Inocybe istriaca sp. nov. from Brijuni National Park (Croatia) and Its Position within Inocybaceae Revealed by Multigene Phylogenetic Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Morphological Study
2.2. DNA Extraction, PCR Amplification, and Sequencing
2.3. Sequence Alignment and Phylogenetic Analysis
| Taxa | Strain/Voucher | Country | ITS | LSU | RPB2 | TEF1 | Phylog. Dataset | Refs. |
|---|---|---|---|---|---|---|---|---|
| Auritella hispida | TH10009 PT | Cameroon | KT378203 | KT378207 | KT378215 | MK426179 | 1 | [8,20] |
| Auritella spiculosa | TH9866 PT | Cameroon | KT378204 | KT378206 | KT378214 | MK426182 | 1 | [8,20] |
| Crepidotus prostratus | PBM3463/PERTH:08242135 | Australia | HQ728537 | HQ728538 | HQ728540 | MK426172 | 1 | [8,20] |
| Inocybe adorabilis | SMNS-STU-F-0901582 HT | Austria | OK057159 | OK057159 | OK078903 | — | 1, 2 | [21] |
| Inocybe adorabilis | SMNS-STU-F-0901641 PT | Austria | OK057161 | OK057161 | — | — | 2 | [21] |
| Inocybe aeruginascens | JG310508/TENN063936 | Germany | GU949591 | MH220256 | MH249787 | — | 1 | [22] |
| Inocybe agglutinata | WTU:1094 PBM1352 | USA | KY990521 | AY038312 | AY509113 | — | 1 | [14,23,24] |
| Inocybe agroterae | SMNS-STU-F-0901680 HT | Germany | ON003436 | ON003436 | — | — | 2 | [6] |
| Inocybe alcis | SMNS-STU-F-0901712 IT | Finland | OP164083 | OP164083 | — | — | 2 | [25] |
| Inocybe aphroditeana | SMNS-STU-F-0901678 HT | Germany | ON003432 | ON003432 | — | — | 2 | [6] |
| Inocybe asterospora cf. | ZRL20152002 | China | LT716046 | KY418862 | KY419008 | KY419064 | 1 | [26] |
| Inocybe astraiana | SMNS-STU-F-0901240 HT | Germany | MN512321 | MN512321 | — | — | 2 | [27] |
| Inocybe athenana | SMNS-STU-F-0901238 HT | Germany | MN512320 | MN512320 | — | — | 2 | [27] |
| Inocybe audens | SMNS-STU-F-0901251 HT | Germany | MW647616 | MW647616 | — | — | 2 | [28] |
| Inocybe aurantiobrunnea | SMNS-STU-F-0001816 IT | Spain | OP164016 | OP164016 | — | — | 2 | [25] |
| Inocybe beatifica | SMNS-STU-F-0901261 HT | Germany | MW845857 | — | — | — | 2 | [29] |
| Inocybe bellidiana | SMNS-STU-F-0901473 HT | Germany | MW845860 | MW845860 | — | — | 2 | [29] |
| Inocybe cacaocolor | PBM3790/TENN:067022 IT | Australia | KJ778845 | KJ756464 | KJ756422 | — | 1 | [30] |
| Inocybe caesaraugustae | AH 56200 HT | Spain | OL352083 | — | — | — | 2 | [31] |
| Inocybe carissima | SMNS-STU-F-0901701 HT | Germany | OP164058 | OP164058 | — | — | 2 | [25] |
| Inocybe carolinensis | PBM3906/TENN067756 PT | USA | KP636853 | KP171055 | KM555147 | — | 1 | [32] |
| Inocybe chalcodoxantha | WTU F-043333 IT | Canada | NR_119900 | — | — | — | 2 | [33] |
| Inocybe coriacea | SMNS-STU-F-0901683 HT | Germany | ON003439 | ON003439 | — | — | 2 | [6] |
| Inocybe corydalina | AM10687 TURA6488 | Russia Belgium | MH216083 | AY038314 | AY337370 | — | 1 | [32] |
| Inocybe cuniculina | KR-M-0043257 HT | Netherlands | MN625273 | MN625273 | — | — | 2 | [27] |
| Inocybe curcumina | KR-M-0042332 HT | Germany | MH366621 | — | — | — | 2 | [34] |
| Inocybe cygnea | SMNS-STU-F-0901671 HT | Germany | ON003447 | ON003447 | — | — | 2 | [6] |
| Inocybe derbschii | KR-M-0005011 HT | Germany | MG012466 | — | — | — | 2 | [34] |
| Inocybe devina | SMNS-STU-F-0901659 HT | Germany | ON003423 | ON003423 | — | — | 2 | [6] |
| Inocybe drenthensis | SMNS-STU-F-0901477 HT | Netherlands | MW845869 | MW845869 | — | — | 2 | [29] |
| Inocybe dryadiana | SMNS-STU-F-0901259 HT | Germany | MW845873 | MW845873 | — | — | 2 | [29] |
| Inocybe dulciolens | PBM2646/TENN 062477 HT | USA | MH216088 | MH220265 | MH249796 | — | 1 | [32] |
| Inocybe dvaliniana | SMNS-STU-F-0901559 HT CNF 1/8916 IT | Austria | MW647624 | MW647624 | OQ587951 | — | 1, 2 | [28], This study |
