Abstract
The genus Scleroderma contains gasteroid basidiomycetes, which form globose spores with echinulate to reticulate ornamentation on the surface. Based on the morphological observations in combination with molecular data, four new species, S. erubescens, S. separatum, S. squamulosum, and S. vinaceum, were described from Yunnan, southwestern China. Images of fresh basidiomata and scanning electron microscope (SEM) images of basidiospores are provided. Phylogenetic analyses based on ITS sequences show that these four new taxa belong to the Scleroderma section Scleroderma.
1. Introduction
Scleroderma Pers. (Sclerodermataceae, Boletales) is an easily recognizable genus of gasteroid fungi, characterized by the subglobose, pyriform, or subturbinate basidiomata, a firm peridium, which dehisces at maturity, and globose, colored, and ornamented basidiospores [1].
Species of Scleroderma play a vital role in maintaining plant diversity, ecosystem stability, and afforestation [2,3,4,5,6,7], and were found to be one of the most species-rich groups throughout the tropics [8]. They form ectomycorrhizae with plants in the Cistaceae, Dipterocarpaceae, Fagaceae, Juglandaceae, Myrtaceae, Mimosaceae, and Pinaceae families [1,5,9,10,11,12].
Currently, there are 198 entries of Scleroderma listed in the Index Fungorum online database (http://www.indexfungorum.org/Names/Names.asp; accessed on 2 October 2022), and approximately 40 species were recognized [13]. In China, 16 Scleroderma species have been reported mainly based on morphology prior to this study: S. areolatum Ehrenb., S. bovista Fr., S. cepa Pers., S. citrinum Pers., S. dictyosporum Pat., S. flavidum Ellis and Everh., S. floridanum Guzmán, S. nitidum Berk., S. paradoxum G.W. Beaton, S. polyrhizum (J.F. Gmel.) Pers., S. sinnamariense Mont., S. texense Berk., S. verrucosum (Bull.) Pers., S. yunnanense Y. Wang, S. venenatum Y.Z. Zhang, and S. suthepense Kumla, Suwannarach, and Lumyong [13,14,15,16,17,18]. Among these, S. areolatum, S. bovista, S. cepa, S. citrinum, S. flavidum, S. polyrhizum, and S. verrucosum, are used as medicinal mushrooms in China, they are considered to be beneficial in the treatment of hemostasis, swelling, and detoxification, as well as to be antibacterial and insecticidal [19], while S. yunnanense is a popular edible fungus [16].
In recent years, during the ongoing efforts to inventory the ectomycorrhizal (ECM) species of broad-leaved forests in Yunnan, as well as the pecan (Carya illinoinensis) orchards, a few specimens of Scleroderma were collected in Yunnan, southwestern China. Based on morphological and molecular analyses, several collections were found to be not identical with the known species. Therefore, the aims of this study are to describe the new species of Scleroderma from Yunnan based on the macro- and microscopic characters, and to infer the phylogenetic positions of the newly described species based on ITS sequences.
2. Materials and Methods
2.1. Collections Studied
The Scleroderma specimens were collected in Yunnan, China, and stored in the Herbarium of Kunming Institute of Botany, the Chinese Academy of Sciences (KUN, with HKAS numbers, Table 1).

Table 1.
Taxon information, GenBank accession numbers (new species are in bold), and references of the sequences used in the present study.
2.2. Morphological Analysis
Macroscopic characters were described based on field records. The color codes were based on Kornerup and Wanscher [31]. A tiny piece of tissue was sectioned from dried specimen and immersed in 5% KOH solution on a glass slide; then, the microscopic characters were observed using a Leica DM2500 microscope (Bensheim, Germany). The characters, such as peridium, gleba, capillitium, and basidiospores, were observed and measured. Fragments of gleba from dried specimens were fixed on an aluminum stub with double-sided adhesive tape, coated with gold palladium, and then the surface ornamentation of basidiospores were observed under a ZEISS Sigma 300 scanning electron microscope (SEM).
The notations [n/m/p] indicate that the measurements were taken on n basidiospores, from m basidiomata and p collections. For the basidiospore size dimensions, spore ornamentation was not included, and the spore ornamentation was measured separately. The dimensions of the basidiospores were provided with the notation (a–) b–c (–d). The range b–c indicates the minimum and maximum values of 90% of the measurements. The a and d in parentheses indicate the extreme values.
2.3. Phylogenetic Analysis
Genomic DNA was extracted using the CTAB method [32]. The universal primer pair ITS1F/ITS4 [33,34] was used to amplify the ITS region. The PCR reactions were carried out in a total volume of 25 µL, containing 12.5 µL of Kodaq 2× PCR MasterMix (Tolo Biotechnology Co., Ltd., Shanghai, China), 1 µL forward primer (5 µM), 1 µL reverse primer (5 µM), and 1–2 µL template DNA (depending on the concentration of the template); ddH2O was added until the total volume reached 25 µL. Parameters for the PCR reaction program were as follows: initial denaturation at 94 °C for 5 min, followed by 35 cycles of denaturation at 94 °C for 50 s, annealing at 51 °C for 60 s, and an extension at 72 °C for 60 s; then, a final extension at 72 °C for 8 min. Successful PCR products were sent to Tsingke Biotechnology Co., Ltd. (Beijing, China) for sequencing, and both ends were sequenced using the same primers as used for the PCR. Sequences of both directions were assembled and manually edited using SeqMan 6.01 [35] and BioEdit V7.0.9 [36]. Initial analyses with all available Scleroderma species suggest that the sequences of putative novel species belong to Scleroderma section Scleroderma. Thus, reliable sequences of species within S. sect. Scleroderma, as well as sequences of representative species of other sections were included as ingroups [11,12,13,16,20,21,24,27]. Pisolithus arhizus, P. tinctorius, and P. albus were used as the outgroup taxa according to previous studies [11,22,26,30]. Multiple sequence alignment were performed in MAFFT 7.130b [37]. AliView 1.23 [38] was used for further manual adjustments to ensure the accuracy of alignments.
Bayesian inference (BI) analyses and maximum likelihood (ML) analyses were conducted to infer the phylogenetic positions of the Scleroderma taxa. Nucleotide substitution models based on the Akaike information criterion (AIC) were obtained and GTR + GAMMA was selected as the best-fit model using MrModeltest 2.3 [39]. BI analysis was conducted using MrBayes 3.2.7a [40], running for 1 million generations and trees were sampled every 100 generations, and the initial 25% of sampled data were discarded as burn-in. ML analyses were performed using RAxML 8.2.12 [41]. Statistical support was calculated with 1000 bootstrap replicates. The phylogenetic trees were drawn using FigTree 1.4.3 [42] and edited in Adobe Illustrator CS3 v.11.0.2 (https://www.adobe.com; accessed on 7 June 2021).
