 lizard species [18,19], is one of the most diverse tetrapod genera in the world, distributed in the southern part of South America [19,20]. Its high diversity has been related to the Andean uplift that fragmented species distribution, promoting speciation [20,21]. Presently, various genetic analyses support the role of different geographic barriers (e.g., rivers and mountains) in this genus diversification [22,23,24]. Similarly, the diversity of different South American taxa has also been associated with changes in landscape, climate, and drainage from the Andean formation, e.g., [25,26,27]. However, no other genus in the Andean mountains has a similar diversity to Liolaemus, even the genera belonging to the Liolaemidae family [19]. Therefore, geographic barriers per se do not explain the Liolaemus diversity, and other factors, such as reproductive barriers involving organismal traits, i.e., sexual signals, may play a prominent role in Liolaemus speciation [28]. In this context, if populations of Liolaemus species show relevant differences in sexual signals, this should be reflected in population discrimination. Such discrimination can be a starting point of an ethological isolating barrier disrupting the species cohesion [29], which may contribute to Liolaemus diversity.
Chemical signals, or scents, are relevant in the speciation process of many taxa [30]. In Liolaemus, scents, further than allow individual discrimination [31,32,33], may facilitate isolation among sympatric congeneric species [31,34,35]. The composition of precloacal secretions, a pheromone source in Liolaemus lizards [36,37,38], differs between sexes [39], among males of a population, of different populations, and among congeneric species [40,41], suggesting that scents may evolve fast [34,35]. Thus, considering that scents of nearby populations can differ [41], possibly populations may discriminate between homo- and heterotypic conspecific scents, i.e., from the same and different populations, respectively, and most likely, homotypic scents would be selected (e.g., [42,43]). If so, species cohesion would be disrupted in the absence of geographic barriers.
This study explored evidence of ethological isolating barriers in Liolaemus species by investigating population scent discrimination in closely located populations of two species, L. tenuis and L. lemniscatus. These are common species of central Chile [19], and evidence indicates that scents play an essential role in different daily contexts [31,34,44,45,46]. Therefore, for these species, scent population discrimination may be relevant.
2. Materials and Methods
Individuals of L. tenuis and L. lemniscatus were collected from the south bank of the Maipo River in central Chile (Figure 1), a well-recognized geographic barrier for many lizard species [23,47,48,49]. The aim was to collect closely located populations without separation by geographic barriers. Collected populations may have gene flow supported by genetic analyses of nearby populations to the studied ones, which suggests that the collected populations would be from a single genetic lineage [23,50].
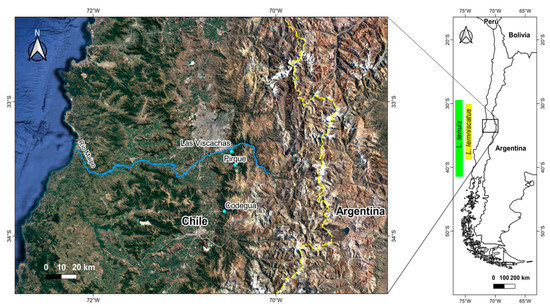
Figure 1.
Geographic localization of the studied populations (cyan dots) of Liolaemus tenuis and L. lemniscatus in central Chile. The total distributional range of both species in Chile is shown: green, L. tenuis; yellow, L. lemniscatus.
Considering that male–male competition and mate attraction signals can be relevant in speciation [6,7,8], population discrimination was explored by exposing lizards to rival or mate scents from different origins, i.e., homo- and heterotypic populations, quantifying their responses to the different scents. Three experiments were performed: Experiment I was designed to assess male rival scent discrimination. Experiment II explored lizards’ mate scent discrimination. Using a two-choice stimulus test [51], individuals were exposed simultaneously to scents of potential mates from different populations to stimulate them to explore scents, which may allow lizards to assess the best mate choice. Finally, in Experiment III, an extension of Experiment II, individuals were exposed longer to these scents to determine whether they would prefer any of these, quantified by the association with each scent source [51]. Below, these experiments are described.
2.1. Experiment I—Scent Discrimination of Homo- vs. Heterotypic Rivals
Animals and their maintenance. In January 2001, adult males of both species were collected from different populations (Figure 1); 19 L. tenuis (9 from Codegua (34.03° S; 70.57° W; Datum WGS84, 765 m), 3 from Las Vizcachas (33.62° S; 70.52; 890 m), and 7 from Pirque (33.73° S; 70.49° W; 850 m)) and 16 L. lemniscatus (8 from Las Vizcachas and 8 from Codegua). Following previous studies [34,52], in the laboratory, lizards were placed in an indoor vivarium with a glass roof, exposing them to the sun and natural photoperiod (13:11 L:D). Additionally, the vivarium was equipped with halogen lamps to maintain similar temperatures to those recorded on a typical summer day (between 12 °C to 36 °C). Lizards were housed individually in plastic enclosures (37 × 30 × 15 cm) with a 3 cm sand-layer substrate. Enclosures had a lid of plastic mesh and were equipped with a bowl to keep water permanently and a rock to provide shelter and a basking place. Food (mealworms) was supplied three times a week, dusted with vitamins.
Experimental design. Animals remained without perturbation in their enclosures for a week, allowing habituation to experimental conditions and scent releasing, because enclosures were used as a source of substrate-borne scents [34]. For the experiments, the tested lizard was taken from its enclosure and kept in a cloth bag for 10 min to reduce handling associated stress [34]; after that, the bag was placed in the experimental enclosure, allowing the lizard to move freely. The bag was removed once the individual was utterly on the substrate, and the trial started. The experimental enclosure corresponded to one used by a homo- or a heterotypic male. Lizards were tested in a counterbalanced design with scents from different populations to avoid any potential effect of the presentation order. Just before the trial, the enclosure resident was removed with the rock and the bowl for water. The focal population of L. tenuis was Codegua, which was exposed to homotypic scents and two heterotypic scent sources from Pirque and Las Vizcachas. In the case of L. lemniscatus, both populations, Codegua and Las Vizcachas, were exposed to homo- and heterotypic scents.
