Community Richness and Diversity of Endophytic Fungi Associated with the Orchid Guarianthe skinneri Infested with “Black Blotch” in the Soconusco Region, Chiapas, Mexico
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Disinfestation and Preservation of Biological Material for Mass Sequencing
2.3. DNA Extraction and PCR
2.4. Statistical Analysis and Bioinformatics
3. Results
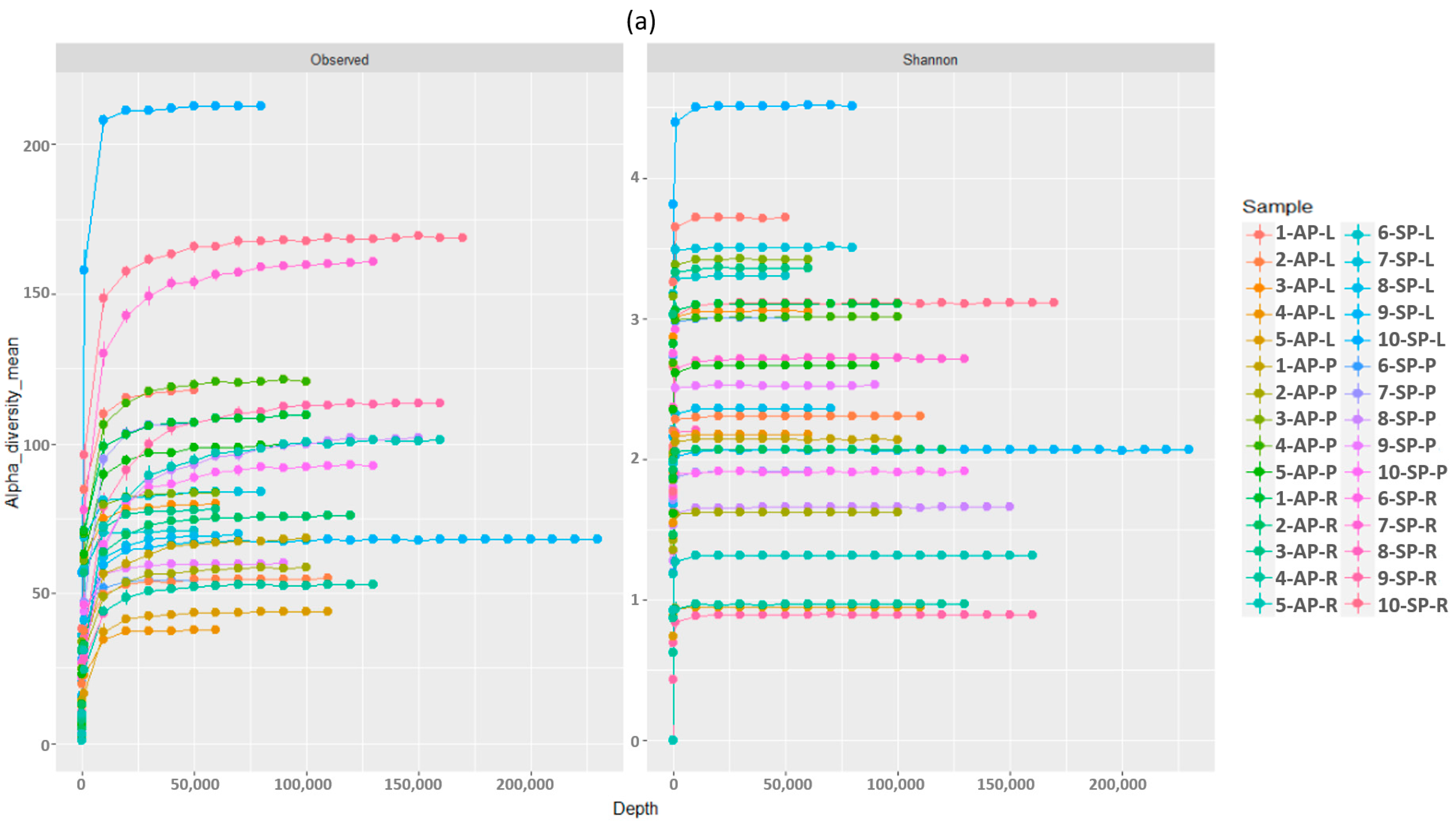
3.1. Fungal Diversity Overview
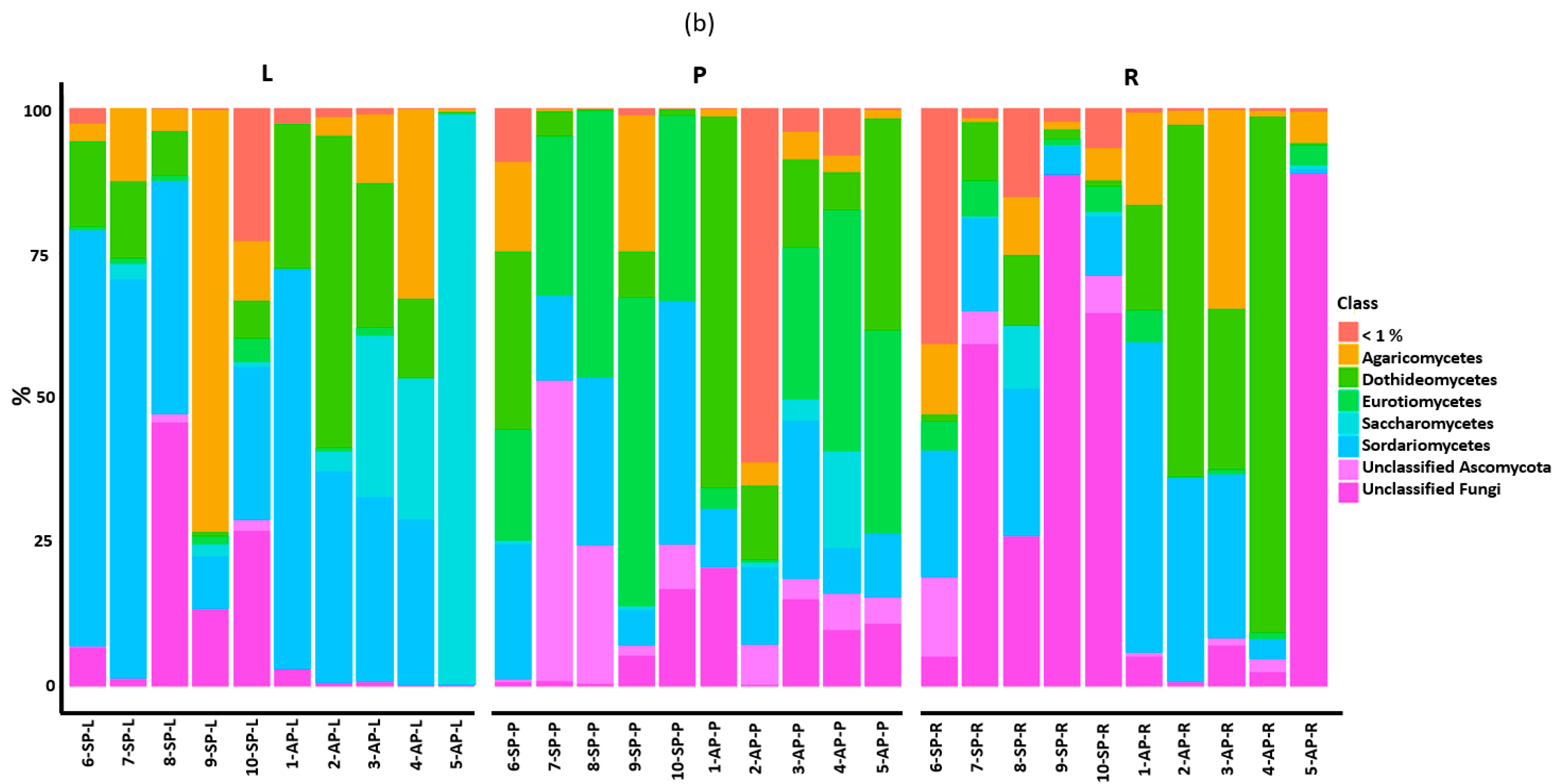
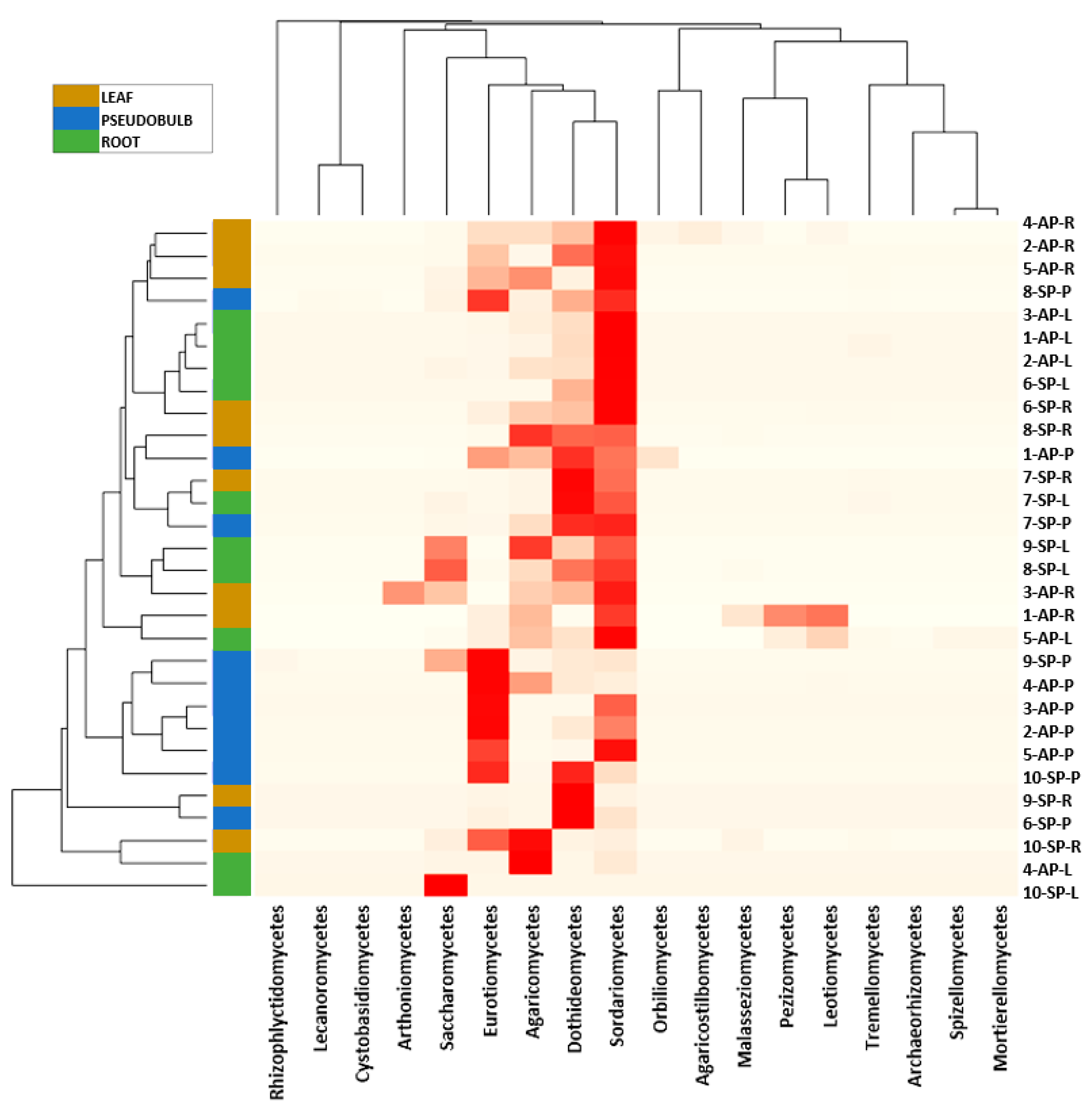
3.2. Taxonomic Assignment
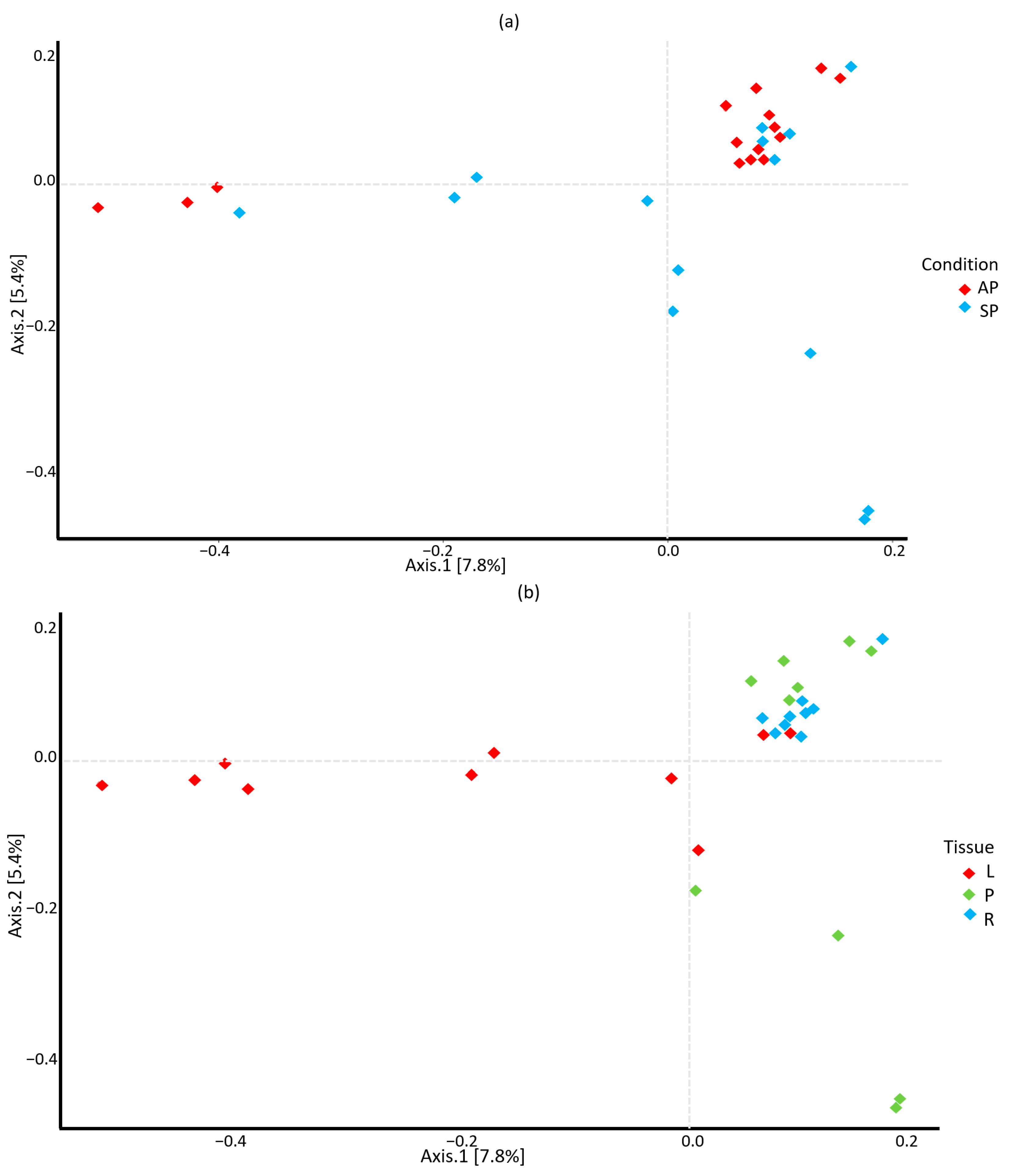
3.3. Beta Diversity of Tissue-Hosted Fungal Communities
3.4. Beta Diversity of Associated Fungal Communities by Asymptomatic and Symptomatic Plant Tissues
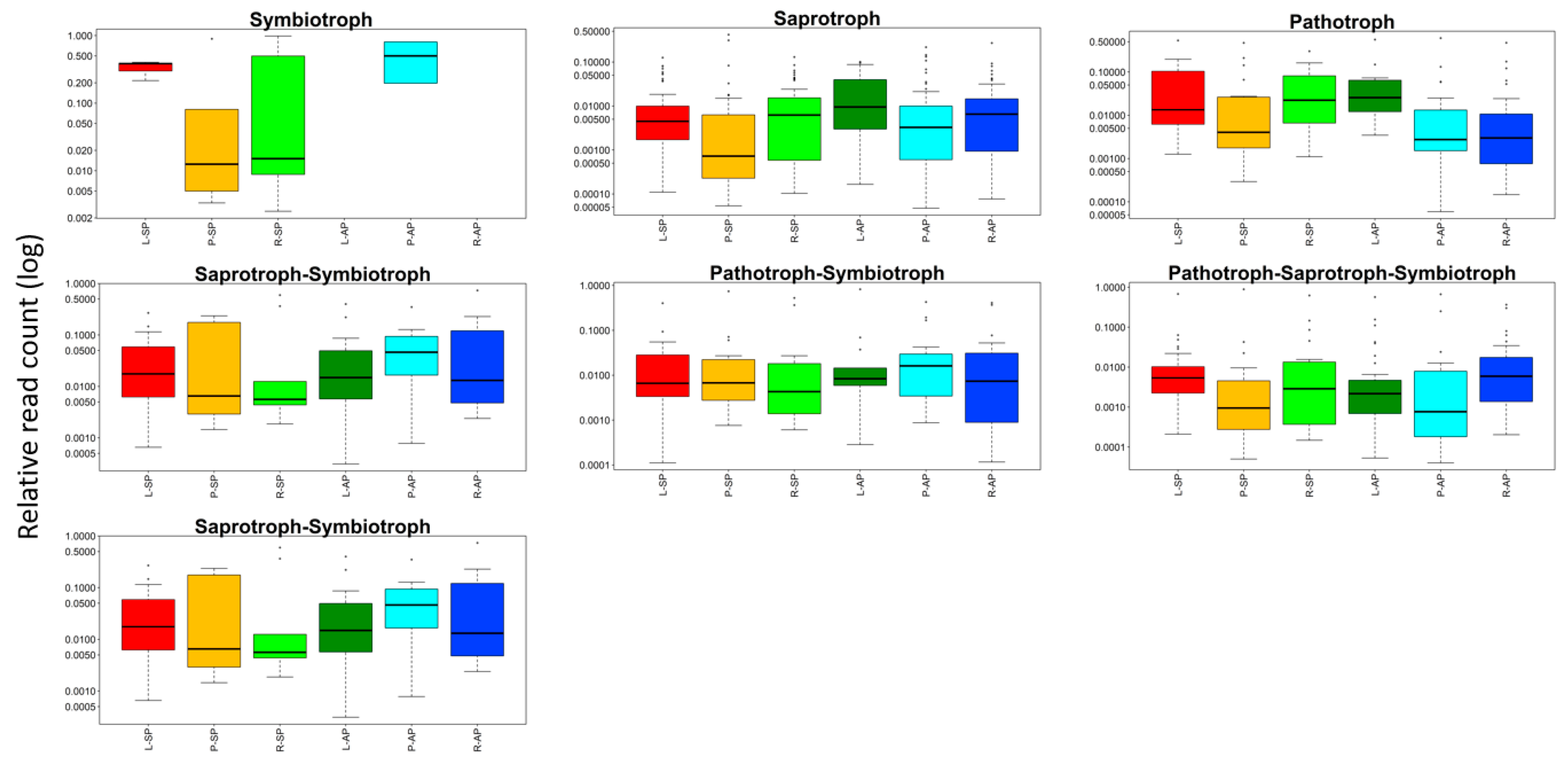
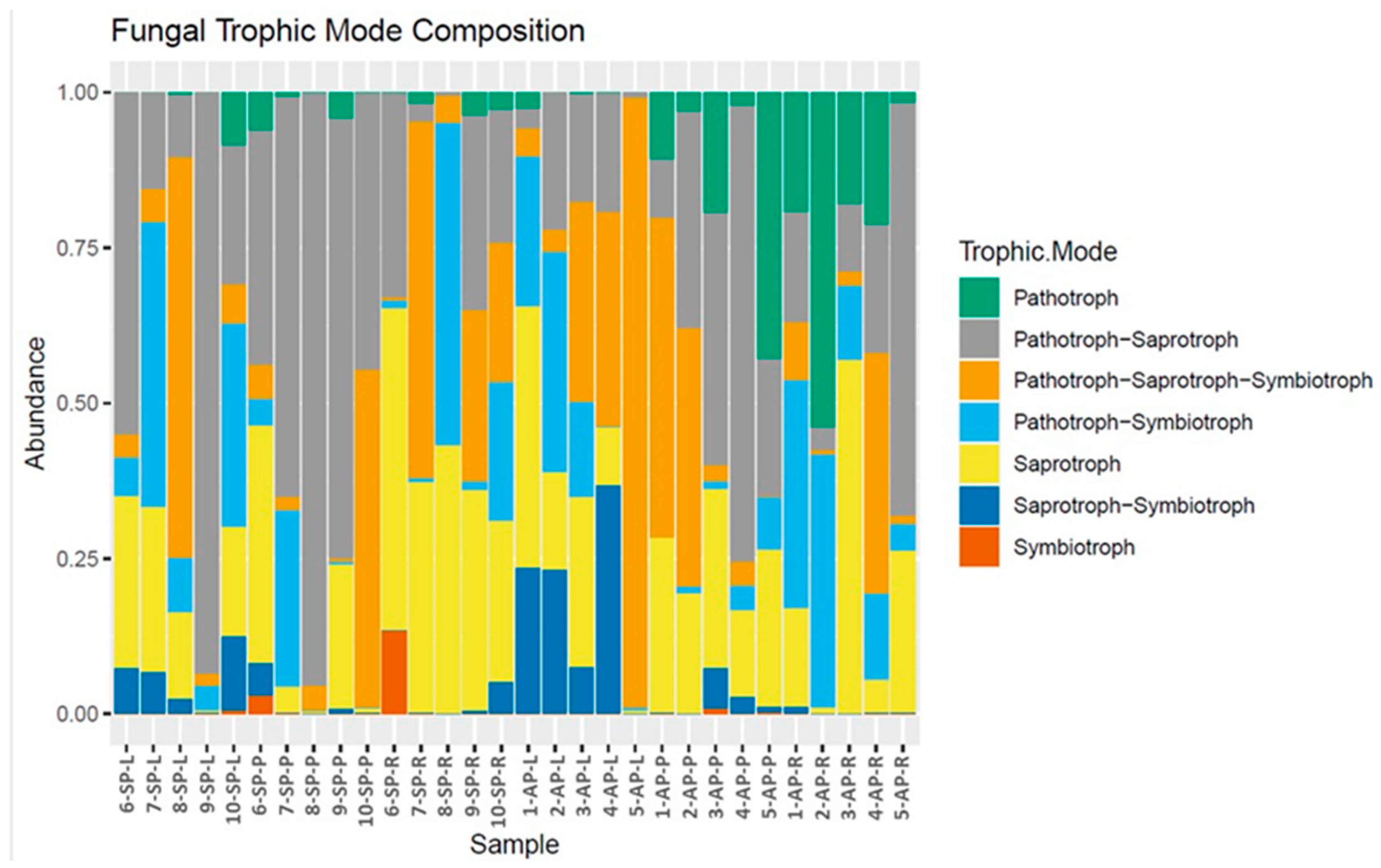
3.5. Analysis of Functional Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peay, K.; Kennedy, P.G.; Talbot, J.M. Dimensions of biodiversity in the Earth mycobiome. Nat. Rev. Genet. 2016, 14, 434–447. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities with Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Xing, Y.