3. Results
3.1. Phylogenetic Analysis
The newly generated ITS sequences were deposited in GenBank (www.ncbi.nlm.nih.gov/genbank/; accessed on 10 July 2021, Table 1). The ITS data set included 68 sequences of Scleroderma specimens, including 51 sequences obtained from GenBank database and 17 sequences newly generated in this study (Table 1).
The topologies of the trees obtained in the BI and ML analyses were not significantly different from each other, and the result from the Bayesian analysis is shown in Figure 1, with the ML bootstrap (MLB) and Bayesian posterior probabilities (BPP) shown above the branches. The ITS tree consisted of four main clades (Figure 1): clade A (MLB = 100%, BPP = 1.00), clade B (MLB = 100%, BPP = 1.00), clade C (MLB = 63%, BPP = 0.87), and clade D (MLB = 82%, BPP = 0.99). The collections referring to the four species from Yunnan clustered in four well-supported groups in clade D: S. squamulosum (MLB = 100%, BPP = 1.00), S. vinaceum (MLB = 100%, BPP = 1.00), S. separatum (MLB = 100%, BPP = 1.00), and S. erubescens (MLB = 100%, BPP = 0.99) (Figure 1).
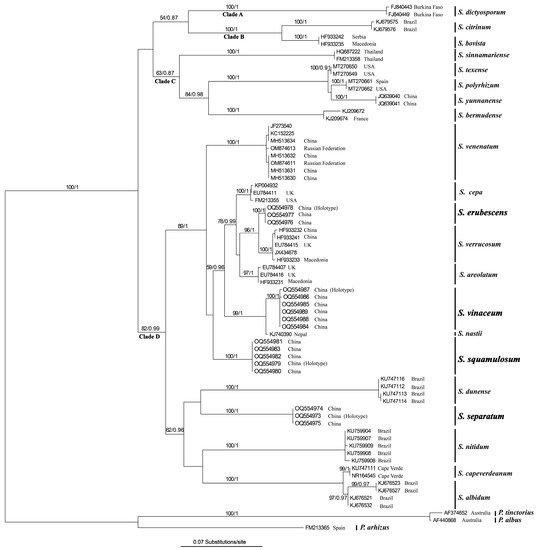
Figure 1.
Phylogenetic tree inferred from maximum likelihood (ML) analysis based on the dataset of ITS sequences of Scleroderma species. The ML bootstrap numbers (MLB) ≥ 50% and Bayesian posterior probabilities (BPP) ≥ 0.95 are shown above the branches. New taxa described in the present study are in bold.
3.2. Taxonomy
Scleroderma erubescens Z.W. Ge, R. Wu & L.-R. Zhou, sp. nov.;
MycoBank No.: MB 847686;

Figure 2.
Basidiomata of Scleroderma species. (A,B) Scleroderma erubescens ((A) Z. W. Ge 4356; (B) Z. W. Ge 4828, holotype). (C–E) Scleroderma separatum ((C) Z. W. Ge 4148, holotype; (D) Z. W. Ge 5394; (E) L. R. Zhou 31). (F,G) Scleroderma squamulosum ((F) Y. J. Hao 373, holotype; (G) L. P. Tang 821). (H,I) Scleroderma vinaceum ((H) J. Qin 197; (I) Z. W. Ge 5651). Scale bars = 1 cm.
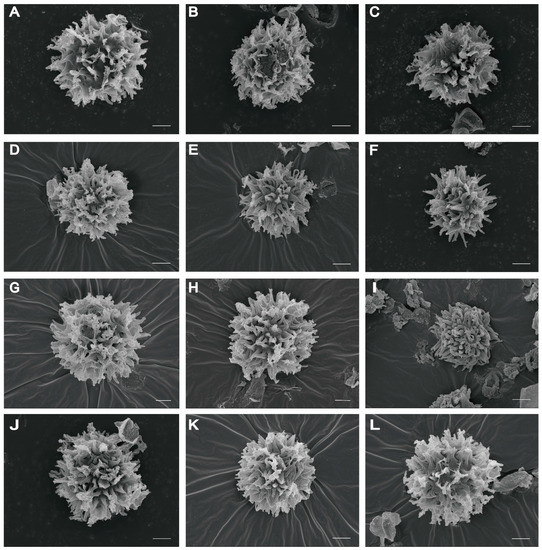
Figure 3.
Basidiospores of Scleroderma species under SEM. (A–C) Scleroderma erubescens ((A,B) Z. W. Ge 4356; (C) Z. W. Ge 4828, holotype). (D–F) Scleroderma separatum ((D,E) Z. W. Ge 4148, holotype; (F) Z. W. Ge 5394). (G,H) Scleroderma squamulosum ((G,H) Y. J. Hao 373, holotype). (I–L) Scleroderma vinaceum ((I) Z. W. Ge 2789; (J) Z. W. Ge 5651; (K) J. Qin 197; (L) X. T. Zhu 387). Scale bars = 2 µm.
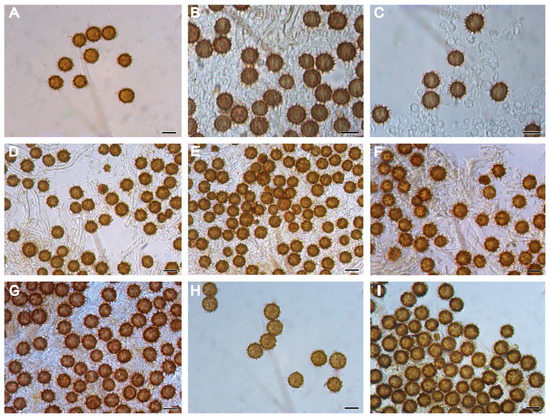
Figure 4.
Basidiospores of Scleroderma species in KOH under LM. (A–C) Scleroderma erubescens ((A) Z. W. Ge 4356; (B,C) Z. W. Ge 4828, holotype). (D,E) Scleroderma separatum ((D,E) L. R. Zhou 31). (F,G) Scleroderma squamulosum ((F) Y. J. Hao 373, holotype; (G) Z. W. Ge 2935). (H,I) Scleroderma vinaceum ((H,I) Z. W. Ge5651). Scale bars = 10 µm.