Once the tested male was in the experimental enclosure, the latency to the first tongue flick was recorded, i.e., the time elapsed since the individual was placed in the enclosure until it made the first tongue flick [52]. After this display, the lizard’s behavior was filmed for 10 min with an 8 mm digital video camera (Sony DRC-TRV310) placed 50 cm above the enclosure. Following previous studies [34], potential bias in behavior due to variations in body temperatures was controlled by recording the cloacal temperature of the tested individual at the end of the trial. If values were not close (±2 °C) to the mean selected body temperature of the species ~35–36 °C [53], the trial was discarded and repeated later. This protocol also ensured that the experimental enclosures had similar temperatures; thus, scent efficacy was similar across trials [54]. After the experiment, the tested and scent-donor males were placed back in their enclosures, and the rock and the bowl for water were placed back in the donor’s enclosure. Individuals were subjected to one trial (homo- or heterotypic stimulus) per day and had an intertrial of at least two days.
A total of 53 trials were performed, 21 for L. tenuis and 32 for L. lemniscatus. From the videos, the following variables were recorded [33,52]: 1—Number of tongue flicks. The number of times the lizard protruded and retracted the tongue, which touched the air or a surface. Tongue flicks allow lizards to explore the chemical environment by collecting scents (volatile and nonvolatile compounds) and delivering them to the vomeronasal organ, i.e., vomerolfaction [55]. 2—Motion time. Total time that the lizard moved, including adjustments of body posture, head movements (scanning), displacements of the body’s center of gravity, and displays that occurred at too low frequency to be analyzed independently (marking behaviors, tail waving, slow motion, defecation, and digging). 3—Head displays. Time exhibiting up and down head movements combined or not with forelimb flexions.
2.2. Experiment II—Scent Discrimination of Homo- vs. Heterotypic Mates
In December 2003, adults of both sexes from two populations of both species were collected: L. tenuis: 22 from Codegua (11♂ and 11♀) and 19 from Las Vizcachas (10♂ and 9♀) and L. lemniscatus: 35 from Codegua (21♂ and 14♀) and 18 from Las Vizcachas (11♂ and 7♀). In the laboratory, lizards were maintained as in the previous experiment, except that the rocks of the maintenance enclosures were replaced by inverted clay pots, which were later used as scent sources. In addition, there was a new vivarium that did not have a glass roof. Thus, halogen lamps provided the light, keeping the summer photoperiod.
Experiments were performed in a round enclosure of 50 cm in diameter with a layer of 2 cm clean sand at the bottom. Following designs in which animals were exposed simultaneously to two scent sources (e.g., [51,56,57]), the arena was virtually divided into two halves, placing symmetrically a clay pot on each side, one of a homo- and the other of a heterotypic potential mate. The location of pots was randomized across trials. The lizard was filmed for 20 min after the first tongue flick.
A total of 94 trials were performed, 41 for L. tenuis and 53 for L. lemniscatus. From the videos, five variables were quantified: 1—time spent at each side of the experimental enclosure, 2—time spent on each clay pot, 3—the number of tongue flicks performed on each side of the enclosure, 4—the number of tongue flicks directed to each clay pot, and finally, 5—time moving on each side of the experimental enclosure. In the cases when the lizard remained between the pots, its position was determined by considering where the head was pointing. On the other hand, considering that the substrate (sand) of the experimental enclosures did not have scents, differences between the variables involving exploration of the sides and the pots (i.e., variables 1–2 and 3–4) may provide information on the exploration of neutral zones, although close to a scent source. Finally, after each trial, enclosures were thoroughly rinsed with soap and clean water, and the sand was discarded and replaced.
The scent donors of the two populations used for each trial were selected, considering they did not differ by more than 10% in their body size. The same restriction was applied to the next experiment.
2.3. Experiment III—Scent Preference for Homo- vs. Heterotypic Mates
In February 2004, new adult individuals of both sexes from the two populations of each species were collected: L. tenuis: 16 from Codegua (6♂ and 10♀) and 13 from Las Vizcachas (7♂ and 6♀) and L. lemniscatus: 16 from Codegua (8♂ and 8♀) and 13 from Las Vizcachas (8♂ and 5♀). In the laboratory, lizards were maintained as in Experiment II. To assess scent preferences, the tested lizard remained for 4.5 hrs in the round enclosure described above. Every 30 min, it was observed behind a blind at which side of the enclosure the lizard was located. Scent preference was determined by calculating the percentage of time that lizards were at each side of the enclosure (e.g., [57]). A total of 58 trials were performed, 29 by species.
Individuals were kept in good conditions in the laboratory, and at the end of each of the three experiments, lizards were released at their capture sites.
2.4. Statistics
Data for each experiment by species were analyzed using General Linear Models for repeated measures (scent origin; homo- vs. heterotypic), followed by Fisher LSD tests. For Experiment I, analyses determined the effect of scent origin on four responses. However, the head display was only analyzed for L. lemniscatus, as this behavior was exhibited in low frequency by L. tenuis, and its time of exhibition was included in motion time. The normality of head display in L. lemniscatus was improved by log-transforming the data. The statistical analyses of L. lemniscatus also included the effect of the population (Codegua vs. Las Viscachas) and its interaction with scent origin.
For Experiments II and III, tests assessed the effects of scent origin, the sex and population of origin of the tested lizard, and their interactions on the recorded variables. In Experiment III, the percentages of time associated with homo- and heterotypic scents were transformed using the square root of the arcsine. For these two experiments, preliminary t-tests for independent samples indicated no differences between the body sizes of scents donors. Therefore, this variable was not incorporated into the analyses.
Statistical analyses were performed with STATISTICA (StatSoft, Inc. Tulsa, OK, USA, 2001).
3. Results
3.1. Experiment I—Scent Discrimination of Homo- vs. Heterotypic Rivals
Liolaemus tenuis. Scent origin did not modulate the latency to the first tongue flick nor motion time (Table 1A; Figure 2A and 2C, respectively), but it did affect the number of tongue flicks; lizards explored the hetero- more than the homotypic scents and did not show differences in the exploration to both heterotypic scents (Figure 2B).

Table 1.
