High Species Richness of Decapod Crustaceans on an Urban Rocky Shore Beach
Abstract
1. Introduction
2. Materials and Methods
2.1. Area of Study
2.2. Sampling Methodology
2.3. Morphological Identification
2.4. Molecular Identification
2.5. Statistical Analysis
3. Results
3.1. Species Richness of Decapod Crustaceans
3.2. Spatial Study of the Decapod Crustacean Communities
4. Discussion
4.1. Variability of the Assemblages through the Longitudinal and Vertical Gradient
4.2. Biological Remarks
4.3. Species Richness of Decapod Crustaceans in an Urban Rocky Shore Beach
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Grave, S.; Pentcheff, N.D.; Ahyong, S.T.; Chan, T.-Y.; Crandall, K.A.; Dworschak, P.C.; Felder, D.L.; Feldmann, R.M.; Fransen, C.H.J.M.; Goulding, L.Y.D.; et al. A Classification of Living and Fossil Genera of Decapod Crustaceans. Raffles Bull. Zool. 2009, 21, 1–109. [Google Scholar]
- García Raso, J.E.; Cuesta, J.A.; Abelló, P.; Macpherson, E. Updating changes in the Iberian decapod crustacean fauna (excluding crabs) after 50 years. Sci. Mar. 2018, 82, 207–229. [Google Scholar] [CrossRef]
- García Raso, J.E.; Mateo Ramírez, A. Clase Malacaostraca, Orden Decapoda. IDE@—SEA 2015, 80, 1–17. [Google Scholar]
- Templado, J.; Ballesteros, E.; Galparsoro, I.; Borja, A.; Serrano, A.; Martín, L.; Brito, A. Inventario Español de Hábitats y Especies Marinos; Ministry of Agriculture, Food and Environment: Madrid, Spain, 2012; pp. 1–229. [Google Scholar]
- Nunes-Ruivo, L. Crustacea Decapoda (I-Galatheidea et Brachyura). Résultas Sci. Camp. N.R.P. 1961, 4, 1–36. [Google Scholar]
- Forest, J. Crustacés Décapodes: Campagnes du “Professeur Lacaze-Duthiers” aux Baléares, juin 1953 et août 1954. Vie Milieu 1965, 16, 325–413. [Google Scholar]
- Zariquiey Álvarez, R. Crustáceos Decápodos Ibéricos. Inv. Pesq. 1968, 32, 1–510. [Google Scholar]
- Sardá, F.; Valladares, F.J.; Abelló, P. Crustáceos Decápodos y Estomatópodos capturados durante la Campaña “Golfo de Cádiz’81”. Res. Exp. Cient. 1982, 10, 89–100. [Google Scholar]
- García Raso, J.E. Brachyura of the coast of Southern Spain (Crustacea Decapoda). Spixiana 1984, 7, 105–113. [Google Scholar]
- García Raso, J.E. Consideraciones taxonómicas sobre algunas especies de Crustáceos Decápodos infralitorales de la Isla Chafarinas. In Proceedings of the III Congreso Ibérico de Entomología, Granada, Spain, 1988; pp. 57–64. [Google Scholar]
- González Gurriarán, E.; Méndez, M. Crustáceos Decápodos das Costas de Galicia; Do Castro: A Coruña, Spain, 1985; 256p. [Google Scholar]
- Almaça, C. Evolutionary and zoogeographical remarks on the Mediterranean fauna of brachyuran crabs. In Mediterranean Marine Ecosystems. NATO Conference Series (I Ecology); Moraitou-Apostolopoulou, M., Kiortsis, V., Eds.; Springer: Boston, MA, USA, 1985; Volume 8, pp. 347–366. [Google Scholar]
- Sanz, A. Crustacea Decapoda costeros del Faro de Cullera (Mediterraneo occidental). Bol. Asoc. Esp. Entom. 1986, 10, 13–19. [Google Scholar]
- García Raso, J.E.; Fernández Muñoz, R. Estudios de una comunidad de Crustáceos Decápodos de fondos “coralígenos” de alga calcárea Mesophyllum lichenoides del sur de España. Inv. Pesq. 1987, 51 (Suppl. S1), 301–322. [Google Scholar]
- García Raso, J.E.; González Gurriarán, E.; Sardá, F. Estudio comparativo de la fauna de Crustáceos Decápodos Braquiuros de tres áreas de la Península Ibérica (Galicia, Málaga y Cataluña). Inv. Pesq. 1987, 51 (Suppl. S1), 43–55. [Google Scholar]
- Holthuis, L.B. Végétaux et Invert. In Méditerranée et Mer Noire, 1st ed.; Fisher, W., Bauchot, M.L., Eds.; FAO: Rome, Italy, 1987; Volume 1, pp. 189–292. [Google Scholar]
- Abelló, P.; Valladares, F.J.; Castellón, A. Analysis of the structure of decapod crustacean assemblages the Catalán Coast (North-West Mediterranean). Mar. Biol. 1988, 98, 39–49. [Google Scholar] [CrossRef]
- Marco-Herrero, E.; Abelló, P.; Drake, P.; García Raso, J.E.; González-Gordillo, J.I.; Guerao, G.; Palero, F.; Cuesta, J.A. Annotated checklist of brachyuran crabs (Crustacea Decapoda) of the Iberian Peninsula (SW Europe). Sci. Mar. 2015, 79, 243–256. [Google Scholar] [CrossRef]
- Mateo Ramírez, Á. Comunidades de Crustáceos Decápodos Asociados a Fondos Superficiales de Fanerógamas Marinas y Su Relación Con Biotopos Colindantes en el Sur de España. Ph.D. Thesis, University of Malaga, Malaga, Spain, 2015. [Google Scholar]
- González-Gordillo, J.I.; Cuesta Mariscal, J.A.; Pablos, F. Adiciones al conocimiento de los crustáceos decápodos de las zonas mediolitoral e infralitoral de las costas suratlánticas andaluzas (Suroeste España). Cah. Biol. Mar. 1990, 31, 417–429. [Google Scholar]
- Snelling, B. The distribution of intertidal crabs in the Brisbane River. Aust. J. Mar. Freshwat. Res. 1959, 10, 67–83. [Google Scholar] [CrossRef]
- Abele, L.G. Species diversity of decapod crustaceans in marine habitats. Ecology 1974, 55, 156–161. [Google Scholar] [CrossRef]
