Diversity of True Bugs (Hemiptera: Heteroptera) on Common Ragweed (Ambrosia artemisiifolia) in Southern Slovakia
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Essl, F.; Biró, K.; Brandes, D.; Broennimann, O.; Bullock, J.M.; Chapman, D.S.; Chauvel, B.; Dullinger, S.; Fumanal, B.; Guisan, A.; et al. Biological Flora of the British Isles: Ambrosia artemisiifolia. J. Ecol. 2015, 103, 1069–1098. [Google Scholar] [CrossRef]
- Chauvel, B.; Cadet, E. Introduction and spread of an invasive species: Ambrosia artemisiifolia in France. Acta Bot. Gall. Bull. De La Société Bot. De Fr. 2011, 158, 309–327. [Google Scholar] [CrossRef]
- Guillemin, J.P.; Chauvel, B. Effects of the seed weight and burial depth on the seed behavior of common ragweed (Ambrosia artemisiifolia). Weed Biol. Manag. 2011, 11, 217–223. [Google Scholar] [CrossRef]
- Kazinczi, G.; Novák, R. Integrated Methods for Suppression of Common Ragweed; National Food Chain Safety Office, Directorate of Plant Protection Soil Conservation and Agri-environment: Budapest, Hungary, 2014; p. 226. ISBN 978-963-89968-4-8. [Google Scholar]
- Weber, E. Invasive Plant Species of the World: A Reference Guide to Environmental Weeds, CABI 2nd ed.; CABI Publishing: Wallingford, UK, 2017; p. 596. [Google Scholar]
- Kazinczi, G.; Béres, I.; Pathy, Z.; Novák, R. Common ragweed (Ambrosia artemisiifolia L.): A review with special regards to the results in Hungary: II. Importance and harmful effect, allergy, habitat, allelopathy, and beneficial characteristics. Herbologia 2008, 9, 93–118. [Google Scholar]
- Storms, W.; Meltzer, E.O.; Nathan, R.A.; Selner, J.C. The economic impact of allergic rhinitis. J. Allergy Clin. Immunol. 1997, 99, 820–824. [Google Scholar] [CrossRef]
- Simard, M.J.; Benoit, D.L. Distribution and abundance of an allergenic weed, common ragweed (Ambrosia artemisiifolia L.), in rural settings of southern Quebec, Canada. Can. J. Plant Sci. 2010, 90, 549–557. [Google Scholar] [CrossRef]
- Zisenis, M. Alien plant species: A real fear for urban ecosystems in Europe? Urban Ecosyst. 2015, 18, 355–370. [Google Scholar] [CrossRef]
- Bordas-Le Floch, V.; Le Mignon, M.; Bouley, J. Identification of Novel Short Ragweed Pollen Allergens Using Combined Transcriptomic and Immunoproteomic Approaches. PLoS ONE 2015, 10, e013625. [Google Scholar] [CrossRef]
- Smith, M.; Cecchi, L.; Skjøth, C.A.; Karrer, G.; Šikoparija, B. Common ragweed: A threat to environmental health in Europe. Environ. Int. 2013, 61, 115–126. [Google Scholar] [CrossRef]
- Hamaoui-Laguel, L.; Vautard, R.; Liu, L.; Solmon, F.; Viovy, N.; Khvorostyanov, D.; Essl, F.; Chuine, I.; Colette, A.; Semenov, M.; et al. Effects of climate change and seed dispersal on airborne ragweed pollen loads in Europe. Nat. Clim. Chang. 2015, 5, 766–771. [Google Scholar] [CrossRef]
- Gerber, E.; Schaffner, U.; Gassmann, A.; Hinz, H.L.; Seier, M.; Müller-Schärer, H. Prospects for biological control of Ambrosia artemisiifolia in Europe: Learning from the past. Weed Res. 2011, 51, 559–573. [Google Scholar] [CrossRef]
- Cassis, G. True Bugs (Insecta: Hemiptera: Heteroptera): Evolution, Classification, Biodiversity and Biology, Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Dolling, W.R. Hemiptera. Oxford Natural History Museum Publications; Oxford University Press: Oxford, UK, 1991; p. 274. ISBN 9780198540168. [Google Scholar]
- Zurbrügg, C.; Frank, T. Factors influencing bug diversity (Insecta: Heteroptera) in semi-natural habitats. In Arthropod Diversity and Conservation. Topics in Biodiversity and Conservation; Hawksworth, D.L., Bull, A.T., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 15, p. 525. [Google Scholar] [CrossRef]
- Mitchell, P.L. Heteroptera as vectors of plant pathogens. Neotrop. Entomol. 2004, 33, 519–545. [Google Scholar] [CrossRef]
- Mitchell, P.L.; Zeilinger, A.; Medrano, E.G.; Esquivel, J.F. Pentatomoids as vectors of plant pathogens. In Invasive Stink Bugs and Related Species (Pentatomoidea): Biology, Higher Systematics, Semiochemistry, and Management; McPherson, J.E., Ed.; CRC Press: Boca Raton, FL, USA, 2018; pp. 611–640. [Google Scholar]
- Torma, A.; Bozsó, M.; Tölgyesi, C.; Gallé, R. Relationship of different feeding groups of true bugs (Hemiptera: Heteroptera) with habitat and landscape features in Pannonic salt grasslands. J. Insect Conserv. 2017, 21, 645–656. [Google Scholar] [CrossRef]
- Gibicsár, S.; Keszthelyi, S. Topographical Based Significance of Sap-Sucking Heteropteran in European Wheat Cultivations: A Systematic Review. Diversity 2023, 15, 109. [Google Scholar] [CrossRef]
- Laterza, I.; Dioli, P.; Tamburini, G. Semi-natural habitats support populations of stink bug pests in agricultural landscapes. Agric. Ecosyst. Environ. AGR 2023, 342, 108223. [Google Scholar] [CrossRef]
- Abukenova, V.S.; Slavchenko, N.P.; Kartbayeva, G.T.; Myrzabayev, A.B.; Yeshmagambetova, A.B.; Duzbayeva, N.M.; Kabbassova, M.T.; Abukenova, A.K. Composition and Ecological Structure of the Fauna of Litter and Soil True Bugs (Insecta, Heteroptera) in Kazakh Upland (Central Kazakhstan) Pine Forests. Diversity 2022, 14, 618. [Google Scholar] [CrossRef]
- Maceljski, M.; Igrc, J. The Phytophagous Insect Fauna of Ambrosia artemisiifolia in Yugoslavia. In Proceedings of the VII International Symposium on Biological Control of Weeds, Rome, Italy, 6–11 March 1988. [Google Scholar]
- Kiss, B.; Koczor, S.; Rédei, D. Occurrence and feeding of hemipterans on common ragweed (Ambrosia artemisiifolia) in Hungary. Bull. Insectol. 2008, 61, 195–196. [Google Scholar]
- Franin, K.; Franin, G.K.; Maričić, B.; Marcelić, Š.; Pavlović, M.; Kos, T.; Barić, B.; Laznik, Z. True bugs (Heteroptera) assemblage and diversity in the ecological infrastructures around the Mediterranean vineyards. Bull. Insectology 2021, 74, 65–78. [Google Scholar]
- Limonta, L.; Gaini, P.; Dioli, P. First Results on Heteroptera (Hemiptera) of Dry Grassland in Malpaga-Basella Nature Reserve (Italy). Diversity 2022, 14, 981. [Google Scholar] [CrossRef]
- Zaťko, M. Land Structure. In Landscape Atlas of the Slovak Republic; Abaffy, D., Miklós, L., Eds.; Slovak Environmental Agency, Banská Bystrica: Slovakia, Bratislava, 2002; p. 344. [Google Scholar]
- Costa, L.D.F. Further Generalizations of the Jaccard Index. 2022. Available online: https://hal.science/hal-03384438v4 (accessed on 17 February 2023).