Diagnosis: Differs from the similar S. verrucosum in having smaller basidiomata that turn reddish purple to blackish red after injury, smaller basidiospores with longer spikes, a thinner peridium, and hyphae with clamp connections.
Holotype: CHINA. Yunnan Province: Kunming City, Luquan Yi and Miao Autonomous County, Pingshan Township, Xiaowan Village, in a forest intermixed by Castanea mollissima, Carya illinoinensis, and a few Pinus yunnanensis; 25°28′55″ N, 102°29′55″ E, alt. 1680 m, 19 July 2019, Z. W. Ge 4828 (KUN-HKAS 126621, holotype!). GenBank: ITS = OQ554978.
Etymology: The species epithet refers to the basidiomata turning reddish purple to blackish red when bruised.
Description: Basidiomata epigeous, 10–20 mm in diam., 12–35 mm tall; upper part globose to subglobose, turning reddish purple to blackish red (11D5) when bruised. Peridium is leathery, thin, roughly 0.5 mm thick, intact at the early stage, irregularly rupturing at the apex at maturity. Surface is light yellow (5C5), brownish yellow (5E7), and pale yellow (3B4); becoming brown (5E6) to earthy brown (5D6) when dried, cracked into tiny squamules, exposing the whitish inner layer, fading to yellow white (3A2) toward the gleba. Gleba firm, gray–white (4C7) when young, turning gray–black (3E1) with age. Pseudostipe subcylindrical, 3–15 mm high, 3–15 mm in diam., pinched or folded at base, attached to the substrate via aggregated white rhizomorphs.
Basidiospores [100/5/3] (6.5–) 7.0–11.0 (–12.0) µm in diam. (not including ornamentation), 9.0 µm on average; globose, rarely subglobose, echinulate; gray–black to dark brown (2F5) in KOH; spiny under LM and SEM, and ornamentation 1.0–3.0 µm high (Figure 3A–C and Figure 4A–C). Peridium composed of subcylindric hyphae, evenly and tightly arranged, from light yellow (3B3) to earthy brown (5D6), often inflated at the septa, hyphae from the peridium toward the gleba light yellow (5A2). Gleba made up of globose to moniliform expanded cells measuring 2.0–5.0 µm. Hyphae of rhizomorphs bifurcating. Clamp connections present on gleba hyphae.
Habit and Habitat: Solitary, scattered to gregarious on soil in forests intermixed by Castanea spp., Carya illinoinensis, and Pinus spp.
Additional specimens examined: CHINA. Yunnan Province: Nujiang Lisu Autonomous Prefecture, Fugong County, Shiyueliang Township, 3 August 2011, X.T. Zhu 363 (KUN-HKAS 73740, paratype), alt. 1700 m; Baoshan City, Longyang District, Taibaoshan Forest Park, in a forest dominated by Pinus yunnanensis and Castanea spp.; 17 September 2019, Z. W. Ge 4356 (KUN-HKAS 126622, paratype), alt. 1700 m.
Notes: Scleroderma erubescens is characterized by its small basidiomata turning reddish purple to blackish red after injury and basidiospores with irregular spiky ornamentation. Scleroderma verrucosum, originally described from France, forms the sister species to S. erubescens (Figure 1). Both species have thin peridium covered with tiny squamules and conspicuous echinulate basidiospores. However, S. erubescens has smaller basidiomata, which turn reddish purple to blackish red after injury, and a thinner peridium and hyphae with clamp connections. In addition, S. erubescens has smaller basidiospores and slightly longer spines (measuring 1.0–3.0 µm) [14,15,17,22,43].
Scleroderma erubescens is also similar to S. nitidum, originally described from Nepal, in having small basidiospores and basidiomata with thin, yellowish brown peridium, and echinulate basidiospores [43]. However, S. nitidum is sessile (or only has small pseudostipe), lacks clamp connections, and its mature gleba is dark purpuraceous or grayish brown [43].
Scleroderma texense Berk., originally described from Texas, USA, resembles S. erubescens by the sizes of the basidiospores and the presence of clamp connections. However, S. texense has subreticulate basidiospores, a rubescent endoperidium, and the mature basidiomata have a strongly squamulose surface [43]. In addition, the basidiomata of S. erubescens are smaller than those of S. texense (60–100 mm diam.).
Scleroderma areolatum, originally described from Germany, is another species similar to S. erubescens by having spiny basidiospores, sessile or sharply stipitate basidiomata, and dehiscence through an irregular, lacerate apical pore, whitish to dark reddish brown gleba, strongly rubescent context, and the absence of clamp connections. However, the basidiospores of S. erubescens are smaller than those of S. areolatum (9–) 10–15 (–18) µm diam.), and the peridium of S. erubescens turns reddish purple to blackish red when bruised [43].
Scleroderma cepa, originally described from Europe, is also similar to S. erubescens in sizes of basidiospore ornamentation, but has larger basidiospores measuring (7–) 8–13 (–14) µm, sessile or pseudostipitate basidiomata, stellate dehiscence, and the absence of clamp connections. In addition, the peridium of S. erubescens is thicker, and its basidiomata turn reddish purple to blackish red when bruised. [43,44].
Scleroderma separatum Z.W. Ge, R. Wu & L.-R. Zhou, sp. nov.;
MycoBank No.: MB 847687;
Diagnosis: Differs from Scleroderma albidum in having warty, peelable squamules on the peridium, smaller basidospores, and clamp connections at the hyphae of the rhizomorphs.
Holotype: CHINA. Yunnan Province: Yuxi City, Xinping Yi and Dai Autonomous County, Guishan Township, Douga Village, in the orchards of Carya illinoinensis (surrounded by forest dominated by Pinus yunnanensis); 24°3′43″ N, 102°1′13″ E, alt. 1520 m, 2 August 2018, Z. W. Ge 4148 (KUN-HKAS 126618, holotype!). GenBank: ITS = OQ554973.
Etymology: The species epithet refers to the peelable squamules on the peridium.
Description: Basidiomata are epigeous, 14–28 mm in diam., 15–42 mm in height; the upper part is globose, from subglobose to irregularly oblate. Peridium is leathery, thin, 0.5–1.5 mm thick, intact when young, rupturing at the apex, and leaving an irregular opening at maturity. Surface is rough, from tan (3D3) to ochraceous–brown (5E2); apex cracking into furfuraceous to slightly warty squamules, which can be shed completely to expose the hay (5C4) to greenish yellow (3B4) background. Gleba gray–black (13E1), compact, and powdery when mature. Stipe is subcylindric, 5–30 mm in length and 3–5 mm in diam., with numerous white rhizomorphs at the base.
