Landscape and Marine Environmental Factors Jointly Regulate the Intertidal Species Richness and Community Structure in the Islands of South Korea
Abstract
1. Introduction
2. Materials and Methods
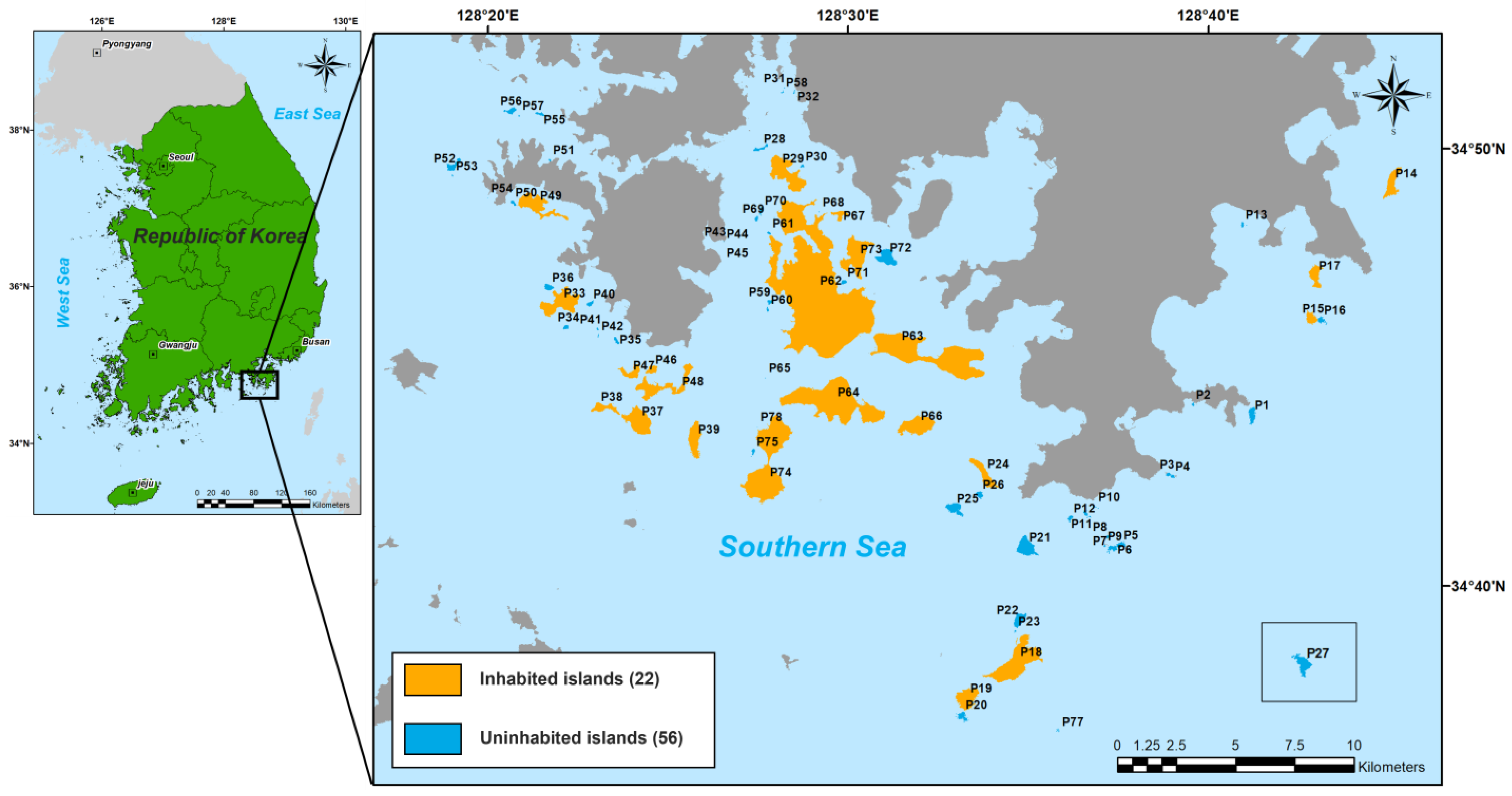
2.1. Study Area
2.2. Species Richness, Funational Diversity, and Community Structure Factors
2.3. Landscape and Marine Environmental Factors
2.4. Statistical Analysis
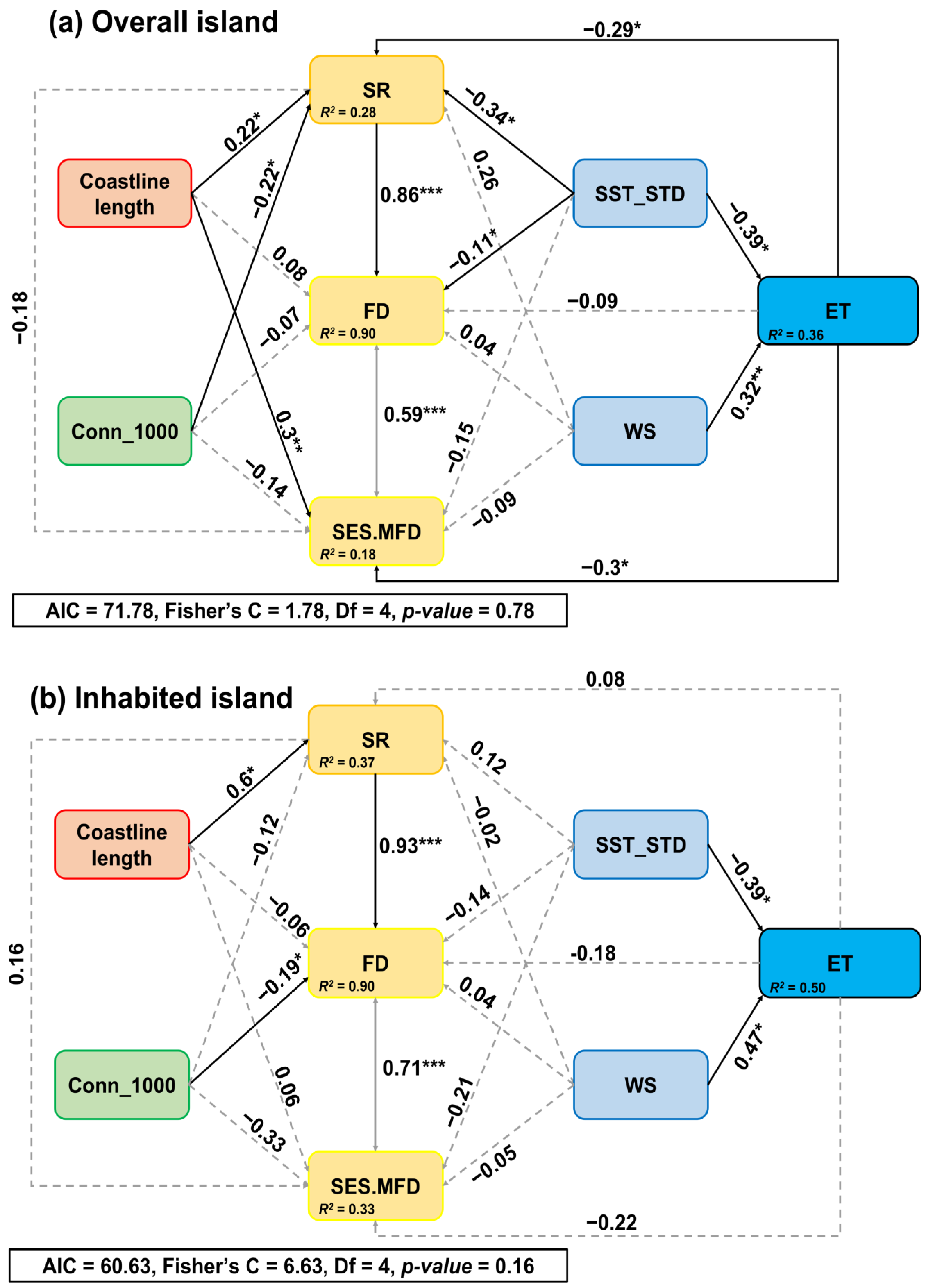
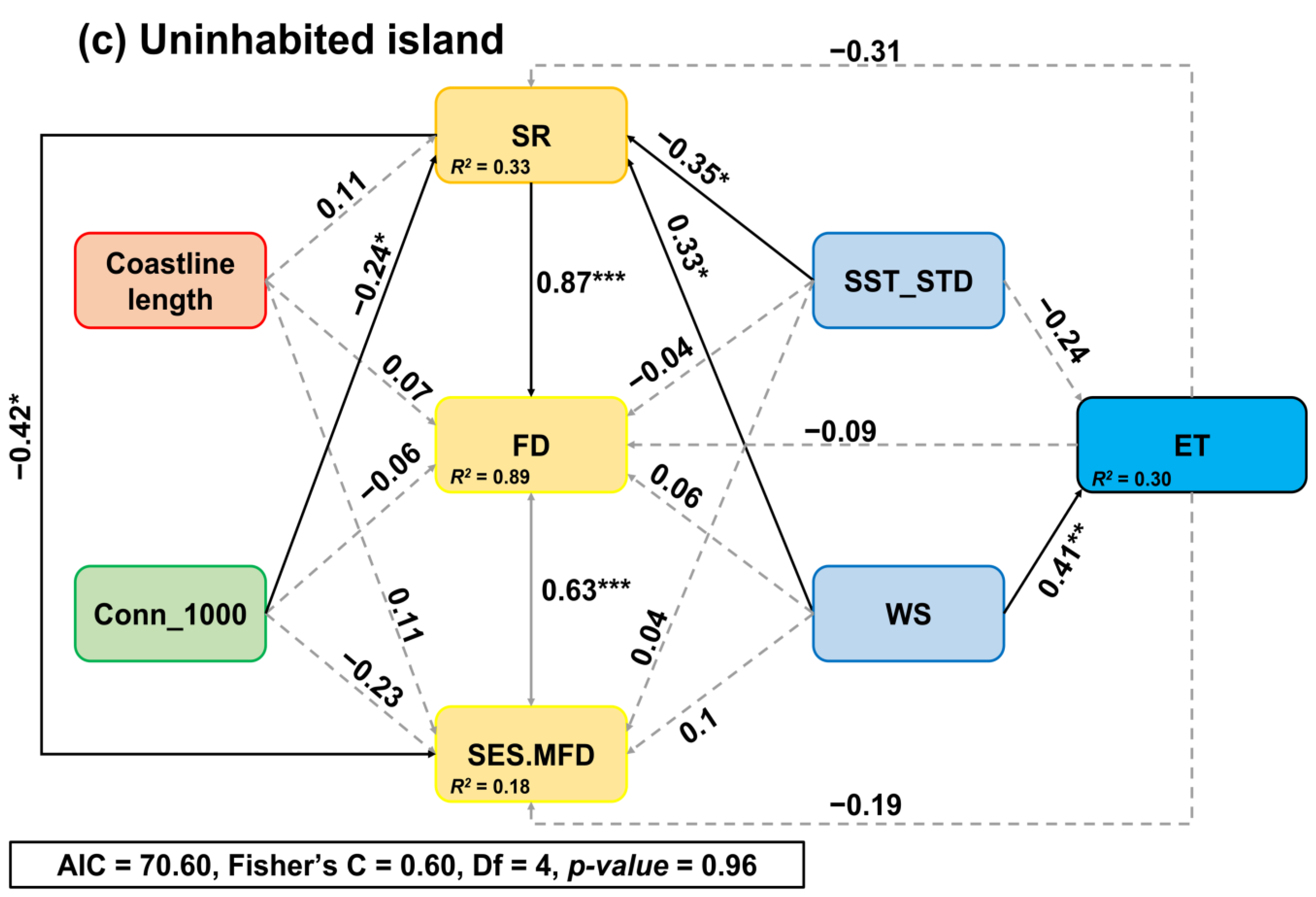
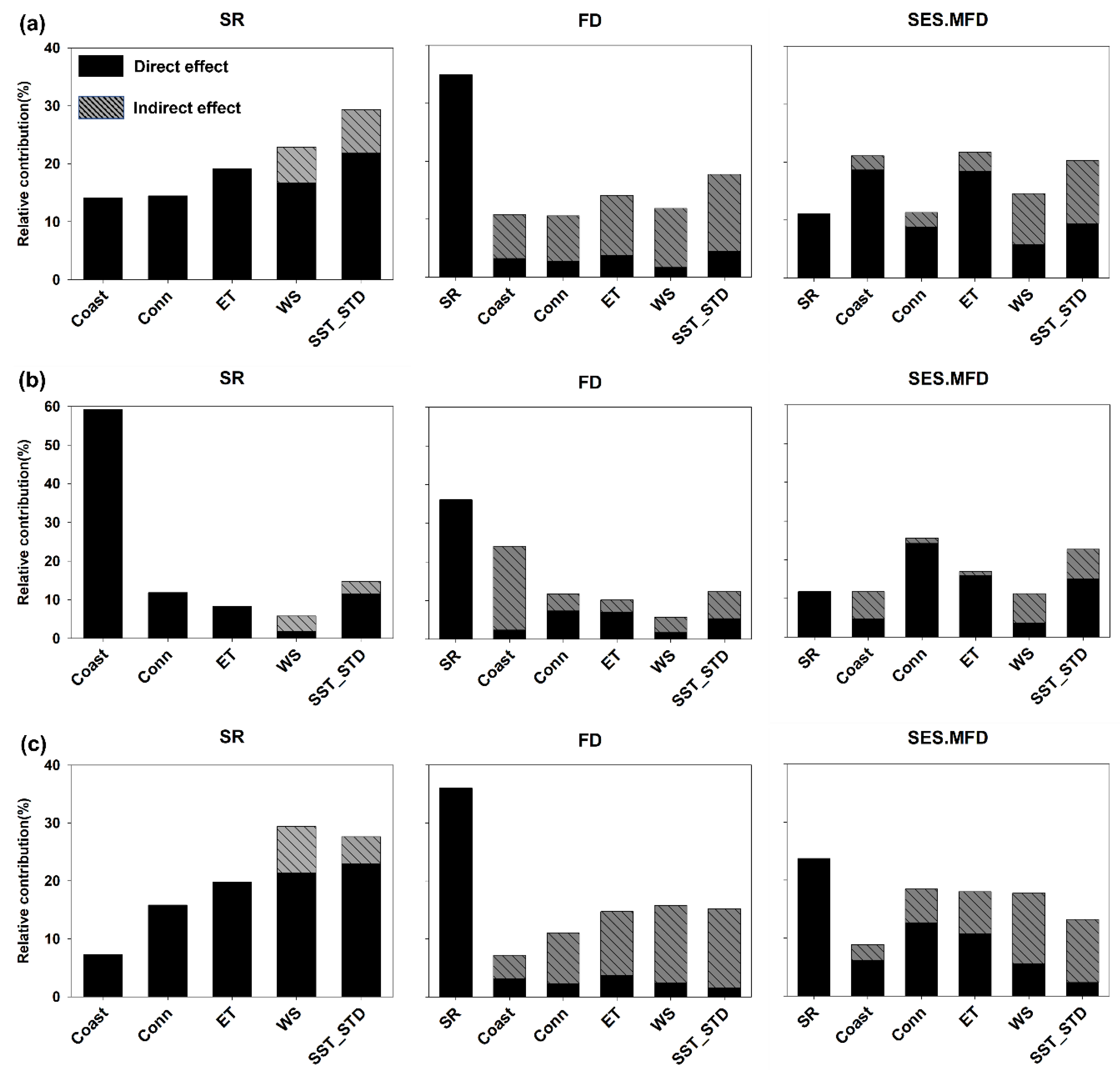
3. Results
4. Discussion
4.1. Drivers of SR of the Intertidal Organisms across Overall Islands
4.2. Drivers of FD and SES.MFD of Intertidal Organisms across Overall Islands
4.3. Drivers of the Intertidal SR, FD, and SES.MFD between the Inhabited and Uninhabited Islands