| Inocybe elysii | SMNS-STU-F-0901682 HT | Germany | ON003438 | ON003438 | — | — | 2 | [6] |
| Inocybe erinaceomorpha | JV14756F/TURA7645 | Sweden | MH216089 | MH220266 | MH249797 | — | 1 | [32] |
| Inocybe flavoalbida | PBM3768/TENN:067000 IT | Australia | KJ729873 | KJ729901 | KJ729932 | MK426183 | 1, 2 | [30] |
| Inocybe flocculosa | EL10605 | Finland | AM882992 | AM882992 | — | — | 2 | [34] |
| Inocybe flocculosa cf. | ZRL20151789 | China | LT716045 | KY418861 | KY419007 | KY419063 | 1 | [26] |
| Inocybe freyae | SMNS-STU-F-0901673 HT | Germany | ON003431 | ON003431 | — | — | 2 | [6] |
| Inocybe fuscicothurnata | PBM3980/TENN:068940 | USA | MF487844 | KY990485 | MF416408 | MK426184 | 1 | [23] |
| Inocybe fuscidula | EL9505 | Finland | AM882886 | AM882886 | — | — | 2 | [35] |
| Inocybe ghibliana | SMNS-STU-F-0901256 HT | Germany | MW845878 | MW845878 | — | — | 2 | [29] |
| Inocybe glaucescens | LVK12144/TENN073754 HT | USA | MH216097 | MH220273 | MH249804 | — | 1 | [32] |
| Inocybe grammopodia | KR-M-0044138 | Germany | MH366590 | — | — | — | 2 | [34] |
| Inocybe griseotarda | J. Poirier n 19901119-01 HT | France | MF361839 | — | — | — | 2 | [36] |
| Inocybe griseovelata | EL20906 | France | FN550931 | FN550931 | — | — | 2 | [35] |
| Inocybe griseovelata | SMNS-STU-F-0901568 ET | Germany | MW845942 | MW845942 | — | — | 2 | [29] |
| Inocybe grusiana | SMNS-STU-F-0901262 HT | Germany | MW845884 | MW845884 | — | — | 2 | [29] |
| Inocybe heterosemen | XC98091209 IT | France | OK057119 | — | — | — | 1, 2 | [21] |
| Inocybe humidicola | PBM3719/TENN:066955 | Australia | KP171126 | KJ801181 | KJ811575 | MK426185 | 1 | [8,30] |
| Inocybe inodora | EL2405 | Norway | AM882834 | AM882834 | — | — | 2 | [35] |
| Inocybe inodora | SMNS-STU-F-0901438 | Austria | MT101874 | MT101874 | — | — | 1, 2 | [37] |
| Inocybe iseranensis | TR gmb 00981 HT | France | OK057141 | OK057141 | — | — | 1, 2 | [21] |
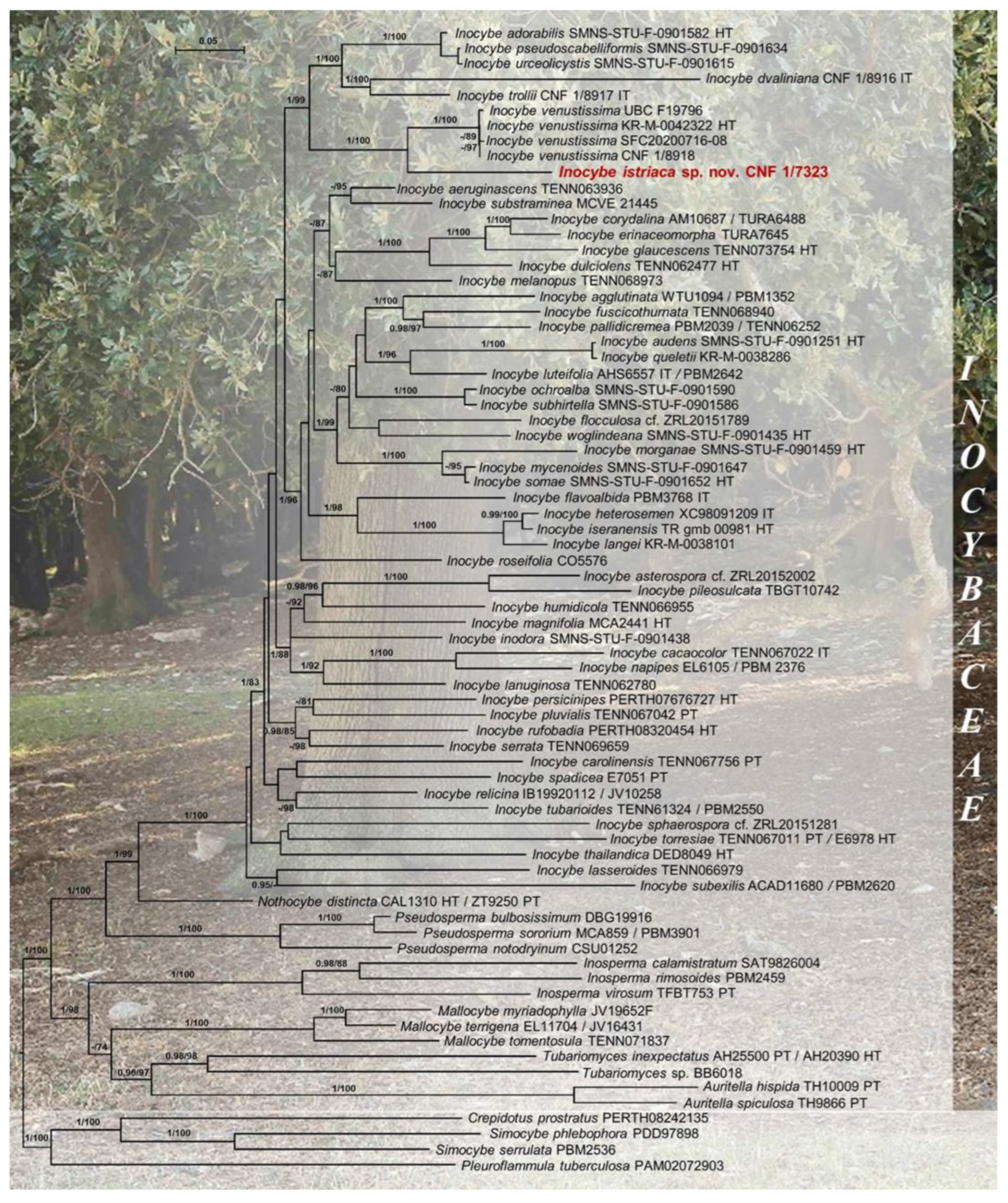
| Inocybe istriacasp. nov. | CNF 1/7323 HT | Croatia | OQ550176 | OQ550175 | OQ587954 | OQ596331 | 1, 2 | This study |
| Inocybe knautiana | SMNS-STU-F-0901491 HT | Germany | MW845887 | MW845887 | — | — | 2 | [29] |
| Inocybe kuberae | SMNS-STU-F-0901668 HT | Germany | ON003427 | ON003427 | — | — | 2 | [6] |
| Inocybe lampetiana | SMNS-STU-F-0901494 HT | Germany | MW845891 | MW845891 | — | — | 2 | [29] |
| Inocybe langei | KR-M-0038101 | Germany | OK057121 | OK057121 | — | — | 1, 2 | [21] |
| Inocybe langei | SMNS-STU-F-0900983 | Germany | OK057205 | OK057205 | — | — | 2 | [21] |
| Inocybe lanuginosa | PBM3023/TENN:062780 | USA | HQ232480 | KP170923 | KM245992 | MK426186 | 1 | [8,30] |
| Inocybe lasseroides | PBM3749/TENN:066979 | Australia | KP171145 | KP170924 | KM245993 | MK426187 | 1 | [8,38] |
| Inocybe laurina | SMNS-STU-F-0901247 HT | Germany | MN512325 | MN512325 | — | — | 2 | [27] |
| Inocybe lechiana | SMNS-STU-F-0901268 HT | Austria | MN512330 | MN512330 | — | — | 2 | [28] |
| Inocybe lucis | SMNS-STU-F-0901616 HT | Germany | ON003441 | ON003441 | — | — | 2 | [6] |
| Inocybe luteifolia | AHS6557 IT PBM2642 | USA | FJ436331 | EU307814 | EU307816 | MK426188 | 1, 2 | [8,39] |
| Inocybe magnifolia | MCA2441 HT | Guyana | JN642228 | JN642244 | EU600899 | MK426189 | 1 | [40] |
| Inocybe melanopus | PBM3975/TENN:068973 | USA | — | MH220276 | MH249807 | MK426190 | 1 | [8,32] |
| Inocybe morganae | SMNS-STU-F-0901459 HT | Austria | OK057143 | — | — | — | 1, 2 | [21] |
| Inocybe morganae | SMNS-STU-F-0901608 | Germany | OK057201 | OK057201 | — | — | 2 | [21] |
| Inocybe mortenii | DB19-9-20-5 PT | Austria | OP164049 | OP164049 | — | — | 2 | [25] |