Results of the General Linear Models for repeated measures to test male scent discrimination of homo- vs. heterotypic rivals (scent origin) in two species. A. Liolaemus tenuis. Three responses of the focal population (Codegua) were recorded when individuals were exposed to scents from different origins. Sample size: 9. B. Liolaemus lemniscatus. The analyses included the effect of the tested population (Codegua and Las Viscachas) and its interaction with scent origin upon 4 responses. Total sample size: 16, 8 males from each population. Presented values are the F-statistics [degree of freedom] (p-value); statistically significant tests (p < 0.05) are shown in bold.
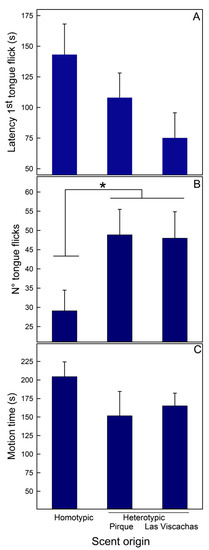
Figure 2.
Mean (+SE) values of the three behaviors recorded in males of Liolaemus tenuis from Codegua when tested for rival scent discrimination. Scents were from the same population (homotypic) and two heterotypic populations, Pirque and Las Viscachas: (A)—Latency to the first tongue flick, (B)—Number of tongue flicks, and (C)—Motion time (s). * = p < 0.05.
Liolaemus lemniscatus. Scent origin was the only factor that modulated some responses (Table 1B). First, males showed shorter latency to the first tongue flick when scents were from hetero- rather than homotypic rivals (Figure 3A). Second, males exhibited head displays for an extended period when they were exposed to homotypic scents (Figure 3D). None of the other variables, i.e., number of tongue flicks and motion time, were affected by scent origin (Figure 3B and 3C, respectively).
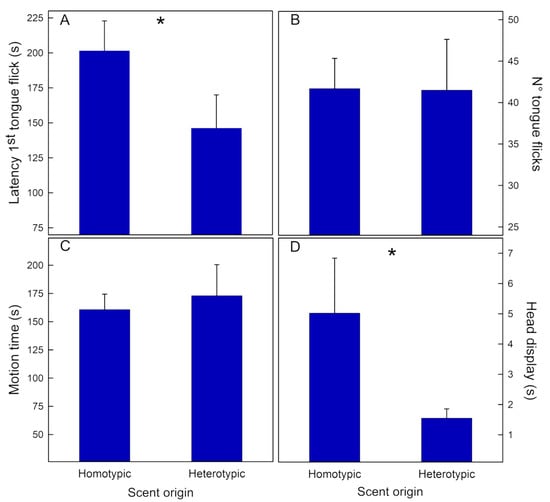
Figure 3.
Mean (+SE) values of the four behaviors recorded in males of Liolaemus lemniscatus when tested for rival scent discrimination. Scents were from conspecifics of different origins, homotypic vs. heterotypic. Pooled data of the two studied populations (Codegua and Las Viscachas): (A)—Latency to the first tongue flick (s), (B)—Number of tongue flicks, (C)—Motion time(s), and (D)—Head display (s). * = p < 0.05.
3.2. Experiment II—Scent Discrimination of Homo- vs. Heterotypic Mates
Liolaemus tenuis. All the recorded variables were modulated by scent origin (Table 2A). Lizards, independently of their sex or population of origin, spent more time, tongue flicked more, and moved more in the area and pot with homotypic scents (Figure 4A,B). In addition, sex and population of origin modulated the motion time (Table 2A); males moved more than females (2.09 ± 0.17 vs. 1.54 ± 0.12 min, respectively), and lizards from Codegua moved more than those from Las Viscachas (2.01 ± 0.17 vs. 1.58 ± 0.12 min, respectively).

Table 2.
Results of the General Linear Models for repeated measures to test discrimination between homo- vs. heterotypic potential mate (scent origin). Analyses included the effects of the sex (male vs. female) and the population (Pop.; Codegua vs. Las Viscachas) of the tested individuals and their interactions upon five recorded responses. A. Liolaemus tenuis. B. Liolaemus lemniscatus. Shown values are the F-statistics [degree of freedom] (p-value); statistically significant tests (p < 0.05) are shown in bold.
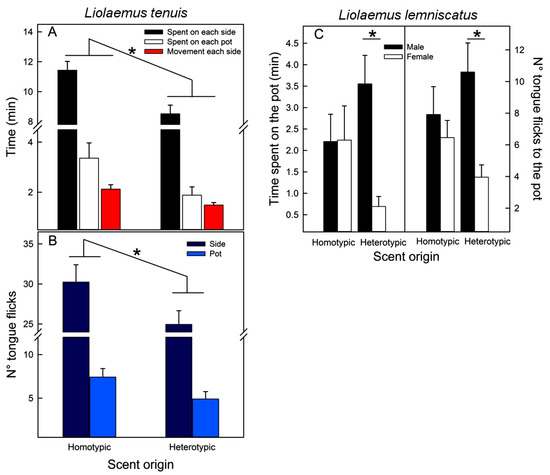
Figure 4.
Mean (+SE) values of behaviors exhibited by lizards when tested for scent discrimination of potential mates from different origins, homo- and heterotypic. Liolaemus tenuis. Data from both populations and sexes were pooled: (A)—Time (min) spent on each side of the enclosure, on the pot with scents, and moving in each side of the enclosure. (B)—Number of tongue flicks displayed at each side of the enclosure and directed to the pot. (C)—Liolaemus lemniscatus. Data from both populations were pooled. Effect of the interaction between scent origin (homo- vs. heterotypic) and the sex of the tested individual upon the time (min) spent in the pot with scents from different origins (left) and the number of tongue flicks directed to those pots (right). * = p < 0.05.
Liolaemus lemniscatus. Scent origin only modulated the exploration of the pots, and only in interaction with the sex of the tested lizards (Table 2B); females spent less time and directed fewer tongue flicks to pots with heterotypic scents than males (Figure 4C). However, the a posteriori tests could not exclude that both sexes behave similarly to pots with different scents (Figure 4C).