-M.; Chen, J.; Cui, J.-L.; Chen, X.-M.; Guo, S.-X. Antimicrobial Activity and Biodiversity of Endophytic Fungi in Dendrobium devonianum and Dendrobium thyrsiflorum from Vietman. Curr. Microbiol. 2011, 62, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Christian, N.; Herre, E.A.; Clay, K. Foliar endophytic fungi alter patterns of nitrogen uptake and distribution in Theobroma cacao. New Phytol. 2019, 222, 1573–1583. [Google Scholar] [CrossRef]
- Douanla-Meli, C.; Langer, E.; Mouafo, F.T. Fungal endophyte diversity and community patterns in healthy and yellowing leaves of Citrus limon. Fungal Ecol. 2013, 6, 212–222. [Google Scholar] [CrossRef]
- Hamzah, T.N.T.; Lee, S.Y.; Hidayat, A.; Terhem, R.; Faridah-Hanum, I.; Mohamed, R. Diversity and Characterization of Endophytic Fungi Isolated From the Tropical Mangrove Species, Rhizophora mucronata, and Identification of Potential Antagonists Against the Soil-Borne Fungus, Fusarium solani. Front. Microbiol. 2018, 9, 1707. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Shin, K.C.; Lee, J.K. Fungal Community Analyses of Endophytic Fungi from Two Oak Species, Quercus mongolica and Quercus serrata, in Korea. Mycobiology 2021, 49, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Yin, D.; Zhai, Y.; He, A.; Qiu, L.; Liu, Q.; Ma, N.; Shen, H.; Jia, Q.; Liang, Z.; et al. Diversity of endophytic fungal community in Huperzia serrata from different ecological areas and their correlation with Hup A content. BMC Microbiol. 2022, 22, 191. [Google Scholar] [CrossRef]
- Rim, S.O.; Roy, M.; Jeon, J.; Montecillo, J.A.V.; Park, S.-C.; Bae, H. Diversity and Communities of Fungal Endophytes from Four Pinus Species in Korea. Forests 2021, 12, 302. [Google Scholar] [CrossRef]
- Sanz-Ros, A.V.; Müller, M.M.; Martín, R.S.; Diez, J.J. Fungal endophytic communities on twigs of fast and slow growing Scots pine (Pinus sylvestris L.) in northern Spain. Fungal Biol. 2015, 119, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Tibpromma, S.; Karunarathna, S.C.; Bhat, J.D.; Suwannarach, N.; Stephenson, S.L.; Elgorban, A.M.; Al-Rejaie, S.; Xu, J.; Mortimer, P.E. Using Culture-Dependent and Molecular Techniques to Identify Endophytic Fungi Associated with Tea Leaves (Camellia spp.) in Yunnan Province, China. Diversity 2022, 14, 287. [Google Scholar] [CrossRef]