| Inocybe mycenoides | SMNS-STU-F-0901647 | Germany | OK057156 | OK057156 | OK078899 | — | 1 | [21] |
| Inocybe napipes | EL6105 PBM 2376 | Norway | AM882926 | AY239024 | AY337390 | — | 1 | [14,35] |
| Inocybe ochroalba | SMNS-STU-F-0901590 | Finland | OK057137 | OK057137 | OK078918 | — | 1 | [21] |
| Inocybe ochroalba | EL5704 | Sweden | AM882882 | AM882882 | — | — | 2 | [34] |
| Inocybe orioli | SMNS-STU-F-0901703 HT | Germany | OP164074 | OP164074 | — | — | 2 | [25] |
| Inocybe orionis | SMNS-STU-F-0901455 HT | Germany | MW845898 | MW845898 | — | — | 2 | [29] |
| Inocybe pallidicremea | PBM2039 PBM2744/TENN:06252 | USA | KY990553 | AY380385 | AY337388 | MK426191 | 1 | [8,14] |
| Inocybe perchtana | SMNS-STU-F-0901245 HT | Austria | MN512326 | MN512326 | — | — | 2 | [27] |
| Inocybe persicinipes | PBM2197/PERTH:07676727 HT | Australia | KF977215 | EU600837 | EU600836 | MK426192 | 1 | [8,41] |
| Inocybe pholiotinoides | SMNS-STU-F-0901702 | Germany | OP164095 | — | — | — | 2 | [25] |
| Inocybe pileosulcata | TBGT:10742 | India | KP308810 | KP170979 | KM406218 | MK426193 | 1 | [8,30,38] |
| Inocybe pipilikae | SMNS-STU-F-0901539 HT | Austria | MW647629 | MW647629 | — | — | 2 | [28] |
| Inocybe pluvialis | PBM3228/TENN:067042 PT | Australia | KF871777 | KF853401 | KF891954 | MK426194 | 1 | [8,30] |
| Inocybe pseudodestricta | KR-M-0043223 | Netherlands | MH366594 | — | — | — | 2 | [34] |
| Inocybe pseudodestricta | PRM716231 HT | Czechia | MG012468 | — | — | — | 2 | [34] |
| Inocybe pseudoscabelliformis | SMNS-STU-F-0901634 | Germany | OK057172 | OK057172 | OK078908 | — | 1 | [21] |
| Inocybe pusio cf. | DB16-8-14-24 | Germany | MH366588 | — | — | — | 2 | [34] |
| Inocybe queletii | KR-M-0038286 | Germany | MT101893 | — | — | — | 1, 2 | [37] |
| Inocybe relicina | IB19920112 JV10258 | New Zealand Finland | AF325664 | AY038324 | AY333778 | — | 1 | [24,42] |
| Inocybe roseifolia | CO5576 | USA | MH578026 | MK421968 | MH577441 | MK426195 | 1 | [8,32] |
| Inocybe roseipes cf. | MCVE 9856 | Italy | JF908143 | — | — | — | 2 | [43] |
| Inocybe rufobadia | NLB885/PERTH:08320454 HT | Australia | KF977213 | KF915290 | KF991385 | MK426196 | 1 | [8,30] |
| Inocybe scolopacis | SMNS-STU-F-0901527 HT | Germany | MW845913 | MW845913 | — | — | 2 | [29] |
| Inocybe serrata | PBM3235/TENN:069659 | Australia | KP636810 | KP171012 | KM555111 | MK426197 | 1 | [8,30] |
| Inocybe soliana | SMNS-STU-F-0901664 HT | Germany | ON003425 | ON003425 | — | — | 2 | [6] |
| Inocybe somae | SMNS-STU-F-0901652 HT | Germany | OK057148 | OK057148 | OK078901 | — | 1 | [21] |
| Inocybe spadicea | PBM2203/E7051 PT | Australia | KP636866 | EU600865 | — | MK426198 | 1 | [8,30,41] |
| Inocybe sphaerospora cf. | ZRL20151281 | China | LT716044 | KY418860 | KY419006 | KY419062 | 1 | [26] |
| Inocybe subexilis | ACAD11680 PBM2620 | Canada USA | MH578001 | EU307845 | EU307847 | MK426199 | 1 | [8,39] |
| Inocybe subhirtella | SMNS-STU-F-0901586 | Germany | OK057133 | OK057133 | OK078915 | — | 1 | [21] |
| Inocybe substraminea | MCVE 21445 | Italy | JF908170 | — | — | — | 1, 2 | [43] |
| Inocybe tarda | SMNS-STU-F-0901730 ET | Germany | OP164094 | OP164094 | — | — | 2 | [25] |
| Inocybe thailandica | DED8049 HT | Thailand | GQ893013 | GQ892968 | KM656129 | MK426200 | 1 | [8,38] |
| Inocybe tiburtina | SMNS-STU-F-0901565 HT | Germany | MW845939 | MW845939 | — | — | 2 | [29] |
| Inocybe torresiae | TENN:067011 PT PBM2157/E6978 HT | Australia | KP641635 | EU600874 | EU600873 | — | 1 | [30,38,41] |
| Inocybe trollii | CNF 1/8917 IT | Germany | OQ550174 | OQ550177 | OQ587952 | OQ596333 | 1 | This study |
| Inocybe trollii | SMNS-STU-F-0901674 HT | Germany | ON003430 | ON003430 | — | — | 2 | [6] |