3.3. Experiment III—Scent Preference for Homo- vs. Heterotypic Mates
Individuals of L. tenuis, independent of their sex and population of origin, spent more time with homotypic scents (Table 3; Figure 5). In contrast, the scent preference in L. lemniscatus was influenced by the population of origin of the tested lizards; the lizards from Las Viscachas spent more time with homotypic scents than the Codegua population, which stayed for longer with heterotypic scents (Figure 5). The a posteriori tests, however, could not exclude that both populations behave similarly to pots with scents of different origin, although the Codegua population showed a tendency to prefer heterotypic scents (p = 0.052; Figure 5).

Table 3.
Results of the General Linear Models for repeated measures to test preference for homo- vs. heterotypic potential mate (scent origin), including the effect of the sex (male vs. female) and the population (Codegua vs. Las Viscachas) of the tested individuals and their interactions with scent origin. Shown values are the F-statistics (degree of freedom) (p-value); statistically significant tests (p < 0.05) are shown in bold.
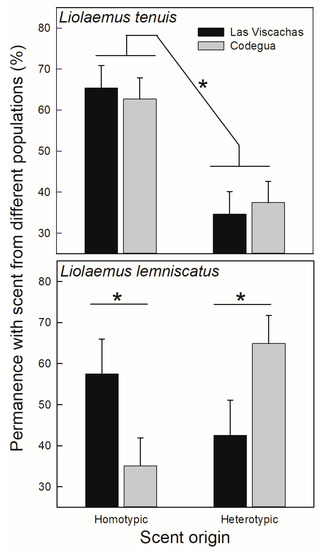
Figure 5.
Mean untransformed (+SE) values of the permanence (%) at each side of the enclosure contained clay pots with conspecific scents from different origins (homo- vs. heterotypic) to test scent preference. Data for two populations, Codegua and Las Viscachas, are presented for each species, Liolaemus tenuis (upper panel) and L. lemniscatus (lower panel). * = p < 0.05.
4. Discussion
The studied Liolaemus species showed discrimination between homo- and heterotypic scents, and L. tenuis showed a clear preference for homotypic potential mates. These results suggest a scent differentiation across populations, probably mirrored by differences in chemoreception. Considering that the tested populations of these species were closely located, enough to be treated as similar (e.g., [58]), that they were not separated by geographic barriers (e.g., rivers), and that they most likely were from a unique genetic lineage [23,50], the recorded population discrimination suggests that ethological isolating barriers involving the chemical modality may evolve relatively fast in these species, which may facilitate reproductive isolation even without geographic barriers. The two species, however, differed in the strength of their population discrimination and, therefore, in the degree of reproductive isolation that the chemical modality may convey.
- Scent discrimination of homo- vs. heterotypic rivals (Experiment I)
The responses triggered by competitors from different origins have been relevant to investigate reproductive isolation across populations or incipient species (e.g., [11,12,59,60]). In this study, males of both Liolaemus species discriminated between potential rivals from different populations, suggesting that scents may contribute to population isolation (e.g., [43]).
The higher rate of tongue flicks exhibited by L. tenuis toward heterotypic scents suggests that these scents were perceived as different from those of the own population, although not as different as those from congeneric males, which elicited less exploration than homotypic scents [34,61]. Most likely, individuals of different populations share a “species signature” [34], allowing recognition of heterotypic males as conspecifics. However, the two heterotypic populations were not discriminated. Most likely, scents may also provide a “population signature” (e.g., [62]), and when scents are not from the own population, they are treated as different, independently of how dissimilar they are from the own “population signature”. Thus, lizards seem to exhibit a dichotomous response, i.e., own vs. different.
In the case of L. lemniscatus, male scents from both populations triggered similar tongue-flick rates. This response is likely a consequence of assessing and discriminating volatile scent compounds by olfaction rather than by vomerolfaction, which has already been proposed for some Liolaemus species [33,36], including L. lemniscatus [52]. This olfactory assessment triggered in the lizards a sooner exploration with the tongue of the heterotypic male scents and a more extended exhibition of head displays toward homotypic scents, which indicates that males of the own population were perceived as more threatening rivals [33,34].
- 2.
- Discrimination and preference for homo- vs. heterotypic potential mates (Experiments II and III)
The simultaneous exposition of scents of potential mates from different populations triggered in L. tenuis individuals higher exploration (Experiment II) and a preference for homotypic mates (Experiment III), indicating that the potential mates from the own population were more attractive. These results suggest that the studied populations may experience ethological isolating barriers mediated by chemical modality and that gene flow may be restricted even without geographic barriers (e.g., [43]). Genetic analyses of L. tenuis along its geographic distribution have consistently revealed that the species might constitute a diverse cryptic species complex [23,47,63]. Thus, unraveling this complex should combine genetic and scent chemical analyses, ideally including scent discrimination tests (e.g., [64]).
In L. lemniscatus, the simultaneous exposure to scents from potential mates of different populations did not determine clear-cut results; there was weak or nonexistent population discrimination and preference. During the brief scent exposition (20 min, Experiment II), the sex of the tested individual modulated scent exploration, since females spent less time investigating heterotypic scents than males. However, because both sexes behaved similarly with homo- and heterotypic scents, population discrimination is not well supported. Similarly, the prolonged scent exposure (4.5 hrs, Experiment III) revealed that populations differed in their scent preferences. Las Viscachas lizards spent more time with homotypic scents than Codegua lizards, which preferred heterotypic scents; thus, both populations were more attracted to Las Viscachas scents. However, because both populations behaved similarly with homo- and heterotypic scents, population preference is not well supported. Therefore, L. lemniscatus may have a weak ethological isolating barrier associated with the chemical modality, mainly determined by male scent discrimination. Consequently, L. lemniscatus may have relatively strong species cohesion, and gene flow across populations may only be slightly restricted, unless other sensory modalities, e.g., vision [65], may have a more relevant role in reproductive isolation in this species.
- 3.
- Liolaemus diversity and ethological isolating barriers associated with the chemical sensory modality.
Scents may evolve fast in lizards (e.g., [66]), which is supported by scent variation across populations in different species (e.g., [41,67,68,69]). This scent variation, however, does not necessarily determine population discrimination (e.g., [42,58]) and may play a minor role in the evolution of reproductive isolation [70]. Nevertheless, there is also evidence in the species of different genera showing that scent variation across populations is mirrored by population scent discrimination, leading to propose that such scent variations may promote reproductive isolation, i.e., Podarcis [42,58,71], Psammodromus [72], and Aspidoscelis [43]. However, even if these population’s scent discriminations promote reproductive isolation, this does not determine high diversification in these genera, at least compared with Liolaemus diversity. For example, the highest diversity of the three mentioned genera is exhibited by Aspidoscelis, with 44 recognized species [18], and some of these have many subspecies [73], with evidence suggesting that Aspidoscelis diversity would be a consequence of hybridization and introgression across taxa [73].