- Zarza, E.; López-Pastrana, A.; Damon, A.; Guillén-Navarro, K.; García-Fajardo, L.V. Fungal diversity in shade-coffee plantations in Soconusco, Mexico. PeerJ 2022, 10, e13610. [Google Scholar] [CrossRef]
- Verma, A.; Shameem, N.; Jatav, H.S.; Sathyanarayana, E.; Parray, J.A.; Poczai, P.; Sayyed, R.Z. Fungal Endophytes to Combat Biotic and Abiotic Stresses for Climate-Smart and Sustainable Agriculture. Front. Plant Sci. 2022, 13. [Google Scholar] [CrossRef]
- Sarsaiya, S.; Shi, J.; Chen, J. A comprehensive review on fungal endophytes and its dynamics on Orchidaceae plants: Current research, challenges, and future possibilities. Bioengineered 2019, 10, 316–334. [Google Scholar] [CrossRef] [PubMed]
- Carbajal-Valenzuela, I.A.; Muñoz-Sanchez, A.H.; Hernández-Hernández, J.; Barona-Gómez, F.; Truong, C.; Cibrián-Jaramillo, A. Microbial Diversity in Cultivated and Feral Vanilla Vanilla planifolia Orchids Affected by Stem and Rot Disease. Microb. Ecol. 2021, 84, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Novotná, A.; Benítez, Á.; Herrera, P.; Cruz, D.; Filipczyková, E.; Suárez, J.P. High diversity of root-associated fungi isolated from three epiphytic orchids in southern Ecuador. Mycoscience 2018, 59, 24–32. [Google Scholar] [CrossRef]