| Inocybe tubarioides | TENN61324 PBM2550 | USA | EU439453 | AY732211 | EU307855 | MK426201 | 1 | [8,39] |
| Inocybe tyrii | SMNS-STU-F-0901679 HT | Germany | ON003434 | ON003434 | — | — | 2 | [6] |
| Inocybe urceolicystis | SMNS-STU-F-0901615 | Finland | OK057175 | OK057175 | OK078914 | — | 1 | [21] |
| Inocybe venustissima | KR-M-0042322 HT | Austria | MH366625 | — | — | — | 1, 2 | [34] |
| Inocybe venustissima | KR-M-0042323 PT | Austria | MH366626 | — | — | — | 2 | [34] |
| Inocybe venustissima | KR-M-0042323 PT CNF 1/8918 | Austria | OQ550173 | OQ550172 | OQ587953 | OQ596332 | 1 | This study |
| Inocybe venustissima | SFC20200716-08 | South Korea | ON059521 | — | — | — | 1, 2 | [44] |
| Inocybe venustissima (as I. auricoma) | UBC F19796 | Canada | HQ604526 | HQ604526 | — | — | 1, 2 | unpubl. |
| Inocybe woglindeana | SMNS-STU-F-0901435 HT | Germany | MT101882 | MT101882 | — | — | 1, 2 | [37] |
| Inocybe zethi | SMNS-STU-F-0901456 HT | Germany | ON003440 | ON003440 | — | — | 2 | [6] |
| Inosperma calamistratum | SAT9826004 | USA | JQ801387 | JQ815410 | JQ846467 | MK426204 | 1 | [8,45] |
| Inosperma rimosoides | PBM2459 | USA | DQ404391 | AY702014 | DQ385884 | DQ435790 | 1 | [8,46] |
| Inosperma virosum | TBGT753 PT | India | KT329452 | KT329458 | KT329446 | MK426208 | 1 | [8,47] |
| Mallocybe myriadophylla | JV19652F | Finland | DQ221106 | AY700196 | AY803751 | DQ435791 | 1 | [46] |
| Mallocybe terrigena | EL11704 JV16431 | Sweden Finland | AM882864 | AY380401 | AY333309 | — | 1 | [14,35] |
| Mallocybe tomentosula | PBM4138/TENN:071837 | USA | MG773814 | MK421969 | MH577506 | MK426210 | 1 | [8,32] |
| Nothocybe distincta | CAL1310 HT ZT9250 PT | India | KX171343 | EU604546 | EU600904 | MK426212 | 1 | [8,41,48] |
| Pseudosperma bulbosissimum | DBG19916 | USA | MH024849 | MH024885 | MH249788 | MK426213 | 1 | [8,32] |
| Pseudosperma fascinosum | SMNS-STU-F-0901666 HT | Germany | ON003426 | — | — | — | 2 | [6] |
| Pseudosperma huginii | STU:SMNS-STU-F-0901564 HT | Austria | MW647628 | — | — | — | 2 | [28] |
| Pseudosperma notodryinum | CO4463/CSU 01252 | USA | MH578028 | MK421970 | MH577509 | MK426216 | 1, 2 | [8,32] |
| Pseudosperma sororium | MCA859 PBM3901 | USA | JQ408772 | MH220278 | MH249810 | MK426218 | 1 | [8,32] |
| Simocybe phlebophora | PBM3089/PDD:97898 | New Zealand | MK421963 | MK421967 | MK415449 | — | 1 | [8] |
| Simocybe serrulata | PBM2536 | USA | DQ494696 | AY745706 | DQ484053 | GU187755 | 1 | [49] |
| Tubariomyces inexpectatus | AH25500 PT AH20390 HT | Spain | GU907095 | EU569855 | GU907088 | — | 1 | [38,41,50] |
| Tubariomyces sp. | BB6018 | Zambia | MK421965 | EU600887 | EU600886 | MK426220 | 1 | [8,38,41] |
3. Results
3.1. Molecular Phylogenetic Analyses
3.2. Taxonomy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittermeier, R.A.; Turner, W.R.; Larsen, F.W.; Brooks, T.M.; Gascon, C. Global Biodiversity Conservation: The Critical Role of Hotspots. In Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas; Zachos, F.E., Habel, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 3–22. ISBN 978-3-642-20992-5. [Google Scholar]
- Médail, F.; Monnet, A.C.; Pavon, D.; Nikolic, T.; Dimopoulos, P.; Bacchetta, G.; Arroyo, J.; Barina, Z.; Albassatneh, M.C.; Domina, G.; et al. What Is a Tree in the Mediterranean Basin Hotspot? A Critical Analysis. For. Ecosyst. 2019, 6, 17. [Google Scholar] [CrossRef]
- Branković, Č.; Güttler, I.; Gajić-Čapka, M. Evaluating Climate Change at the Croatian Adriatic from Observations and Regional Climate Models’ Simulations. Clim. Dyn. 2013, 41, 2353–2373. [Google Scholar] [CrossRef]
- Brijuni National Park Official Website. Available online: https://www.np-brijuni.hr/en/brijuni (accessed on 12 January 2023).