Basidiospores [100/6/3] (4.5–) 5.0–7.0 (–8.5) µm in diam., 6.5 µm on average; globose, occasionally subglobose, gray–black (4F4) in KOH, with spinose ornamentation (up to 1.2–2.5 µm high) under LM and SEM (Figure 3D–F and Figure 4D,E). Peridial squamules consisting of evenly arranged, yellowish brown hyphae, about 5 µm in diam.; inner layer of the peridium composed of loosely arranged, colorless hyphae, expanded at the septa, and intensively gelatinized. Gleba are compact and composed of colorless hyphae, 2.5–6.0 µm in width. Clamp connections present on the rhizomorphic hyphae.
Habit and Habitat: Solitary, scattered to gregarious on soil under leaves and litter of Carya illinoinensis, and surrounded by a forest dominated by Pinus yunnanensis.
Known distribution: So far known from Yunnan, southwestern China.
Additional specimens examined: CHINA. Yunnan Province: Yuxi City, Xinping Yi and Dai Autonomous County, Guishan Township, Douga Village, 2 August 2018, L. R. Zhou 31 (KUN-HKAS 126619, paratype), alt. 1520 m; Dali Bai Autonomous Prefecture, Yangbi Yi Autonomous County, Pingpo Township, in a pecan (Carya illinoinensis) orchard, 25 August 2020, Z. W. Ge 5394 (KUN-HKAS 126620, paratype), alt. 1520 m.
Notes: Scleroderma separatum is characterized by small basidiospores with crowded and sharp spines, easily detachable peridium squamules, the presence of clamp connections on the rhizoma hyphae, and the well-developed stipe. Scleroderma separatum is phylogenetically close to S. dunense B.D.B. Silva, Sulzbacher, Grebenc, Baseia & M.P. Martín (Figure 1), a common species originally described from Rio Grande do Norte of Brazil, occurring near Coccoloba sp. Both species have echinulate basidiospores and hyphae with clamp connections. However, S. dunense is sessile or short pseudostipitate (less than 2 mm); has larger basidiospores (8.3–10.7 × 8.0–10.8 µm) and shorter basidiospore spines (0.9–1.3 µm) [25].
Scleroderma separatum is also similar to S. albidum Pat. because of the small basidiomata with pale yellowish brown, thin peridium which changes color to reddish brown when bruised, and echinulate basidiospores [45]. However, S. albidum, originally described from France, has stellate dehiscence in old specimens, has grayish green gleba interspersed with yellowish tramal veins, larger basidiospores (12.0–14.5 µm in diam.), and its hyphae lack clamp connections [45].
Scleroderma cepa has similar sized basidiomata and ornamented basidiospores, but differs from S. separatum by having larger basidiospores (8–13 µm) and the absence of clamp connections on the rhizomorph hyphae [43,44].
Scleroderma nitidum is also similar in size of basidiomata and changes the color of the endoperidium to reddish when bruised. However, S. nitidum is sessile, has larger basidiospores (7.0–11.0 µm in diam.), and lacks clamp connections.
Scleroderma erubescens is similar in forming basidiospores with spinose ornamentation and the presence of clamp connections. However, S. erubescens has larger basidiospores, measuring (6.5–) 7.0–11.0 (–12.0) µm, and a thinner (0.1–0.5 mm) peridium.
Scleroderma squamulosum Z.W. Ge, R. Wu & L.-R. Zhou, sp. nov.;
MycoBank No.: MB 847688;
Diagnosis: Differs from the similar species Scleroderma areolatum in having a well-developed stipe, light-colored basidiomata, and smaller basidospores, with abundant attachments.
Holotype: CHINA. Yunnan Province: Nujiang Lisu Autonomous Prefecture, Lushui city, at the roadside of national highway, which runs 49 km from Pianma Town to Lushui City, on the soil under Fagaceae and Pinus yunnanensis; alt. 3100 m, 6 August 2011, Y. J. Hao 373 (KUN-HKAS 71482, holotype!). GenBank: ITS = OQ554979.
Etymology: “squamulosum” refers to the scattered, tiny, and thin squamules on the stipes.
Description: Basidiomata are epigeous, from pyriform to oblate. They are 20–42 mm in diam., up to 30–65 mm in height, and the upper part is subglobose. Peridium is leathery, thin, 1.5–3.5 mm thick; it is intact when young, splitting up at the apex, and forming an irregular apical pore at maturity. Surface is rough, brownish yellow (5E7) to hay (5D4)-colored, more or less cracked into small, irregularly shaped, furfuraceous squamules, exposing the gray –pink (10C2) inner layer; fading to gray–pink (10C2) toward the gleba. Gleba are compact when young, white to whitish, becoming gray (5F1), from grayish black (4E2) to dark gray–black (3E1) with age, and finally powdery. Stipe is well-developed, firm, and cylindrical; 24–40 mm in length, 5–20 mm in diam.; covered with tiny, scattered, brownish yellow (5E6) squamules, with well-developed, white to whitish rhizomorphs aggregated at the stipe base.
Basidiospores [100/8/5] (7.5–) 8.0–11.5 (–12.0) µm in diam., including ornamentation (6.0–9.0 µm in diam. excluding ornamentation), 9.0 µm on average; globose, occasionally subglobose, and echinulate under LM and SEM (Figure 3G,H and Figure 4F,G); mauve brown (5F7) in KOH under LM, densely covered with spines or narrow pyramidal warts tapering to sharp points; up to 1.5–2.5 µm in height, sometimes with appendages, which occasionally connect the warts together. Peridium is composed of dichotomously branched, colorless hyphae, and inflated near the septa. Gleba composed of septate colorless hyphae, 3.0–5.0 µm in diam. Clamp connections are absent in all tissues.
Habit and Habitat: Solitary, scattered to gregarious on soil under leaves and litter of mixed broad-leaved forest dominated by species of Fagaceae.
Known distribution: So far known from Yunnan, southwestern China.