The discrimination of nearby populations in the two Liolaemus species suggests that scents and/or their recognition may evolve fast (e.g., [34]), and thus, the potential establishment of ethological isolating barriers, although at different rates across species. In this scenario, these ethological barriers may contribute to Liolaemus diversification, even without geographic barriers, which may explain the restricted geographic distribution of various Liolaemus species [19]. Recently, it was shown that the number of precloacal glands, one scent source in Liolaemus [31], is unrelated to this genus diversity [74]. Therefore, variability in the scent composition and variability should be a factor to be explored regarding Liolaemus diversification.
5. Conclusions
Speciation rates significantly differ across taxa [2,28], and unraveling the causes behind such variation is one of the fundamental questions in speciation research [75], which is a challenging task, as speciation is a multicause process [2]. The present study provides a starting point to investigate the role of ethological isolating barriers associated with the chemical modality in disrupting species’ cohesion, thus facilitating speciation in Liolaemus, in synergy or not with geographical barriers (e.g., [20]). The question remains open, however, as to why the studied species exhibit different levels of population discrimination and preference. Considering these species have evolved along similar demographic trajectories [50] and their studied populations were exposed to the same environmental conditions, a factor that modulates scent composition (e.g., [66,76,77]), higher behavioral similarities could be expected. Future investigations may tackle this question and explore population discrimination in species from different clades, since studies show hybridizations and introgressions in some clades, e.g., [78,79,80], implying that scent discrimination may not be relevant for all species.
Funding
Financial support was provided by IFS (International Foundation of Science, Sweden), Projects: 2933-1/2.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the current Chilean laws governing animal capture and maintenance imposed by Servicio Agrícola y Ganadero (Permits N° 2320; 3327).
Data Availability Statement
Data presented in this article are available on request from the corresponding author.
Acknowledgments
I thank Marco Mangiacotti for the invitation to participate in the special issue “Ecology and Evolution of Chemical Communication in Lizards”, Daniel Benitez and Julio Labra for their help during fieldwork, Eduardo Aguilera for his invaluable assistance with filming, animal care, and fieldwork, Hermann M. Niemeyer and Mario Penna for allowing use of their facilities to develop this study, two reviewers for their valuable comments made for a previous version, Juan E. Contardo for kindly making the map of the population localities, and Thomas F. Hansen for very insightful comments on an early version of the manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Matute, D.R.; Cooper, B.S. Comparative studies on speciation: 30 years since Coyne and Orr. Evolution 2021, 75, 764–778. [Google Scholar] [CrossRef] [PubMed]
- Coyne, J.A.; Orr, H.A. Speciation; Sinauer Associates: Sunderland, UK, 2004. [Google Scholar]
- Mayr, E. Systematics and the Origin of Species; Columbia University Press: New York, NY, USA, 1942. [Google Scholar]
- Gavrilets, S.; Vose, A. Dynamic patterns of adaptive radiation: Evolution of mating preferences. In Speciation and Patterns of Diversity; Butlin, R.K., Bridle, J.R., Schluter, D., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 102–126. [Google Scholar]
- Butlin, R.K.; Ritchie, M.G. Behaviour and speciation. In Behaviour and Evolution; Slater, P.J.B., Halliday, T.R., Barrett, P., Eds.; Cambridge University Press: New York, NY, USA, 1994; pp. 43–79. [Google Scholar]
- Panhuis, T.M.; Butlin, R.K.; Zuk, M.; Tregenza, T. Sexual selection and speciation. Trends Ecol. Evol. 2001, 16, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Mendelson, T.C.; Safran, R.J. Speciation by sexual selection: 20 years of progress. Trends Ecol. Evol. 2021, 36, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- West-Eberhard, M.J. Sexual selection, social competition, and speciation. Q. Rev. Biol. 1983, 58, 155–183. [Google Scholar] [CrossRef]
- Roriz, A.K.P.; Japyassú, H.F.; Cáceres, C.; Vera, M.T.; Joachim-Bravo, I.S. Pheromone emission patterns and courtship sequences across distinct populations within Anastrepha fraterculus (Diptera-Tephritidae) cryptic species complex. Bull. Entomol. Res. 2019, 109, 408–417. [Google Scholar] [CrossRef]
- Roberts, N.S.; Mendelson, T.C. Identifying female phenotypes that promote behavioral isolation in a sexually dimorphic species of fish Etheostoma zonale. Curr. Zool. 2021, 67, 225–236. [Google Scholar] [CrossRef]
- Uy, J.A.C.; Irwin, D.E.; Webster, M.S. Behavioral isolation and incipient speciation in birds. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 1–24. [Google Scholar] [CrossRef]
- Lipshutz, S.E.; Overcast, I.A.; Hickerson, M.J.; Brumfield, R.T.; Derryberry, E.P. Behavioural response to song and genetic divergence in two subspecies of white-crowned sparrows (Zonotrichia leucophrys). Mol. Ecol. 2017, 26, 3011–3027. [Google Scholar] [CrossRef]
- Mason, N.A.; Burns, K.J.; Tobias, J.A.; Claramunt, S.; Seddon, N.; Derryberry, E.P. Song evolution, speciation, and vocal learning in passerine birds. Evolution 2017, 71, 786–796. [Google Scholar] [CrossRef]
- Ellis, E.A.; Oakley, T.H. High rates of species accumulation in animals with bioluminescent courtship displays. Curr. Biol. 2016, 26, 1916–1921. [Google Scholar] [CrossRef]
- Mendelson, T.C.; Shaw, K.L. Rapid speciation in an arthropod. Nature 2005, 433, 375–376. [Google Scholar] [CrossRef]
- Knight, M.E.; Turner, G.F. Laboratory mating trials indicate incipient speciation by sexual selection among populations of the cichlid fish Pseudotropheus zebra from Lake Malawi. Proc. R. Soc. B. 2004, 271, 675–680. [Google Scholar] [CrossRef]
- Maan, M.E.; Seehausen, O.; Söderberg, L.; Johnson, L.; Ripmeester, E.A.P.; Mrosso, H.D.J.; Taylor, M.I.; van Dooren, T.J.M.; van Alphen, J.J.M. Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proc. R. Soc. B. 2004, 271, 2445–2452. [Google Scholar] [CrossRef]
- Uetz, P.; Freed, P.; Aguilar, R.; Hošek, J. The Reptile Database. 2023. Available online: http://www.reptile-database.org/ (accessed on 15 June 2023).