- Mešić, A.; Haelewaters, D.; Tkalčec, Z.; Liu, J.; Kušan, I.; Catherine Aime, M.; Pošta, A. Inocybe brijunica sp. nov., a New Ectomycorrhizal Fungus from Mediterranean Croatia Revealed by Morphology and Multilocus Phylogenetic Analysis. J. Fungi 2021, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Bandini, D.; Oertel, B.; Eberhardt, U. Noch Mehr Risspilze (3): Einundzwanzig Neue Arten Der Familie Inocybaceae. Mycol. Bavarica 2022, 22, 31–138. [Google Scholar]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Rajeshkumar, K.C.; Aptroot, A.; Zhang, G.Q.; et al. Outline of Fungi and Fungus-like Taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Matheny, P.B.; Hobbs, A.M.; Esteve-Raventós, F. Genera of Inocybaceae: New Skin for the Old Ceremony. Mycologia 2020, 112, 83–120. [Google Scholar] [CrossRef]
- Clémençon, H. Cytology and Plectology of the Hymenomycetes, 2nd ed.; Cramer: Stuttgart, Germany, 2012. [Google Scholar]
- Erb, B.; Matheis, W. Pilzmikroskopie; Kosmos: Stuttgart, Germany, 1982. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 1990, 315–322. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS Primers with Enhanced Specificity for Basidiomycetes—Application to the Identification of Mycorrhizae and Rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid Genetic Identification and Mapping of Enzymatically Amplified Ribosomal DNA from Several Cryptococcus Species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Matheny, P.B. Improving Phylogenetic Inference of Mushrooms with RPB1 and RPB2 Nucleotide Sequences (Inocybe; Agaricales). Mol. Phylogenet. Evol. 2005, 35, 1–20. [Google Scholar] [CrossRef]
- Rehner, S. Primers for Elongation Factor 1-α (EF1-α). 2001. Available online: http://ocid.NACSE.ORG/research/deephyphae/EF1primer.pdf (accessed on 11 February 2022).
- Rehner, S.A.; Buckley, E. A Beauveria Phylogeny Inferred from Nuclear ITS and EF1-Alpha Sequences: Evidence for Cryptic Diversification and Links to Cordyceps Teleomorphs. Mycologia 2005, 97, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Haelewaters, D.; Toome-Heller, M.; Albu, S.; Aime, M.C. Red Yeasts from Leaf Surfaces and Other Habitats: Three New Species and a New Combination of Symmetrospora (Pucciniomycotina, Cystobasidiomycetes). Fungal Syst. Evol. 2019, 5, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Misawa, K.; Kuma, K.I.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Matheny, P.B.; Henkel, T.W.; Séné, O.; Korotkin, H.B.; Dentinger, B.T.M.; Aime, M.C. New Species of Auritella (Inocybaceae) from Cameroon, with a Worldwide Key to the Known Species. IMA Fungus 2017, 8, 287–298. [Google Scholar] [CrossRef]
- Bandini, D.; Oertel, B.; Eberhardt, U. More Smooth-Spored Species of Inocybe (Agaricales, Basidiomycota): Type Studies and 12 New Species from Europe. Persoonia Mol. Phylogeny Evol. Fungi 2022, 48, 91–149. [Google Scholar] [CrossRef]
- Matheny, P.B.; Norvell, L.L.; Giles, E.C. A Common New Species of Inocybe in the Pacific Northwest with a Diagnostic PDAB Reaction. Mycologia 2013, 105, 436–446. [Google Scholar] [CrossRef]
- Matheny, P.B.; Swenie, R.A. The Inocybe geophylla Group in North America: A Revision of the Lilac Speciessurrounding I. lilacina. Mycologia 2018, 110, 618–634. [Google Scholar] [CrossRef]
- Matheny, P.B.; Liu, Y.J.; Ammirati, J.F.; Hall, B.D. Using RPB1 Sequences to Improve Phylogenetic Inference among Mushrooms (Inocybe, Agaricales). Am. J. Bot. 2002, 89, 688–698. [Google Scholar] [CrossRef]
- Bandini, D.; Brandrud, T.E.; Dima, B.; Dondl, M.; Fachada, V.; Hussong, A.; Mifsud, S.; Oertel, B.; Rodríguez Campo, F.J.; Thüs, H.; et al. Fibre Caps across Europe: Type Studies and 11 New Species of Inocybe (Agaricales, Basidiomycota). Integr. Syst. 2022, 5, 1–85. [Google Scholar] [CrossRef]