Additional specimens examined: CHINA. Yunnan Province: Xishuangbanna Dai Autonomous Prefecture, Jinghong City, Dadugang, 22 July 2007, L. P. Tang 342, (KUN-HKAS 54573, paratype), alt. 2380 m, in natural forest dominated by species in Fagaceae; Dehong Dai and Jingpo Autonomous Prefecture, Yingjiang County, Xima Township, Tongbiguan Nature Reserve, 17 July 2009, L. P. Tang 821 (KUN-HKAS 56778, paratype), in natural forest dominated by species in Fagaceae, alt. 2170 m; Baoshan City, Longling County, Zhen’an Town, Daxue Mountain, Luobo Pass, 30 July 2014, X. B. Liu 464 (KUN-HKAS 87110, paratype), alt. 2500 m; Yuxi City, Xinping Yi and Dai Autonomous County, Ailao Mountain, 26 June 2011, Z. W. Ge 2935 (KUN-HKAS 70439, paratype).
Notes: Scleroderma squamulosum is characterized by small basidiospores with crowded spines or narrow pyramidal warts and well-developed white to whitish rhizomorphs, as well as well-developed stipe covered by scattered, small squamules. Scleroderma venenatum, a widely distributed poisonous species originally described from Guizhou province of China, is close to S. squamulosum according to the ITS phylogeny (Figure 1). However, S. squamulosum has larger basidiomata, better developed stipe, and smaller basidospores with more attachments. In addition, the basidiospores of S. squamulosum are mauve brown under LM, while those of S. venenatum are golden yellow [13].
Scleroderma squamulosum is also similar to S. areolatum in small basidiomata with thin yellowish peridium and echinulate basidiospores [7]. However, S. areolatum is sessile (or only with tiny pseudostipe), has brownish violet to dark olivaceous gleba, and larger basidiospores measuring 11.0–16.0 µm (not including the height of spore ornaments).
Scleroderma dictyosporum Pat., originally described from France, is similar in size of basidiomata and basidiospores, with the presence of tightly packed squamules on the peridium, and a large number of white rhizomorphs anchored to the ground at the base [7]. However, the stipe of S. squamulosum is better developed than in S. dictyosporum, and the basidiospores of the former are brownish in color, while those of the latter are golden yellow [7].
Scleroderma erubescens, a new species described in this study, is also similar in the ornamentation and size of basidiospores. However, basidiomata of S. erubescens turn reddish purple to blackish red when bruised, having clamp connections in the gleba which is composed of globose to moniliform expanded cells measuring 2.0–5.0 µm. In addition, the stipe of S. squamulosum is better developed than that of S. erubescens, and the peridium of S. squamulosum is thicker than in S. erubescens.
Scleroderma separatum is similar in ornamentation of basidiospores, the peelable squamules on the peridium, and the hyphae of rhizomorphs with clamp connections. However, S. squamulosum has a better developed stipe than S. separatum (5–30 mm in length and 3–5 mm in diam.), smaller basidiospores, measuring 5–7 µm in diam., and the peridium is thicker than S. separatum (0.5–1.5 mm thick).
Scleroderma separatum is also similar to Scleroderma nitidum in size of basidiomata and basidiospores, and lack of clamp connections. However, S. nitidum is sessile, with starwise dehiscence in the old basidiomata, and the endoperidium changes color to reddish when bruised.
Scleroderma vinaceum Z.W. Ge, R. Wu & L.-R. Zhou, sp. nov.;
MycoBank No.: MB 847689;
Diagnosis: Differs from the similar species Scleroderma verrucosum in the peridium, changing color to vinaceous after damage, with a longer stipe and smaller basidiomata.
Holotype: CHINA. Yunnan Province: Nujiang Lisu Autonomous Prefecture, Gongshan County, Puladi Township, Bujiuwa Village, in forest dominated by Castanea spp.; 27.57673846° N, 98.80763979° E, alt. 1520 m, 1 August 2011, X.T. Zhu 346 (KUN-HKAS 73723, holotype!). GenBank: ITS = OQ554987.
Etymology: “vinaceum” refers to the basidiomata turning vinaceous when bruised.
Description: Basidiomata are epigeous, 10–20 mm in diam., 15–42 mm in height; upper part is subglobose to oblate. Peridium is leathery, thin, 1–2.5 mm thick, intact in the early stage, splitting up at the apex, and forming an irregular apical pore at maturity. Surface is earthy brown (5E4) to dark brown (5F5), cracking into tiny squamules, exposing the whitish to light yellow (5A2) background, which turns vinaceous after damage. Gleba are light gray (7E1) and compact when young, dark gray –green (2E4) and loose powdery when mature. Stipe is well-developed, cylindrical, firm, light gray, 4–10 mm in length and 3–5 mm in diam.; base with numerous white to whitish rhizomorphs.
Basidiospores [100/9/6] (6.0–) 7.0–10.0 (–10.5) µm in diam., 8.0 µm on average, globose, echinulate, gray–brown (4F6) in KOH; covered with dense spines or anemone shaped warts under LM and SEM (Figure 3I–L and Figure 4H–I); spines 1.0–3.0 µm high. Hyphae of the surface are inflated, yellow, and 2.0–3.0 µm in diam.; hyphae from the peridium toward the gleba are septate, colorless, and inflated, at 4.0–6.0 µm in diam. Clamp connections are absent.
Habit and Habitat: Solitary; scattered to gregarious on soil under leaves and the litter of broad-leaved forest dominated by species in Fagaceae.
Additional specimens examined: CHINA. Yunnan Province: Nujiang Lisu Autonomous Prefecture, Lushui City, 6 August 2010, T. Guo 63 (KUN-HKAS 69055, paratype), alt. 2825, in natural forest dominated by species within Fagaceae; Nujiang Lisu Autonomous Prefecture, Fugong County, 5 August 2011, J. Qin 197 (KUN-HKAS 73183, paratype), alt. 2400 m, in natural forest dominated by species within Fagaceae; Nujiang Lisu Autonomous Prefecture, Lushui City, Luzhang Township, 5 August 2011, X. T. Zhu 387 (KUN-HKAS 73764, paratype), alt. 2400 m, in natural forest dominated by species within Fagaceae; Chuxiong Yi Autonomous Prefecture, Chuxiong city, Zixi Mountain, 18 September 2010, Z. W. Ge 2789 (KUN-HKAS 61712, paratype), alt. 2400 m, in natural forest dominated by species within Fagaceae; Chuxiong Yi Autonomous Prefecture, Lufeng city, Wutai Mountain, 12 August 2021, Z. W. Ge 5651 (KUN-HKAS 126617, paratype), alt. 2320 m, in natural forest dominated by species within Fagaceae.
