Abstract
The development of large-scale mining activity along the Central Andes of Argentina (CAA) has generated significant amounts of waste materials containing heavy metals. Phytoremediation is a promising eco-friendly, low-cost, and effective technology for the removal of heavy metals. The present study aimed to identify two native dominant species from the CCA, Adesmia subterranea and A. pinifolia, as metal-tolerant plant species for the first time, by evaluating the germination and early seedling growth at different concentrations (ppm) of Cd (3, 4.5 and 6), Ni (150, 225 and 300), As (20, 30 and 40), and Hg (0.8, 1.2 and 1.6) Early seedling growth was found to be more sensitive to heavy metals than germination. Ni and As exhibited the greatest inhibitory effect on both species’ germination percentages. In contrast, with Cd and Hg, no inhibitory effect was recorded. Root length, metal tolerance index, and fresh and dry weight were stimulated with Hg. However, the phytotoxic effect was greater as the concentration of Ni, As, and Cd increased. As an overall conclusion, the order of toxicity for these species can be classified as Ni > As > Cd > Hg. Therefore, Adesmia species could be considered as candidates for phytoremediation of soils contaminated with Hg and low concentrations of Cd.
1. Introduction
Large-scale mining activity has been developed along the Central Andes Mountains (between 2500 and 5000 m asl) in Argentina, especially the metallic mining production of copper, gold, and silver [1,2]. Like most extractive anthropogenic activities, mining generates large quantities of waste materials, leaching piles, and tailings containing mainly Cd, Cu, Ni, Pb, Hg, Zn, and As [3,4,5]. In arid and semi-arid regions, these mining wastes pose a serious problem since they can be dispersed to the surrounding ecosystem by the wind and water [6,7], and continuously accumulate in the environment, causing long-term deleterious effects on both human and ecosystem health [8,9]. Hence, heavy metal-contaminated soils must be cleaned up to minimize their impact on ecosystems.
Phytoremediation is a promising eco-friendly, low-cost, and effective technology for the removal of heavy metals from the environment [10,11]. This technique is based on the capacity of plants and their associated microbes to remove heavy metals or lower their bioavailability in the soil through degradation, accumulation, and stabilization of the circulating pollutants [12,13]. Identifying and selecting suitable plant species is crucial for the success of a phytoremediation program [14]. The selected species must have the capacity to tolerate the heavy metal at different stages of the life cycle through different strategies [15]. Seed germination and early seedling growth are the plant development stages most sensitive to heavy metal stress [16,17] since plant defense mechanisms have not been completely developed at those stages [18]. However, several plant species have been evaluated in terms of their tolerance to the phytotoxic effects of heavy metals and have been proposed as metal-tolerant, with the potential for phytoremediation. Recently, Kalinhoff and Calderón [19] reported Bidens pilosa and Heliocarpus americanus as being metal-tolerant plants with potential for phytoremediation in a Hg mining area, since these species showed germination percentages between 40% and 50% and good growth up to 4 mg/l Hg. Another study reported that seeds of Nama aff. stenophylla can germinate (62–79%) even in the presence of high concentrations of Cd, As, Fe, Zn, and Pb (0.44, 1.44, 1.56, 37.25, and 8.09 mg/l, respectively), indicating tolerance to these heavy metals [20]. Nedjimi [21] stated that the exposure of Peganum harmala to increasing concentrations of Zn (0, 100, 200, and 300 µM) showed a low inhibitory effect on germination (<70%) and hypocotyl length.
One of the factors limiting the efficiency of the phytoremediation technique is plant adaptation to the environmental conditions of the target region [22]. The Central Andes region in Argentina is an Andean desert environment, characterized by a cold and dry climate, with scarce precipitation (120–300 mm/year) and high solar radiation [23]. Due to the harsh climatic conditions of this region, only native plant species are capable of overcoming these constraints, making them the most suitable to be considered for phytoremediation [24]. Numerous species of Fabaceae have been proposed as promising resources for phytoremediation due to their deep root system and their capability to establish a symbiotic relationship with soil nitrogen-fixing rhizobacteria. These features allow Fabaceae species to colonize marginal lands and nutrient-poor soils, like metal-contaminated soils [25,26,27]. Therefore, two Fabaceae species, Adesmia atacamensis, native to the Andes Mountains of Peru, and Adesmia horrida, native to the northern Andes Mountains of Argentina, have been reported to be suitable for the phytoextraction or phytostabilization of heavy metals (including Cu, Fe, Pb, and Zn) of mine tailings [28,29]. However, the potential of high-altitude native plants from the Central Andes Mountains of Argentina for phytoremediation has been explored only a little. Thus, the use of germination tests under different metal stresses could be helpful for the rapid identification of new plants with potential for phytoremediation.
The present study aimed to identify two native dominant species from the Central Andes Mountains of Argentina, Adesmia subterranea Clos and Adesmia pinifolia Gillies ex Hook and Arn, as metal-tolerant plant species for the first time, through the evaluation of germination and early seedling growth when exposed to heavy metals (Hg, Ni, Cd, and As).
2. Materials and Methods
2.1. Plant Material
A. subterranea Clos is a compact wooden cushion plant (up to 50 cm in height) that occurs between 3200 and 4000 m asl distributed along the Central Andes Mountains of Argentina and Chile [30,31]. A. pinifolia Gillies ex Hook and Arn is a shrub 1.5–2 m in height that grows between 1500 and 3700 m asl along the Andes Mountains of Argentina and Chile [30,31].
2.2. Seed Collection and Storage
Seeds of A. subterranea and A. pinifolia were randomly hand-collected from different communities in the Manzano Histórico Nature Reserve (33°36′37.00″ S 69°32′60″ W) and Cordon del Plata Provincial Park (32°58′47.53″ S 69°21′.59″ W), respectively. The fruit capsules were cleaned, and the seeds were then stored in the dark at 4 °C in a cold storage room until the germination test.