- Zhao, R.L.; Li, G.J.; Sánchez-Ramírez, S.; Stata, M.; Yang, Z.L.; Wu, G.; Dai, Y.C.; He, S.H.; Cui, B.K.; Zhou, J.L.; et al. A Six-Gene Phylogenetic Overview of Basidiomycota and Allied Phyla with Estimated Divergence Times of Higher Taxa and a Phyloproteomics Perspective. Fungal Divers. 2017, 84, 43–74. [Google Scholar] [CrossRef]
- Bandini, D.; Oertel, B.; Schüssler, C.; Eberhardt, U. Noch Mehr Risspilze: Fünfzehn Neue Und Zwei Wenig Bekannte Arten Der Gattung Inocybe. Mycol. Bavarica 2020, 20, 13–101. [Google Scholar]
- Bandini, D.; Oertel, B.; Eberhardt, U. Noch Mehr Risspilze (2): Dreizehn Neue Arten Der Familie Inocybaceae. Mycol. Bavarica 2021, 21, 27–98. [Google Scholar]
- Bandini, D.; Oertel, B.; Eberhardt, U. A Fresh Outlook on the Smooth-Spored Species of Inocybe: Type Studies and 18 New Species. Mycol. Prog. 2021, 20, 1019–1114. [Google Scholar] [CrossRef]
- Matheny, P.B.; Bougher, L.N. Fungi of Australia Inocybaceae; Australian Biological Resources Study; CSIRO Publishing: Canberra, VC, Australia; Melbourne, VC, Australia, 2017. [Google Scholar]
- Munoz, G.; Pancorbo, F.; Turegano, Y.; Esteve, F. New Species and Combinations of Inocybe with Lilac or Violet Colours in Europe. Fungi Iber. 2022, 2, 7–26. [Google Scholar] [CrossRef]
- Matheny, P.B.; Kudzma, L.V. New Species of Inocybe (Inocybaceae) from Eastern North America. J. Torrey Bot. Soc. 2019, 146, 213–235. [Google Scholar] [CrossRef]
- Schoch, C.L.; Robbertse, B.; Robert, V.; Vu, D.; Cardinali, G.; Irinyi, L.; Meyer, W.; Nilsson, R.H.; Hughes, K.; Miller, A.N.; et al. Finding Needles in Haystacks: Linking Scientific Names, Reference Specimens and Molecular Data for Fungi. Database 2014, 2014, bau061. [Google Scholar] [CrossRef]
- Bandini, D.; Oertel, B.; Ploch, S.; Ali, T.; Vauras, J.; Schneider, A.; Scholler, M.; Eberhardt, U.; Thines, M. Revision of Some Central European Species of Inocybe (Fr.:Fr.) Fr. Subgenus Inocybe, with the Description of Five New Species. Mycol. Prog. 2019, 18, 247–294. [Google Scholar] [CrossRef]
- Ryberg, M.; Nilsson, R.H.; Kristiansson, E.; Töpel, M.; Jacobsson, S.; Larsson, E. Mining Metadata from Unidentified ITS Sequences in GenBank: A Case Study in Inocybe (Basidiomycota). BMC Evol. Biol. 2008, 8, 50. [Google Scholar] [CrossRef]
- Bizio, E.; Ferisin, G.; Dovana, F. Note Sul Campo Di Variabilita Di Inocybe. Riv. Micol. 2017, 60, 59–70. [Google Scholar]
- Bandini, D.; Vauras, J.; Weholt, Ø.; Oertel, B.; Eberhardt, U. Inocybe woglindeana, a New Species of the Genus Inocybe, Thriving in Exposed Habitats with Calcareous Sandy Soil. Karstenia 2020, 58, 41–59. [Google Scholar] [CrossRef]
- Horak, E.; Matheny, P.B.; Desjardin, D.E.; Soytong, K. The Genus Inocybe (Inocybaceae, Agaricales, Basidiomycota) in Thailand and Malaysia. Phytotaxa 2015, 230, 201. [Google Scholar] [CrossRef]
- Kropp, B.R.; Matheny, P.B.; Nanagyulyan, S.G. Phylogenetic Taxonomy of the Inocybe splendens Group and Evolution of Supersection “Marginatae”. Mycologia 2010, 102, 560–573. [Google Scholar] [CrossRef]
- Matheny, P.B.; Aime, M.C.; Smith, M.E.; Henkel, T.W. New Species and Reports of Inocybe (Agaricales) from Guyana. Kurtziana 2012, 37, 23–39. [Google Scholar]
- Matheny, P.B.; Aime, M.C.; Bougher, N.L.; Buyck, B.; Desjardin, D.E.; Horak, E.; Kropp, B.R.; Lodge, D.J.; Soytong, K.; Trappe, J.M.; et al. Out of the Palaeotropics? Historical Biogeography and Diversification of the Cosmopolitan Ectomycorrhizal Mushroom Family Inocybaceae. J. Biogeogr. 2009, 36, 577–592. [Google Scholar] [CrossRef]
- Peintner, U.; Bougher, N.L.; Castellano, M.A.; Moncalvo, J.M.; Moser, M.M.; Trappe, J.M.; Vilgalys, R. Multiple Origins of Sequestrate Fungi Related to Cortinarius (Cortinariaceae). Am. J. Bot. 2001, 88, 2168–2179. [Google Scholar] [CrossRef]