- Abele, L.G. Comparative species composition and relative abundance of decapod crustaceans in marine habitats of Panamá. Mar. Biol. 1976, 38, 263–278. [Google Scholar] [CrossRef]
- Bertness, M.D. Predation, physical stress, and the organization of a tropical rocky intertidal hermit crab community. Ecol. Soc. Am. 1981, 62, 411–425. [Google Scholar] [CrossRef]
- Le Roux, P.J. The Population Ecology and Feeding Biology of Rocky-Shore Crabs on the Cape Peninsula. Ph.D. Thesis, University of Cape Town, Cape Town, South Africa, 1991. [Google Scholar]
- Flores, A.A.V.; Paula, J. Intertidal distribution and species composition of brachyuran crabs at two rocky shores in Central Portugal. Hydrobiologia 2001, 449, 171–177. [Google Scholar] [CrossRef]
- Vaghela, A.; Kundu, R. Spatiotemporal variations of hermit crab (Crustacea: Decapoda) inhabiting rocky shore along Saurashtra coast, western coast of India. Indian J. Geo-Mar. Sci. 2012, 41, 146–151. [Google Scholar]
- González-Ortegón, E.; Cuesta, J.A. An illustrated key of Palaemon and Palaemonetes (Crustacea: Decapoda: Caridea) from European waters, including the alien species Palaemon macrodactylus. J. Mar. Biol. Assoc. 2006, 86, 93–102. [Google Scholar] [CrossRef]
- Ng, P.K.L.; De Forges, B. Revision of the spider crab genus Maja Lamarck, 1801 (Crustacea: Brachyura: Majoidea: Majidae), with descriptions of seven new genera and 17 new species from the Atlantic and Indo-West Pacific. Raffles B. Zool. 2015, 63, 110–225. [Google Scholar]
- d’Udekem d’Acoz, C. The genus Hippolyte Leach, 1814 (Crustacea: Decapoda: Caridea: Hippolytidae) in the East Atlantic Ocean and the Mediterranean Sea, with a checklist of all species in the genus. Zool. Verh. 1996, 303, 1–133. [Google Scholar]
- Forest, J. Le genere Macropodia Leach dans les eaux atlantiques européennes (Crustacea Brachyura Majidae). Cah. Biol. Mar. 1964, 19, 323–342. [Google Scholar]
- Van Noort, G.J.; Adema, J.P.H.M. The genus Macropodia Leach, 1814 in the North Sea and adjacent waters, with the description of a new species. Zool. Med. 1985, 59, 363–379. [Google Scholar]
- Holthuis, L.B.; Gottlieb, E. An annotated list of the Decapod Crustacea of the Mediterranean coast of Israel, with an appendix listing the Decapoda of the eastern Mediterranean. B Res. Counc. Israel 1958, 7B, 1–126. [Google Scholar]
- Estoup, A.; Largiadèr, C.R.; Perrot, E.; Chourrout, D. Rapid one-tube DNA extraction for reliable PCR detection of fish polymorphic markers and transgenes. Mol. Mar. Biol. Biotech. 1996, 5, 295–298. [Google Scholar]
- Crandall, K.A.; Fitzpatrick, J.F.J. Crayfish molecular systematics: Using a combination of procedures to estimate phylogeny. Syst. Biol. 1996, 45, 1–26. [Google Scholar] [CrossRef]
- Schubart, C.D.; Cuesta, J.A.; Felder, D.L. Glyptograpsidae, a new brachyuran family from Central America: Larval and adult morphology, and a molecular phylogeny of the Grapsoidea. J. Crust. Biol. 2002, 22, 28–44. [Google Scholar] [CrossRef]
- Clark, R.K.; Gorley, R.N. PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research); PRIMER-E: Plymouth, UK, 2006; 190p. [Google Scholar]
- Manning, R.B.; Holthuis, L.B. West African brachyuran crabs (Crustacea: Decapoda). Smithson. Contrib. Zool. 1981, 306, 1–379. [Google Scholar] [CrossRef]
- Ramalhosa, P.; Canning- Clode, J.; Biscoito, M. First record of Pisa carinimana (Decapoda: Epialtidae) from Madeira Island (Northeastern Atlantic Ocean). Bocagiana 2014, 239, 1–7. [Google Scholar]
- Garcia Raso, J.E.; González-Ortegón, E.; Palero, F.; Cuesta, J.A. A new cryptic species of Inachus Weber, 1795 (Decapoda: Brachyura: Inachidae) from European waters and an updated identification key to the species of Inachus with two protogastric tubercles. J. Crust. Biol. 2022, 42, 1–13. [Google Scholar] [CrossRef]
- Velásquez, C.; Jaramillo, E.A.; Camus, P.; Manzano, M.; Sánchez, R. Biota del intermareal rocoso expuesto de la Isla Grande de Chiloé, Archipiélago de Chiloé, Chile: Patrones de diversidad e implicancias ecológicas y biogeográficas. Rev. Biol. Mar. Oceanogr. 2016, 51, 33–50. [Google Scholar] [CrossRef]
- López de la Rosa, I.; García Raso, J.E.; Rodríguez, A. Evolution of a decapod community (Crustacea) of shallow soft bottoms with seaweeds from southern Europe. J. Mar. Biol. Ass. UK 2002, 82, 85–95. [Google Scholar] [CrossRef]
- Mateo Ramírez, Á.; García Raso, J.E. Temporal changes in the structure of the crustacean decapod assemblages associated with Cymodocea nodosa meadows from the Alboran Sea (Western Mediterranean Sea). Mar. Ecol. 2012, 33, 302–316. [Google Scholar] [CrossRef]
- Scipione, M.B.; Gambi, M.C.; Lorenti, M.; Russo, G.F.; Zuppo, V. Vagile fauna of the leaf stratum of Posidonia oceanica and Cymodocea nodosa in the Mediterranean Sea. In Seagrass Biology: Proceedings of an International Workshop, Rottnest Island, Australia, 25–29 January 1996; Kuo, J., Phillips, R.C., Walker, D.I., Kirkman, H., Eds.; Faculty of Sciences, University of Western Australia: Rottnest Island, Australia; pp. 249–260.
