Paleoclimate and Paleoenvironment Reconstructions from Middle Eocene Successions at Beni-Suef, Egypt: Foraminiferal Assemblages and Geochemical Approaches
Abstract
1. Introduction
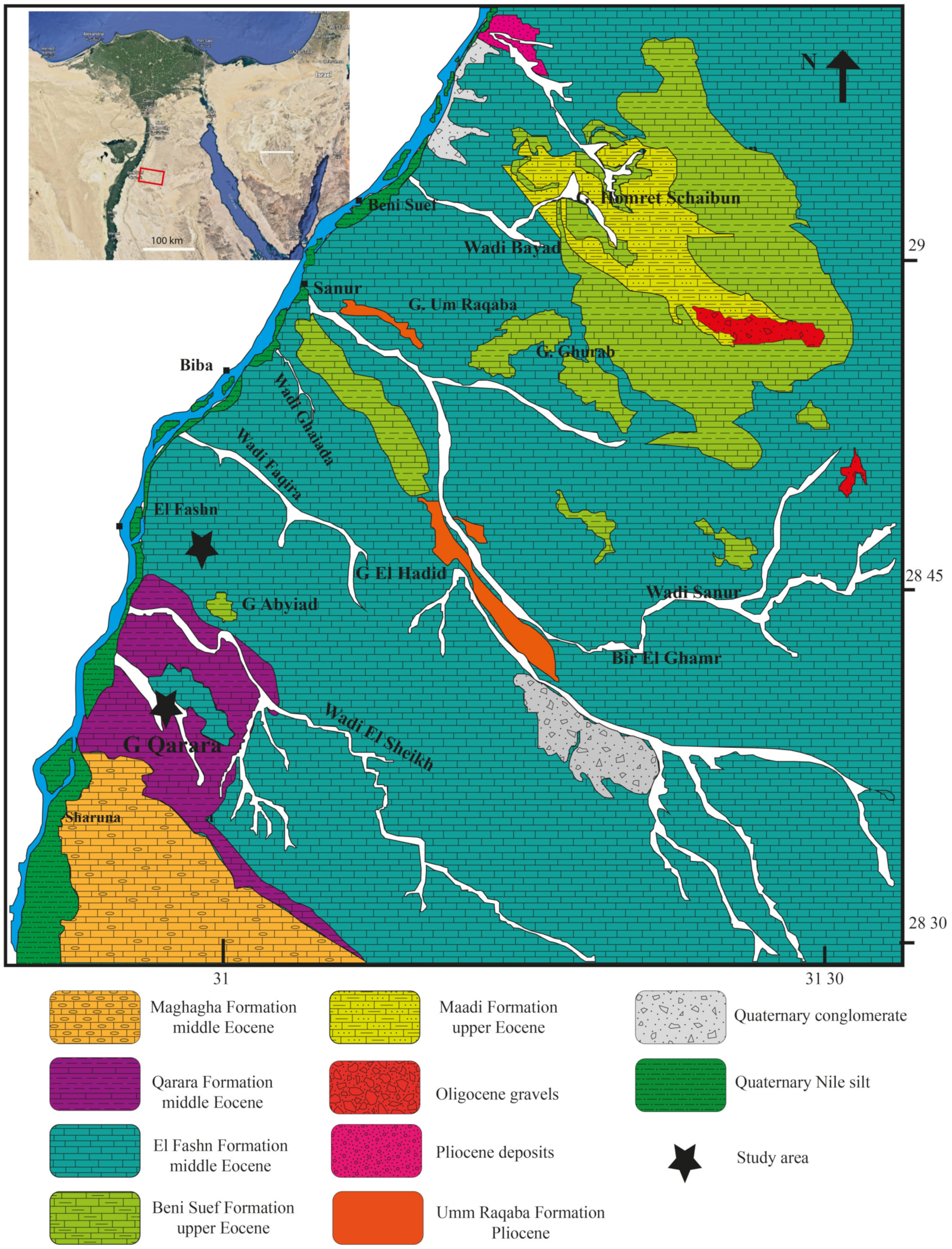
2. Geologic Setting and Stratigraphy
2.1. Qarara Formation
2.2. El Fashn Formation