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Negoita, L.; Fridley, J.D.; Lomolino, M.V.; Mittelhauser, G.; Craine, J.M.; Weiher, E. Isolation-driven functional assembly of plant communities on islands. Ecography 2016, 39, 1066–1077. [Google Scholar] [CrossRef]
- Auffret, A.G.; Aggemyr, E.; Plue, J.; Cousins, S.A. Spatial scale and specialization affect how biogeography and functional traits predict long-term patterns of community turnover. Funct. Ecol. 2017, 31, 436–443. [Google Scholar] [CrossRef]
- Ottaviani, G.; Keppel, G.; Marcantonio, M.; Mucina, L.; Wardell-Johnson, G. Woody species in resource-rich microrefugia of granite outcrops display unique functional signatures. Austral Ecol. 2019, 44, 575–580. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; Volume 1. [Google Scholar]
- Si, X.; Cadotte, M.W.; Zeng, D.I.; Baselga, A.; Zhao, Y.; Li, J.; Wu, Y.; Wang, S.; Ding, P.; Meiri, S. Functional and phylogenetic structure of island bird communities. J. Anim. Ecol. 2017, 86, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Cadotte, M.W.; Davies, T.J.; Antonelli, A.; Ding, P.; Svenning, J.C.; Faurby, S. Phylogenetic and functional clustering illustrate the roles of adaptive radiation and dispersal filtering in jointly shaping late—Quaternary mammal assemblages on oceanic islands. Ecol. Lett. 2022, 25, 1250–1262. [Google Scholar] [CrossRef]
- Davidar, P.; Yoganand, K.; Ganesh, T. Distribution of forest birds in the Andaman islands: Importance of key habitats. J. Biogeogr. 2001, 28, 663–671. [Google Scholar] [CrossRef]