- García Raso, J.E.; Martín, M.J.; Díaz, V.; Cobos, V.; Manjón-Cabeza, M.E. Diel and seasonal changes in the structure of a Decapod (Crustacea: Decapoda) community of Cymodocea nodosa from Southeastern Spain (West Mediterranean Sea). In Issues of Decapod Crustacean Biology. Developments in Hydrobiology; Thessalou-Legaki, M., Ed.; Springer: Dordrecht, Germany, 2006; Volume 184, pp. 59–68. [Google Scholar]
- García Muñoz, J.E.; Manjón-Cabeza, M.E.; García Raso, J.E. Decapod crustacean assemblages from littoral bottoms of the Alborán Sea (Spain, west Mediterranean Sea): Spatial and temporal variability. Sci. Mar. 2008, 72, 437–449. [Google Scholar]
- Stevčić, C.; Pérez-Miguel, M.; Drake, P.; Tovar-Sánchez, A.; Cuesta, J.A. Macroinvertebrate communities on rocky shores: Impact due to human visitors. Estuar. Coast. Shelf. S 2018, 211, 127–136. [Google Scholar] [CrossRef]

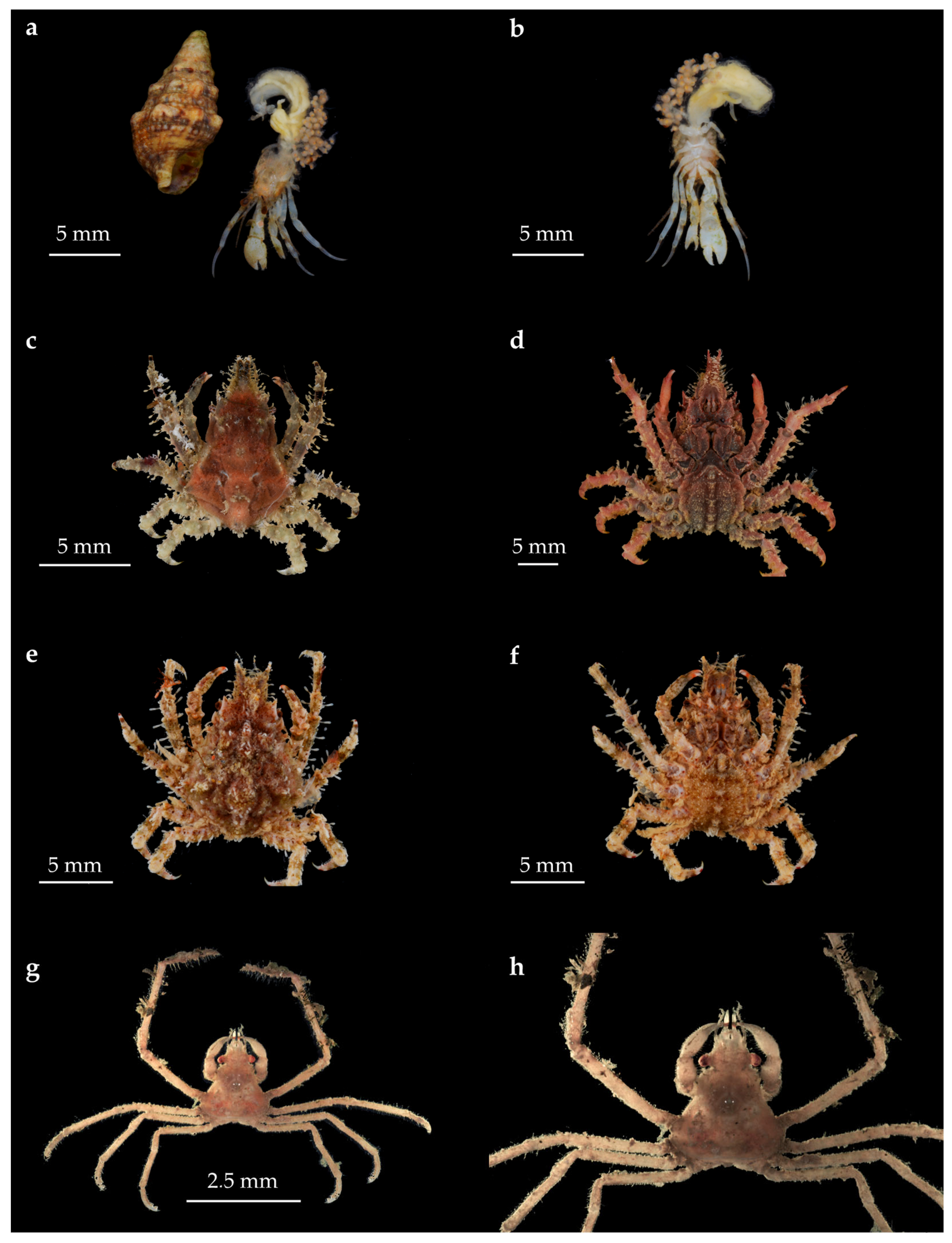
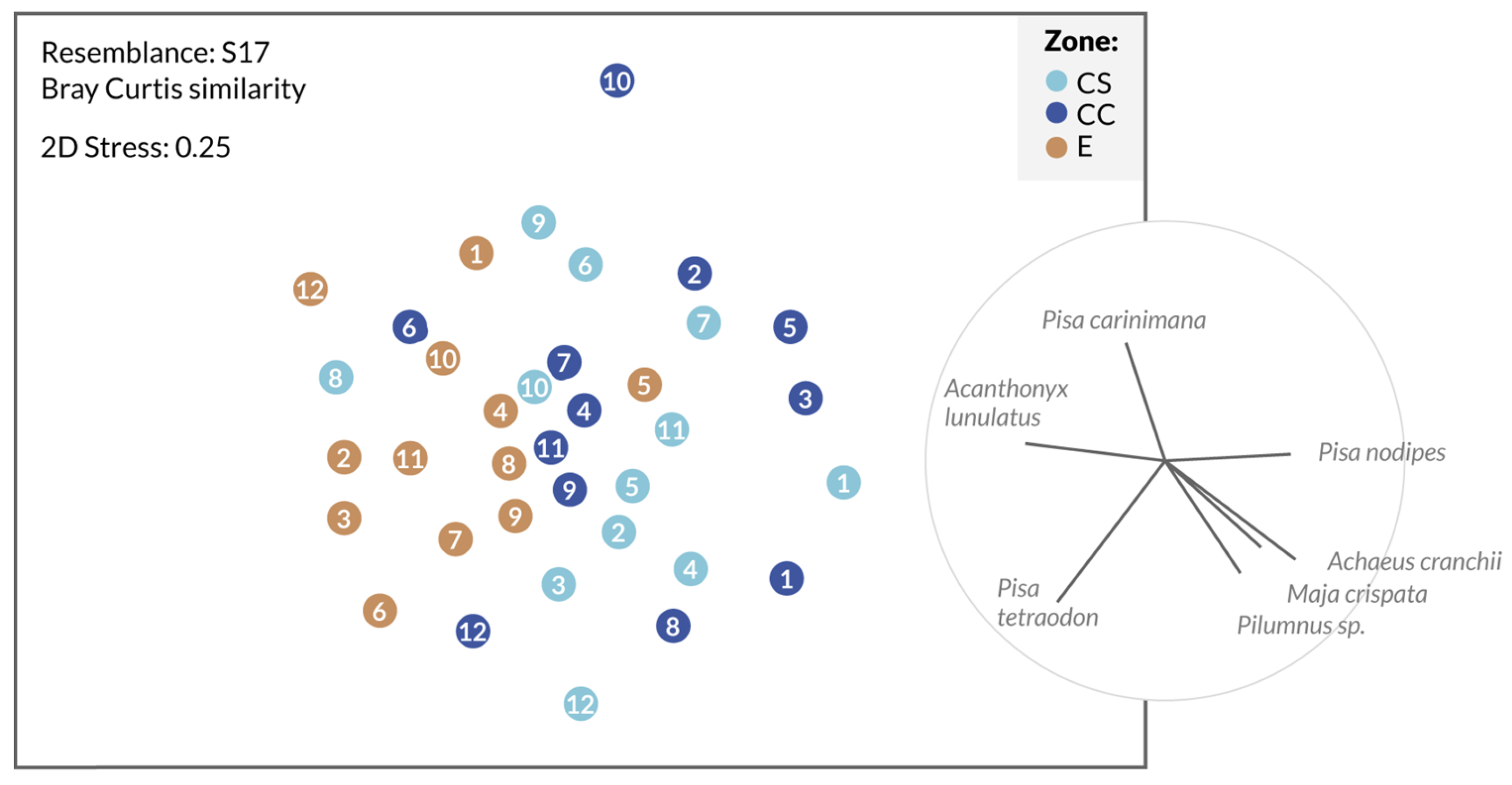
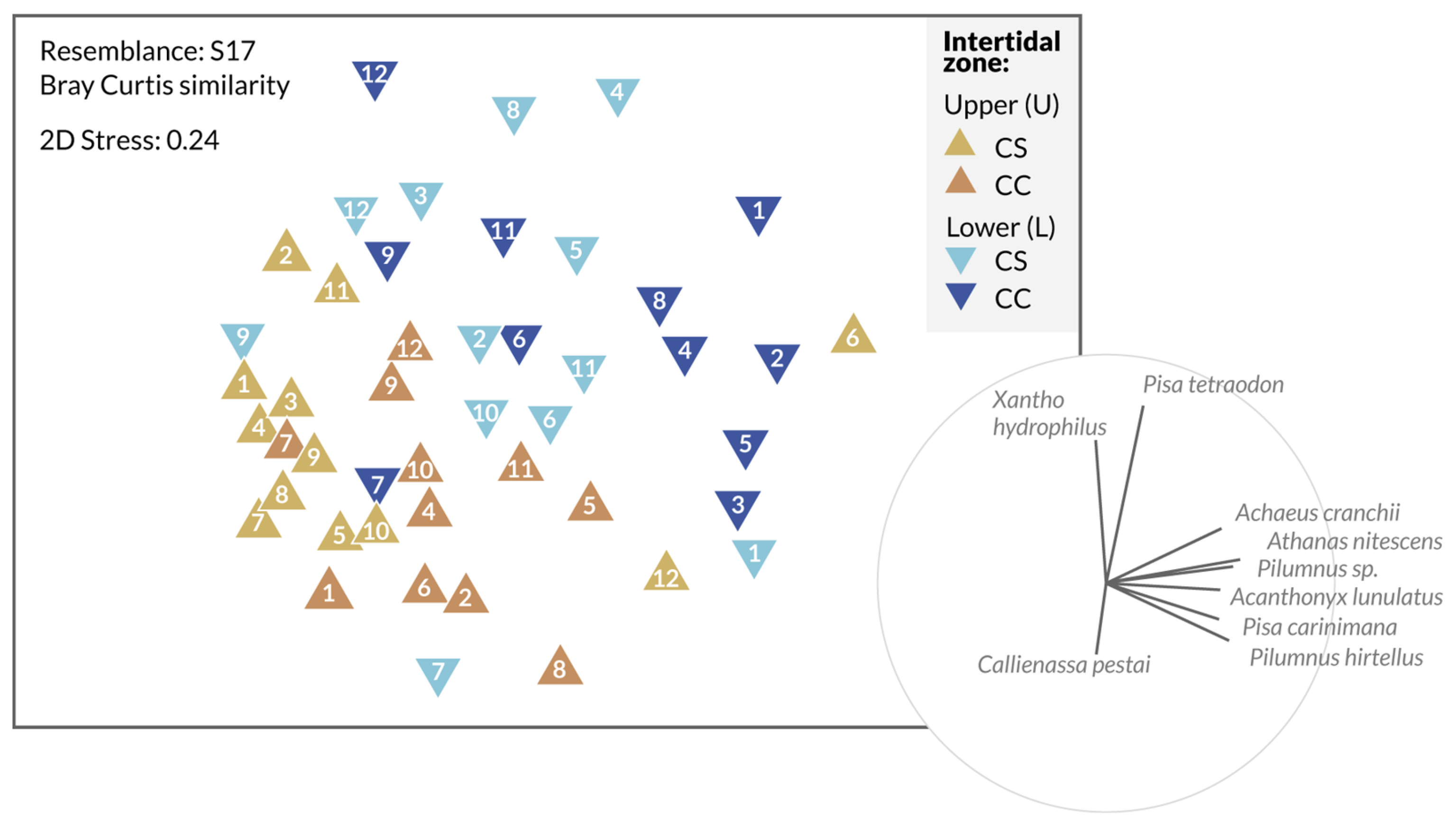
| Zone | Coordinates | Substrate | Area (km2) |
|---|---|---|---|
| CCL | 36°32′02.4″ N/6°18′54.0″ W | Hard bottom with boulder stones. Heterogeneous geomorphology. Abundant pools and algae. | 0.022 |
| CCU | 36°31′58.1″ N/6°18′36.9″ W | Hard bottom with boulder stones, Heterogeneous geomorphology. Abundant pools and algae. | 0.024 |
| CSL | 36°31′47.0″ N/6°19′04.1″ W | Hard bottom with boulder stones. Heterogeneous geomorphology. Abundant pools and algae. | 0.025 |
| CSU | 36°31′43.3″ N/6°18′40.2″ W | Hard/Sandy bottom with boulder stones. Heterogeneous geomorphology. Abundant pools with a low number of algae. | 0.023 |
| E | 36°31′38.3″ N/6°18′54.5″ W | Hard/Sandy bottom. Homogeneous geomorphology. Few pools with a low number of algae. | 0.019 |
| Species | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Jan | Feb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sicionidae | ||||||||||||
| Sicyonia carinata (Brünnich, 1768) | X | |||||||||||
| Hippolytidae | ||||||||||||
| Lysmata seticaudata (Risso, 1816) | X | O | O | O | OX | X | ||||||
| Hippolyte inermis Leach, 1815 | O | O | O | O | O | OX | ||||||
| Hippolyte leptocerus (Heller, 1863) | O | O | X | X | NX | X | X | NX | X | |||
| Eualus cranchii (Leach, 1817) | O | O | OX | O | OX | O | OX | O | X | X | X | NX |
| Alpheidae | ||||||||||||
| Alpheus dentipes Guérin, 1832 | X | OX | OX | OX | OX | OX | OX | X | X | X | X | X |
| Athanas nitescens (Leach, 1814) | X | OX | OX | OX | O | OX | OX | X | X | X | X | X |
| Processidae | ||||||||||||
| Processa sp. | X | |||||||||||
| Palaemonidae | ||||||||||||
| Palaemon elegans Rathke, 1837 | NX | OX | OX | OX | OX | OX | OX | X | X | X | X | X |