- Abdala, C.; Laspiur, A.; Scrocchi, G.; Semhan, R.; Lobo, F.; Valladares, P. Las Lagartijas de la Familia Liolaemidae. Sistemática, Distribución e Historia Natural de una de las Familias de Vertebrados más Diversas del Cono Sur de Sudamérica; Vol. 1, Vol. 2; RIL Editores; Universidad de Tarapacá: Santiago, Chile, 2021. [Google Scholar]
- Esquerré, D.; Brennan, I.G.; Catullo, R.A.; Torres-Pérez, F.; Keogh, J.S. How mountains shape biodiversity: The role of the Andes in biogeography, diversification, and reproductive biology in South America’s most species-rich lizard radiation (Squamata: Liolaemidae). Evolution 2019, 73, 214–230. [Google Scholar] [CrossRef]
- Esquerré, D.; Ramírez-Álvarez, D.; Pavón-Vázquez, C.J.; Troncoso-Palacios, J.; Garín, C.F.; Keogh, J.S.; Leaché, A.D. Speciation across mountains: Phylogenomics, species delimitation and taxonomy of the Liolaemus leopardinus clade (Squamata, Liolaemidae). Mol. Phylogenet. Evol. 2019, 139, 106524. [Google Scholar] [CrossRef]
- Torres-Pérez, F.; Mendez, M.A.; Benavides, E.; Moreno, R.A.; Lamborot, M.; Palma, R.E.; Ortiz, J.C. Systematics and evolutionary relationships of the mountain lizard Liolaemus monticola (Liolaemini): How morphological and molecular evidence contributes to reveal hidden species diversity. Biol. J. Linn. Soc. 2009, 96, 635–650. [Google Scholar] [CrossRef]
- Muñoz-Mendoza, C.; D’Elía, G.; Panzera, A.; Méndez T, M.A.; Villalobos-Leiva, A.; Sites, J.W.; Victoriano, P.F. Geography and past climate changes have shaped the evolution of a widespread lizard from the Chilean hotspot. Mol. Phylogenet. Evol. 2017, 116, 157–171. [Google Scholar] [CrossRef]
- Cianferoni, F.; Yáñez, R.P.; Eduardo, R.E.; Garin, C.F.; Torres-Pérez, F. Deep divergences within Liolaemus nigroviridis (Squamata, Liolaemidae) lineages associated with sky islands in central Chile. Zootaxa 2013, 3619, 59–69. [Google Scholar] [CrossRef]
- Boschman, L.M.; Condamine, F.L. Mountain radiations are not only rapid and recent: Ancient diversification of South American frog and lizard families related to Paleogene Andean orogeny and Cenozoic climate variations. Glob. Planet. Chang. 2022, 208, 103704. [Google Scholar] [CrossRef]
- Vallejos Garrido, P.; Pino, K.; Espinoza-Aravena, N.; Pari, A.; Inostroza-Michael, O.; Toledo-Muñoz, M.; Castillo-Ravanal, B.; Romero-Alarcón, V.; Hernández, C.; Palma, R.; et al. The importance of the Andes in the evolutionary radiation of Sigmodontinae (Rodentia, Cricetidae), the most diverse group of mammals in the Neotropics. Sci. Rep. 2023, 13. [Google Scholar] [CrossRef]
- Chaves, J.A.; Weir, J.T.; Smith, T.B. Diversification in Adelomyia hummingbirds follows Andean uplift. Mol. Ecol. 2011, 20, 4564–4576. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Colli, G.R.; Grundler, M.R.; Costa, G.C.; Prates, I.; Rabosky, D.L. No link between population isolation and speciation rate in squamate reptiles. Proc. Natl. Acad. Sci.-Biol. 2022, 119, e2113388119. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.J.; Wilson, R.A. Cohesion, gene flow, and the nature of species. J. Philos. 2010, 107, 61–79. [Google Scholar] [CrossRef]
- Smadja, C.; Butlin, R.K. On the scent of speciation: The chemosensory system and its role in premating isolation. Heredity 2009, 102, 77–97. [Google Scholar] [CrossRef]
- Labra, A. Multi-contextual use of chemosignals by Liolaemus lizards. In Chemical Signals in Vertebrates XI; Hurst, J.L., Beynon, R.J., Roberts, S.C., Wyatt, T.D., Eds.; Springer Link: New York, NY, USA, 2008; pp. 357–365. [Google Scholar]
- Labra, A. Comunicación, el mediador de las interacciones sociales en animales. In Comportamiento Social de la Fauna Nativa de Chile; Ebensperger, L.A., Labra, A., Eds.; Ediciones UC: Santiago, Chile, 2020; pp. 263–273. [Google Scholar]
- Labra, A. Chemoreception and the assessment of fighting abilities in the lizard Liolaemus monticola. Ethology 2006, 112, 993–999. [Google Scholar] [CrossRef]
- Labra, A. Chemical stimuli and species recognition in Liolaemus lizards. J. Zool. 2011, 285, 215–221. [Google Scholar] [CrossRef]
- Labra, A. The chemical-speciation hypothesis in Liolaemus: A response to Pincheira-Donoso. J. Zool. 2012, 288, 234–236. [Google Scholar] [CrossRef]
- Valdecantos, S.; Labra, A. Testing the functionality of precloacal secretions from both sexes in the South American lizard, Liolaemus chiliensis. Amphibia-Reptilia 2017, 38, 209–216. [Google Scholar] [CrossRef]
- Labra, A. Sistemas de comunicación en reptiles. In Herpetología de Chile; Vidal, M.A., Labra, A., Eds.; Science Verlag: Santiago, Chile, 2008; pp. 547–577. [Google Scholar]
- Labra, A.; Brann, J.H.; Fadool, D.A. Heterogeneity of voltage-and chemosignal-activated response profiles in vomeronasal sensory neurons. J. Neurophysiol. 2005, 94, 2535–2548. [Google Scholar] [CrossRef]