- Osmundson, T.W.; Robert, V.A.; Schoch, C.L.; Baker, L.J.; Smith, A.; Robich, G.; Mizzan, L.; Garbelotto, M.M. Filling Gaps in Biodiversity Knowledge for Macrofungi: Contributions and Assessment of an Herbarium Collection DNA Barcode Sequencing Project. PLoS ONE 2013, 8, e62419. [Google Scholar] [CrossRef]
- Yoo, S.; Cho, Y.; Kim, J.S.; Kim, M.; Lim, Y.W. Fourteen Unrecorded Species of Agaricales Underw. (Agaricomycetes, Basidiomycota) from the Republic of Korea. Mycobiology 2022, 50, 219–230. [Google Scholar] [CrossRef]
- Kropp, B.R.; Matheny, P.B.; Hutchison, L.J. Inocybe Section Rimosae in Utah: Phylogenetic Affinities and New Species. Mycologia 2013, 105, 728–747. [Google Scholar] [CrossRef]
- Brandon Matheny, P.; Wang, Z.; Binder, M.; Curtis, J.M.; Lim, Y.W.; Henrik Nilsson, R.; Hughes, K.W.; Hofstetter, V.; Ammirati, J.F.; Schoch, C.L.; et al. Contributions of Rpb2 and Tef1 to the Phylogeny of Mushrooms and Allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 2007, 43, 430–451. [Google Scholar] [CrossRef]
- Pradeep, C.K.; Vrinda, K.B.; Varghese, S.P.; Korotkin, H.B.; Matheny, P.B. New and Noteworthy Species of Inocybe (Agaricales) from Tropical India. Mycol. Prog. 2016, 15, 24. [Google Scholar] [CrossRef]
- Latha, K.P.D.; Manimohan, P.; Matheny, P.B. A New Species of Inocybe Representing the Nothocybe Lineage. Phytotaxa 2016, 267, 40. [Google Scholar] [CrossRef]
- Matheny, P.B.; Curtis, J.M.; Hofstetter, V.; Aime, M.C.; Moncalvo, J.M.; Ge, Z.W.; Yang, Z.L.; Slot, J.C.; Ammirati, J.F.; Baroni, T.J.; et al. Major Clades of Agaricales: A Multilocus Phylogenetic Overview. Mycologia 2006, 98, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, P.; Manjón, J.L.; Matheny, P.B.; Esteve-Raventós, F. Tubariomyces, a New Genus of Inocybaceae from the Mediterranean Region. Mycologia 2010, 102, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian Inference of Phylogenetic Trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree of Life (ITOL) v5: An Online Tool for Phylogenetic Tree Display and Annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- Kuyper, T.W. A Revision of the Genus Inocybe in Europe I. Subgenus Inosperma and the Smooth-Spored Species of Subgenus Inocybe; Persoonia-Supplement; Naturalis Biodiversity Center: Leiden, The Netherlands, 1986; Volume 3, ISBN 9071236021. [Google Scholar]
- Stangl, J. Die Gattung Inocybe in Bayern; Hoppea: Regensburg, Germany, 1989; Volume 46, ISBN 0247900044. [Google Scholar]
- Grund, D.W.; Stuntz, D.E. Nova Scotian Inocybes. I. Mycologia 1968, 60, 406–425. [Google Scholar] [CrossRef]
- Carteret, X.; Reumaux, P. Miettes Sur Les Inocybes (6ème Série), Études de Quelques Nains des Feuillus de La Plaine, Accompagnée d’ Une Clé de Détermination Des Taxons de La Section Lilacinae R. Heim. Bull. Soc. Mycol. Fr. 2012, 127, 1–53. [Google Scholar]
- Velenovský, J. České Houby 1–5; České Botanické Společnosti: Prague, Czech Republic, 1920. [Google Scholar]
- Kuyper, T.W. Studies in Inocybe I.—Revision of the New Taxa of Inocybe Described by Velenovský. Persoonia 1985, 12, 375–400. [Google Scholar]
- Ferrari, E. Inocybe Dai Litorali Alla Zona Alpina. In Fungi non Delineati 54/55; Edizioni Candusso: Alassio, Italy, 2010. [Google Scholar]
- Konrad, P.A. Notes Critiques Sur Quelques Champignons Du Jura. Bull. Soc. Mycol. Fr. 1929, 45, 375–400. [Google Scholar]
- Alessio, C.L.; Rebaudengo, E. Inocybe. Iconographia Mycologica; Suppl. 3; Museo Tridentino di Scienze Naturali: Trento, Italy, 1980; Volume 29. [Google Scholar]


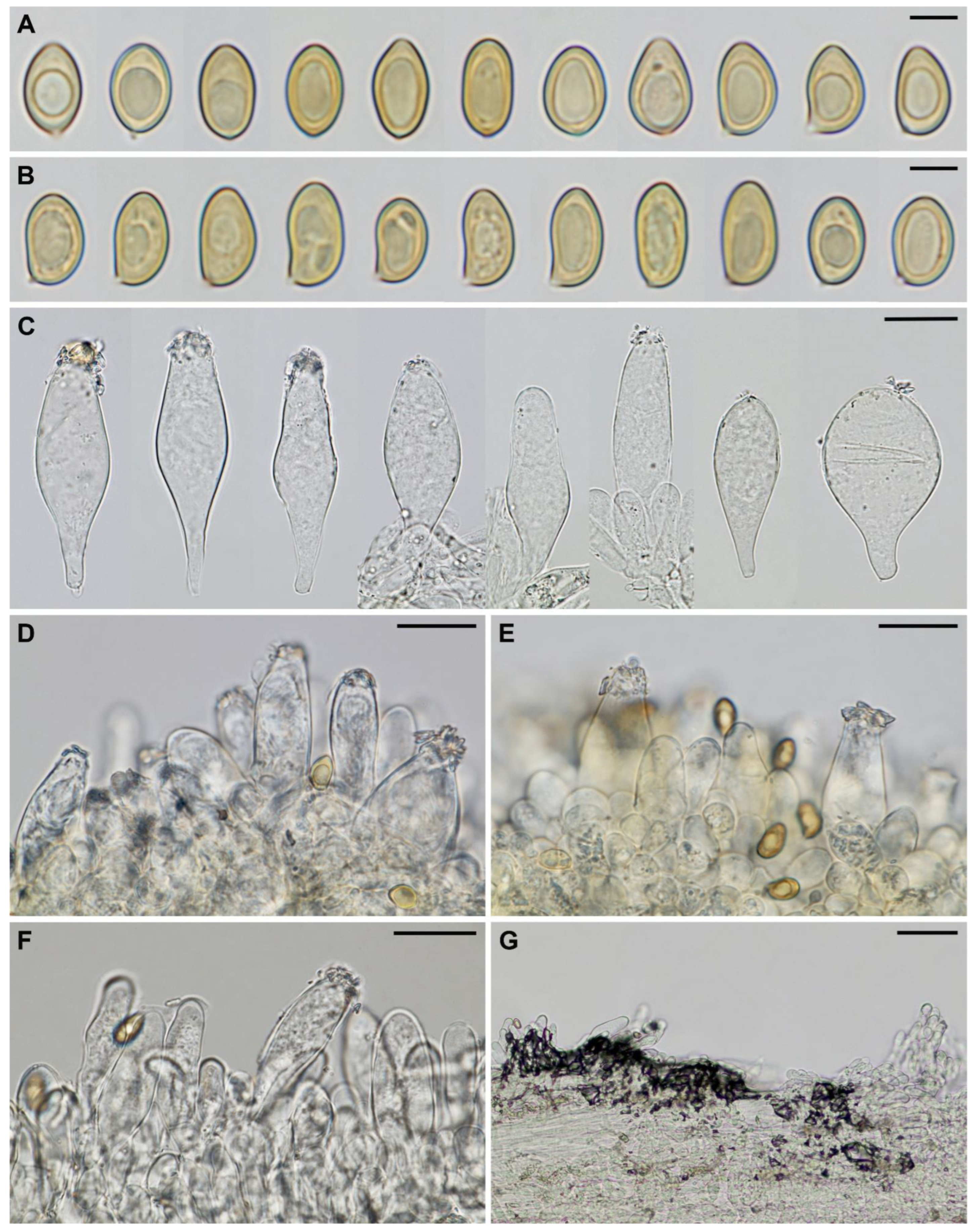

| Species | Spore Size (μm) | Pleurocystidia Size (μm) | Pleuroc. Thick at Apex (μm), Walls Colour (KOH) | Habitat | References |
|---|---|---|---|---|---|