Notes: Scleroderma vinaceum is characterized by small basidiomata with a long stipe, basidiospores with dense spines or sea-anemone-shaped warts, and the vinaceous color change of peridium when bruised. Scleroderma nastii Raut., a species collected from a Quercus forest in a subtropical region of Nepal, also has small basidiomata and similar sized basidiospores; it is close to S. vinaceum according to the ITS phylogeny (Figure 1). However, S. vinaceum has larger basidiomata and basidiospores with spiny ornamentation under LM, and the spines under SEM are irregular, while the basidiospores of S. nastii are irregularly reticulate under SEM [27].
Scleroderma vinaceum is similar to S. verrucosum in the small basidiomata with thin yellowish peridium and echinulate basidiospores [43]. However, S. verrucosum has a shorter stipe, brownish violet to dark olivaceous gleba, and larger basidiospores (9.0–12.0 µm) with shorter spines (0.5–2.0 µm).
Scleroderma bermudense Coker, originally described from Bermuda Island, is also similar in size of basidiomata and basidiospores, with a yellow or brownish peridium covered with tightly packed squamules, and changes color to violaceous red when cut [43]. However, S. bermudense has clamp connections and subreticulate basidiospores; old basidiomata with stelliform dehiscence [43]. In addition, the stipe base of S. vinaceum is better developed than that of S. bermudense.
Scleroderma venenatum, a widely distributed poisonous species described from China, has similar sized basidiomata. However, S. venenatum is sessile, and has larger basidiospores and clamp connections. In addition, the basidiospores of S. venenatum are golden yellow in KOH under LM [13], while those of S. vinaceum are vinaceous brown.
Key to known species of Scleroderma in China
- 1 Basidiospores echinulate; neither subreticulate nor reticulate.............................................2
- 1 Basidiospores subreticulate or reticulate................................................................................13
- 2 Basidiomata with well-developed stipe....................................................................................3
- 2 Basidiomata sessile or short pseudostipitate...........................................................................7
- 3 Peridium turns to vinaceous, reddish purple to blackish red when bruised........................4
- 3 Peridium does not change color when bruised......................................................................5
- 4 Clamp connections present; peridium turns to reddish purple to blackish red when bruised..........................................................................................................................S. erubescens
- 4 Clamp connections absent, peridium turns to vinaceous when bruised............S. vinaceum
- 5 Clamp connections present........................................................................................................6
- 5 Clamp connections absent..................................................................................S. squamulosum
- 6 Basidiomata whitish to yellowish, with large squamules.................................S. yunnanense
- 6 Basidiomata tan to ochraceous brown, with warty and peelable squamules...S. separatum
- 7 Peridium does not change color when bruised......................................................................8
- 7 Peridium turns to pale pink, pale pinkish brown to pale brown when bruised.........S. cepa
- 8 Peridium thick, usually >1 mm..................................................................................S. flavidum
- 8 Peridium thin, usually <1 mm....................................................................................................9
- 9 Basidiomata pseudostipitate....................................................................................................10
- 9 Basidiomata sessile....................................................................................................................11
- 10 Basidiospores (8.0–) 9.0–12.0 (–14.0) µm in diam..............................................S. verrucosum
- 10 Basidiospores (6.0–) 7.0–11.0 (–12.0) µm in diam....................................................S. nitidum
- 11 Gleba brownish violet to dark olivaceous with abundant yellowish trama veins; basidiospores 11.0–17.0 µm in diam....................................................................................S. areolatum
- 11 Gleba ash grey, grey to dark grey without yellowish trama veins; basidiospores 9.0–16.0 µm...........................................................................................................................................12
- 12 Basidiospores 9.0–13.0 µm in diam............................................S. venenatum var. venenatum
- 12 Basidiospores 12.0–16.0 µm in diam.....................................S. venenatum var. macrosporum
- 13 Basidiospores subreticulate....................................................................................................14
- 13 Basidiospores reticulate..........................................................................................................18
- 14 Peridium thin, usually <1 mm, about 0.5–0.6 mm thick.....................................S. suthepense
- 14 Peridium thick, usually >1 mm..............................................................................................15
- 15 Basidiomata sessile..................................................................................................................16
- 15 Basidiomata with well-developed stipe................................................................................17
- 16 Basidiomata with imbricate scales and tomentose surface; basidiospores 10.4–13.6 µm in diam.........................................................................................................................S. floridanum
- 16 Basidiomata without imbricate scales and tomentose surface; basidiospores (6.0–) 7.0–11.0 (–12.0) µm in diam....................................................................................................S. texense
- 17 Dehiscence stilliform, basidiospores 5.0–13.0 µm in diam..............................S. polyrhizum
- 17 Irregularly dehiscent at the top; basidiospores 2.5–7.5 µm in diam...........S. sinnamariense
- 18 Peridium thick, usually >1 mm..............................................................................................19
- 18 Peridium thin, usually ≤1 mm...............................................................................................20
- 19 Peridium 2–5 mm thick, yellowish brown to pale orangish yellow, coarsely scaly; endoperidium rubescent when bruised; basidiospores (9.0–) 11.0–14.0 (–17.0) µm in diam.................................................................................................................................S. citrinum
- 19 Peridium 1.5–2.5 mm thick, light yellow, endoperidium does not change colour when bruised, basidiospores 9.0–16.0 µm in diam...........................................................S. paradoxum
- 20 Sessile or with a short-pseudostipe or a short-fasciculate base formed by compact mycelia; basidiospores (10.0–)11.0–13.0 (–15.0) µm in diam.; clamp connections present……………..................................................................................................................S. bovista
- 20 With a short or thick pseudostipe; basidiospores 5.5–9.0 µm in diam.; clamp connections absent..............................................................................................................S. dictyosporum
4. Discussion
Based on the macro- and micromorphological characters, in combination with the ITS phylogeny, four new species of Scleroderma were described from southwestern China, which improved our understanding the species diversity of Scleroderma. Macroscopically, the size of the basidiomata, the color of the peridium and gleba, the thickness of the peridium, and the type of dehiscence of basidiomata are of important characters in delimitating Scleroderma species. Microscopically, the size and ornamentation of the basidiospores, and the presence/absence of clamp connections are very useful in distinguishing species of this genus. In addition, the geographic distribution is also helpful in distinguishing certain species.