- Glen, S. “Jaccard Index/Similarity Coefficient” From StatisticsHowTo.com: Elementary Statistics for the Rest of Us! 2023. Available online: https://www.statisticshowto.com/jaccard-index/ (accessed on 17 February 2023).
- Malenovský, I.; Baňař, P.; Kment, P.A. Contribution to the faunistic of the Hemiptera (Cicadomorpha, Fulgoromorpha, Het-eroptera, and Psylloidea) associated with dry grassland sites in southern Moravia (Czech Republic). Acta Musei Morav. Sci. Biol. 2011, 96, 41–187. [Google Scholar]
- Saulich, A.K.; Musolin, D.L. Seasonal Development of Plant Bugs (Heteroptera, Miridae): Subfamily Mirinae, Tribe Mirini. Entomol. Rev. 2020, 100, 135–156. [Google Scholar] [CrossRef]
- Yazıcı, G.; Yildirim, E. Prefered host plants species by Miridae (Hemiptera: Heteroptera) species in Erzurum Province of Turkey. Entomofauna 2017, 38, 193–212. [Google Scholar]
- Collyer, E. Biology of some Predatory Insects and Mites Associated with the Fruit Tree Red Spider Mite (Metatetranychus ulmi (Koch)) in South-Eastern England II. Some Important Predators of the Mite. Hortic. Sci. 1953, 28, 85–97. [Google Scholar] [CrossRef]
- Chinajariyawong, A.; Harris, V.E. Inability of Deraeocoris signatus (Distant) (Hemiptera: Miridae) to survive and reproduce on cotton without prey. Aust. J. Entomol. 1987, 26, 37–40. [Google Scholar] [CrossRef]
- Sanchez, J.-A.; Martinez-Cascales, J.I.; Lacasa, A. Abundance and wild host plants of predator mirids (Heteroptera: Miridae) in horticultural crops in the Southeast of Spain. IOBC/WPRS Bull. 2003, 26, 147–151. [Google Scholar]
- Eyles, A.C. New genera and species of the Lygus-Complex (Hemiptera: Miridae) in the New Zealand subregion compared with subgenera (now genera) studied by Leston (1952) and Niastama, Reuter, New Zealand. J. Zool. 1999, 26, 303–354. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Varis, A.L. Host plants of the European tarnished plant bug Lygus rugulipennis Poppius (Het., Miridae). J. Appl. Entomol. 1991, 111, 484–498. [Google Scholar] [CrossRef]
- Hradil, K.; Psota, V.; Štastná, P. Species diversity of true bugs on apples in terms of plant protection. Plant Prot. Sci. 2013, 49, 73–83. [Google Scholar] [CrossRef]
- Kivan, M.; Dirik, E. Edirne ili buğday ekiliş alanlarında tespit edilen Heteroptera türleri. Türkiye Entomoloji Bülteni 2016, 6, 357–369. [Google Scholar] [CrossRef]
- Rabitsch, W. Alien True Bugs of Europe (Insecta: Hemiptera: Heteroptera). Zootaxa 2008, 1827, 1–44. [Google Scholar] [CrossRef]
- Stehlík, J.L. Results of the investigations on Hemiptera in Moravia made by the Moravian Museum (Tingidae). Acta Musei Morav. Sci. Biol. 2002, 87, 87–149. [Google Scholar]
- Pekár, S.; Hrušková, M. How granivorous Coreus marginatus (Heteroptera: Coreidae) recognises its food. Acta Ethol. 2006, 9, 26–30. [Google Scholar] [CrossRef]
- Aukema, B. Rhopalus tigrinus (Rhopalidae) en Eurydema ornatum (Pentatomidae) nieuw voor de Nederlandse fauna (Heteroptera). Entomol. Ber. 1993, 53, 19–22. [Google Scholar]
- Stewart, A.J.A.; Bantock, T.M.; Beckmann, B.C.; Botham, M.S.; Hubble, D.; Roy, D.B. The role of ecological interactions in determining species ranges and range changes. Biol. J. Linn. Soc. Lond. 2015, 115, 647–663. [Google Scholar] [CrossRef]
- Vilímová, J.; Rohanová, M. The external morphology of eggs of three Rhopalidae species (Hemiptera: Heteroptera) with a review of the eggs of this family. Acta Entomol. Musei Natl. Pragae 2010, 50, 75–95. [Google Scholar]
- Stehlík, J.L.; Vavřínová, I. Results of the investigations on Hemiptera in Moravia made by the Moravian Museum (Coreoidea 2). Acta Musei Morav. Sci. Biol. 1989, 74, 175–200. [Google Scholar]
- Honěk, A.; Štys, P.; Martinková, Z. Arthropod community of dandelion (Taraxacum officinale) capitula during seed dispersal. Biologia 2013, 68, 330–336. [Google Scholar] [CrossRef]
- Ellis, W.N. (2001–2022) Leafminers and Plant Galls of Europe. Plant Parasites of Europe: Leafminers, Gallers and Fungi. Available online: https://bladmineerders.nl (accessed on 20 November 2022).