2.3. Heavy Metal Treatments
Test solutions were applied as heavy metal salts: Cd (ClCd2·H2O), Ni (NiSO4·6H2O), As (Na2HAsO4·7H2O), and Hg (Hg(NO3)2·H2O. For germination trials, three concentrations (the maximum legally allowed in agricultural soils in ArgentinaNational Hazardous Waste Law 24,051 and 150%, and 200% higher values) of each metal evaluated were tested: Cd (3, 4.5, and 6 ppm), Ni (150, 225, and 300 ppm), As (20, 30, and 40 ppm), and Hg (0.8, 1.2, and 1.6 ppm). Distilled water was used as control (C). Heavy metal treatment solutions were solidified with 0.8% (w/v) Bacto agar and adjusted to pH 5.5 using HCl (2N) prior to autoclaving [32].
2.4. Germination Assay
Intact and fully developed seeds were selected for the germination test. Immediately before use, seeds were surface-sterilized in sodium hypochlorite (1%) solution for 5 min to prevent fungal or bacterial contamination, and finally washed three times with tap water. To break seed dormancy, seeds were soaked in concentrated sulfuric acid (H2SO4) for 5 min, rinsed several times with tap water, and dried at room temperature [33].
Each treatment consisted of four replicates of 25 seeds each, which were placed on the surface of the agar in Petri dishes (9 cm diameter). Then, they were incubated in a growth chamber (Servicio Mecatrónico, Model Master 4200) under light (12 h light/12 h dark) and constant temperature (20/23 °C) conditions; the selected temperatures are within the optimal germination temperatures for the species [33]. Petri dishes were randomly distributed in the incubators and their positions were changed daily. Seed germination was recorded daily, and the seeds were considered germinated when the radicle was approximately 1 mm in length. The experiment ended after 15 days.
2.5. Germination Response Variables
Germination percentage: Germination percentage (GP) of seeds exposed to the heavy metal treatments was calculated as the number of germinated seeds/total number of seeds × 100.
Mean time to germinate (MTG) was estimated for each species using the following formula [34]:
where n is the number of seeds germinated between scoring intervals, d is the incubation period in days at that time point, and N is the total number of seeds germinated in the treatment.
2.6. Metal Tolerance
In order to evaluate the effect of the heavy metal treatment on roots, root length (RL) was measured and the metal tolerance index (MTI) was calculated after 15 days of seedling growth. Root length was measured using a stainless-steel digital caliper (Wemberley, accuracy 0.01 mm) and tolerance values of root elongation were calculated using the following formula [35]:
where RL is radicle length in metal treatment plants and RLc is radicle length in the control.
2.7. Fresh Weight and Dry Weight of Early Seedlings
After 15 days of exposure to heavy metal treatments, early seedling growth was measured as the fresh weight (FW) and dry weight (DW) of the entire seedling. To determine the DW, the seedlings were placed in an oven at 70 ºC for 3 days until a constant weight was reached.
2.8. Statistical Analyses
Data were subjected to two-way ANOVA (heavy metal treatments and species as factors) using INFOSTAT [36], followed by the DGC post hoc test at p < 0.05 to detect significant differences between means.
3. Results
3.1. Germination Percentage
Germination percentage (GP) was significantly influenced by species (p < 0.0001), heavy metal treatments (p < 0.0001), and their interaction (two-way ANOVA p < 0.005). A. subterranea was significantly more sensitive to all the heavy metals evaluated than A. pinifolia (GP: 79.5% and 90.69%, respectively). Among the heavy metals evaluated, Ni exhibited the greatest inhibitory effect on A. pinifolia seed germination (Figure 1). GP decreased gradually with increasing metal concentration, with a maximum germination decline of 17.7% and 15% compared to the control observed at Ni 300 ppm and Ni 225 ppm, respectively. The same tendency was observed for the As treatments, with the highest reduction being recorded at 30 ppm (5%) and 40 ppm (11%). In contrast, A. pinifolia maintained similar GP values in Cd treatments compared to the control (Figure 1). At Hg 1.2 ppm, no inhibitory effect was detected. The seed germination of A. subterranea was significantly reduced with exposure to As and Ni at different concentrations (Figure 2). The highest reduction (26%) in GP was recorded for the Ni 300 ppm treatment, followed by As 40 ppm (23%). By contrast, GP was not significantly affected by the Hg and Cd treatments compared to the control (Figure 2).
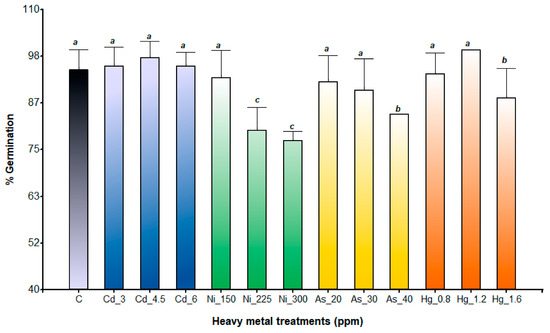
Figure 1.
Germination percentage of A. pinifolia seeds exposed to Cd (3, 4.5, and 6 ppm), Ni (150, 225, and 300 ppm), As (20, 30, and 40 ppm), and Hg (0.8, 1.2, and 1.6 ppm) and C (control). Values are expressed as means ± SD. Different letters denote significant differences between treatments (p < 0.05) according to the DGC test.
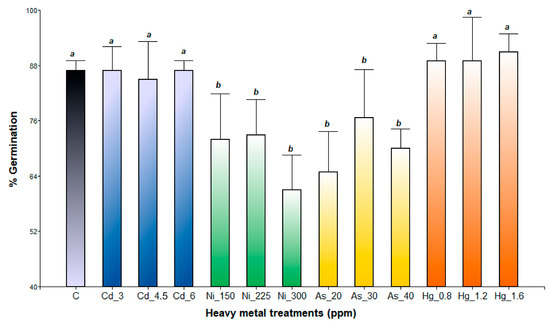
Figure 2.