| Palaemon serratus (Pennant, 1777) | O | O | X | OX | X | OX | O | X | ||||
| Crangonidae | ||||||||||||
| Philocheras fasciatus (Risso, 1816) | X | |||||||||||
| Callianassidae | ||||||||||||
| Callianassa pestai (Montagu, 1808) | X | |||||||||||
| Diogenidae | ||||||||||||
| Clibanarius erythropus (Latreille, 1818) | X | X | OX | OX | OX | OX | X | X | X | X | X | X |
| Paguridae | ||||||||||||
| Anapagurus pusillus Henderson, 1888 | X | |||||||||||
| Cestopagurus timidus (Roux, 1830) | X | OX | OX | NX | O | OX | O | X | X | X | X | |
| Pagurus anachoretus Risso, 1827 | X | X | OX | OX | OX | OX | OX | OX | X | X | X | X |
| Galatheidae | ||||||||||||
| Galathea squamifera Leach, 1814 | O | O | O | X | X | X | ||||||
| Porcellanidae | ||||||||||||
| Pisidia longimana (Risso, 1816) | X | NX | X | X | X | X | X | X | X | X | X | NX |
| Porcellana platycheles (Pennant, 1777) | NX | NX | X | X | OX | OX | OX | NX | NX | NX | X | NX |
| Ethusidae | ||||||||||||
| Ethusa mascarone (Herbst, 1785) | O | O | O | OX | O | |||||||
| Pirimelidae | ||||||||||||
| Pirimela denticulate (Montagu, 1808) | O | O | X | |||||||||
| Polybiidae | ||||||||||||
| Liocarcinus navigator Herbst, 1794 | O | XO | O | O | O | O | X | O | ||||
| Portunidae | ||||||||||||
| Carcinus maenas (Linnaeus, 1758) | O | X | X | O | O | |||||||
| Xanthidae | ||||||||||||
| Xantho hydrophilus (Herbst, 1790) | OX | OX | OX | OX | O | X | X | X | X | X | X | |
| Xantho pilipes A. Milne-Edwards, 1867 | X | OX | OX | OX | O | OX | X | X | X | X | ||
| Xantho poressa (Olivi, 1792) | X | X | OX | OX | OX | OX | OX | X | X | X | X | X |
| Eriphiidae | ||||||||||||
| Eriphia verrucosa (Forskall, 1775) | X | O | X | OX | O | X | X | |||||
| Pilumnidae | ||||||||||||
| Pilumnus hirtellus (Linnaeus, 1761) | OX | OX | OX | OX | OX | NX | OX | X | X | X | X | X |
| Pilumnus sp. | X | OX | OX | OX | NX | OX | X | X | X | X | X | X |
| Pinnotheridae | ||||||||||||
| Afropinnotheres monodi Manning, 1993 | X | |||||||||||
| Grapsidae | ||||||||||||
| Pachygrapsus marmoratus (Fabricius, 1787) | X | X | OX | OX | OX | X | X | X | X | X | X | X |
| Pachygrapsus transversus (Gibbes, 1850) | X | X | X | NX | X | X | X | X | X | X | X | X |
| Varunidae | ||||||||||||
| Brachynothus atlanticus Forest, 1957 | X | X | X | X | ||||||||
| Epialtidae | ||||||||||||
| Acanthonyx lunulatus (Risso, 1816) | X | X | OX | OX | OX | OX | O | X | X | X | X | X |
| Pisa carinimana Miers, 1879 | X | NX | O | X | X | X | ||||||
| Pisa nodipes Leach, 1815 | O | X | X | OX | O | |||||||
| Pisa tetraodon (Pennant, 1777) | OX | OX | OX | OX | OX | OX | NX | X | X | X | OX | NX |
| Majidae | ||||||||||||
| Maja crispata Risso, 1827 | X | O | X | X | O | O | X | X | ||||