- Chi, Y.; Sun, J.; Fu, Z.; Xie, Z. Spatial pattern of plant diversity in a group of uninhabited islands from the perspectives of island and site scales. Sci. Total Environ. 2019, 664, 334–346. [Google Scholar] [CrossRef]
- Avila, S.P.; Melo, C.; Berning, B.; Sá, N.; Quartau, R.; Rijsdijk, K.F.; Ramalho, R.S.; Cordeiro, R.; De Sá, N.C.; Pimentel, A.; et al. Towards a ‘sea-level sensitive’ dynamic model: Impact of island ontogeny and glacio-eustasy on global patterns of marine island biogeography. Biol. Rev. 2019, 94, 1116–1142. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, H.; Singh, N.; Tang, Y.; Cai, Z. Intertidal zone effects on occurrence, fate and potential risks of microplastics with perspectives under COVID-19 pandemic. Chem. Eng. J. 2022, 429, 132351. [Google Scholar] [CrossRef]
- Somero, G.N. Linking biogeography to physiology: Evolutionary and acclimatory adjustments of thermal limits. Front. Zool. 2005, 2, 1. [Google Scholar] [CrossRef]
- Stickle, W.B.; Carrington, E.; Hayford, H. Seasonal changes in the thermal regime and gastropod tolerance to temperature and desiccation stress in the rocky intertidal zone. J. Exp. Mar. Biol. Ecol. 2017, 488, 83–91. [Google Scholar] [CrossRef]
- Wethey, D.S.; Brin, L.D.; Helmuth, B.; Mislan, K.A.S. Predicting intertidal organism temperatures with modified land surface models. Ecol. Model. 2011, 222, 3568–3576. [Google Scholar] [CrossRef]
- National Oceanic and Atmospheric Administration. What Is the Intertidal Zone? The Intertidal Zone Is the Area Where the Ocean Meets the Land between High and Low Tides, 2023. Available online: https://oceanservice.noaa.gov/facts/intertidal-zone.html (accessed on 4 April 2023).