Germination percentage of A. subterranea seeds exposed to Cd (3, 4.5, and 6 ppm), Ni (150, 225, and 300 ppm), As (20, 30, and 40 ppm), and Hg (0.8, 1.2, and 1.6 ppm) and C (control). Values are expressed as means ± SD. Different letters denote significant differences between treatments (p < 0.05) according to the DGC test.
3.2. Mean Time to Germinate
The statistical interaction between the fixed factors (species and heavy metal treatments) significantly affected the MTG (p < 0.0001). In A. pinifolia, MTG values were similar for all the heavy metal treatments (p = 0.1953), ranging between 1.60 ± 0.08 and 1.98 ± 0.31 days (Figure 3). In contrast, A. subterranea seeds under all the As treatment conditions had the longest MTG (Figure 4). The number of days to germinate increased with increasing As concentrations (2.14 ± 0.45 days at As 20 ppm, 2.33 ± 0.12 days at As 30 ppm, and 4.20 ± 0.14 days at As 40 ppm). Likewise, in Ni treatments, MTG was also significantly reduced (1.96 ± 0.15 days at Ni 150 ppm, 1.70 ± 0.04 days at Ni 225 ppm, and 1.99 ± 0.17 days at Ni 300 ppm). The lowest MGT values were recorded in seeds germinated in the Cd, Hg, and control treatments (Figure 4).
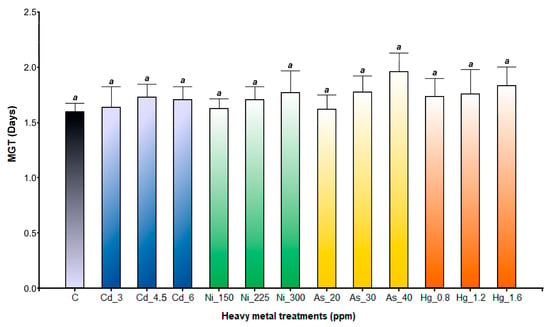
Figure 3.
Mean time to germinate in A. pinifolia seeds exposed to Cd (3, 4.5, and 6 ppm), Ni (150, 225, and 300 ppm), As (20, 30, and 40 ppm), and Hg (0.8, 1.2, and 1.6 ppm) and C (control). Values are expressed as mean ± SD. Different letters denote significant differences between treatments (p < 0.05) according to the DGC test.
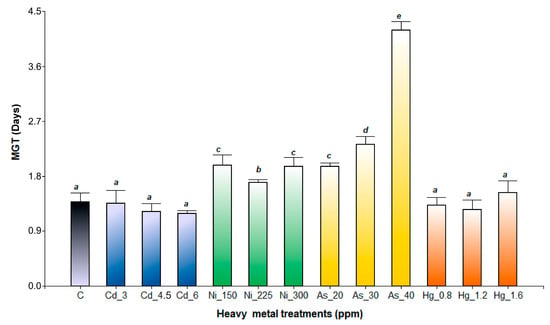
Figure 4.
Mean time to germinate in A. subterranea seeds exposed to Cd (3, 4.5, and 6 ppm), Ni (150, 225, and 300 ppm), As (20, 30, and 40 ppm), and Hg (0.8, 1.2, and 1.6 ppm) and C (control). Values are expressed as mean ± SD. Different letters denote significant differences between treatments (p < 0.05) according to the DGC test.
3.3. Radicle Length
Radicle length was affected by species, heavy metal treatments, and their interaction (two-way ANOVA p < 0.0001), (Table 1). For the Ni treatments, the radicle length of A. pinifolia was significantly inhibited compared with that of the control, resulting in the lowest values of all treatments (Table 1). The increasing Ni concentration had a pronounced effect on the reduction of radicle length (96.5% at 150 ppm, 96.7% at 225 ppm, and 96.5% at 300 ppm). The same trend was observed in the As and Cd treatments, in which radicle length was also affected, reaching values significantly lower than those of the control (Table 1). However, radicle length increased significantly in all the Hg treatments compared with the control, especially the 1.2 ppm treatment (10.67 cm). The inhibition effect of heavy metals on the radicle length of A. pinifolia decreased in the order Ni > As > Cd > Hg.

Table 1.
Effects of heavy metal (Cd, Ni, As, and Hg) treatments on radicle length and metal tolerance index (MTI) of A. pinifolia and A. subterranea after 15 days of exposure.
In the case of A. subterranea, both the Ni and As treatments reduced root length significantly compared with the control. The maximum toxicity was observed at Ni 300 ppm, with a reduction of 95.3%, and at As 40 ppm, with a reduction of 91.9%. Similarly, all Cd treatments significantly decreased root length, but with no significant differences between concentrations (Table 1). In contrast, for the Hg treatments, radicle length increased significantly compared with the control, reaching the highest values in the Hg 1.2 ppm treatment (9.38 cm). The inhibition effect on radicle length in this species by heavy metals decreased in the order Ni > As > Cd > Hg.
3.4. Metal Tolerance Index
Metal tolerance index (MTI) was significantly affected by metal treatments, species, and their interaction (p < 0.0001) (Table 1). The increasing Ni concentration reduced the MTI of A. pinifolia significantly with respect to the control and the remaining heavy metal treatments, with values ranging from 2.60% (Ni 300 ppm) through 3.23% (Ni 225 ppm) to 3.41% (Ni 150 ppm). The same tendency was observed in the Cd and As treatments. In contrast, seeds exposed to the Hg treatments had a significantly higher MTI than control seeds. The maximum value (114.68%) was recorded at Hg 1.2 ppm (Table 1). The order of toxicity of heavy metals was Ni > As > Cd > Hg. The MTI of A. subterranea in all Ni treatments and As 40 ppm was drastically reduced with respect to the control, reaching the lowest value at Ni 300 ppm (4.61%). The Hg treatments did not show any phytotoxic effect, with MTI being significantly higher than that of the control (141% at Hg 0.8 ppm, 157.5% Hg 1.2 ppm, and 152% at 1.6 ppm) (Table 1). The order of toxicity of heavy metals was Ni > As > Cd > Hg.