| Inachidae | ||||||||||||
| Achaeus cranchii Leach, 1817 | OX | NX | OX | O | OX | OX | X | X | X | |||
| Achaeus gracilis (Costa, 1839) | X | X | O | O | O | O | X | |||||
| Inachus gaditanus Garcia Raso, González-Ortegón, Palero and Cuesta, 2022 | OX | O | X | OX | O | X | O | OX | ||||
| Macropodia czerniavskii (Brandt, 1880) | X | NX | OX | X | X | X | X | X | ||||
| Macropodia longirostris (Fabricius, 1775) | O | O | O | X | ||||||||
| Macropodia rostrata (Linnaeus, 1716) | OX | O | X | O | O | OX | X | X | X | X | X |
| Group C Average Similarity: 74.05 | ||||||
|---|---|---|---|---|---|---|
| Species | Av. Ab. | Av. Sim. | Sim/SD | %Contrib | Cum% | |
| X. poressa | 1.00 | 6.64 | 8.69 | 8.97 | 8.97 | |
| P. platycheles | 1.00 | 6.64 | 8.69 | 8.97 | 17.93 | |
| P. longimana | 1.00 | 6.64 | 8.69 | 8.97 | 26.90 | |
| P. elegans | 1.00 | 6.64 | 8.69 | 8.97 | 35.86 | |
| P. anachoretus | 1.00 | 6.64 | 8.69 | 8.97 | 44.83 | |
| P. transversus | 1.00 | 6.64 | 8.69 | 8.97 | 53.79 | |
| P. marmoratus | 1.00 | 6.64 | 8.69 | 8.97 | 62.76 | |
| C. erythropus | 1.00 | 6.64 | 8.69 | 8.97 | 71.73 | |
| C. timidus | 1.00 | 6.64 | 8.69 | 8.97 | 80.69 | |
| Pilumnus sp.1 | 0.88 | 4.90 | 1.75 | 6.62 | 87.31 | |
| P. tetraodon | 0.67 | 2.78 | 0.87 | 3.75 | 91.06 | |
| Group E Average similarity: 79.28 | ||||||
| Species | Av. Ab. | Av. Sim. | Sim/SD | %Contrib | Cum% | |
| X. poressa | 1.00 | 7.01 | 9.62 | 8.84 | 8.84 | |
| P. platycheles | 1.00 | 7.01 | 9.62 | 8.84 | 17.68 | |
| P. longimana | 1.00 | 7.01 | 9.62 | 8.84 | 26.52 | |
| P. elegans | 1.00 | 7.01 | 9.62 | 8.84 | 35.35 | |
| P. anachoretus | 1.00 | 7.01 | 9.62 | 8.84 | 44.19 | |
| P. transversus | 1.00 | 7.01 | 9.62 | 8.84 | 53.03 | |
| P. marmoratus | 1.00 | 7.01 | 9.62 | 8.84 | 61.87 | |
| C. erythropus | 1.00 | 7.01 | 9.62 | 8.84 | 70.71 | |
| C. timidus | 1.00 | 7.01 | 9.62 | 8.84 | 79.55 | |
| Pilumnus sp.1 | 0.83 | 4.67 | 1.44 | 5.89 | 85.43 | |
| P. tetraodon | 0.83 | 2.57 | 1.44 | 5.77 | 91.20 | |
| Groups C and E Average dissimilarity: 26.65 | ||||||
| Species | Group C Av. Ab. | Group E Av. Ab. | Av. Dissim. | Dissim/SD | %Contrib | %Cum |
| A. lunulatus | 0.00 | 0.58 | 1.97 | 1.16 | 7.38 | 7.38 |
| A. nitescens | 0.00 | 0.58 | 1.94 | 1.17 | 7.27 | 14.65 |
| X. hydrophilus | 0.46 | 0.33 | 1.65 | 0.96 | 6.19 | 20.84 |
| A. cranchii | 0.38 | 0.25 | 1.45 | 0.87 | 5.44 | 26.28 |
| H. leptocerus | 0.13 | 0.42 | 1.43 | 0.87 | 5.35 | 31.63 |
| P. tetraodon | 0.67 | 0.83 | 1.37 | 0.79 | 5.16 | 36.78 |
| M. rostrata | 0.38 | 0.08 | 1.33 | 0.80 | 4.99 | 41.77 |
| X. pilipes | 0.33 | 0.08 | 1.23 | 0.74 | 4.61 | 46.38 |
| M. czerniavskii | 0.33 | 0.08 | 1.19 | 0.75 | 4.46 | 50.84 |
| E. cranchii | 0.33 | 0.00 | 1.14 | 0.70 | 4.26 | 55.10 |