- Gierlasiński, G.; Taszakowski, A.; Lis, B. Stilt bugs (Hemiptera: Heteroptera: Berytidae) of Poland: Checklist, distribution, bionomics. Fragm. Faunist. 2019, 62, 1–25. [Google Scholar] [CrossRef]
- Laukkanen, L.; Mutikainen, P.; Muola, A.; Leimu, R. Plant-species diversity correlates with genetic variation of an oligophagous seed predator. PLoS ONE 2014, 9, e94105. [Google Scholar] [CrossRef]
- Linnavuori, R.E. Studies on the Cimicomorpha and Pentatomomorpha (Hemiptera: Heteroptera) of Khuzestan and the adjacent provinces of Iran. Acta Entomol. Musei Natl. Pragae 2011, 51, 21–48. [Google Scholar]
- Stehlík, J.L.; Vavřínová, I. Results of the investigations on Hemiptera in Moravia made by the Moravian Museum (Lygaeidae I). Acta Musei Morav. Sci. Biol. 1997, 81, 231–298. [Google Scholar]
- Redei, D. True Bug (Heteroptera) Assemblages of Medicinal and Aromatic Plants. Ph.D. Thesis, Corvinus University, Budapest, Hungary, 2007. [Google Scholar]
- Stehlík, J.L.; Vavřínová, I. Results of the investigations on Hemiptera in Moravia made by the Moravian Museum (Lygaeidae III). Acta Musei Morav. Sci. Biol. 1998, 83, 21–70. [Google Scholar]
- Stehlík, J.L. Results of the investigations on Hemiptera in Moravia made by the Moravian Museum (Pentatomoidea 4). Acta Musei Morav. Sci. Biol. 1995, 70, 147–175. [Google Scholar]
- Halászfy, É. Poloskák II. Heteroptera II. In Magyarország Állatvilága (Fauna Hungariae); Akadémiai Kiadó: Budapest, Hungary, 1959; pp. 1–87. [Google Scholar]
- Kment, P.; Hradil, K.; Baňař, P.; Balvín, P.; Cunev, J.; Ditrich, T.; Jindra, Z.; Roháčová, M.; Straka, M.; Sychra, J. New and interesting records of true bugs (Hemiptera: Heteroptera) from the Czech Republic and Slovakia. Acta Musei Morav. Sci. Biol. 2013, 98, 495–541. [Google Scholar]
- Stehlík, J.L. Results of the investigations on Hemiptera in Moravia made by the Moravian Museum (Pentatomoidea 5). Acta Musei Morav. Sci. Biol. 1986, 71, 147–178. [Google Scholar]
- Linnavuori, R.E. Studies on the Acanthosomatidae, Scutelleridae and Pentatomidae (Heteroptera) of Gilan and the adjacent provinces in northern Iran. Acta Entomol. Musei Natl. Pragae 2008, 48, 1–21. [Google Scholar] [CrossRef]
- Gözüaçik, C.; Konuksal, A. Investigations on Heteroptera (Hemiptera) Species in Cereal Agroecosystem of Turkish Republic of Northern Cyprus. Kafkas Univ. Inst. Nat. Appl. Sci. J. 2019, 12, 56–67. [Google Scholar]
- Hemala, V.; Cunev, J.; Franc, V. On the occurrence of the stink bug Eysarcoris ventralis (Hemiptera: Heteroptera: Pentatomidae) in Slovakia, with notes on its distribution in neighboring countries. Klapalekiana 2014, 50, 51–59. [Google Scholar]
- Stehlík, J.L. Results of the investigations on Hemiptera in Moravia made by the Moravian Museum (Pentatomoidea 3). Acta Musei Morav. Sci. Biol. 1984, 69, 163–185. [Google Scholar]
- Ghahari, H.; Moulet, P.; Rider, D.A. An annotated catalog of the Iranian Pentatomoidea (Hemiptera: Heteroptera: Pentatomomorpha). Zootaxa 2014, 3837, 1–95. [Google Scholar] [CrossRef]
- Khadka, A.; Hodges, A.C.; Leppla, N.C.; Tillman, P.G. Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) Nymph Survival and Adult Feeding Preferences for Crop Plants in Florida. Fla. Entomol. 2021, 104, 136–139. [Google Scholar] [CrossRef]
- Noge, K.; Tamogami, S. Isovaleronitrile co-induced with its precursor, l-leucine, by herbivory in the common evening primrose stimulates foraging behavior of the predatory blue shield bug. Biosci. Biotechnol. Biochem. 2018, 82, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Stehlík, J.L. Results of the investigations on Hemiptera in Moravia made by the Moravian Museum (Pentatomoidea VII). Acta Musei Morav. Sci. Biol. 1995, 79, 85–96. [Google Scholar]
- Burgess, L.; Dueck, J.; Mckencie, D. Insect vectors of the yeast Nematospora coryli in mustard, Brassica juncea, crops in southern Saskatchewan. Can. Entomol. 1993, 115, 25–30. [Google Scholar] [CrossRef]
- Judd, S.; Hodkinson, I. The Biogeography and Regional Biodiversity of the British Seed Bugs (Hemiptera: Lygaeidae). J. Biogeogr. 1998, 25, 227–249. [Google Scholar] [CrossRef]
- Wheeler, A.G. Biology of the Plant Bugs (Hemiptera: Miridae): Pests, Predators, Opportunists; Cornell University Press: Ithaca, NY, USA, 2001; 528p. [Google Scholar]