| Inocybe istriaca | 8.4–10.2–11.9 × 5.2–6.2–7.2 | 50–65–80 × 14–20–34 | up to 1.5, (sub)hyaline | Mediterranean forest of Quercus ilex, edge with grassland | This study |
| I. adorabilis | 8.0–8.9–9.9 × 4.6–5.1–5.6 | 37–54–69 × 11–15–22 | up to 3.5(–4.5), yellowish-greenish | subalpine forest, Picea abies | [21] |
| I. audens | 7.8–9.2–10.5 × 5.0–5.8–6.7 | 41–60–72 × 11–16–25 | up to 5.0(–6.0), (sub)hyaline to light yellowish-greenish | under coniferous trees, Picea abies, Abies alba, Larix, etc. | [28] |
| I. chalcodoxantha | 7.5–10 × 5–6.5, mostly 9 × 5.5 | 50–72 × 13–21 | 1.0–3.3, hyaline | under conifers, in moss or needles | [57] |
| I. heterosemen | 6.5–7.6–8.1 × 3.5–4.2–4.8 | 29–38–49 × 12–16–20 | up to 2.5(–3.5), yellowish-greenish | mostly deciduous forests, Salix, Betula pubescens, Populus tremula, Alnus, etc. | [21,58] |
| I. inodora | 9.0–11.0–12.8 × 5.2–6.2–7.4 * 10.0–14.0 × 5.5–7.0 ** | 44–59–68 × 12–18–25 | up to 3.0(–4.0), yellowish-greenish | mostly under deciduous trees | [37], [55] **, [56] *, [59,60] |
| I. iseranensis | 7.5–8.3–9.4 × 4.7–5.0–5.7 | 37–46–58 × 14–16–18 | up to 1.5(–2.5), yellowish-greenish | alpine regions, Salix herbacea, Betula nana, B. pubescens | [21,61] |
| I. langei | 6.4–7.0–8.0 × 3.8–4.4–5.0 * 7.0–9.0 × 4.5–5.0(–5.5) ** | 35–47–57 × 9–12–15 * 40–60 × 13–20 ** | up to 3.0(–3.5), (pale) yellowish-greenish | mostly with deciduous, but also coniferous trees, Quercus, Salix, Alnus, Picea, Pinus, etc. | [21] *, [56] ** |
| I. morganae | 8.6–9.7–11.2 × 4.9–5.6–6.1 | 35–52–66 × 10–16–27 | up to 1.5(–2.0), yellowish-greenish | montane regions, Picea abies | [21] |
| I. queletii | (8–)8.5–12(–13) × 5.8–7 | 56–75 × 13–22(–25) | up to 3, hyaline | montane regions, Abies alba | [55,56,62] |
| I. substraminea | (9–)10–12(–13.5) × 5–6 | 55–75 × 15–22 | n/a | submontane forest, Fagus sylvatica | [63] |
| I. trollii | 8.0–9.6–11.1 × 5.0–5.6–6.6 | 44–53–60 × 11–14–19 | up to 2.0(–2.5), yellow-green | under Pinus sylvestris, Corylus avellana, Populus sp. | [6] |
| I. venustissima | 7.3–8.9–10.9 × 4.6–5.3–6.7 | 35–50–76 × 11–16–23 | up to 1.5(–2.0), weak, pale yellowish-greenish | montane to subalpine forests, Picea abies (Austria); Tsuga heterophylla (Canada); Larix kaempferi (Korea) | [34,44] |
| I. woglindeana | 8.0–10.2–13.0 × 4.9–5.9–7.1 * 9.0–11.3–14.3 × 5.3–6.3–7.4 ** | 35–57–77 × 12–19–31 * 51–67–82 × 15–20–30 ** | up to 1.0, pale yellow | mostly under deciduous trees, always Salix (S. caprea), mixed with Betula, Populus, Pinus sylvestris, etc. | [37] Germany *, Finland ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pošta, A.; Bandini, D.; Mešić, A.; Pole, L.; Kušan, I.; Matočec, N.; Malev, O.; Tkalčec, Z. Inocybe istriaca sp. nov. from Brijuni National Park (Croatia) and Its Position within Inocybaceae Revealed by Multigene Phylogenetic Analysis. Diversity 2023, 15, 755. https://doi.org/10.3390/d15060755
Pošta A, Bandini D, Mešić A, Pole L, Kušan I, Matočec N, Malev O, Tkalčec Z. Inocybe istriaca sp. nov. from Brijuni National Park (Croatia) and Its Position within Inocybaceae Revealed by Multigene Phylogenetic Analysis. Diversity. 2023; 15(6):755. https://doi.org/10.3390/d15060755
Chicago/Turabian StylePošta, Ana, Ditte Bandini, Armin Mešić, Lucia Pole, Ivana Kušan, Neven Matočec, Olga Malev, and Zdenko Tkalčec. 2023. "Inocybe istriaca sp. nov. from Brijuni National Park (Croatia) and Its Position within Inocybaceae Revealed by Multigene Phylogenetic Analysis" Diversity 15, no. 6: 755. https://doi.org/10.3390/d15060755
APA StylePošta, A., Bandini, D., Mešić, A., Pole, L., Kušan, I., Matočec, N., Malev, O., & Tkalčec, Z. (2023). Inocybe istriaca sp. nov. from Brijuni National Park (Croatia) and Its Position within Inocybaceae Revealed by Multigene Phylogenetic Analysis. Diversity, 15(6), 755. https://doi.org/10.3390/d15060755