- Kim, H.H.; Lee, C.B. On the relative importance of landscape variables to plant diversity and phylogenetic community structure on uninhabited islands, South Korea. Landsc. Ecol. 2021, 36, 209–221. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, H.S.; Lee, H.I.; Lee, S.W.; Lee, Y.J.; Lee, C.B. Relative importance of landscape and climate factors to the species diversity of plant growth forms along an East Asian Archipelago. Forests 2022, 13, 218. [Google Scholar] [CrossRef]
- Davison, J.; Moora, M.; Öpik, M.; Ainsaar, L.; Ducousso, M.; Hiiesalu, I.; Jairus, T.; Johnson, N.; Jourand, P.; Kalamees, R.; et al. Microbial island biogeography: Isolation shapes the life history characteristics but not diversity of root-symbiotic fungal communities. ISME J. 2018, 12, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Li, S.P.; Wang, P.; Chen, Y.; Wilson, M.C.; Yang, X.; Ma, C.; Lu, J.; Chen, X.; Wu, J.; Shu, W.; et al. Island biogeography of soil bacteria and fungi: Similar patterns, but different mechanisms. ISME J. 2020, 14, 1886–1896. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, Y.J.; Lim, H.M.; Han, Y.G.; Choi, M.J.; Nam, S.H. A faunistic study of insects of uninhabited islands in the Docho-myeon, Sinan-gun, Jeollanam-do. Korean J. Environ. Ecol. 2011, 25, 673–684. [Google Scholar]
- Lee, H.J.; Cho, K.M.; Hong, S.K.; Kim, J.E.; Kim, K.W.; Lee, K.A.; Moon, K.O. Management plan for UNESCO Shinan Dadohae Biosphere Reserve (SDBR), Republic of Korea: Integrative perspective on ecosystem and human resources. J. Ecol. Environ. 2010, 33, 95–103. [Google Scholar] [CrossRef]
- Hallyeo National Marine Park. A Report of Hallyeo Haesang National Marine Park (Tongyeong and Geoje) on the Fact-Finding Survey Uninhabited and Inhabited Islands; Hallyeo National Marine Park Eastern Management Office: Namhae-gun, Republic of Korea, 2016. [Google Scholar]
- Korea National Park. Hallyeo Haesang National Marine Park and Taean Coastal National Park Are A Paradise for Wildlife; Korea National Park: Seoul, Republic of Korea, 2015. [Google Scholar]
- Dong, J.Y.; Zhao, L.; Yang, X.; Sun, X.; Zhang, X. Functional trait responses of macrobenthos to anthropogenic pressure in three temperate intertidal communities. Front. Mar. Sci. 2021, 8, 756814. [Google Scholar] [CrossRef]
- Root, R.B. The niche exploitation pattern of the blue-gray gnatcatcher. Ecol. Monogr. 1967, 37, 317–350. [Google Scholar] [CrossRef]
- Emery, K.A.; Kramer, V.R.; Schooler, N.K.; Michaud, K.M.; Madden, J.R.; Hubbard, D.M.; Miller, R.J.; Dugan, J.E. Habitat partitioning by mobile intertidal invertebrates of sandy beaches shifts with the tides. Ecosphere 2022, 13, e3920. [Google Scholar] [CrossRef]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Swenson, N.G.; Erickson, D.L.; Mi, X.; Bourg, N.A.; Forero-Montaña, J.; Ge, X.; Howe, R.; Lake, J.K.; Liu, X.; Ma, K.; et al. Phylogenetic and functional alpha and beta diversity in temperate and tropical tree communities. Ecology 2012, 93, S112–S125. [Google Scholar] [CrossRef]
- Chun, J.H.; Lee, C.B. Partitioning the regional and local drivers of phylogenetic and functional diversity along temperate elevational gradients on an East Asian peninsula. Sci. Rep. 2018, 8, 2853. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Biological Resources. Biodiversity of the Korean Peninsula, 2011. Available online: https://species.nibr.go.kr/ (accessed on 9 March 2023).
- National Biodiversity Center. National List of Species, 2018. Available online: https://www.kbr.go.kr/ (accessed on 10 March 2023).