- Lu, Y.; Jiao, Z.; Li, G.; Wyckhuys, K.A.G.; Wu, K. Comparative overwintering host range of three Adelphocoris species (Hemiptera: Miridae) in northern China. Crop Prot. 2011, 30, 1455–1460. [Google Scholar] [CrossRef]
- Pan, H.; Lu, Y.; Wyckhuys, K.A.G. Early-Season Host Switching in Adelphocoris spp. (Hemiptera: Miridae) of Differing Host Breadth. PLoS ONE 2013, 8, e59000. [Google Scholar] [CrossRef]
- Puchkov, V.G. Glavneishie klopy-slepnyaki—Vrediteli sel’skokhozyaistvennykh kul’tur (Major Capsid Bugs Important as Agricultural Pests); Naukova Dumka: Kyiv, Ukraine, 1966. [Google Scholar]
- Šafářová, D.; Lauterer, P.; Starý, M.; Válová, P.; Navrátil, M. Insight into epidemiological importance of phytoplasma vectors in vineyards in South Moravia, Czech Republic. Plant Prot. Sci. 2018, 54, 234–239. [Google Scholar] [CrossRef]
- Korbášová, Z. Výskyt a Variabilita Fytoplasmy Stolburu v Hyalestes obsoletus a Dalších Potenciálních Vektorech. Diploma Thesis, Palackého University, Olomouc, Czech Republic, 20 April 2012. [Google Scholar]
- Emmett, B.J.; Baker, L.A.E. Insect Transmission of Fireblight. Plant Dis. 1971, 20, 41–45. [Google Scholar] [CrossRef]
- Březíková, M.; Linhartová, Š. First report of potato stolbur phytoplasma in hemipterans in southern Moravia—Short Communication. Plant Prot. Sci. 2007, 43, 73–76. [Google Scholar] [CrossRef]
- Sobko, O.A.; Matsishina, N.V.; Fisenko, P.V.; Kim, I.V.; Didora, A.S.; Boginskay, N.G.; Volkov, D.I. Viruses in the agrobiocenosis of the potato fields. IOP Conf. Ser. Earth Environ. Sci. 2021, 677, 052093. [Google Scholar] [CrossRef]
- Sobko, O.; Matsishina, N.; Fisenko, P.; Kim, I.; Boginskaya, N. Phytoviruses in the Potato Field Tripartite Agroecosystem. In Fundamental and Applied Scientific Research in the Development of Agriculture in the Far East (AFE-2021). Lecture Notes in Networks and Systems; Muratov, A., Ignateva, S., Eds.; Springer: Cham, Switzerland, 2022; Volume 353, p. 1176. [Google Scholar] [CrossRef]
- Orságová, H.; Březíková, M.; Schlesingerová, G. Presence of phytoplasmas in hemipterans in Czech vineyards. Bull. Insectology 2011, 64, S119–S120. [Google Scholar]
- Ratnadass, A.; Deguine, J.P. Three-way interactions between crop plants, phytopathogenic fungi, and mirid bugs. Agron. Sustain. Dev. 2020, 6640, 46. [Google Scholar] [CrossRef]
- Hiruki, C. Paulownia witches’-broom disease important in East Asia. Acta Horti. 1999, 496, 63–68. [Google Scholar] [CrossRef]
- Kumar, R.; Tiwari, R.K.; Sundaresha, S.; Kaundal, P.; Raigond, B. Potato Viruses and Their Management. In Sustainable Management of Potato Pests and Diseases; Chakrabarti, S.K., Sharma, S., Shah, M.A., Eds.; Springer: Singapore, 2022; p. 922. [Google Scholar] [CrossRef]
- Lacomme, C.; Glais, L.; Bellstedt, D.U.; Dupuis, B.; Karasev, A.V. (Eds.) Potato Virus Y: Biodiversity, Pathogenicity, Epidemiology, and Management; Springer International Publishing: Basel, Switzerland, 2017; p. XI. 261p. [Google Scholar]
- Agindotan, B.O.; Shiel, P.J.; Berger, P.H. Simultaneous detection of potato viruses, PLRV, PVA, PVX and PVY from dormant potato tubers by TaqMan real-time RT-PCR. J. Virol. Methods 2007, 142, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Gibbs, A.J.; Adams, I.P.; Wilson, C.; Botermans, M.; Fox, A.; Kreuze, J.; Boonham, N.; Kehoe, M.A.; Jones, R.A.C. Potato Virus A Isolates from Three Continents: Their Biological Properties, Phylogenetics, and Prehistory. Phytopathology 2021, 111, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, F. Candidatus Phytoplasma solani (Stolbur phytoplasma), CABI Compendium. CABI Compendium 2022. [Google Scholar] [CrossRef]
- Kosovac, A.; Ćurčić, Ž.; Stepanović, J.; Rekanović, E.; Duduk, B. Epidemiological role of novel and already known ‘Ca. P. solani’ cixiid vectors in rubbery taproot disease of sugar beet in Serbia. Sci. Rep. 2023, 13, 1433. [Google Scholar] [CrossRef]
- Jensen, D.D. Herbaceous Host Plants of Western X-Disease Agent. Phytopathology 1971, 61, 1465. [Google Scholar] [CrossRef]
- Fránová, J.; Špak, J.; Šimková, M. First report of a 16SrIII-B subgroup phytoplasma associated with leaf reddening, virescence and phyllody of purple coneflower. Eur. J. Plant Pathol. 2013, 136, 7–12. [Google Scholar] [CrossRef]