3.5. Fresh Weight and Dry Weight of Early Seedlings
Two-way ANOVA showed that the heavy metal treatments (p < 0.0001), species (p < 0.0001, and their interaction (p = 0.0397) significantly affected the FW of early seedlings (Table 2). Increases in Ni and As concentration decreased the FW of A. pinifolia significantly compared to the control. The highest reduction was observed at (Ni 150 ppm (269.2 mg), Ni 225 ppm (242.7 mg), Ni 300 ppm (245.28 mg), and As 40 ppm (279.35 mg). Similarly, Cd significantly reduced the FW compared to the control; however, no significant difference was observed with increasing concentrations. In contrast, the highest seedling FW was recorded for the Hg 1.2 ppm treatments (948.83 mg), with no differences from the control (961.78 mg) (Table 2). Early seedling FW of A. subterranea decreased significantly with increasing concentrations of Ni and As, reaching the minimum value in the Ni 300 ppm treatment (160.38 mg). All the Hg treatments increased the FW values, with no differences from the control. The highest FW was recorded for the Hg 1.6 ppm treatment (841.78 mg). For the Cd treatment, FW values were significantly lower than those of the control and Hg treatments, but higher than the Ni and As treatments, with no differences among concentrations (Table 2).

Table 2.
Effects of heavy metal (Cd, Ni, As, and Hg) treatments on fresh weight (FW) and dry weight (DW) of A. pinifolia and A. subterranea after 15 days of exposure.
Dry weight (DW) was significantly affected by metal treatments (p < 0.001), species (p < 0.0001), and their interaction (p = 0.0345) (Table 2). Increases in As concentration significantly reduced the DW of A. pinifolia compared to the control. The lowest value was recorded at As 40 ppm (48.25 mg) and As 30 ppm (64.4 mg). Similarly, DW values were significantly reduced by Cd and Ni compared to the control, being similar between treatments. On the other hand, the early seedling DW was highest for the control and Hg 1.2 ppm, with significant differences from the remaining treatments (Table 2). The DW of A. subterranea under exposure to As, Ni, and Cd was significantly lower than that in the control and Hg treatments. Contrarily, Hg treatments did not affect the DW, with values not differing from the control (Table 2).
4. Discussion
Some heavy metals are essential micronutrients (i.e., Cu, Mg, Zn, and Ni); however, at high concentrations, they can induce toxic effects in plants. Other non-essential metals and metalloids (i.e., Cd, Hg, Pb, and As), which do not play any beneficial role in plant growth, can cause toxicity symptoms and metabolic damage to plants at very low concentrations [37,38]. Therefore, the evaluation of heavy metal tolerance during germination and early seedling development may give an insight into the tolerance of these species at later stages of their life cycle [32,39]. In the present study, we evaluated the effect of different concentrations of heavy metals (Hg, Ni, Cd, and As) on seed germination, early seedling growth, and root elongation of two endemic species of the Central Andean Mountains of Argentina, A. pinifolia and A. subterranea, with the aim to establish their phytoremediation potential.
Nickel is considered an essential microelement for plant growth and development, but excessive Ni levels in the soil can be toxic to plants. The common indicators of Ni phytotoxicity to plants include inhibition of germination and reduction in the germination speed index [40,41]. In our study, the Ni treatments had an adverse effect on seed germination and MGT. We observed a major decrease (of at least 39%) in germination for A. subterranea and 23% for A. pinifolia under 300 ppm Ni treatment conditions as compared to the control. Increasing Ni concentrations significantly delayed the germination process of A. subterranea, but did not statistically significantly affect the MGT of A. pinifolia. These results are in agreement with previous records reported in the literature, in that Ni at high concentrations has a negative effect on seed germination in various species. For example, Vyas [42] reported a 37% decrease in the germination of Rauwolfia serpentina when exposed to 200 ppm Ni. In addition, the germination of Coronilla varia and Trifolium arvense was reduced by 18% and 92.5%, respectively, under exposure to 200 ppm Ni [32]. These results could be attributed to the influence of Ni on the performance of α-amylase and protease functions, which, in turn, affect the digestion and utilization of food reserves in germinating seeds [43,44]. In addition to germination, the most important observable consequence of Ni toxicity in plants is root elongation suppression and growth inhibition [45]. In the case of A. pinifolia and A. subterranea, increasing Ni concentrations significantly reduced radicle length (0.24 cm and 0.28 cm, respectively) and MTI (2.6 and 4.61%, respectively) compared to the control (9.30 cm and 5.96 cm, respectively). Elevated concentrations of Ni can significantly impact cell division in root meristems, altering root length [40]. Similarly, a root length decrease due to the presence of Ni was also reported for Phacelia tanacetifolia, Cucumis sativus, and Brassica nigra [46]; Sorghum bicolor [47]; and Salsola vermiculata [48].
Arsenic has no known biological role in plants, being generally toxic. The main effects reported are a reduction in or inhibition of seed germination, root elongation, growth, and various earlier developmental processes that occur during the initial stages of seedling development [49]. It has been well documented that the same concentrations of heavy metals may have different effects among species or even varieties [43,50,51]. In A. pinifolia, the germination parameters evaluated were significantly affected only at the highest As concentration evaluated (40 ppm), with a reduction in germination by 11% in comparison to the control. A. subterranea was more sensitive to As, being affected at all concentrations. Germination decreased by 23% compared to the control and took 4.20 days. Our results are supported by previous findings, which state that germination was reduced remarkably in certain species subjected to As, like Oryza sativa [52], Cymbopogon jwarancusa [53], and Helichrysum tyrrhenicum [54]. High concentrations of As might have an adverse effect on α-amylase, which is an important enzyme responsible for seed germination, ultimately leading to a decrease in the percentage of germination [51]. The inhibition of As was stronger at the early seedling stage than during germination. Root length, MTI, and biomass displayed a decreasing trend with increasing As concentrations in the media. The inhibition of root length for both species, A. pinifolia and A. subterranea, was significantly affected at up to 40 ppm, with a 91% decrease compared to the controls. In contrast, the inhibition of seedling biomass was stronger for A. subterranea than for A. pinifolia, showing that the latter species is less tolerant of As. Reduced root length and seedling growth in response to As exposure has been frequently reported in Chenopodium quinua, Cistus salviifolius, and Cymbopogon jwarancusa [53,55,56].