- García-Roa, R.; Carreira, S.; López, P.; Martín, J. Genders matters: Sexual differences in chemical signals of Liolaemus wiegmannii lizards (Iguania, Liolaemidae). Biochem. Syst. Ecol. 2016, 69, 108–114. [Google Scholar] [CrossRef]
- Escobar, C.A.; Labra, A.; Niemeyer, H.M. Chemical composition of precloacal secretions of Liolaemus lizards. J. Chem. Ecol. 2001, 27, 1677–1690. [Google Scholar] [CrossRef]
- Escobar, C.; Escobar, C.A.; Labra, A.; Niemeyer, H.M. Chemical composition of precloacal secretions of two Liolaemus fabiani populations: Are they different? J. Chem. Ecol. 2003, 29, 629–638. [Google Scholar] [CrossRef]
- Runemark, A.; Gabirot, M.; Svensson, E. Population divergence in chemical signals and the potential for premating isolation between islet-and mainland populations of the Skyros wall lizard (Podarcis gaigeae). J. Evol. Biol. 2011, 24, 795–809. [Google Scholar] [CrossRef]
- Raya-García, E.; Suazo-Ortuño, I.; Campos-García, J.; Martín, J.; Alvarado-Díaz, J.; Mendoza-Ramírez, E. Chemical signal divergence among populations influences behavioral discrimination in the whiptail lizard Aspidoscelis lineattissimus (Squamata: Teiidae). Behav. Ecol. Sociobiol. 2020, 74, 144. [Google Scholar] [CrossRef]
- Labra, A. The peculiar case of an insectivorous iguanid lizard that detects chemical cues from prey. Chemoecology 2007, 17, 103–108. [Google Scholar] [CrossRef]
- Labra, A.; Niemeyer, H.M. Intraspecific chemical recognition in the lizard Liolaemus tenuis. J. Chem. Ecol. 1999, 25, 1799–1811. [Google Scholar] [CrossRef]
- Labra, A.; Escobar, C.A.; Aguilar, P.M.; Niemeyer, H.M. Sources of pheromones in the lizard Liolaemus tenuis. Rev. Chil. Hist. Nat. 2002, 75, 141–147. [Google Scholar] [CrossRef]
- Victoriano, P.F. Phylogeography of Chilean lizards: Histories of genetic diversification on the western slope of southern Andes. In Lizards of Patagonia. Diversity, Systematics, Biogeography and Biology of the Reptiles at the End of the World; Morando, M., Avila, L.J., Eds.; Springer: Cham, Switzerland, 2020; pp. 255–291. [Google Scholar]
- Lamborot, M.; Alvarez-Sarret, E. Karyotypic variation within and between populations of Liolaemus monticola (Tropiduridae) separated by the Maipo River in the Coastal Range of Central Chile. Herpetologica 1993, 49, 435–449. [Google Scholar]
- Lamborot, M.; Eaton, L. The Maipo River as a biogeographical barrier to Liolaemus monticola (Tropiduridae) in the mountain ranges of central Chile. J. Zool. Syst. Evol. Res. 1997, 35, 105–111. [Google Scholar] [CrossRef]
- Victoriano, P.F.; Ortiz, J.C.; Benavides, E.; Adams, B.J.; Sites, J.W. Comparative phylogeography of codistributed species of Chilean Liolaemus (Squamata: Tropiduridae) from the central-southern Andean range. Mol. Ecol. 2008, 17, 2397–2416. [Google Scholar] [CrossRef]
- Dougherty, L.R. Designing mate choice experiments. Biol. Rev. 2020, 95, 759–781. [Google Scholar] [CrossRef] [PubMed]
- Labra, A.; Niemeyer, H.M. Variability in the assessment of snake predation risk by Liolaemus lizards. Ethology 2004, 110, 649–662. [Google Scholar] [CrossRef]
- Labra, A.; Pienaar, J.; Hansen, T.F. Evolution of thermal physiology in Liolaemus lizards: Adaptation, phylogenetic inertia, and niche tracking. Am. Nat. 2009, 174, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; López, P. Effects of global warming on sensory ecology of rock lizards: Increased temperatures alter the efficacy of sexual chemical signals. Funct. Ecol. 2013, 27, 1332–1340. [Google Scholar] [CrossRef]
- Halpern, M.; Martínez-Marcos, A. Structure and function of the vomeronasal system: An update. Prog. Neurobiol. 2003, 70, 245–318. [Google Scholar] [CrossRef]
- Olsson, M.; Madsen, T.; Nordby, J.; Wapstra, E.; Ujvari, B.; Wittsell, H. Major histocompatibility complex and mate choice in sand lizards. Proc. R. Soc.. B. 2003, 270, S254–S256. [Google Scholar] [CrossRef]
- Martín, J.; López, P. Chemoreception, symmetry and mate choice in lizards. Proc. R. Soc. B 2000, 267, 1265–1269. [Google Scholar] [CrossRef]
- Gabirot, M.; López, P.; Martín, J. Differences in chemical sexual signals may promote reproductive isolation and cryptic speciation between Iberian wall lizard populations. Int. J. Evol. Biol. 2012, 2012, 698520. [Google Scholar] [CrossRef]
- Weir, J.T.; Price, T.D. Song playbacks demonstrate slower evolution of song discrimination in birds from Amazonia than from temperate North America. PLoS Biol. 2019, 17, e3000478. [Google Scholar] [CrossRef]
- Price, T. Sexual selection and natural selection in bird speciation. Philos. Trans. R. Soc. Lond. B 1998, 353, 251–260. [Google Scholar] [CrossRef]
- Labra, A.; Escobar, C.A.; Niemeyer, H.M. Chemical discrimination in Liolaemus lizards: Comparison of behavioral and chemical data. In Chemical Signals in Vertebrates IX; Marchelewska-Koj, A., Lepri, J.J., Müller-Schwarze, D., Eds.; Springer Link: New York, NY, USA, 2001; pp. 439–444. [Google Scholar]