3. Materials and Methods
4. Results
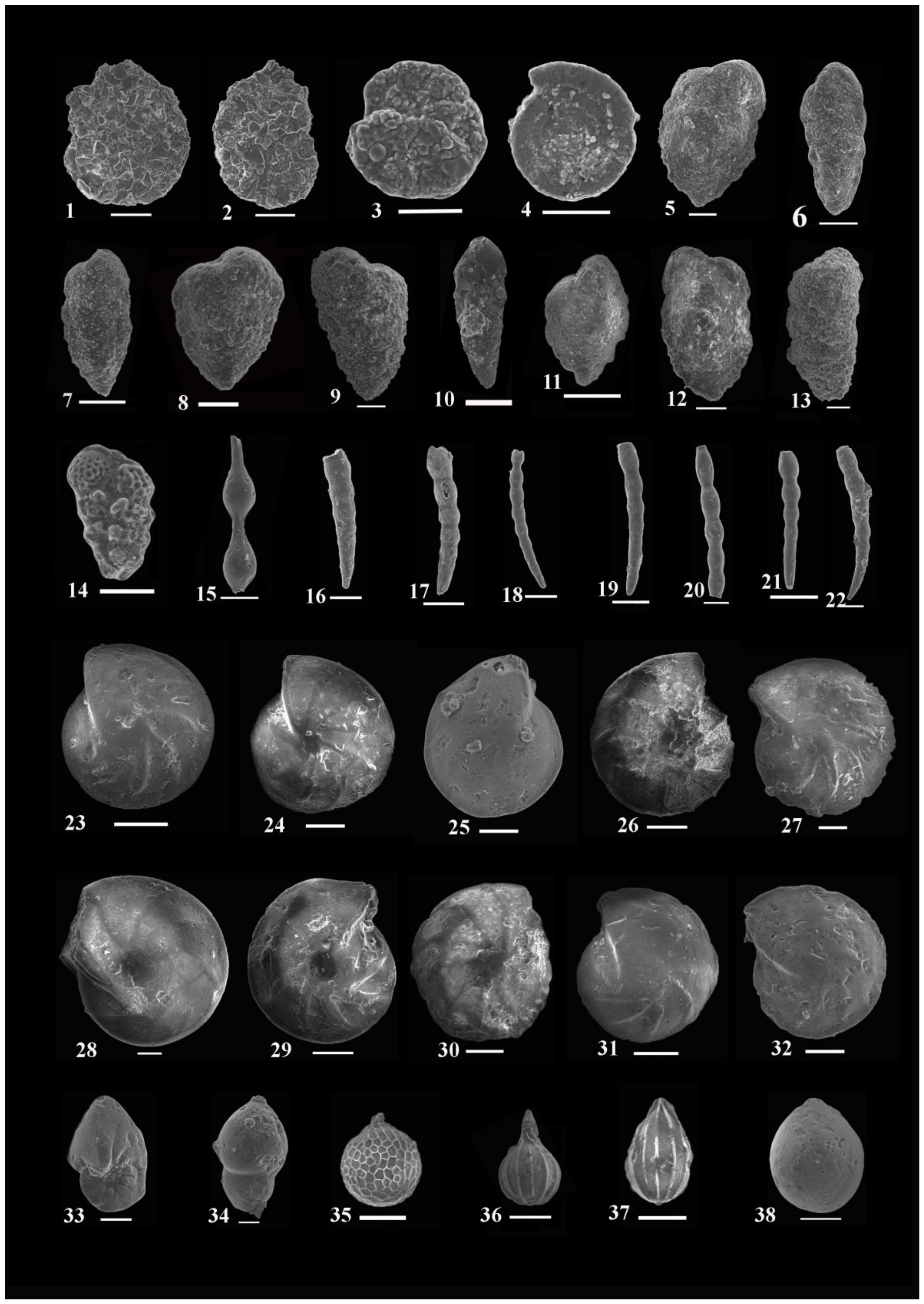
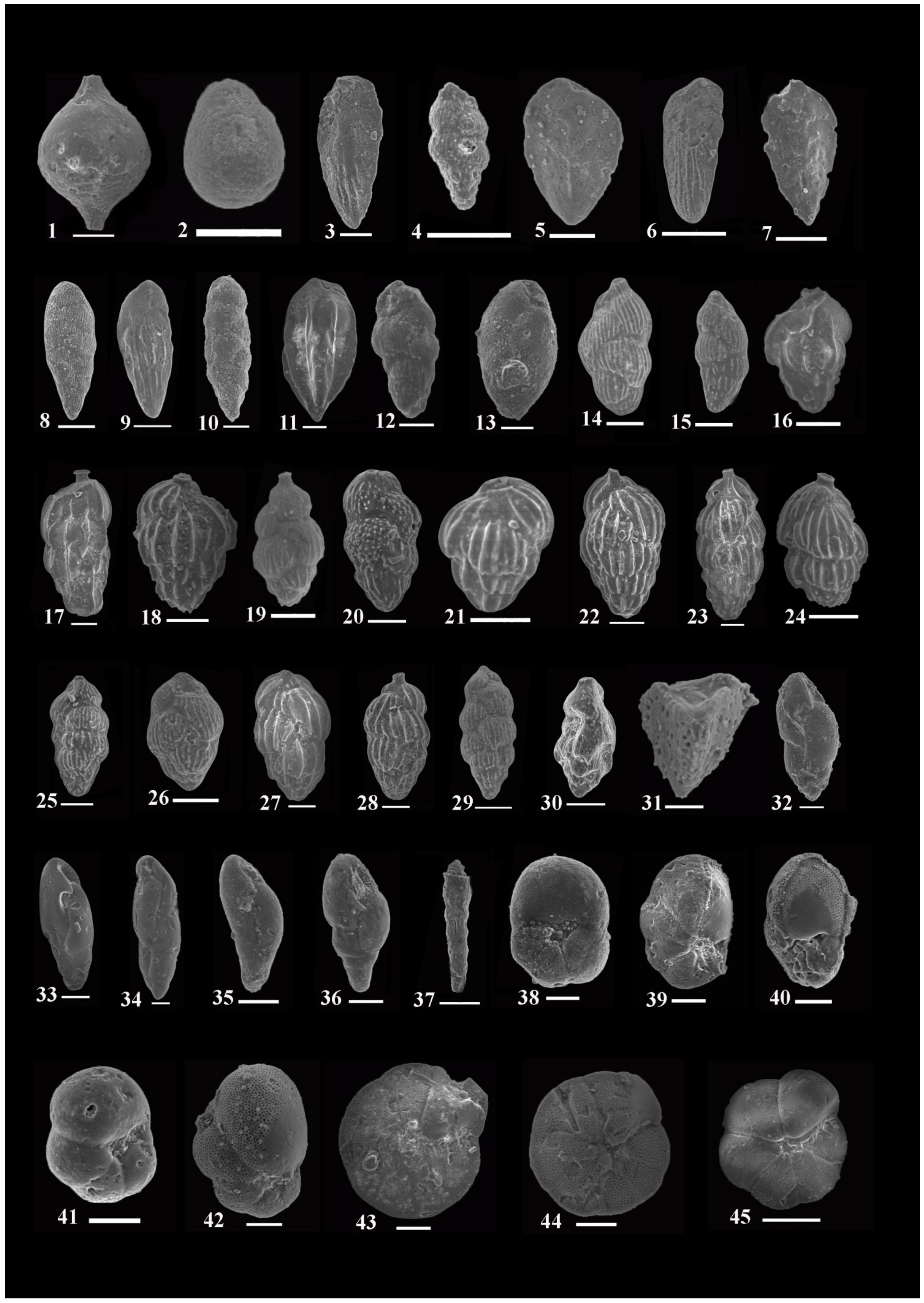
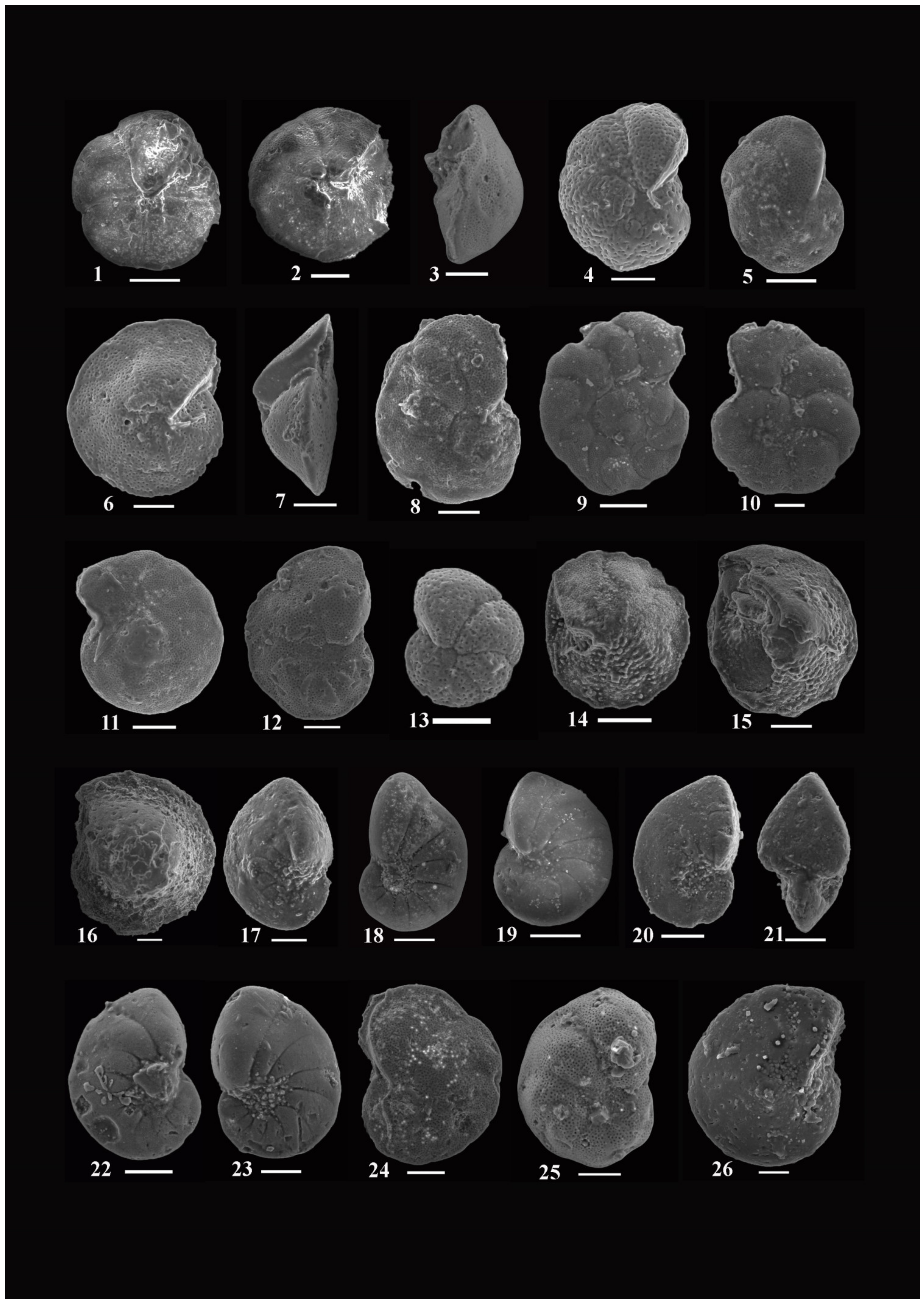