Mercury is a highly toxic nonessential metal in plants, even at relatively low concentrations, [57]. However, some species have shown the ability to germinate and tolerate Hg at certain levels. Rodríguez-Alonso et al. [58] reported that Quercus ilex was able to tolerate medium–high Hg concentrations (25–50 µM), reaching germination percentages over 65%. Pusa jai Kisan, an Indian mustard (Brassica juncea L.) genotype, maintained germination percentages above 80% after being exposed to various Hg concentrations (5, 10, 25, and 50 µM) [59]. These results are in agreement with our findings, showing that in some cases, Hg can stimulate germination. A. pinifolia and A. subterranea managed to tolerate up to 1.6 ppm, the degree of germination ranged between 88 and 100%, and the mean germination time was unaffected, indicating a high tolerance. Mercury generally inhibited plant growth, causing a considerable decline in the root length, shoot length, and biomass of seedlings in a dose-dependent manner. In contrast, all the Hg concentrations tested in this work stimulated radicle elongation in both species. When subjected to Hg 1.2 ppm treatment, A. pinifolia exhibited a 17% greater root length than the control. Similarly, A. subterranea demonstrated a remarkable increase in root growth (57.8%) compared to the control. On the other hand, seedling growth was unaffected by Hg treatments, reaching the same values as the control. The absence of negative impacts caused by Hg on seed germination and seedling growth may be attributed to a hormetic effect. Plants exposed to low concentrations of non-essential and toxic metal ions were found to frequently undergo hormetic growth stimulation [60,61]. This finding is related to the fact that a small amount of mercury in plant cells could induce oxidative stress and enhance antioxidant enzyme activity [62]. The induction of growth at low concentrations of Hg has been reported for other species such as Zinger officinale (≤1.5 mg/kg Hg) [63] and Cyrtomium macrophyllum (5–200 mg/kg Hg) [64].
Cadmium is considered one of the most toxic heavy metals, with negative effects on plant growth and development, even at very low concentrations [65]. Nevertheless, in response to all the Cd treatments, A. pinifolia and A. subterranea were able to germinate by over 96% and 87%, respectively, without differing from the control, and the time to germinate was not reduced. These results suggest that the concentrations evaluated did not disrupt the respiratory function or depletion of seed reserves. These results are consistent with previous findings for Triticum aestivum (CV. Seh06 and Far06) [43], Ambrosia artemisiifolia [32], and Eruca sativa [66]. Despite the Cd tolerance of germination, root development was more sensitive to the toxic effect of Cd. The reason for the occurrence of this harmful impact could be due to the direct contact of metals with the developing seedling after radicle protrusion [67]. Root length and MTI of both species were significantly inhibited, with the phytotoxic effect increasing as the Cd concentration increased. However, the critical concentration that resulted in significant inhibitions varied between species: for A. pinifolia, it was observed at 4.5 ppm and for A. subterranea, at 6 ppm. The same trend was observed for biomass. Raising the concentration of Cd usually results in growth suppression, since this metal has a tendency to accumulate within plant tissues and impede crucial physiological processes [68]. Similar results were found for three varieties of Indian mustard (Brassicaceae) [69], Arabidopsis thaliana [70], and Albizia lebbeck [71] under Cd treatments, with fresh and dry weight and heavy metal tolerance index decreasing with increasing concentrations. The documented increase in the impact of cadmium could be due to its influence on hydrolytic enzymes, reducing the availability of food for the developing radicle and plumule.
5. Conclusions
Screening for germination and early seedling development is a fast and practical way to obtain information about how plant species respond to heavy metals. The present study showed that A. pinifolia and A. subterranea respond differentially to heavy metal type and concentration. The Hg treatments tested had no toxic effect on seed germination or early seedling growth in either species. Therefore, Adesmia species could be a potential option for remediating soils contaminated with Hg. Ni and As showed a detrimental effect on germination; moreover, the most significant impact was observed on root growth. This consequence may potentially affect the successful establishment and growth of Adesmia. However, more studies are needed to characterize the response of these species at later stages, especially for A. subterranea, which showed a higher sensitivity to Ni and As. Cd had no toxic effects on seed germination; nevertheless, further growth and development exhibited certain toxicity at the highest concentration evaluated (6 ppm). Therefore, both species could be successfully grown at low concentrations of Cd. As an overall conclusion, according to our results, the order of toxicity for A. pinifolia and A. subterranea can be classified as Ni > As > Cd > Hg. We recommend conducting further tests at later stages of development for a better understanding of the tolerance of these species.
Author Contributions
V.P. performed all biological assays. V.P., C.A.P. and G.E.F. conceptualized the study, formal analysis, and wrote the article. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially funded by ANPCyT (PICT-2021-I-A-01048), and CICITCA-UNSJ (Argentina) PDTS No. 80020220200052SJ.
Institutional Review Board Statement
The seed collecting was approved by Dirección de Recursos Naturales Renovables-Secretaría de Ambiente y Ordenamiento Territorial (RIT-2021-183-E-GDEMZA-DRNR#SAYOT).
Data Availability Statement
Not applicable.