- Wyatt, T. Pheromones and signature mixtures: Defining species-wide signals and variable cues for identity in both invertebrates and vertebrates. J. Comp. Physiol. A 2010, 196, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Panzera, A.; Leaché, A.D.; D’Elía, G.; Victoriano, P.F. Phylogenomic analysis of the Chilean clade of Liolaemus lizards (Squamata: Liolaemidae) based on sequence capture data. Peer J. 2017, 5, e3941. [Google Scholar] [CrossRef] [PubMed]
- Zozaya, S.M.; Higgie, M.; Moritz, C.; Hoskin, C.J. Are pheromones key to unlocking cryptic lizard diversity? Am. Nat. 2019, 194, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Labra, A.; Carazo, P.; Desfilis, E.; Font, E. Agonistic interactions in a Liolaemus lizard: Structure of head bob displays. Herpetologica 2007, 63, 11–18. [Google Scholar] [CrossRef]
- Baeckens, S.; Martín, J.; García-Roa, R.; Pafilis, P.; Huyghe, K.; Van Damme, R. Environmental conditions shape the chemical signal design of lizards. Funct. Ecol. 2018, 32, 566–580. [Google Scholar] [CrossRef]
- Mangiacotti, M.; Fumagalli, M.; Scali, S.; Zuffi, M.A.; Cagnone, M.; Salvini, R.; Sacchi, R. Inter-and intra-population variability of the protein content of femoral gland secretions from a lacertid lizard. Curr. Zool. 2016, 63, 657–665. [Google Scholar] [CrossRef]
- Ortega, J.; Martín, J.; Crochet, P.A.; López, P.; Clobert, J. Seasonal and interpopulational phenotypic variation in morphology and sexual signals of Podarcis liolepis lizards. PLoS ONE 2019, 14, e0211686. [Google Scholar] [CrossRef]
- Ibáñez, A.; Menke, M.; Quezada, G.; Gustavo Jiménez-Uzcátegui; Schulz, S.; Steinfartz, S. Diversity of compounds in femoral secretions of Galápagos iguanas (genera: Amblyrhynchus and Conolophus), and their potential role in sexual communication in lek-mating marine iguanas (Amblyrhynchus cristatus). Peer J. 2017, 5, e3689. [Google Scholar] [CrossRef]
- MacGregor, H.E.A.; Lewandowsky, R.A.M.; d’Ettorre, P.; Leroy, C.; Davies, N.W.; While, G.M.; Uller, T. Chemical communication, sexual selection, and introgression in wall lizards. Evolution 2017, 71, 2327–2343. [Google Scholar] [CrossRef]
- Martín, J.; López, P. Interpopulational differences in chemical composition and chemosensory recognition of femoral gland secretions of male lizards Podarcis hispanica: Implications for sexual isolation in a species complex. Chemoecology 2006, 16, 31–38. [Google Scholar] [CrossRef]
- Martín, J.; López, P.; Iraeta, P.; Díaz, J.A.; Salvador, A. Differences in males’ chemical signals between genetic lineages of the lizard Psammodromus algirus promote male intrasexual recognition and aggression but not female mate preferences. Behav. Ecol. Sociobiol. 2016, 70, 1657–1668. [Google Scholar] [CrossRef]
- Barley, A.J.; Nieto-Montes de Oca, A.; Reeder, T.W.; Manríquez-Morán, N.L.; Monroy, J.C.; Hernández-Gallegos, O.; Thomson, R.C. Complex patterns of hybridization and introgression across evolutionary timescales in Mexican whiptail lizards (Aspidoscelis). Mol. Phyl. Evol. 2019, 132, 284–295. [Google Scholar] [CrossRef]
- Murali, G.; Meiri, S.; Roll, U. Chemical signalling glands are unlinked to species diversification in lizards. Evolution, 2023; in press. [Google Scholar] [CrossRef]
- Butlin, R.; Debelle, A.; Kerth, C.; Snook, R.R.; Beukeboom, L.W.; Cajas, R.C.; Diao, W.; Maan, M.E.; Paolucci, S.; Weissing, F.J. What do we need to know about speciation? Trends Ecol. Evol. 2012, 27, 27–39. [Google Scholar] [CrossRef]
- Zozaya, S.M.; Teasdale, L.C.; Moritz, C.; Higgie, M.; Hoskin, C.J. Composition of a chemical signalling trait varies with phylogeny and precipitation across an Australian lizard radiation. J. Evol. Biol. 2022, 35, 919–933. [Google Scholar] [CrossRef]
- Campos, S.M.; Pruett, J.A.; Soini, H.A.; Zúñiga-Vega, J.J.; Goldberg, J.K.; Vital-García, C.; Hews, D.K.; Novotny, M.V.; Martins, E.P. Volatile fatty acid and aldehyde abundances evolve with behavior and habitat temperature in Sceloporus lizards. Behav. Ecol. 2020, 31, 978–991. [Google Scholar] [CrossRef]
- Esquerré, D.; Keogh, J.S.; Demangel, D.; Morando, M.; Avila, L.J.; Sites Jr, J.W.; Ferri-Yáñez, F.; Leaché, A.D. Rapid radiation and rampant reticulation: Phylogenomics of South American Liolaemus lizards. Syst. Biol. 2022, 71, 286–300. [Google Scholar] [CrossRef]
- Morando, M.; Avila, L.J.; Baker, J.; Sites, J.W. Phylogeny and phylogeography of the Liolaemus darwinii complex (Squamata: Liolaemidae): Evidence for introgression and incomplete lineage sorting. Evolution 2004, 58, 842–861. [Google Scholar] [CrossRef]
- Morando, M.; Medina, C.D.; Minoli, I.; Pérez, C.H.F.; Sites, J.W.J.; Avila, L.J. Diversification and evolutionary histories of Patagonian steppe lizards. In Lizards of Patagonia. Diversity, Systematics, Biogeography and Biology of the Reptiles at the End of the World; Morando, M., Avila, L.J., Eds.; Springer Nature: Cham, Switzerland, 2020; pp. 217–254. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).