- Rančić, D.; Paltrinieri, S.; Toševski, I.; Petanović, R.; Stevanović, B.; Bertaccini, A. First report of multiple inflorescence disease of Cirsium arvense and its association with a 16SrIII-B subgroup phytoplasma in Serbia. Plant Pathol. 2005, 54, 561. [Google Scholar] [CrossRef]
- Pavlovic, S.; Pljevljakusic, D.; Starovic, M.; Stojanovic, S.; Josic, D. First Report of 16SrIII-B Phytoplasma Subgroup Associated with Virescence of Arnica montana in Serbia. Plant Dis. 2012, 96, 1691. [Google Scholar] [CrossRef] [PubMed]
- Edwin, B.T.; Mohankumar, C. Kerala wilt disease phytoplasma: Phylogenetic analysis and identification of a vector, Proutista moesta. Physiol. Mol. Plant Pathol. 2007, 71, 41–47. [Google Scholar] [CrossRef]
- Tingey, W.M.; Pillimer, E.A. Lygus Bugs: Crop Resistance and Physiological Nature of Feeding Injury. Bull. Entomol. Soc. Am. 1977, 23, 277–287. [Google Scholar] [CrossRef]
- Strong, F.E. Physiology of Injury Caused by Lygus hesperus. J. Econ. Entomol. 1970, 63, 808–814. [Google Scholar] [CrossRef]
- Laimer, M.; Bertaccini, A. Phytoplasma Elimination from Perennial Horticultural Crops. In Phytoplasmas: Plant Pathogenic Bacteria–II; Bertaccini, A., Weintraub, P., Rao, G., Mori, N., Eds.; Springer: Singapore, 2019; pp. 185–206. [Google Scholar] [CrossRef]
- Müller, D.; Leyser, O. Auxin, cytokinin and the control of shoot branching. Ann. Bot. 2011, 107, 1203–1212. [Google Scholar] [CrossRef]
- Suzuki, M.; Takahashi, T.; Komeda, Y. Formation of corymb-like inflorescences due to delay in bolting and flower development in the corymbosa2 mutant of Arabidopsis. Plant Cell Physiol. 2002, 43, 298–306. [Google Scholar] [CrossRef]
- Weintraub, P.; Beanland, L. Insect vectors of phytoplasmas. Annu. Rev. Entomol. 2006, 51, 91–111. [Google Scholar] [CrossRef]
- Backus, E.A. Sensory systems, and behaviors which mediate hemipteran plant-feeding: A taxonomic overview. J. Insect Physiol. 1988, 34, 151–165. [Google Scholar] [CrossRef]
- Nipah, J.O.; Jones, P.; Hodgetts, J.; Dickinson, M. Detection of phytoplasma DNA in embryos from coconut palms in Ghana, and kernels from maize in Peru. Bull. Insectol. 2007, 60, 385–386. [Google Scholar]
- Olivier, C.Y.; Galka, B.; Sèguin-Swartz, G. Detection of aster yellows phytoplasma DNA in seed and seedlings of canola (Brassica napus and B. rapa) and AY strain identification. Can. J. Plant Pathol. 2010, 32, 298–305. [Google Scholar] [CrossRef]
- Péricart, J. Hemipteres Lygaeidae euro-mediterraneens 1-3. In Faune de France; Fédération Française des Sociétés de Sciences Naturelles: Paris, France, 1998; Volume 84A–C, pp. 453, 468, 487. [Google Scholar]
- Schuh, R.T.; Slater, J.A. True Bugs of the World (Hemiptera: Heteroptera): Classification and Natural History; Cornell University Press: Ithaca, NY, USA, 1995; p. 336. [Google Scholar]
- White, D.T.; Billington, S.J.; Walsh, K.B.; Scott, P.T. DNA sequence analysis supports the association of phytoplasmas with papaya (Carica papaya) dieback, yellow crinkle, and mosaic. Australas. Plant Pathol. 1997, 26, 28–36. [Google Scholar] [CrossRef]
- Wieczorek, A.; Wright, M. PCR detection of phytoplasma from witches’ broom disease on Protea spp. (Proteaceae) and associated arthropods. Acta Hortic. 2003, 602, 161–166. [Google Scholar] [CrossRef]
- Hogenhout, S.A.; Oshima, K.; Ammar, E.; Kakizawa, S.; Heather, N.; Kingdom, H.N.; Namba, S. Phytoplasmas: Bacteria that manipulate plants and insects. Mol. Plant Pathol. 2008, 9, 403–423. [Google Scholar] [CrossRef]
- Lattin, J.D. Bionomics of the Nabidae. Annu. Rev. Entomol. 1989, 34, 383–400. [Google Scholar] [CrossRef]
- Hammond, R.B.; Stinner, B.R. Soybean Foliage Insects in Conservation Tillage Systems: Effects of Tillage, Previous Cropping History, and Soil Insecticide Application. Environ. Entomol. 1987, 16, 524–531. [Google Scholar] [CrossRef]
- Ridgway, R.L.; Jones, S.L. Plant Feeding by Geocoris pallens and Nabis americoferus. Ann. Entomol. Soc. Am. 1968, 61, 232–233. [Google Scholar] [CrossRef]
- Lentz, G.L.; Chambers, A.Y.; Hayes, R.M. Effects of Systemic Insecticide-Nematicides on Midseason Pest and Predator Populations in Soybean. J. Econ. Entomol. 1983, 76, 836–840. [Google Scholar] [CrossRef]