Acknowledgments
V.P. hold a fellowship from CONICET and Facultad de Ciencias Agrarias UNCuyo. GEF is researcher from CONICET. To M. Piñeiro and S. Manrique for the support. To Fundación ArgenINTA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brenning, A.; Azócar, G.F. Minería y glaciares rocosos: Impactos ambientales, antecedentes políticos y legales, y perspectivas futuras. Rev. Geogr. Norte. Gd. 2010, 47, 143–158. [Google Scholar] [CrossRef]
- [SSPE] Subsecretaría de Planificación Económica. Secretaría de Política Económica y Planificación del Desarrollo. Ministerio de Hacienda y Finanzas Públicas. Informes de Cadenas de Valor: Minería Metalífera y Rocas de Aplicación. 2016. ISSN 2525-2221. Available online: https://www.argentina.gob.ar/sites/default/files/sspe_cadena_de_valor_mineria.pdf (accessed on 10 July 2022).
- Mendez, M.; Maier, R.M. Phytostabilization of Mine Tailings in Arid and Semiarid Environments-An Emerging Remediation Technology. Environ. Health Perspect. 2008, 3, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, H.J.P.; Doronila, A.I.; Nicolas, M.; Ebbs, S.D.; Kolev, S.D. Growth of selected plant species in biosolids-amended mine tailings. Min. Eng. 2015, 80, 25–32. [Google Scholar] [CrossRef]
- Yan, B.; Xu, D.M.; Chen, T.; Yan, Z.A.; Li, L.L.; Wang, M.H. Leachability characteristic of heavy metals and associated health risk study in typical copper mining-impacted sediments. Chemosphere 2020, 239, 124748. [Google Scholar] [CrossRef]
- Schwegler, F. Air quality management: A mining perspective. WIT Trans. Ecol. Environ. 2006, 86, e1339. [Google Scholar] [CrossRef]
- Al-Taani, A.A.; Nazzal, Y.; Howari, F.M. Assessment of heavy metals in roadside dust along the Abu Dhabi–Al Ain National Highway, UAE. Environ. Earth Sci. 2019, 78, 411. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Kiran; Bharti, R.; Sharma, R. Effect of heavy metals: An overview. Mater. Today Proc. 2022, 51, 880–885. [Google Scholar] [CrossRef]
- Liu, S.; Yang, B.; Liang, Y.; Xiao, Y.; Fang, J. Prospect of phytoremediation combined with other approaches for remediation of heavy metal-polluted soils. Environ. Sci. Pollut. Res. 2020, 27, 16069–16085. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Imran, A.; Naz, I. A critical review on the phytoremediation of heavy metals from environment: Performance and challenges. Chemosphere 2021, 291, 132979. [Google Scholar] [CrossRef]
- Cameselle, C.; Gouveia, S.; Urréjola, S. Benefits of phytoremediation amended with DC electric field. Application to soils contaminated with heavy metals. Chemosphere 2019, 229, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Enhancing phytoremediation of soils polluted with heavy metals. Curr. Opin. Biotechnol. 2022, 74, 21–31. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Yang, H.; Li, X.; Cui, Z. Physiological responses of Suaeda glauca and Arabidopsis thaliana in phytoremediation of heavy metals. J. Environ. Manag. 2018, 223, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, M.; Rajakaruna, N.; Rizwan, M.; Madawala, H.M.S.P.; Ok, Y.S.; Vithanage, M. Heavy metal-induced oxidative stress on seed germination and seedling development: A critical review. Environ. Geochem. Health 2019, 41, 1813–1831. [Google Scholar] [CrossRef]
- Samma, M.K.; Zhou, H.; Cui, W.; Zhu, K.; Zhang, J.; Shen, W. Methane alleviates copper-induced seed germination inhibition and oxidative stress in Medicago sativa. Biometals 2017, 30, 97–111. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, S.; Shan, X.; Zhu, Y.-G. Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere 2005, 61, 293–301. [Google Scholar] [CrossRef]
- Kalinhoff, C.; Calderón, N.-T. Mercury Phytotoxicity and Tolerance in Three Wild Plants during Germination and Seedling Development. Plants 2022, 11, 2046. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Espinosa, L.; Briones-Gallardo, R.; Flores, J.; del Castillo, E. Effect of heavy metals on seed germination and seedling development of Nama aff. stenophylla collected on the slope of a mine tailing dump. Int. J. Phytoremediat。 2020, 22, 1448–1461. [Google Scholar] [CrossRef]
- Nedjimi, B. Germination characteristics of Peganum harmala L. (Nitrariaceae) subjected to heavy metals: Implications for the use in polluted dryland restoration. Int. J. Environ. Sci. Technol. 2020, 17, 2113–2122. [Google Scholar] [CrossRef]
- Mahar, A.; Wang, P.; Ali, A.; Awasthi, M.K.; Lahori, A.H.; Wang, Q.; Li, R.; Zhang, Z. Challenges and opportunities in the phytoremediation of heavy metals contaminated soils: A review. Ecotoxicol. Environ. Saf. 2016, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Roig, F.A.; Carretero, E.M. La vegetación puneña en la provincia de Mendoza, Argentina. Phytocoenologia 1998, 28, 565–608. [Google Scholar] [CrossRef]
- Arreghini, S.; de Cabo, L.; Serafini, R.; de Iorio, A.F. Effect of the combined addition of Zn and Pb on partitioning in sediments and their accumulation by the emergent macrophyte Schoenoplectus californicus. Environ. Sci. Pollut. Res. 2017, 24, 8098–8107. [Google Scholar] [CrossRef]