- Bungtongdee, N.; Sopalun, K.; Laosripaiboon, W.; Iamtham, S. The chemical composition, antifungal, antioxidant and antimutagenicity properties of bioactive compounds from fungal endophytes associated with Thai orchids. J. Phytopathol. 2018, 167, 56–64. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Cabrera, T. Ficha Técnica de Cattleya Skinneri. En: Palacios E. (compilador). Cuarenta y ocho Especies de la Flora de Chiapas Incluidas en el PROY-NOM-059-ECOL-2000; SNIB CONABIO. Proyecto. W008; Instituto de Historia Natural y Ecología Bases de datos: Chiapas, Mexico, 2006; SNIB CONABIO. Proyecto. W008. México. [Google Scholar]
- Fabiola, H.-R.; Patricia, F.-P.S.; Anne, D.; Karina, G.-N.; Verónica, G.-F. Etiology of the “black blotch” caused by Lasiodiplodia in pseudobulbs of Guarianthe skinneri (Orchidaceae), in the Region of Soconusco, Chiapas, Mexico. J. Plant Pathol. 2022, 104, 1083–1095. [Google Scholar] [CrossRef]
- Johnson, G.I.; Mead, A.J.; Cooke, A.W.; Dean, J.R. Mango stem end rot pathogens—Fruit infection by endophytic colonisation of the inflorescence and pedicel. Ann. Appl. Biol. 1992, 120, 225–234. [Google Scholar] [CrossRef]
- Vidal, N.; Vidal, E.; Vidal, L.; Colorado, J.; Ruiz, R.; Ruiz, J. Anónaceas. Plantas antiguas. Estudios Recientes. Parte 2; Universidad Autónoma Chapingo: Texcoco, Mexico; pp. 205–227.
- Roma, R.; Damon, A.; Sánchez, W. The cacao (Theobroma cacao L.) Policulture and the Tuzantán qato’ok Culture, Chiapas, Mexico. An Ethnoecological Approach. Ethnoscientia 2022, 4, 14–32. [Google Scholar] [CrossRef]
- García, E. Modificaciones al Sistema de Clasificación Climática de Kõeppen (Para Adaptarlo a las Condiciones de la República Mexicana), 2nd ed.; Universidad Nacional Autónoma de México, Instituto de Geografía: Ciudad de México, México, 1973. [Google Scholar]
- Semarnat; Maag Consultoria Ambiental. Manifestación de Impacto Ambiental Modalidad Regional ‘Camino: Ejido 3 de Mayo-Santa Rita, en una Longitud de 4.00 km (del km 0+000 al km 4+000)’ en el Municipio de Mapastepec, Chiapas. Chiapas. Available online: https://apps1.semarnat.gob.mx:8443/dgiraDocs/documentos/chis/estudios/2020/07CH2020VD051.pdf (accessed on 5 April 2023).