| P. carinimana | 0.17 | 0.25 | 1.09 | 0.70 | 4.08 | 59.19 |
| E. verrucosa | 0.21 | 0.17 | 1.03 | 0.66 | 3.87 | 63.06 |
| P. hirtellus | 0.17 | 0.17 | 0.92 | 0.62 | 3.45 | 66.51 |
| P. serratus | 0.17 | 0.17 | 0.92 | 0.62 | 3.45 | 69.95 |
| Pilumnus sp.1 | 0.88 | 0.83 | 0.91 | 0.57 | 3.40 | 73.35 |
| B. atlanticus | 0.04 | 0.25 | 0.85 | 0.61 | 3.21 | 76.56 |
| P. nodipes | 0.25 | 0.00 | 0.82 | 0.57 | 3.09 | 79.65 |
| M. crispata | 0.21 | 0.00 | 0.66 | 0.51 | 2.49 | 82.14 |
| I. gaditanus | 0.21 | 0.00 | 0.66 | 0.51 | 2.49 | 84.62 |
| G. squamifera | 0.17 | 0.00 | 0.52 | 0.45 | 1.94 | 86.56 |
| A. dentipes | 0.08 | 0.08 | 0.51 | 0.42 | 1.91 | 88.47 |
| A. gracilis | 0.08 | 0.08 | 0.48 | 0.42 | 1.81 | 90.28 |
| Group U Average Similitary: 78.61 | ||||||
|---|---|---|---|---|---|---|
| Species | Av. Ab. | Av. Sim. | Sim/SD | %Contrib | %Cum | |
| X. poressa | 1.00 | 8.20 | 7.69 | 10.44 | 10.44 | |
| P. platycheles | 1.00 | 8.20 | 7.69 | 10.44 | 20.87 | |
| P. longimana | 1.00 | 8.20 | 7.69 | 10.44 | 31.31 | |
| P. elegans | 1.00 | 8.20 | 7.69 | 10.44 | 41.74 | |
| P. anachoretus | 1.00 | 8.20 | 7.69 | 10.44 | 52.18 | |
| P. transversus | 1.00 | 8.20 | 7.69 | 10.44 | 62.62 | |
| P. marmoratus | 1.00 | 8.20 | 7.69 | 10.44 | 73.05 | |
| C. erythropus | 1.00 | 8.20 | 7.69 | 10.44 | 83.49 | |
| C. timidus | 1.00 | 8.20 | 7.69 | 10.44 | 93.92 | |
| Group L Average Similarity: 73.85 | ||||||
| Species | Av. Ab. | Av. Sim. | Sim/SD | %Contrib | %Cum | |
| X. poressa | 1.00 | 6.36 | 8.80 | 8.61 | 8.61 | |
| P. platycheles | 1.00 | 6.36 | 8.80 | 8.61 | 17.23 | |
| P. longimana | 1.00 | 6.36 | 8.80 | 8.61 | 25.84 | |
| P. elegans | 1.00 | 6.36 | 8.80 | 8.61 | 34.45 | |
| P. anachoretus | 1.00 | 6.36 | 8.80 | 8.61 | 43.07 | |
| P. transversus | 1.00 | 6.36 | 8.80 | 8.61 | 51.68 | |
| P. marmoratus | 1.00 | 6.36 | 8.80 | 8.61 | 60.29 | |
| C. erythropus | 1.00 | 6.36 | 8.80 | 8.61 | 68.91 | |
| C. timidus | 1.00 | 6.36 | 8.80 | 8.61 | 77.52 | |
| Groups U and L Average Dissimilarity: 66.26 | ||||||
| Species | Group C Av. Ab. | Group E Av. Ab. | Av. Dissim. | Dissim/SD | %Contrib | %Cum |
| P. tetraodon | 0.13 | 0.71 | 2.35 | 1.36 | 8.82 | 8.82 |
| P. hirtellus | 0.46 | 0.79 | 1.91 | 1.03 | 7.18 | 16.00 |
| A. nitescens | 0.29 | 0.54 | 1.82 | 1.02 | 6.84 | 22.84 |
| A. dentipes | 0.46 | 0.54 | 1.80 | 0.99 | 6.76 | 29.59 |
| Pilumnus sp.1 | 0.13 | 0.46 | 1.62 | 0.93 | 6.09 | 35.68 |
| X. hydrophilus | 0.17 | 0.42 | 1.60 | 0.88 | 6.00 | 41.69 |
| A. lunulatus | 0.25 | 0.38 | 1.51 | 0.87 | 5.65 | 47.34 |
| A. cranchii | 0.13 | 0.38 | 1.38 | 0.82 | 5.17 | 52.51 |
| M. rostrata | 0.17 | 0.33 | 1.36 | 0.79 | 5.09 | 57.60 |
| X. pilipes | 0.13 | 0.25 | 1.10 | 0.67 | 4.14 | 61.75 |