- Stam, P.A.; Elmosa, H. The role of predators and parasites in controlling populations of Earias insulana, Heliothis armigera and Bemisia tabaci on cotton in the Syrian Arab Republic. Entomophaga 1990, 35, 315–327. [Google Scholar] [CrossRef]
- Fiallo-Olivé, E.; Pan, L.L.; Liu, S.S.; Navas-Castillo, J. Transmission of Begomoviruses and Other Whitefly-Borne Viruses: Dependence on the Vector Species. Phytopathology 2020, 110, 10–17. [Google Scholar] [CrossRef]
- SHMÚ (Slovak Hydrometeorological Institute). The Ministry of the Environment of the Slovak Republic. 2023. Available online: https://www.shmu.sk/en/ (accessed on 2 May 2023).
- AMET (Association Litschmann & Suchý). Meteorological Stations. 2023. Available online: http://www.amet.cz/ (accessed on 2 May 2023).
- Fauvel, G. Diversity of Heteroptera in agroecosystems: Role of sustainability and bioindication. Agric. Ecosyst. Environ. 1999, 74, 275–303. [Google Scholar] [CrossRef]
- Capinera, J. Relationships between insect pests and weeds: An evolutionary perspective. Weed Sci. 2005, 53, 892–901. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The role of weeds in supporting biological diversity within crop fields. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef]
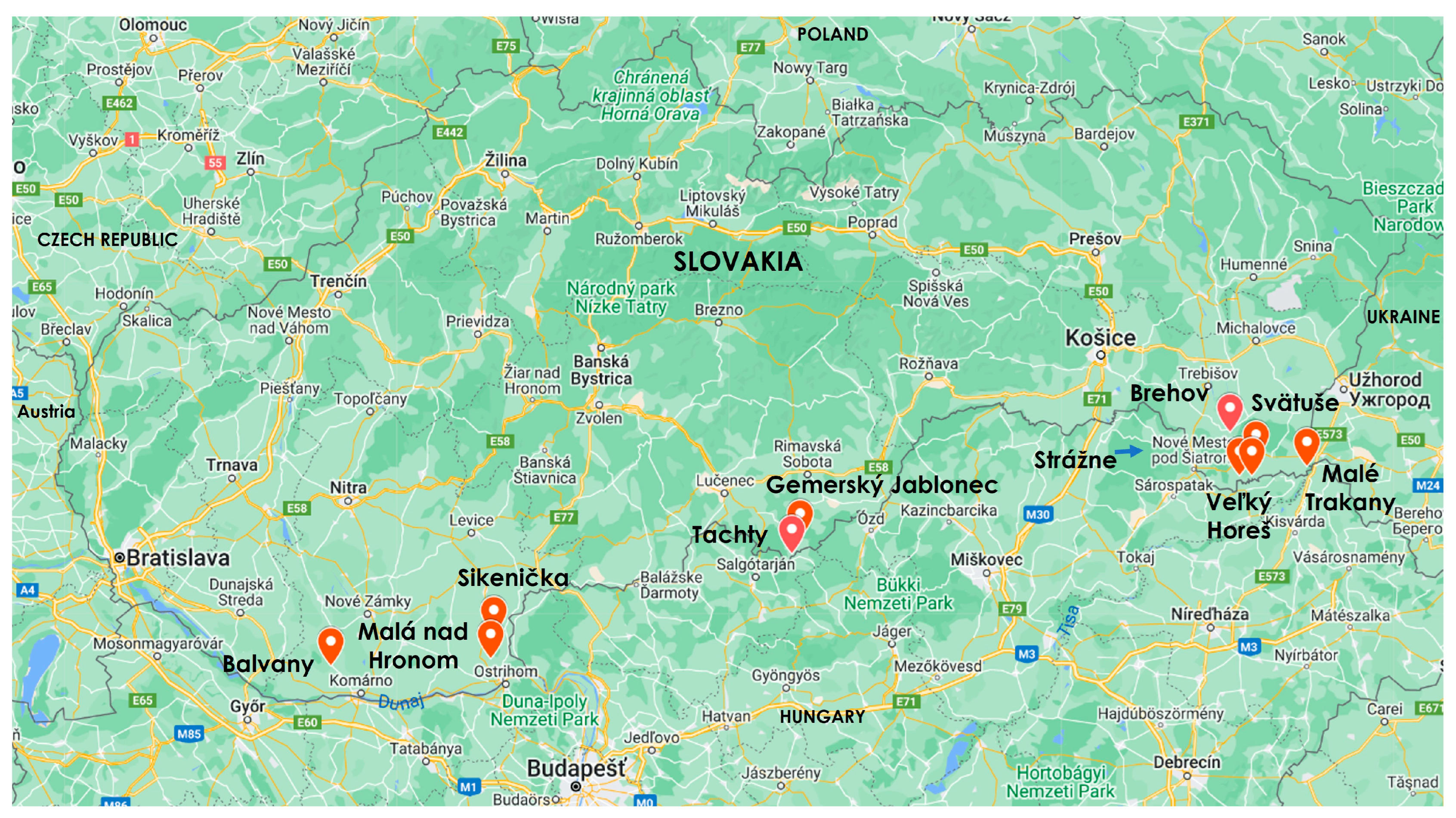









| Locations | Latitude, Longitude | Altitude (m/a.s.l.) | Habitats, Geo Relief | Climate Region |
|---|---|---|---|---|
| Western Slovakia | ||||
| Balvany (Figure 2) | 47°50′24″ N, 17°59′57″ E | 110 | Field margins, plane | Warm, very dry, mild winter |
| Malá nad Hronom (Figure 3) | 47°51′25″ N, 18°40′40″ E | 140 | Weedy agricultural field, hilly country | Warm, very dry, mild winter |
| Sikenička (Figure 4) | 47°55′42″ N, 18°41′34″ E | 140 | Field margins, hilly country | Warm, very dry, mild winter |
| Central Slovakia | ||||
| Gemerský Jablonec (Figure 5) | 48°11′57″ N, 19°59′22″ E | 239 | Mowed meadow, hilly country | Warm, moderate humid, cool winter |
| Tachty (Figure 6) | 48°09′23″ N, 19°56′59″ E | 262 | Field margins, hilly country | Warm, moderate humid, cool winter |
| Eastern Slovakia | ||||
| Brehov (Figure 7) | 48°29′59″ N, 21°48′29″ E | 112 | Field margins, hilly country | Warm, dry, cool winter |
| Malé Trakany (Figure 8) | 48°23′58″ N, 22°07′54″ E | 110 | Field margins, plane | Warm, dry, cool winter |
| Strážne (Figure 9) | 48°22′23″ N, 21°50′47″ E | 100 | Weedy agricultural field, plane | Warm, dry, cool winter |
| Svätuše | 48°25′16″ N, 21°55′10″ E | 110 | Field margins, plane | Warm, dry, cool winter |
| Veľký Horeš (Figure 10) | 48°22′22″ N, 21°54′10″ E | 100 | Weedy agricultural field, plane | Warm, dry, cool winter |
| Infraorder/Family/Species | Food Specialization | References | Balvany | Malá nad Hronom | Sikenička | Gemerský Jablonec | Tachty | Brehov | Malé Trakany | Strážne | Svatuše | Veľký Horeš | TOTAL | Relative Occurrence (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIMICOMORPHA | ||||||||||||||
| NABIDAE | ||||||||||||||
| Nabis (Dolichonabis) limbatus Dahlbom, 1851 | zoophagous | (Wachmann et al. 2006 [30]) | 53 | 185 | 4 | 7 | 14 | 2 | 25 | 12 | 2 | 32 | 336 | 13.46 |
| Nabis rugosus (Linnaeus, 1758) | zoophagous | (Wachmann et al. 2006 [30]) | 1 | 1 | 0.04 | |||||||||
| MIRIDAE | ||||||||||||||
| Adelphocoris lineolatus (Goeze, 1778) | phyto, poly (Fabaceae, eggs are laid to some Asteraceae) | (Puchkov 1972 [31]) | 70 | 149 | 5 | 32 | 20 | 6 | 53 | 35 | 11 | 100 | 481 | 19.27 |
| Adelphocoris vandalicus (Rossi, 1790) | phyto, poly (incl. Asteraceae) | [32] | 1 | 1 | 0.04 | |||||||||
| Adelphocoris seticornis (Fabricius, 1775) | phyto, oligo (Fabaceae) | (Wachmann et al. 2004: [30]) | 1 | 3 | 4 | 0.16 | ||||||||
| Deraeocoris (Deraeocoris) ruber (Linnaeus, 1758) | zoophagous | [33] | 1 | 1 | 0.04 | |||||||||
| Deraeocoris (Camptobrochis) punctulatus (Fallén, 1807) | phytozoophagous (mostly Solanaceae) | [34,35] | 1 | 2 | 3 | 0.12 | ||||||||
| Lygocoris (Lygocoris) pabulinus (Linnaeus, 1761) | phyto, poly | (Southwood & Leston 1959 [36]) | 1 | 1 | 1 | 3 | 0.12 | |||||||
| Lygus pratensis (Linnaeus, 1758) | phyto, poly (incl. Asteraceae) | (Lu & Wu 2008 [31]) | 47 | 37 | 18 | 6 | 5 | 4 | 23 | 12 | 6 | 13 | 171 | 6.85 |
| Lygus rugulipennis Poppius, 1911 | phyto, poly (Asteraceae among the most important) | [37] | 54 | 92 | 2 | 3 | 6 | 1 | 2 | 3 | 1 | 25 | 189 | 7.57 |