- Hong, J.S.; Yoon, S.K. Marine Biology; Lifescience: Seoul, Republic of Korea, 1995. [Google Scholar]
- Hwang, P.A.; Teague, W.J.; Jacobs, G.A.; Wang, D.W. A statistical comparison of wind speed, wave height, and wave period derived from satellite altimeters and ocean buoys in the Gulf of Mexico region. J. Geophys. Res. Oceans 1998, 103, 10451–10468. [Google Scholar] [CrossRef]
- Aggemyr, E.; Auffret, A.G.; Jädergård, L.; Cousins, S.A. Species richness and composition differ in response to landscape and biogeography. Landsc. Ecol. 2018, 33, 2273–2284. [Google Scholar] [CrossRef]
- Spanowicz, A.G.; Jaeger, J.A. Measuring landscape connectivity: On the importance of within-patch connectivity. Landsc. Ecol. 2019, 34, 2261–2278. [Google Scholar] [CrossRef]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Met. Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Portugal, A.B.; Carvalho, F.L.; de Macedo Carneiro, P.B.; Rossi, S.; de Oliveira Soares, M. Increased anthropogenic pressure decreases species richness in tropical intertidal reefs. Mar. Environ. Res. 2016, 120, 44–54. [Google Scholar] [CrossRef]
- Chase, J.M.; Knight, T.M. Scale-dependent effect sizes of ecological drivers on biodiversity: Why standardised sampling is not enough. Ecol. Lett. 2013, 16, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Portillo, J.T.D.M.; Ouchi-Melo, L.S.; Crivellari, L.B.; de Oliveira, T.A.L.; Sawaya, R.J.; Duarte, L.D.S. Area and distance from mainland affect in different ways richness and phylogenetic diversity of snakes in Atlantic Forest coastal islands. Ecol. Evol. 2019, 9, 3909–3917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.M.; Zhou, L.Z. Area, isolation, disturbance and age effects on species richness of summer waterbirds in post-mining subsidence lakes, Anhui, China. Avian Res. 2018, 9, 8. [Google Scholar] [CrossRef]
- Itescu, Y.; Foufopoulos, J.; Pafilis, P.; Meiri, S. The diverse nature of island isolation and its effect on land bridge insular faunas. Glob. Ecol. Biogeogr. 2020, 29, 262–280. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Y.; Tang, S.; Liu, X.; Li, W.; Han, P.; Zeng, D.; Yang, Y.; Wei, G.; Kang, Y.; et al. Nearby large islands diminish biodiversity of the focal island by a negative target effect. Anim. Ecol. 2022, 92, 492–502. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, S.W.; Lee, Y.J.; Lee, H.I. On the relative importance of landscape variables to bird diversity and community structure in Hallyeo Haesang National Marine Park. J. Nat. Res. 2021, 12, 145–158. [Google Scholar] [CrossRef]
- Choi, S.W.; An, J.S.; Yang, H.S. Effect of island geography on plant species on uninhabited islands in southeastern South Korea. Korean J. Environ. Ecol. 2015, 38, 451–459. [Google Scholar] [CrossRef]
- Levitus, S.; Antonov, J.; Boyer, T. Warming of the world ocean, 1955–2003. Geophys. Res. Lett. 2005, 32, L02604. [Google Scholar] [CrossRef]
- Jones, S.J.; Lima, F.P.; Wethey, D.S. Rising environmental temperatures and biogeography: Poleward range contraction of the blue mussel, Mytilus edulis L., in the western Atlantic. J. Biogeogr. 2010, 37, 2243–2259. [Google Scholar] [CrossRef]
- Scrosati, R.A. Low winter extremes in intertidal temperature in Nova Scotia in the absence of an ice foot. Front. Mar. Sci. 2022, 8, 2113. [Google Scholar] [CrossRef]
- IPCC Climate Change. The Physical Science Basis, Contribution of Working Group 1 to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Mieszkowska, N. Intertidal Indicators of Climate and Global Change; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Ding, Z.; Feeley, K.J.; Wang, Y.; Pakeman, R.J.; Ding, P.P. Patterns of bird functional diversity on land-bridge island fragments. J. Anim. Ecol. 2013, 82, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Hao, Z.; Zhang, J. Phylogenetic structure and phylogenetic diversity of angiosperm assemblages in forests along an elevational gradient in Changbaishan, China. J. Plant Ecol. 2014, 7, 154–165. [Google Scholar] [CrossRef]