- Miranda, F. La Vegetación de Chiapas; Gobierno del Estado, Coneculta Chiapas: Tuxtla Gutiérrez, Mexico, 1998; p. 600. [Google Scholar]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Chen, W.; Fungal Barcoding Consortium; Fungal Barcoding Consortium Author List. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Li, L.; Liu, C.; Wen, W.; Li, Q.; Pan, T.; Li, Z.; Qian, G.; He, Y.; Xu, D. Dendrobine biosynthesis in Dendrobium nobile in four different habitats is affected by the variations in the endophytic fungal community. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef]
- Pecoraro, L.; Rasmussen, H.N.; Gomes, S.I.F.; Wang, X.; Merckx, V.S.F.T.; Cai, L.; Rasmussen, F.N. Fungal diversity driven by bark features affects phorophyte preference in epiphytic orchids from southern China. Sci. Rep. 2021, 11, 11287. [Google Scholar] [CrossRef]
- May-Mutul, C.G.; López-Garrido, M.A.; O’connor-Sánchez, A.; Peña-Ramírez, Y.J.; Labrín-Sotomayor, N.Y.; Estrada-Medina, H.; Ferrer, M.M. Hidden Tenants: Microbiota of the Rhizosphere and Phyllosphere of Cordia dodecandra Trees in Mayan Forests and Homegardens. Plants 2022, 11, 3098. [Google Scholar] [CrossRef] [PubMed]
- Haridas, S.; Albert, R.; Binder, M.; Bloem, J.; LaButti, K.; Salamov, A.; Andreopoulos, B.; Baker, S.; Barry, K.; Bills, G.; et al. 101 Dothideomycetes genomes: A test case for predicting lifestyles and emergence of pathogens. Stud. Mycol. 2020, 96, 141–153. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, H.S.; Nguyen, T.T.T.; Lee, H.B. Characterization of Three Species of Sordariomycetes Isolated from Freshwater and Soil Samples in Korea. Mycobiology 2019, 47, 20–30. [Google Scholar] [CrossRef]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. FUNGAL ENDOPHYTES: A Continuum of Interactions with Host Plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gupta, V.K.; Dahms, T.E.; Silva, R.N.; Singh, H.B.; Upadhyay, R.S.; Gomes, E.V.; Tsui, C.K.; Nayak, S.C. Friends or foes? Emerging insights from fungal interactions with plants. FEMS Microbiol. Rev. 2016, 40, 182–207. [Google Scholar] [CrossRef]
- Lacaze, A.; Joly, D.L. Structural specificity in plant–filamentous pathogen interactions. Mol. Plant Pathol. 2020, 21, 1513–1525. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kang, J.; Nontachaiyapoom, S.; Wen, T.; Hyde, K.D. Non-mycorrhizal endophytic fungi from orchids. Curr. Sci. 2015, 109, 72–87. [Google Scholar]
- Cevallos, S.; Herrera, P.; Sánchez-Rodríguez, A.; Declerck, S.; Suárez, J.P. Untangling factors that drive community composition of root associated fungal endophytes of Neotropical epiphytic orchids. Fungal Ecol. 2018, 34, 67–75. [Google Scholar] [CrossRef]
- Ovando, I. Isolation of Endophytic Fungi and Their Mycorrhizal Potential for the Tropical Epiphytic Orchids Cattleya skinneri, C. aurantiaca and Brassavola nodosa. Asian J. Plant Sci. 2005, 4, 309–315. [Google Scholar] [CrossRef]
- dos Reis, J.B.A.; Lorenzi, A.S.; de Vale, H.M.M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022, 204, 675. [Google Scholar] [CrossRef]
- Tedersoo, L.; Bahram, M.; Zinger, L.; Nilsson, R.H.; Kennedy, P.G.; Yang, T.; Anslan, S.; Mikryukov, V. Best practices in metabarcoding of fungi: From experimental design to results. Mol. Ecol. 2022, 31, 2769–2795. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Div. 2008, 28, 1–13. Available online: http://www.fungaldiversity.org/fdp/sfdp/28-1.pdf (accessed on 5 April 2023).