4.1. Systematic Paleontology and Foraminiferal Biostratigraphy
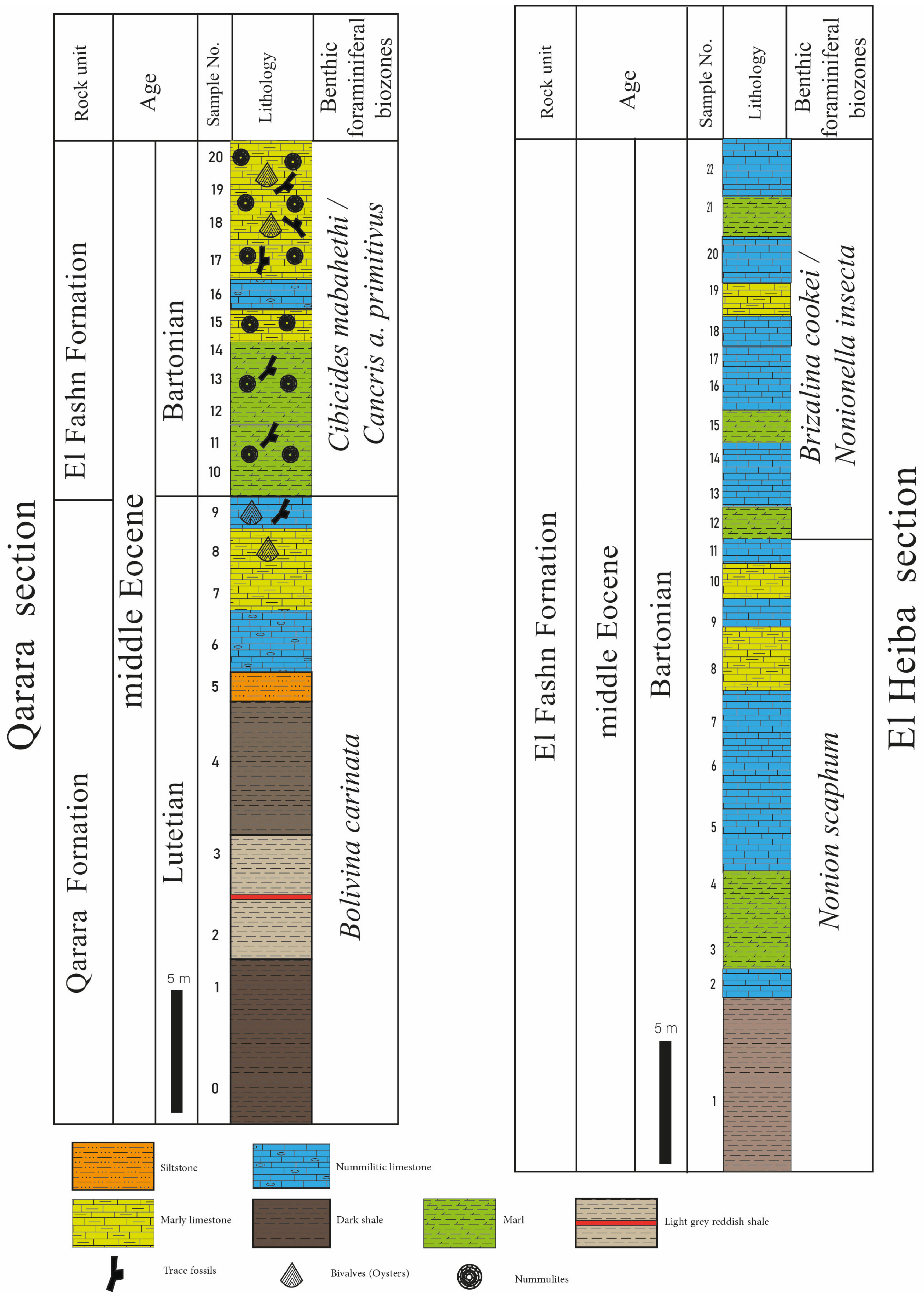
4.1.1. Bolivina carinata Lowest Occurrence Zone
- Age: middle Eocene (Lutetian)
- Definition: Interval from the lowest occurrence (LO) of Bolivina carinata to the LO of Brizalina cookei.