Based on basidiospore ornamentation and the presence/absence of clamp connections, Guzmán et al. [43] grouped Scleroderma species into three sections: (1) sect. Reticulatae, characterized by reticulate basidiospores and the presence of clamp connections; (2) sect. Scleroderma, characterized by spiny ornamentation on basidiospores (echinulate spores) and the absence of clamp connections; (3) sect. Sclerangium, which forms subreticulate basidiospores and clamp connections. In agreement with the results of recent studies [22,25,26,27], the phylogeny inferred from the ITS sequences in this study (Figure 1) showed that these Scleroderma species clustered into three strongly supported clades (clades A, B, and D) and a moderately supported clade (clade C). This result largely agrees with the infrageneric classification of three sections proposed by Guzmán [43], except for S. dictyosporum, which formed an isolated clade (clade A) of its own. Species in clade B, which corresponds to sect. Reticulate, have reticulate basidiospores and possess clamp connections; clade C, corresponds to Guzmán’s sect. Sclerangium, includes species with reticulate basidiospores and clamp connections; clade D, corresponding to sect. Scleroderma, containing species with echinulate basidiospores and no clamp connections, with S. nastii, reported as having subreticulate basidiospores, as an exception. The four new species described here, S. squamulosum, S. vinaceum, S. separatum, and S. erubescens, all belong to sect. Scleroderma, which generally has echinulate basidiospores and lacks clamp connections. However, both S. separatum and S. erubescens have been found to possess clamp connections in the present study. Thus, the section Scleroderma not only contains species without clamp connections, but also contains species with clamp connections.
ECM fungi demonstrate various degrees of host specificity and certain fungal genera exhibit strong host specificity [46,47]. Compared to the other genera (e.g., Chroogomphus, Rhizopogon, and Suillus, which are specifically associated with the pine family), which exhibited strong mycorrhizal host specificity [46,48], Scleroderma exhibits less host specificity. In the present study, in addition to native forests dominated by Pinus yunnanensis (Pinaceae) and Castanea spp. (Fagaceae), we found out that S. erubescens and S. separatum can also be found in pecan (Juglandaceae) orchards. Since pecan trees were not native to China, one may speculate whether the Scleroderma species found in pecan orchards in the present study were introduced with the host trees. However, this seems not very likely, since the senior author performed a regional investigation of ECM fungi associated with pecan trees in the northeastern United States [13], and the comparison among sequences of Scleroderma recovered from the southeastern USA and the sequences generated in the present study showed that the Scleroderma species described in the present study are not the same as those from the USA [13]. Thus, whether there are Scleroderma species introduced to China from the USA requires further investigation.
Author Contributions
Conceptualization, Z.-W.G.; data collection, R.W., L.Z. and Z.-W.G.; methodology, Z.-W.G., R.W. and L.Z.; formal analysis, R.W. and Z.-W.G.; writing—original draft preparation, R.W., Z.-W.G. and L.Z.; writing—review and editing, R.W., Z.-W.G. and H.Q.; funding acquisition, Z.-W.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research is financed by the National Natural Science Foundation of China (no. 31872619), the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (no. 2019HJ209001006), the Digitalization, Development and Application of Biotic Resources (202002AA100007), and the Talent Project of Yunnan (no. 202005AC160037).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The sequences have been deposited in the GenBank database with accession numbers shown in Table 1.
Acknowledgments
We are grateful to Y. J. Hao, L. P. Tang, T. Guo, and J. Qin, as well as X. B. Liu and X. T. Zhu, for providing specimens. Z. J. Gu is thanked for his kind help in taking SEM images. We also thank the reviewers and editors for their constructive advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Guzmán, G. Monografía del género Scleroderma Pers. emend. Fr.(Fungi-Basidiomycetes). Darwiniana 1970, 16, 233–407. [Google Scholar]
- van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Read, D.J.; Perez-Moreno, J. Mycorrhizas and nutrient cycling in ecosystems—A journey towards relevance? New Phytol. 2003, 157, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Kernaghan, G. Mycorrhizal diversity: Cause and effect? Pedobiologia 2005, 49, 511–520. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Bussaban, B.; Lumyong, S. Scleroderma suthepense, a new ectomycorrhizal fungus from Thailand. Mycotaxon 2013, 123, 1–7. [Google Scholar] [CrossRef]
- Danielson, R.M. Ectomycorrhizal associations in jack pine stands in northeastern Alberta. Can. J. Bot. 1984, 62, 932–939. [Google Scholar] [CrossRef]
- Sanon, K.B.; Bâ, A.M.; Delaruelle, C.; Duponnois, R.; Martin, F. Morphological and molecular analyses in Scleroderma species associated with some Caesalpinioid legumes, Dipterocarpaceae and Phyllanthaceae trees in southern Burkina Faso. Mycorrhiza 2009, 19, 571–584. [Google Scholar] [CrossRef]
- Corrales, A.; Koch, R.A.; Vasco-Palacios, A.M.; Smith, M.E.; Ge, Z.-W.; Henkel, T.W. Diversity and distribution of tropical ectomycorrhizal fungi. Mycologia 2022, 114, 919–933. [Google Scholar] [CrossRef]
- Ge, Z.-W.; Brenneman, T.; Bonito, G.; Smith, M.E. Soil pH and mineral nutrients strongly influence truffles and other ectomycorrhizal fungi associated with commercial pecans (Carya illinoinensis). Plant Soil 2017, 418, 493–505. [Google Scholar] [CrossRef]
- Marx, D.H.; Bryan, W.C.; Cordell, C.E. Survival and growth of pine seedlings with Pisolithus ectomycorrhizae after two years on reforestation sites in North Carolina and Florida. For. Sci. 1977, 23, 363–373. [Google Scholar]