- Olson, P.E.; Castro, A.; Joern, M.; DuTeau, N.M.; Pilon-Smits, E.A.H.; Reardon, K.F. Comparison of Plant Families in a Greenhouse Phytoremediation Study on an Aged Polycyclic Aromatic Hydrocarbon-Contaminated Soil. J. Environ. Qual. 2007, 36, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Wani, P.A.; Khan, M.S. Bioremediation: A Natural Method for the Management of Polluted Environment. In Toxicity of Heavy Metals to Legumes and Bioremediation; Zaidi, A., Wani, P., Khan, M., Eds.; Springer: Vienna, Austria, 2012; pp. 101–114. [Google Scholar] [CrossRef]
- Hao, X.; Taghavi, S.; Xie, P.; Orbach, M.J.; Alwathnani, H.A.; Rensing, C.; Wei, G. Phytoremediation of Heavy and Transition Metals Aided by Legume-Rhizobia Symbiosis. Int. J. Phytoremediat. 2014, 16, 179–202. [Google Scholar] [CrossRef] [PubMed]
- Cazón, J.P.; Benítez, L.; Murray, J.; Kirschbaum, A.; Kirschbaum, P.; Donati, E. Environmental Impact on Soil, Water and Plants from the Abandoned Pan de Azúcar Mine. Adv. Mater. Res. 2013, 825, 88–91. [Google Scholar] [CrossRef]
- Lam, E.J.; Keith, B.F.; Montofré, L.; E Gálvez, M. Copper Uptake by Adesmia atacamensis in a Mine Tailing in an Arid Environment. Air, Soil Water Res. 2018, 11, 1178622118812462. [Google Scholar] [CrossRef]
- Kiesling, R. Flora de San Juan, República Argentina: Vol.1, Pteridófitas Gimnospermas, Dicotiledóneas, Dialipétalas (Salicáceas a leguminosas); Vazquez Mazzini: Buenos Aires, Argentina, 1994; pp. 291–301. [Google Scholar]
- Ulibarri, E.A.; Burkart, A. Sinopsis de las especies de Adesmia (Leguminosae-Papilionoideae) de la Argentina. Darwiniana 2000, 38, 59–126. [Google Scholar]
- Bae, J.; Benoit, D.L.; Watson, A.K. Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ. Pollut. 2016, 213, 112–118. [Google Scholar] [CrossRef]
- Parera, C.A.; Ruiz, M. Adesmia subterranea Clos Germination Physiology and Presowing Treatments. J. Range Manag. 2003, 56, 273. [Google Scholar] [CrossRef]
- Brenchley, J.L.; Probert, R.J. Seed germination responses to some environmental factors in the seagrass Zostera capricorni from eastern Australia. Aquat. Bot. 1998, 62, 177–188. [Google Scholar] [CrossRef]
- Wilkins, D.A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat version. InfoStat Transfer Center, FCA, National University of Córdoba, Argentina. 2020. Available online: http://www.infostat.com.ar (accessed on 8 October 2022).
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 56–66. [Google Scholar]
- Tiwari, S.; Sarangi, B.K. Comparative analysis of antioxidant response by Pteris vittata and Vetiveria zizanioides towards arsenic stress. Ecol. Eng. 2017, 100, 211–218. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, J.S. Effects of nickel chloride on germination and seedling growth of different wheat (Triticum aestivum L. em Thell.) cultivars. J. Pharm. Phytochem. 2018, 7, 2227–2234. [Google Scholar]
- Bhalerao, S.A.; Sharma, A.S.; Poojari, A.C. Toxicity of nickel in plants. Int. J. Pure Appl. Biosci. 2015, 3, 345–355. [Google Scholar]
- Da Cunha Neto, A.R.; Carvalho, M.; Morais, G.M.M.; Guaraldo, M.M.D.S.; dos Santos, H.O.; Pereira, W.V.S.; Barbosa, S. Changes in Chromosome Complement and Germination of Lettuce (Lactuca sativa L.) Exposed to Heavy Metal Stress. Water Air Soil Pollut. 2023, 234, 243. [Google Scholar] [CrossRef]
- Vyas, M.K. The toxic effects of nickel and cadmium on germination, seedling growth and biochemical contents of Rauwolfia serpentina Benth. ex Kruz. J. Physiol. Biochem. 2022, 18, 76–87. [Google Scholar]
- Ahmad, M.A.; Gaur, R.; Gupta, M. Comparative biochemical and RAPD analysis in two varieties of rice (Oryza sativa) under arsenic stress by using various biomarkers. J. Hazard. Mater. 2012, 217, 141–148. [Google Scholar] [CrossRef]
- Ashraf, M.Y.; Sadiq, R.; Hussain, M.; Ahmad, M.S.A. Toxic Effect of Nickel (Ni) on Growth and Metabolism in Germinating Seeds of Sunflower (Helianthus annuus L.). Biol. Trace Element Res. 2011, 143, 1695–1703. [Google Scholar] [CrossRef]
- Lešková, A.; Giehl, R.F.H.; Hartmann, A.; Fargašová, A.; von Wirén, N. Heavy Metals Induce Iron Deficiency Responses at Different Hierarchic and Regulatory Levels. Plant Physiol. 2017, 174, 1648–1668. [Google Scholar] [CrossRef] [PubMed]
- Visioli, G.; Conti, F.D.; Gardi, C.; Menta, C. Germination and root elongation bioassays in six different plant species for testing Ni contamination in soil. Bull Environ. Contam Toxicol. 2014, 92, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Ertekin, E.N.; Ertekin, İ.; Bilgen, M. Effects of some heavy metals on germination and seedling growth of sorghum. Kahramanmaraş Sütçü İmam Üniversitesi Tarım Ve Doğa Dergisi 2020, 23, 1608–1615. [Google Scholar] [CrossRef]
- Sanjosé, I.; Muñoz-Rodríguez, A.F.; Ruiz, F.; Navarro, F.; Sánchez-Gullón, E.; Nieva, F.J.; Polo, A.; Infante, M.D.; Castillo, J.M. Metal effects on germination and seedling development in closely-related halophyte species inhabiting different elevations along the intertidal gradient. Mar. Pollut. Bull. 2022, 175, 113375. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M.; Natasha. Arsenic Uptake, Toxicity, Detoxification, and Speciation in Plants: Physiological, Biochemical, and Molecular Aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Moulick, D.; Ghosh, D.; Santra, S.C. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol. Biochem. 2016, 109, 571–578. [Google Scholar] [CrossRef]