- Occurrence: This zone represents the exposed part of the Qarara Formation corresponding to the lower and middle parts of the Qarara section (samples 1 to 9) with a thickness of about 46 m.
- Assemblages: Forty-six species and subspecies are recorded from this zone; the most dominant species are: Bolivina alazanensis venezulana, B. jacksonensis striatella, B. carinata, Nonionella spissa, Cibicides mabahethi, Cibicidoides westi, C. laurisae, Cibicidina carinatus, Eponides ellisorae, Fursenkoina dibolensis, F. squamosa, Lobatula lobatulus, and Uvigerina seriata.
- Correlation: The recorded zone is equivalent to the Quinqueloculina seminula and Haplophragmoides emaclatus/Ammobaculites cubensis zones that were previously recorded from the middle Eocene rocks of the Helwan area and the Nile Valley [20,72]. The interval also correlates to the upper part of the Anomalinoides fayoumensis and Uvigerina nakkadyi/Anomalinoides fayoumensis zones that were defined from the Eastern Desert and Nile Valley, respectively [71,73], as shown in Table 1. On the other hand, it is matched with the lower part of the Bulimina jacksonensis/Uvigerina jacksonensis Zone that was defined by Helal [68] and the upper part of the same zone in addition to the lower part of Cibicidoides truncanus Zone of Elewa et al. [46] in the Fayoum area (Table 1). Regarding the large benthic foraminiferal zonation, this zone could be correlated to the upper part of Nummulites gizehensis Zone and also matches with the lower part of Orbitolites complanatus Zone from the middle Eocene of the Nile valley [70]. Furthermore, it is equivalent to the upper part of Nummulites aff. puchellas Zone, which was recorded from the middle Eocene of Helwan area by Boukhary et al. [74].
4.1.2. Cibicides mabahethi/Cancris auriculus primitivus Concurrent-Range Zone
- Age: middle Eocene (Bartonian)
- Definition: Interval from the LO of Brizalina cookei to the highest occurrence (HO) of Cancris auriculus primitivus.
- Occurrence: This zone corresponds to the whole thickness of the El Fashn Formation exposed in the upper part of the Qarara section (25 m, samples 10 to 20).
- Assemblages: One hundred and forty-three species and subspecies are identified from this zone. The most important and abundant species are: Bolivina alazanensis venezulana, Bolivina carinata, B. jacksonensis, B. jacksonensis striatella, Nonionella insecta, N. spissa, Cancris auriculus primitivus, C. turgidus, Cibicides mabahethi, Cibicidoides laurisae, C. westi, C. yankaulensis, Eponides ellisorae, E. jacksonensis, Neoeponides schreibersi, Fursenkoina dibolensis, F. squamosa, Nonion scaphum,, lenticulina alabamensis, L. alatolimbata, L. costata, L. politus, Pleurostomella cubensis, textularia adamsi, T. arenacea, and T. recta.
- Correlation: The identified zone correlates to the Nonionella africana and Uvigerina coaensis/Uvigerina continusa zones that were reported from the middle Eocene (Bartonian) of the Helwan area [20] and the Nile Valley [72]; see Table 1. It is also coeval to the lower part of the Palmula ansaryi Zone the from north Eastern Desert [17,19] (Table 1). Moreover, this zone is correlated to the middle part of the middle Eocene Bulimina jacksonensis/Uvigerina jacksonensis Zone from Fayoum [68] and the middle to upper part of the Brizalina cookei and Cibicidoides carinatus zones that was identified, respectively, from the north Eastern Desert [73], Nile Valley [71], and Fayoum [46] (Table 1). In addition, it is equivalent to the middle and upper parts of the larger benthic foraminifera Orbitolites complanatus Zone recorded from the Bartonian of the Nile Valley by Mansour et al. [70].
- Age: This zone is correlated to the lower part of the planktonic zone E13 based on the occurrence of the index small and spinose planktonic species Acarinina rohri and Morozovelloides spp., coupled with the coexistence of the large benthic foraminifera Nummulites beaumonti attributing late middle Eocene (Bartonian) age [18,21,23].