- Abdollahzadeh, J.; Javadi, A.; Goltapeh, E.M.; Zare, R.; Phillips, A. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia Mol. Phylogeny Evol. Fungi 2010, 25, 1–10. [Google Scholar] [CrossRef]
- Cabrera, M.G.; Cúndom, M.A. Ocurrencia de Lasiodiplodia theobromae en Cattleya spp. en Corrientes, Argentina. Summa Phytopathol. 2013, 39, 143. [Google Scholar] [CrossRef]
- Picos-Muñoz, P.; García-Estrada, R.; León-Félix, J.; Sañudo-Barajas, A.; Allende-Molar, R. Lasiodiplodia theobromae en cultivos agrícolas de México: Taxonomía, hospedantes, diversidad y control. Rev. Mex. Fitopatol. 2015, 33, 54–74. [Google Scholar]
- Churchland, C.; Grayston, S.J. Specificity of plant-microbe interactions in the tree mycorrhizosphere biome and consequences for soil C cycling. Front. Microbiol. 2014, 5, 261. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lerou, J.; Nuytinck, J.; Gomes, S.I.; Jacquemyn, H.; Sft, V. Root-associated fungi in Orchidaceae: Diversity, phylogeny, ecology, and outstanding questions. bioRxiv 2022, 1–38. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.; Olsson, S.B.; Ravikanth, G.; Shaanker, R.U. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef] [PubMed]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “Secrets” of a Multitalented Biocontrol Agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Lizarazo-Medina, P.X.; Mendoza-Salazar, M.M.; Gutiérrez-Gallo, A.I. Diversity of endophytic mycobiota in Cattleya percivaliana and Cattleya trianaei growing under greenhouse conditions. Actual. Biol. 2015, 37, 67–78. [Google Scholar]
- Ma, X.-Y.; Maharachchikumbura, S.S.N.; Chen, B.-W.; Hyde, K.D.; McKenzie, E.H.C.; Chomnunti, P.; Kang, J.-C. Endophytic Pestalotia taxa in Dendrobium orchids. Phytotaxa 2019, 419, 268–286. [Google Scholar] [CrossRef]
- Sudheep, N.M.; Sridhar, K. Non-mycorrhizal fungal endophytes in two orchids of Kaiga forest (Western Ghats), India. J. For. Res. 2012, 23, 453–460. [Google Scholar] [CrossRef]
- Tsavkelova, E.; Kolomeitseva, G. Fusarium–orchid interactions under greenhouse conditions. S. Afr. J. Bot. 2022, 146, 889–896. [Google Scholar] [CrossRef]
- Salazar-Cerezo, S.; Martinez-Montiel, N.; Cruz-Lopez, M.D.C.; Martinez-Contreras, R.D. Fungal Diversity and Community Composition of Culturable Fungi in Stanhopea trigrina Cast Gibberellin Producers. Front. Microbiol. 2018, 9, 612. [Google Scholar] [CrossRef]
- Vaz, A.B.; Mota, R.C.; Bomfim, M.R.Q.; Vieira, M.L.; Zani, C.L.; Rosa, C.A.; Rosa, L.H. Antimicrobial activity of endophytic fungi associated with Orchidaceae in Brazil. Can. J. Microbiol. 2009, 55, 1381–1391. [Google Scholar] [CrossRef] [PubMed]
- Arias, R.; Romero, A.; Bañuelos, J.; De la Cruz, Y. Inoculation of phosphorus solubilizing fungi and arbuscular mycorrhizae in tomato plants. Rev. Mex. Cienc. Cienc. Agrícolas 2019, 10, 1747–1757. [Google Scholar] [CrossRef]
- López-Guzmán, L.M.; Chacón, S.; Bautista-Gálvez, A. Adiciones al conocimiento sobre la diversidad de los hongos (macromicetes) de Chiapas, México. Sci. Fungorum 2017, 45, 27–35. [Google Scholar] [CrossRef]
- Andrade-Gallegos, R.H.; Sánchez-Vázquez, J.E. La diversidad de hongos en Chiapas: Un reto pendiente. In Diversidad Biológica en Chiapas; ECOSUR-COCYTECH-PLAZA Y VALDES Editores, D.F. México; Plaza y Valdés: Madrid, México, 2004; pp. 33–80. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Ramírez, F.; Damon, A.; Fernández Pavía, S.P.; Guillén-Navarro, K.; Iracheta-Donjuan, L.; Zarza, E.; Castro-Chan, R.A. Community Richness and Diversity of Endophytic Fungi Associated with the Orchid Guarianthe skinneri Infested with “Black Blotch” in the Soconusco Region, Chiapas, Mexico. Diversity 2023, 15, 807. https://doi.org/10.3390/d15070807
Hernández-Ramírez F, Damon A, Fernández Pavía SP, Guillén-Navarro K, Iracheta-Donjuan L, Zarza E, Castro-Chan RA. Community Richness and Diversity of Endophytic Fungi Associated with the Orchid Guarianthe skinneri Infested with “Black Blotch” in the Soconusco Region, Chiapas, Mexico. Diversity. 2023; 15(7):807. https://doi.org/10.3390/d15070807
Chicago/Turabian StyleHernández-Ramírez, Fabiola, Anne Damon, Sylvia Patricia Fernández Pavía, Karina Guillén-Navarro, Leobardo Iracheta-Donjuan, Eugenia Zarza, and Ricardo Alberto Castro-Chan. 2023. "Community Richness and Diversity of Endophytic Fungi Associated with the Orchid Guarianthe skinneri Infested with “Black Blotch” in the Soconusco Region, Chiapas, Mexico" Diversity 15, no. 7: 807. https://doi.org/10.3390/d15070807
APA StyleHernández-Ramírez, F., Damon, A., Fernández Pavía, S. P., Guillén-Navarro, K., Iracheta-Donjuan, L., Zarza, E., & Castro-Chan, R. A. (2023). Community Richness and Diversity of Endophytic Fungi Associated with the Orchid Guarianthe skinneri Infested with “Black Blotch” in the Soconusco Region, Chiapas, Mexico. Diversity, 15(7), 807. https://doi.org/10.3390/d15070807