- Chintey, R.; Prakash, P.; Kumar, K. Evaluation of Germination Attributes, Metal Tolerance Index and Phytotoxicity Index in Green Gram Cultivars [Vigna radiata (L) Wilczek] under Arsenic Toxicity. Res. Jr. Agril. Sci. 2022, 13, 1289–1292. [Google Scholar]
- Zia, Z.; Bakhat, H.F.; Saqib, Z.A.; Shah, G.M.; Fahad, S.; Ashraf, M.R.; Hammad, H.M.; Naseem, W.; Shahid, M. Effect of water management and silicon on germination, growth, phosphorus and arsenic uptake in rice. Ecotoxicol. Environ. Saf. 2017, 144, 11–18. [Google Scholar] [CrossRef]
- Ibrahim, M.; Nawaz, S.; Iqbal, K.; Rehman, S.; Ullah, R.; Nawaz, G.; Almeer, R.; Sayed, A.A.; Peluso, I. Plant-Derived Smoke Solution Alleviates Cellular Oxidative Stress Caused by Arsenic and Mercury by Modulating the Cellular Antioxidative Defense System in Wheat. Plants 2022, 11, 1379. [Google Scholar] [CrossRef]
- Boi, M.E.; Porceddu, M.; Cappai, G.; De Giudici, G.; Bacchetta, G. Effects of zinc and lead on seed germination of Helichrysum microphyllum subsp. tyrrhenicum, a metal-tolerant plant. Int. J. Environ. Sci. Technol. 2020, 17, 1917–1928. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Vieira, C.; Abreu, M.M.; Magalhães, M.C.F. Physiological response of Cistus salviifolius L. to high arsenic concentrations. Environ. Geochem. Health 2020, 42, 2305–2319. [Google Scholar] [CrossRef] [PubMed]
- Parvez, S.; Abbas, G.; Shahid, M.; Amjad, M.; Hussain, M.; Asad, S.A.; Imran, M.; Naeem, M.A. Effect of salinity on physiological, biochemical and photostabilizing attributes of two genotypes of quinoa (Chenopodium quinoa Willd.) exposed to arsenic stress. Ecotoxicol. Environ. Saf. 2020, 187, 109814. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Z.M. Mercury toxicity, molecular response and tolerance in higher plants. Biometals 2012, 25, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alonso, J.; Sierra, M.J.; Lominchar, M.; Millán, R. Effects of mercury on the germination and growth of Quercus ilex L. seedlings. Environ. Sci. Pollut. Res. 2019, 26, 30930–30940. [Google Scholar] [CrossRef]
- Ansari, M.K.A.; Ahmad, A.; Umar, S.; Iqbal, M.; Zia, M.H.; Husen, A.; Owens, G. Suitability of Indian mustard genotypes for phytoremediation of mercury-contaminated sites. South Afr. J. Bot. 2021, 142, 12–18. [Google Scholar] [CrossRef]
- Kranner, I.; Colville, L. Metals and seeds: Biochemical and molecular implications and their significance for seed germination. Environ. Exp. Bot. 2011, 72, 93–105. [Google Scholar] [CrossRef]
- Agathokleous, E. Environmental hormesis, a fundamental non-monotonic biological phenomenon with implications in ecotoxicology and environmental safety. Ecotoxicol. Environ. Saf. 2018, 148, 1042–1053. [Google Scholar] [CrossRef]
- Chen, J.; Shiyab, S.; Han, F.X.; Monts, D.L.; Waggoner, C.A.; Yang, Z.; Su, Y. Bioaccumulation and physiological effects of mercury in Pteris vittata and Nephrolepis exaltata. Ecotoxicology 2009, 18, 110–121. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Lv, Y.; Xu, K.; Lu, S.; Liu, X.; Yang, Y. Effect of soil mercury pollution on ginger (Zingiber officinale Roscoe): Growth, product quality, health risks and silicon mitigation. Ecotoxicol. Environ. Saf. 2020, 195, 110472. [Google Scholar] [CrossRef]
- Xun, Y.; Feng, L.; Li, Y.; Dong, H. Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere 2017, 189, 161–170. [Google Scholar] [CrossRef]
- Muszyńska, E.; Hanus-Fajerska, E.; Ciarkowska, K. Studies on lead and cadmium toxicity in Dianthus carthusianorum calamine ecotype cultivated in vitro. Plant Biol. 2018, 20, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Zhi, Y.; Deng, Z.; Luo, M.; Ding, W.; Hu, Y.; Deng, J.; Li, Y.; Zhao, Y.; Zhang, X.; Wu, W.; et al. Influence of Heavy Metals on Seed Germination and Early Seedling Growth in Eruca sativa Mill. Am. J. Plant Sci. 2015, 6, 582–590. [Google Scholar] [CrossRef]
- Moreira, I.N.; Martins, L.L.; Mourato, M.P. Effect of Cd, Cr, Cu, Mn, Ni, Pb and Zn on seed germination and seedling growth of two lettuce cultivars (Lactuca sativa L.). Plant Physiol. Rep. 2020, 25, 347–358. [Google Scholar] [CrossRef]
- Di Toppi, L.S.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot. 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Chowardhara, B.; Borgohain, P.; Saha, B.; Awasthi, J.P.; Moulick, D.; Panda, S.K. Phytotoxicity of Cd and Zn on three popular Indian mustard varieties during germination and early seedling growth. Biocatal. Agric. Biotechnol. 2019, 21, 101349. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, J.; Jeon, Y.; Kim, H.; Jang, G.; Yoon, Y. Assessing the effects of accumulated Cd(II) on seed germination and root development of Arabidopsis thaliana. Appl. Biol. Chem. 2021, 64, 1–9. [Google Scholar] [CrossRef]
- Farooqi, Z.R.; Iqbal, M.Z.; Kabir, M.; Shafiq, M. Toxic effects of lead and cadmium on germination and seedling growth of Albizia lebbeck (L.) Benth. Pak. J. Bot. 2009, 41, 27–33. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).