| Orthops (Orthops) campestris (Linnaeus, 1758) | phyto, oligo (Apiaceae) | (Wachmann et al. 2004 [30]) | 1 | 3 | 2 | 1 | 7 | 0.28 | ||||||
| Orthotylus sp. | various, indefinite | 50 | 50 | 2.00 | ||||||||||
| Stenodema (Brachystira) calcarata (Fallén, 1807) | phyto, poly (Poaceae rarely Cyperaceae and Juncaceae) | [38] | 2 | 1 | 2 | 5 | 0.20 | |||||||
| Trigonotylus pulchellus (Hahn, 1834) | phyto, poly Amaranthaceae, Fabaceae, Vitis vinifera | (Lodos et al. 2003 [39]) | 3 | 11 | 1 | 1 | 3 | 6 | 2 | 4 | 31 | 1.24 | ||
| TINGIDAE | ||||||||||||||
| Corythucha ciliata (Say, 1832) | phyto, oligo (Platanus spp.) | [40] | 1 | 1 | 0.04 | |||||||||
| Dictyla echii (Schrank, 1782) | phyto, oligo (Boraginaceae) | [41] | 1 | 1 | 0.04 | |||||||||
| PENTATOMOMORPHA | ||||||||||||||
| COREIDAE | ||||||||||||||
| Coreus marginatus marginatus (Linnaeus, 1758) | phyto, poly (Polygonaceae, Asteraceae) | (Putshkov 1962 [42]) | 5 | 1 | 6 | 0.24 | ||||||||
| Gonocerus juniperi (Herrich-Schaeffer, 1839) | phyto, oligo (Cupressaceae) | [40] | 1 | 1 | 0.04 | |||||||||
| RHOPALIDAE | ||||||||||||||
| Brachycarenus tigrinus Schilling, 1829 | phyto, poly (mostly Brassicaceae, Chenopodioideae, Amaranthaceae, Ericaceae) | [43] | 7 | 7 | 0.28 | |||||||||
| Corizus hyoscyami hyoscyami (Linnaeus, 1758) | phyto, poly (incl. Asteraceae) | [44] | 1 | 1 | 0.04 | |||||||||
| Chorosoma schillingii (Schilling, 1829) | phyto, oligo (Poaceae) | [45] | 1 | 1 | 0.04 | |||||||||
| Myrmus miriformis miriformis (Fallén, 1807) | phyto, oligo (Poaceae) | [46] | 2 | 2 | 0.08 | |||||||||
| Stictopleurus abutilon abutilon (Rossi, 1790) | phyto, oligo (Asteraceae) | [47] | 3 | 1 | 4 | 0.16 | ||||||||
| Stictopleurus punctatonervosus (Goeze, 1778) | phyto, oligo (Asteraceae) | [46] | 1 | 4 | 1 | 6 | 0.24 | |||||||
| Rhopalus (Rhopalus) parumpunctatus Schilling, 1829 | phyto, poly (incl. Asteraceae) | [46,48] | 7 | 1 | 1 | 9 | 0.36 | |||||||
| Rhopalus (Rhopalus) subrufus (Gmelin, 1790) | phyto, poly (incl. Asteraceae, some preference for Lamiaceae) | [46,48] | 3 | 3 | 6 | 0.24 | ||||||||
| STENOCEPHALIDAE | ||||||||||||||
| Dicranocephalus medius (Mulsant a Rey, 1870) | phyto, oligo (Euphorbia) | [48] | 3 | 3 | 0.12 | |||||||||
| BERYTIDAE | ||||||||||||||
| Berytinus (Berytinus) minor minor (Herrich-Schaeffer, 1835) | phyto, poly (mainly Fabaceae) | [48,49] | 1 | 1 | 0.04 | |||||||||
| LYGAEIDAE | ||||||||||||||
| Lygaeus equestris (Linnaeus, 1758) | phyto, mono (Vincetoxicum hirundinaria) | [50] | 1 | 1 | 2 | 0.08 | ||||||||
| Nysius ericae ericae (Schilling, 1829) | phyto, poly (incl. Asteraceae) | [48,51] | 63 | 1004 | 1 | 2 | 11 | 19 | 1100 | 44.07 | ||||
| Nysius helveticus (Herrich-Schaeffer, 1850) | phyto, poly (incl. Asteraceae) | [48] | 1 | 1 | 0.04 | |||||||||
| Nysius senecionis senecionis (Schilling, 1829) | phyto, oligo (Asteraceae, preference for Senecio) | [48,52] | 2 | 2 | 0.08 | |||||||||
| OXYCARENIDAE | ||||||||||||||
| Metopoplax origani (Kolenati, 1845) | phyto, oligo (Asteraceae) | [53] | 4 | 5 | 3 | 12 | 0.48 | |||||||
| RHYPAROCHROMIDAE | ||||||||||||||
| Rhyparochromus vulgaris (Schilling, 1829) | phyto, poly (seed feeder) | [48,54] | 2 | 2 | 0.08 | |||||||||
| PENTATOMIDAE | ||||||||||||||
| Aelia acuminata (Linnaeus, 1758) | phyto, oligo (Poaceae) | [55] | 12 | 1 | 1 | 14 | 0.56 | |||||||
| Aelia klugii Hahn, 1833 | phyto, oligo (Poaceae) | [56] | 1 | 1 | 0.04 | |||||||||
| Aelia rostrata (Boheman, 1852) | phyto, oligo (Poaceae) | [57] | 2 | 2 | 0.08 | |||||||||
| Carpocoris (Carpocoris) purpureipennis (De Geer, 1773) | phyto, poly (incl. Asteraceae) | [58,59] | 2 | 1 | 3 | 0.12 | ||||||||
| Dolycoris baccarum (Linnaeus, 1758) | phyto, poly (incl. Asteraceae) | [60] | 4 | 4 | 0.16 | |||||||||
| Eurydema (Eurydema) oleracea (Linnaeus, 1758) | phyto, oligo (Brassicaceae) | [58] | 1 | 4 | 5 | 0.20 | ||||||||
| Eysarcoris ventralis (Westwood, 1837) | phyto, poly (incl. Asteraceae) | [61] | 1 | 1 | 0.04 | |||||||||
| Graphosoma italicum (Müller, 1766) | phyto, oligo (Apiaceae) | [62] | 1 | 1 | 3 | 5 | 0.20 | |||||||
| Peribalus (Peribalus) strictus strictus (Fabricius, 1803) | phyto, poly (incl. Asteraceae) | [63] | 2 | 2 | 0.08 | |||||||||