- Phosri, C.; Martín, M.P.; Watling, R.; Jeppson, M.; Sihanonth, P. Molecular phylogeny and re-assessment of some Scleroderma spp. (Gasteromycetes). An. Jardín Botánico Madr. 2009, 66, 83–91. [Google Scholar] [CrossRef]
- Wilson, A.W.; Binder, M.; Hibbett, D.S. Diversity and evolution of ectomycorrhizal host associations in the Sclerodermatineae (Boletales, Basidiomycota). New Phytol. 2012, 194, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Sun, C.-Y.; Sun, J.; Zhang, K.-P.; Zhang, H.-S.; Guo, X.; Zhou, Y.-J.; Zheng, D.-S.; Li, H.-J. Scleroderma venenatum sp. nov., S. venenatum var. macrosporum var. nov. and S. suthepense new to China. Phytotaxa 2020, 438, 107–118. [Google Scholar] [CrossRef]
- Li, J.-Z. Studies of Scleroderma from China. J. Nat. Sci. Hunan Norm. Univ. 2003, 26, 60–64. [Google Scholar]
- Liu, B.; Fan, I.; Li, J.; Li, T.; Song, B.; Liu, J. Sclerodermatales, Tulostomatales, Phallales et Podaxales. In Flora Fungorum Sinicorum; Science Press: Beijing, China, 2005; Volume 23, pp. 30–31. (In Chinese) [Google Scholar]
- Zhang, C.; Xu, X.-E.; Liu, J.; He, M.; Wang, W.; Wang, Y.; Ji, K. Scleroderma yunnanense, a new species from South China. Mycotaxon 2013, 125, 193–200. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.H.; Yang, Z.L.; Bau, T.; Dai, Y.C. Atlas of Chinese Macrofungal Resources; Central China Farmers’ Publishing House: Zhengzhou, China, 2015; pp. 1194–1198. (In Chinese) [Google Scholar]
- Dai, Y.C.; Yang, Z.L.; Wen, H.A.; Bau, T.; Li, T.H. A revised checklist of edible fungi in China. Mycosystema 2010, 29, 1–21. [Google Scholar] [CrossRef]
- Dai, Y.C.; Yang, Z.L. A revised checklist of medicinal fungi in China. Mycosystema 2008, 27, 801–824. [Google Scholar] [CrossRef]
- Montagner, D.; Coelho, G.; Silveira, A.; Baldoni, D.; Antoniolli, Z. Morphological and molecular analyses in Scleroderma (Basidiomycota) associated with exotic forests in Pampa biome, southern Brazil. Mycosphere 2015, 6, 337–344. [Google Scholar] [CrossRef]
- Brock, P.; Döring, H.; Bidartondo, M.I. How to know unknown fungi: The role of a herbarium. New Phytol. 2009, 181, 719–724. [Google Scholar] [CrossRef]
- Rusevska, K.; Karadelev, M.; Phosri, C.; Dueñas, M.; Watling, R.; Martín, M.P. Rechecking of the genus Scleroderma (Gasteromycetes) from Macedonia using barcoding approach. Turk. J. Bot. 2014, 38, 375–385. [Google Scholar] [CrossRef]
- Mrak, T.; Kühdorf, K.; Grebenc, T.; Štraus, I.; Münzenberger, B.; Kraigher, H. Scleroderma areolatum ectomycorrhiza on Fagus sylvatica L. Mycorrhiza 2017, 27, 283–293. [Google Scholar] [CrossRef]
- Ortiz-Rivero, J.; Watling, R.; Guzmán-Dávalos, L.; Martín, M.P. The many-rooted earthball—Scleroderma geaster and S. polyrhizum revisited, with the description of a new species. Phytotaxa 2021, 510, 1–17. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Richardson, D.; Leroux, J.; Strasberg, D.; Edwards, J.; Roets, F.; Hubka, V.; Taylor, P.; Hey-koop, M. Fungal Planet description sheets: 400–468. Persoonia 2016, 36, 316–458. [Google Scholar] [CrossRef]
- Baseia, I.G.; Silva, B.D.B.; Ishikawa, N.K.; Soares, J.V.C.; França, I.F.; Ushijima, S.; Maekawa, N.; Martín, M.P. Discovery or Extinction of New Scleroderma Species in Amazonia? PLoS ONE 2016, 11, e0167879. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.; Basukala, O.; Shrestha, R.; Poudel, R. Scleroderma nastii sp. nov., a gasteroid mushroom from Phulchoki hill, Nepal. Stud. Fungi 2020, 5, 50–58. [Google Scholar] [CrossRef]
- Kumla, J.; Suwannarach, N.; Bussaban, B.; Matsui, K.; Lumyong, S. Indole-3-acetic acid production, solubilization of insoluble metal minerals and metal tolerance of some sclerodermatoid fungi collected from northern Thailand. Ann. Microbiol. 2014, 64, 707–720. [Google Scholar] [CrossRef]
- Rebriev, Y.A.; Zvyagina, E.A. Scleroderma furfuraceum (Boletales, Agaricomycetes)—A new species from the Russian Far East. Phytotaxa 2022, 555, 169–177. [Google Scholar] [CrossRef]
- Hosaka, K. Phylogeography of the genus Pisolithus revisited with some additional taxa from New Caledonia and Japan. Bull. Natl. Mus. Nat. Sci. Ser. B 2009, 35, 151–167. [Google Scholar]
- Kornerup, A.; Wanscher, J. Methuen Handbook of Colour Fletcher; Fletcher & Son: Norwich, UK, 1981; pp. 1–252. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Swindell, S.R.; Plasterer, T. Sequence Data Analysis Guidebook; Springer: Berlin/Heidelberg, Germany, 1997; pp. 75–89. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 2014, 30, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Nylander, J. MrAIC. pl. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree 1.4. 4 (Computer Program). 2018. Available online: http://tree.bio.ed.ac.uk (accessed on 7 August 2021).
- Guzmán, G.; Cortés-Pérez, A.; Guzmán-Dávalos, L.; Ramírez-Guillén, F.; del Refugio Sánchez-Jácome, M. An emendation of Scleroderma, new records, and review of the known species in Mexico. Rev. Mex. Biodivers. 2013, 84, S173–S191. [Google Scholar] [CrossRef]
- Jeppson, M. Scleroderma cepa Pers. brief notes on its taxonomy, ecology and distribution. Windhalia 1986, 16, 123–126. [Google Scholar]
- Nouhra, E.R.; Caffot, M.L.H.; Pastor, N.; Crespo, E.M. The species of Scleroderma from Argentina, including a new species from the Nothofagus forest. Mycologia 2012, 104, 488–495. [Google Scholar] [CrossRef]
- Bruns, T.D.; Bidartondo, M.I.; Taylor, D.L. Host specificity in ectomycorrhizal communities: What do the exceptions tell us? Integr. Comp. Biol. 2002, 42, 352–359. [Google Scholar] [CrossRef]
- Lofgren, L.; Nguyen, N.H.; Kennedy, P.G. Ectomycorrhizal host specificity in a changing world: Can legacy effects explain anomalous current associations? New Phytol. 2018, 220, 1273–1284. [Google Scholar] [CrossRef]
- Miller, O.K., Jr. The Gomphidiaceae Revisited: A Worldwide Perspective. Mycologia 2003, 95, 176. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).