| E. cranchii | 0.04 | 0.29 | 1.10 | 0.66 | 4.14 | 65.88 |
| M. czerniavskii | 0.08 | 0.25 | 0.97 | 0.64 | 3.66 | 69.54 |
| I. gaditanus | 0.17 | 0.13 | 0.81 | 0.57 | 3.05 | 72.59 |
| M. crispata | 0.08 | 0.17 | 0.73 | 0.53 | 2.74 | 75.34 |
| P. serratus | 0.04 | 0.17 | 0.68 | 0.49 | 2.56 | 77.90 |
| E. verrucosa | 0.08 | 0.13 | 0.68 | 0.48 | 2.55 | 80.45 |
| P. nodipes | 0.04 | 0.17 | 0.66 | 0.49 | 2.48 | 82.93 |
| P. carinimana | 0.08 | 0.13 | 0.60 | 0.48 | 2.24 | 85.17 |
| G. squamifera | 0.04 | 0.13 | 0.52 | 0.43 | 1.96 | 87.13 |
| L. seticaudata | 0.04 | 0.08 | 0.43 | 0.36 | 1.61 | 88.73 |
| C. pestai | 0.08 | 0.04 | 0.42 | 0.36 | 1.59 | 90.32 |
| Species | CCL | CCU | CSL | CSU | E |
|---|---|---|---|---|---|
| Sicyonia carinata | 0 | 1 | 0 | 0 | 0 |
| Lysmata seticaudata | 0 | 0 | 2 | 0 | 0 |
| Hippolyte inermis | 1 | 0 | 0 | 0 | 0 |
| Hippolyte leptocerus | 2 | 0 | 2 | 0 | 5 |
| Eualus cranchii | 3 | 2 | 3 | 0 | 0 |
| Alpheus dentipes | 6 | 7 | 6 | 3 | 0 |
| Athanas nitescens | 8 | 5 | 4 | 3 | 7 |
| Processa sp. | 0 | 0 | 0 | 0 | 1 |
| Palaemon elegans | 12 | 12 | 12 | 12 | 12 |
| Palaemon serratus | 2 | 0 | 4 | 0 | 2 |
| Philocheras fasciatus | 0 | 0 | 0 | 0 | 1 |
| Callianassa pestai | 0 | 0 | 0 | 1 | 0 |
| Clibanarius erythropus | 12 | 12 | 12 | 12 | 12 |
| Anapagurus pusillus | 0 | 1 | 0 | 0 | 0 |
| Cestopagurus timidus | 12 | 12 | 12 | 12 | 12 |
| Pagurus anachoretus | 12 | 12 | 12 | 12 | 12 |
| Galathea squamifera | 2 | 1 | 1 | 0 | 0 |
| Pisidia longimana | 12 | 12 | 12 | 12 | 12 |
| Porcellana platycheles | 12 | 12 | 12 | 12 | 12 |
| Ethusa mascarone | 0 | 1 | 0 | 0 | 0 |
| Pirimela denticulata | 0 | 0 | 0 | 1 | 0 |
| Liocarcinus navigator | 1 | 0 | 0 | 1 | 0 |
| Carcinus maenas | 1 | 0 | 0 | 1 | 0 |
| Xantho hydrophilus | 4 | 1 | 5 | 2 | 4 |
| Xantho pilipes | 4 | 2 | 2 | 1 | 1 |
| Xantho poressa | 12 | 12 | 12 | 12 | 12 |
| Eriphia verrucosa | 1 | 1 | 1 | 2 | 2 |
| Pilumnus hirtellus | 10 | 7 | 10 | 4 | 9 |
| Pilumnus sp. | 7 | 2 | 3 | 0 | 1 |
| Afropinnotheres monodi | 0 | 0 | 0 | 0 | 1 |
| Pachygrapsus marmoratus | 12 | 12 | 12 | 12 | 12 |
| Pachygrapsus transversus | 12 | 12 | 12 | 12 | 12 |
| Brachynothus atlanticus | 1 | 0 | 0 | 0 | 3 |
| Acanthonyx lunulatus | 4 | 3 | 5 | 3 | 5 |
| Pisa carinimana | 3 | 1 | 0 | 1 | 3 |
| Pisa nodipes | 2 | 1 | 2 | 0 | 0 |
| Pisa tetraodon | 8 | 1 | 8 | 3 | 9 |
| Maja crispata | 1 | 0 | 3 | 3 | 0 |
| Achaeus cranchii | 3 | 2 | 3 | 0 | 0 |
| Achaeus gracilis | 2 | 0 | 1 | 0 | 1 |
| Inachus gaditanus | 3 | 2 | 0 | 2 | 0 |
| Macropodia czerniavskii | 3 | 0 | 3 | 2 | 1 |
| Macropodia longirostris | 0 | 0 | 0 | 2 | 0 |
| Macropodia rostrata | 4 | 0 | 4 | 3 | 1 |
| Number of species on each sampling site | 34 | 27 | 29 | 27 | 26 |