- Emerson, B.C.; Gillespie, R.G. Phylogenetic analysis of community assembly and structure over space and time. Trends Ecol. Evol. 2008, 23, 619–630. [Google Scholar] [CrossRef]
- Whittaker, R.J.; Rigal, F.; Borges, P.A.; Cardoso, P.; Terzopoulou, S.; Casanoves, F.; Pla, L.; Guilhaumon, F.; Ladle, R.J.; Triantis, K.A. Functional biogeography of oceanic islands and the scaling of functional diversity in the Azores. Proc. Natl. Acad. Sci. USA 2014, 111, 13709–13714. [Google Scholar] [CrossRef]
- MacArthur, R.; Levins, R. The limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Purvis, A.; Agapow, P.M.; Gittleman, J.L.; Mace, G.M. Nonrandom extinction and the loss of evolutionary history. Science 2000, 288, 328–330. [Google Scholar] [CrossRef]
- Bregman, T.P.; Lees, A.C.; Seddon, N.; MacGregor, H.E.; Darski, B.; Aleixo, A.; Bonsall, M.B.; Tobias, J.A. Species interactions regulate the collapse of biodiversity and ecosystem function in tropical forest fragments. Ecology 2015, 96, 2692–2704. [Google Scholar] [CrossRef] [PubMed]
- Helmus, M.R.; Savage, K.; Diebel, M.W.; Maxted, J.T.; Ives, A.R. Separating the determinants of phylogenetic community structure. Ecol. Lett. 2007, 10, 917–925. [Google Scholar] [CrossRef]
- Chi, Y.; Shi, H.; Wang, Y.; Guo, Z.; Wang, E. Evaluation on island ecological vulnerability and its spatial heterogeneity. Mar. Pollut. Bull. 2017, 125, 216–241. [Google Scholar] [CrossRef]
- Mensah, S.; Veldtman, R.; Assogbadjo, A.E.; Glèlè-Kakaï, R.; Seifert, T. Tree species diversity promotes aboveground carbon storage through functional diversity and functional dominance. Ecol. Evol. 2016, 6, 7546–7557. [Google Scholar] [CrossRef]
- Jung, H.J. Marine Ecology; Seoul National University Press: Seoul, Republic of Korea, 2022. [Google Scholar]


| Functional Category | Functional Trait | Unit | Data Source |
|---|---|---|---|
| Structure size | Body or shell size | mm | National Institute of Biological Resources, South Korea (2011) |
| Body form | Cylindrical | - | |
| Crustacean | |||
| Cnidaria | |||
| Bullate/Saccate | |||
| Bivalved/Gastropod | |||
| Vermiform | |||
| Radial | |||
| Dietary composition | Suspended plankton | % | National Biodiversity Center, South Korea (2018) |
| Seaweed (vegetable) | |||
| Aquatic animal | |||
| Dead organisms | |||
| Foraging behavior | Masticatory eating | % | |
| Filter feeding | |||
| Suction feeding | |||
| Perforation feeding | |||
| Habitat preference | Rocky intertidal zone | % | |
| Tidal flat intertidal zone | |||
| Gravel, Sand intertidal zone | |||
| Subtidal zone | |||
| Supralittoral zone | |||
| Mobility | Movable | - | |
| Immovable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.-K.; Lee, Y.-J.; Lee, C.-B. Landscape and Marine Environmental Factors Jointly Regulate the Intertidal Species Richness and Community Structure in the Islands of South Korea. Diversity 2023, 15, 826. https://doi.org/10.3390/d15070826
Lee M-K, Lee Y-J, Lee C-B. Landscape and Marine Environmental Factors Jointly Regulate the Intertidal Species Richness and Community Structure in the Islands of South Korea. Diversity. 2023; 15(7):826. https://doi.org/10.3390/d15070826
Chicago/Turabian StyleLee, Min-Ki, Yong-Ju Lee, and Chang-Bae Lee. 2023. "Landscape and Marine Environmental Factors Jointly Regulate the Intertidal Species Richness and Community Structure in the Islands of South Korea" Diversity 15, no. 7: 826. https://doi.org/10.3390/d15070826
APA StyleLee, M.-K., Lee, Y.-J., & Lee, C.-B. (2023). Landscape and Marine Environmental Factors Jointly Regulate the Intertidal Species Richness and Community Structure in the Islands of South Korea. Diversity, 15(7), 826. https://doi.org/10.3390/d15070826