4.1.3. Nonion scaphum Lowest Occurrence Zone
- Age: middle Eocene (Bartonian)
- Definition: The present zone is defined from the LO of Nonion scaphum to the LO of Lenticulina alabamensis.
- Occurrence: It represents the lower and middle parts of the El Fashn Formation at the El Heiba section, attaining a thickness of about 34 m (samples 1 to 11).
- Assemblages: Eighty species and subspecies are recorded from this zone. The most common species are: Bolivina carinata, Bulimina jacksonensis, Cibicidoides laurisae, C. westi, Cibicides mabahethi, Eponides jacksonensis, E. cocoaensis, Neoeponides schreibersi, Fursenkoina dibolensis, Uvigerina seriata, Cancris auriculus primitivus, C. danvillensis, Baggina bradyi, Asterigerina brenci, Cibicidina carinatus, Cibicidoides pharoaensis, Halkyardia minima, Lobatula lobatulus, Planulina cocoaensis, Uvigerina batjesi, U. jacksonensis, U. mediterranea, U. peregrina, and U. rippensis.
- Correlation: The present zone is correlated with the Brizalina cookei Zone as proposed by Abd El-Gaied et al. [20] from the middle Eocene sediments in the Helwan area (Table 1). It is matched to the middle part of the Palmula ansaryi Zone in the north Eastern Desert [17,19] and to the lower part of the same zone in the Nile Valley [72]. On the other hand, it is equivalent to the upper part of the Brizalina cookei Zone and the lower part of the Bolivina jacksonensis/Pararotalia audouini Zone recorded from the north Eastern Desert [73], and to the upper part of Brizalina cookei Zone and the lower part of the Nonion scaphum/Pararotalia audouini Zone in the Nile Valley [71], as indicated in Table 1. In the Fayoum area, it is equated to the middle part of the middle Eocene Bulimina jacksonensis/Uvigerina jacksonensis Zone [68], the middle part of Lenticulina costata Zone [69], and the lower part of the Lenticulina alatolimbata Zone [46]. The identified zone could be matched to the lower and middle part of the Nummulites beaumonti Zone, which was identified by Mansour et al. [70] from the Bartonian of the Nile Valley.
4.1.4. Brizalina cookei/Nonionella insecta Concurrent-Range Zone
- Age: middle Eocene (Bartonian)
- Definition: Interval from the LO of Lenticulina alabamensis to the HO of Cibicides mabahethi.
- Occurrence: The present zone is defined from the upper part of the El Fashn Formation, occupying the uppermost part of the El Heiba section with a thickness of about 42 m (samples 12 to 22).
- Assemblages: Seventy-nine species and subspecies are identified from this zone. The most dominant species are: Bolivina carinata, B. jacksonensis striatella, Cibicidoides westi, Cibicides mabahethi, Cibicidina carinatus, Lobatula lobatulus, Eponides ellisorae, Neoeponides schreibersi, Nonionella insecta, Uvigerina peregrine, and U. seriata.
- Correlation: The present zone is equivalent to the Nonion scaphum Zone recorded by Abd El-Gaied et al. [20]) from the Helwan area. It is matched to the upper part of the following zones: the Palmula ansaryi Zone from the north Eastern Desert [17,19] and Lenticulina costata Zone that is recorded from the Fayoum area [69]. It also correlates to the middle and upper parts of the Palmula ansaryi Zone in the Nile Valley [72], Lenticulina alatolimbata Zone defined in the Fayoum area [46], the Bolivina jacksonensis/Pararotalia audouini Zone recorded from north Eastern Desert [73], and the Nonion scaphum/Pararotalia audouini Zone from the Nile Valley [71] (Table 1). In addition, it is equated to the upper middle part of the Bulimina jacksonensis/Uvigerina jacksonensis Zone from Fayoum [68] and to the middle and upper part of the Nummulites beaumonti Zone recognized in the Nile Valley [70].
- Age: Depending on the stratigraphic position, this zone is correlated to the upper part of the planktonic foraminifera zone E13, which witnessed the occurrence of the index small and spinose planktonic species (Acarinina spp., Morozovelloides spp.). Therefore, the recorded zone is assigned to the late middle Eocene (Bartonian) age [18,21,23].