| Habitat | Location | Depth | CD | P/D | SM | N | Ref |
|---|---|---|---|---|---|---|---|
| Rocky intertidal | La Caleta (Cádiz, Spain) | 0 | UB | Monthly/1 year | Visual, hand net, and hand | 44 | P |
| Rocky intertidal | Cabo Raso and Avencas (Lisbon, Portugal) | 0 | NP | 2 seasons (Winter and Summer) | Visual, using transect | 7 | [26] |
| Caulerpa prolifera meadows | Valdelagrana (Cádiz, Spain) | 3–6 | UB | Monthly/2 years | Semi-circular trawl | 49 | [42] |
| Cymodocea nodosa meadows | Mijas (Málaga, Spain) | 1–5 | SCI | Seasonal/1 year | Scuba divers with a manual airlift pump | 34 | [43] |
| Cymodocea nodosa meadows | Island of Ischia (Naples, Italy) | 3 | MPA | Bimonthly/1 year | Scuba divers with hand-towed net | 18 | [44] |
| Cymodocea nodosa meadows | Cabo de Gata (Almeria, Spain) | 10–14 | NP | Seasonal/1 year | Agassiz trawl with a dredge frame | 34 | [45] |
| Posidonia oceanica meadows | Mijas (Málaga, Spain) | 2 | SCI | Seasonal/1 year | Scuba divers using a frame (50 × 50 cm) and an airlift sampler | 34 | [19] |
| Infralitoral macroalgal beds dominated by Halopterys scoparia | Mijas (Málaga, Spain) | 2 | SCA | Seasonal/1 year | Scuba divers using a frame (50 × 50 cm) and an airlift sampler | 35 | [19] |
| Shallow sandy bottoms | Fuengirola and Marbella (Málaga, Spain) | 5 | SCI | Seasonal/1 year | Small heavy rock dredge with a rectangular frame | 18 | [46] |
| Fine sand bottoms | Fuengirola and Marbella (Málaga, Spain) | 15 | SCI | Seasonal/1 year | Small heavy rock dredge with a rectangular frame | 29 | [46] |
| Coarse bottoms | Fuengirola and Marbella (Málaga, Spain) | 15 | SCI | Seasonal/1 year | Small heavy rock dredge with a rectangular frame | 30 | [46] |
| Coralligenous bottoms | Fuengirola and Marbella (Málaga, Spain) | 15 | SCI | Seasonal/1 year | Small heavy rock dredge with a rectangular frame | 41 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Hidalgo, M.; Cervera, J.L.; Cuesta, J.A. High Species Richness of Decapod Crustaceans on an Urban Rocky Shore Beach. Diversity 2023, 15, 716. https://doi.org/10.3390/d15060716
Rodríguez-Hidalgo M, Cervera JL, Cuesta JA. High Species Richness of Decapod Crustaceans on an Urban Rocky Shore Beach. Diversity. 2023; 15(6):716. https://doi.org/10.3390/d15060716
Chicago/Turabian StyleRodríguez-Hidalgo, Mikel, Juan Lucas Cervera, and Jose A. Cuesta. 2023. "High Species Richness of Decapod Crustaceans on an Urban Rocky Shore Beach" Diversity 15, no. 6: 716. https://doi.org/10.3390/d15060716
APA StyleRodríguez-Hidalgo, M., Cervera, J. L., & Cuesta, J. A. (2023). High Species Richness of Decapod Crustaceans on an Urban Rocky Shore Beach. Diversity, 15(6), 716. https://doi.org/10.3390/d15060716