| Piezodorus lituratus (Fabricius, 1794) | phyto, poly (mainly Fabaceae) | [48] | 1 | 1 | 0.04 | |||||||||
| Halyomorpha halys Stål, 1855 | phyto, poly (incl. Asteraceae) | [64] | 2 | 2 | 0.08 | |||||||||
| Zicrona caerulea (Linnaeus, 1758) | zoophagous | [65] | 2 | 1 | 3 | 0.12 | ||||||||
| SCUTELLERIDAE | ||||||||||||||
| Eurygaster testudinaria testudinaria (Geoffroy, 1785) | phyto, poly (mostly Poaceae, Cyperaceae, incl. Asteraceae) | [48,66] | 2 | 2 | 0.08 | |||||||||
| 296 | 1594 | 32 | 54 | 50 | 18 | 134 | 78 | 32 | 208 | 2496 | 100.00 |
| Infraorder/Family/Species | Food Specialization | Carrier/Vector | References | Abundance (pcs) | Abundance (%) |
|---|---|---|---|---|---|
| CIMICOMORPHA | |||||
| MIRIDAE | |||||
| Adelphocoris lineolatus (Goeze, 1778) | phyto, poly (Fabaceae, eggs are laid to some Asteraceae) | Ca. P. solani (16Sr XII) (carrier) | [73] | 481 | 19.27 |
| Aster yellows phytoplasma (16SrI-C and 16SrI-F) (carrier) | [74] | ||||
| Lygocoris (Lygocoris) pabulinus (Linnaeus, 1761) | phyto, poly | Erwinia amylovora (pear shoots) (vector) | [75] | 3 | 0.12 |
| Lygus pratensis (Linnaeus, 1758) | phyto, poly (incl. Asteraceae) | Ca. P. solani (16Sr XII) (mixed sample of L. pratensis and L. rugulipennis) (carrier) | [76] | 171 | 6.85 |
| Potato leaf roll virus (PLRV), Potato virus Y (PVY), Potato virus S (PVS), Potato virus M (PVM), Potato virus A (PVA) (carrier) | [77,78] | ||||
| Lygus rugulipennis (Poppius, 1911) | phyto, poly (Asteraceae among the most important) | Potato leaf roll virus (PLRV), Potato virus M (PVM) (vector) | (Turka 1978 [17]) | 189 | 7.57 |
| X-disease 16SrIII-B (in larva) (carrier) | [79] | ||||
| Ca. P. solani (16Sr XII) (carrier) | [73] | ||||
| Aster yellows phytoplasma (16SrI-C and 16SrI-F) (carrier) | [74] | ||||
| Pseudomonas syringae pv. aptata (sugar beet) (carrier) | (Bilewicz-Pawińska 1967 [69]) | ||||
| Orthops (Orthops) campestris (Linnaeus, 1758) | phyto, oligo (Apiaceae) | Stemphylium radicinum (vector) | (Bech 1967 in [80]) | 7 | 0.28 |
| PENTATOMOMORPHA | |||||
| LYGAEIDAE | |||||
| Nysius ericae ericae (Schilling, 1829) | phyto, poly (incl. Asteraceae) | Nematospora coryli (on Brassica juncea) (carrier) | [67] | 1100 | 44.07 |
| PENTATOMIDAE | |||||
| Halyomorpha halys (Stål, 1855) | phyto, poly (incl. Asteraceae) | Paulownia witches’ broom phytoplasma (16SrI-D) (vector) | [81] | 2 | 0.08 |
| Sites | Balvany | Malá n/Hronomm | Sikenička | Gemerský Jablonec | Tachty | Brehov | Malé Trakany | Strážne | Svätuše | Veľký Horeš |
|---|---|---|---|---|---|---|---|---|---|---|
| Balvany | • | 24 | 50 | 43 | 28 | 33 | 28 | 46 | 17 | 37 |
| Malá n/Hronom | 9/28 * | • | 23 | 22 | 15 | 17 | 27 | 27 | 8 | 21 |
| Sikenička | 6/12 | 7/30 | • | 60 | 40 | 50 | 22 | 67 | 27 | 50 |
| GemerskýJablonec | 6/14 | 7/22 | 6/10 | • | 33 | 40 | 17 | 70 | 12 | 42 |
| Tachty | 4/14 | 4/33 | 4/11 | 4/12 | • | 50 | 33 | 36 | 23 | 38 |
| Brehov | 5/12 | 6/30 | 5/8 | 5/10 | 5/8 | • | 23 | 44 | 18 | 33 |
| MaléTrakany | 7/25 | 11/37 | 5/23 | 5/24 | 7/21 | 6/20 | • | 26 | 25 | 28 |
| Strážne | 6/13 | 8/30 | 6/9 | 7/10 | 4/11 | 5/9 | 6/23 | • | 13 | 46 |
| Svätuše | 5/16 | 5/36 | 4/14 | 4/16 | 5/13 | 4/13 | 8/24 | 4/15 | • | 17 |
| VeľkýHoreš | 6/16 | 7/34 | 6/12 | 6/14 | 5/12 | 5/12 | 7/24 | 6/13 | 5/17 | • |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, P.; Tóthová, M.; Krchňavá, V.; Ščevková, J. Diversity of True Bugs (Hemiptera: Heteroptera) on Common Ragweed (Ambrosia artemisiifolia) in Southern Slovakia. Diversity 2023, 15, 757. https://doi.org/10.3390/d15060757
Tóth P, Tóthová M, Krchňavá V, Ščevková J. Diversity of True Bugs (Hemiptera: Heteroptera) on Common Ragweed (Ambrosia artemisiifolia) in Southern Slovakia. Diversity. 2023; 15(6):757. https://doi.org/10.3390/d15060757
Chicago/Turabian StyleTóth, Peter, Monika Tóthová, Veronika Krchňavá, and Jana Ščevková. 2023. "Diversity of True Bugs (Hemiptera: Heteroptera) on Common Ragweed (Ambrosia artemisiifolia) in Southern Slovakia" Diversity 15, no. 6: 757. https://doi.org/10.3390/d15060757
APA StyleTóth, P., Tóthová, M., Krchňavá, V., & Ščevková, J. (2023). Diversity of True Bugs (Hemiptera: Heteroptera) on Common Ragweed (Ambrosia artemisiifolia) in Southern Slovakia. Diversity, 15(6), 757. https://doi.org/10.3390/d15060757