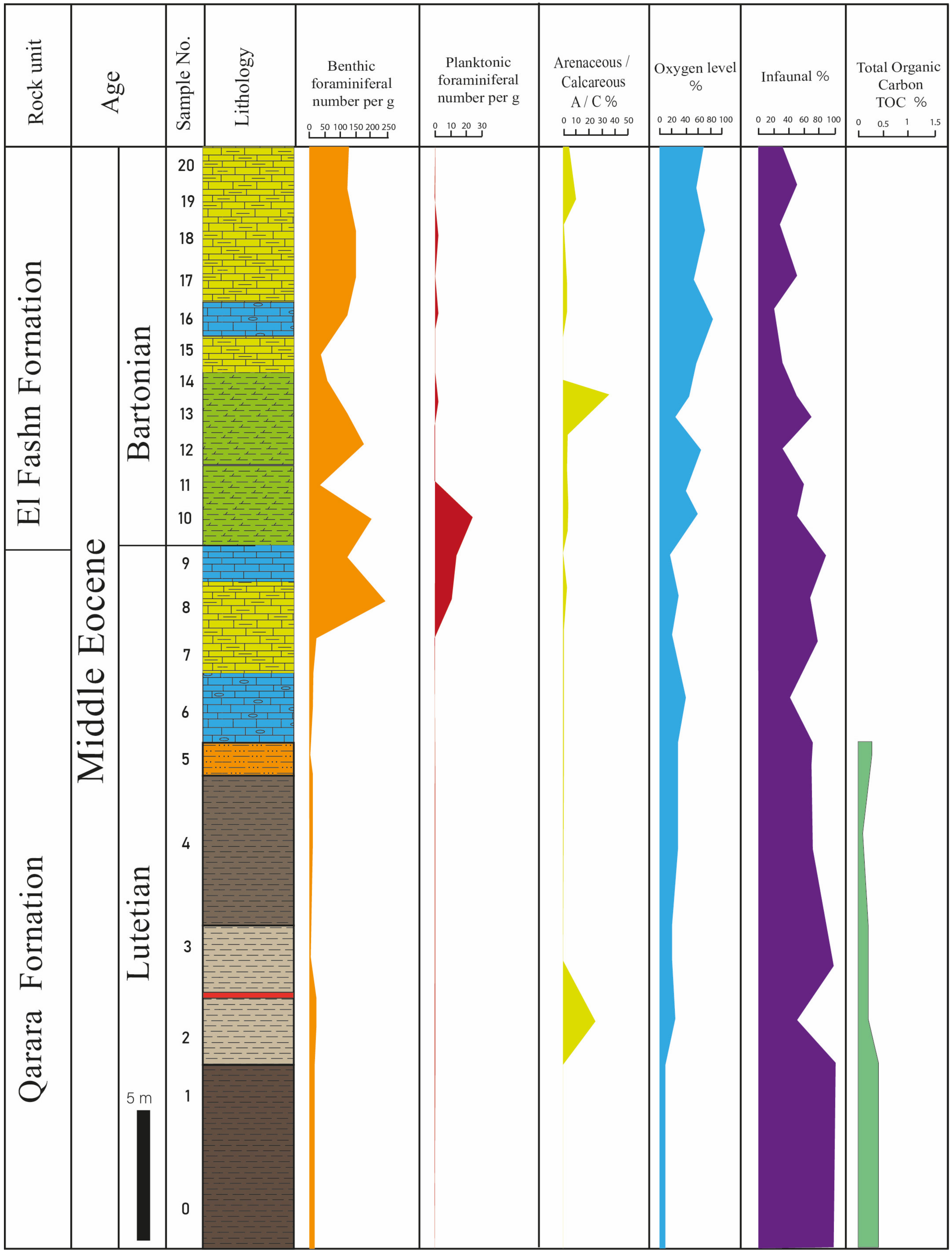
4.2. Geochemistry
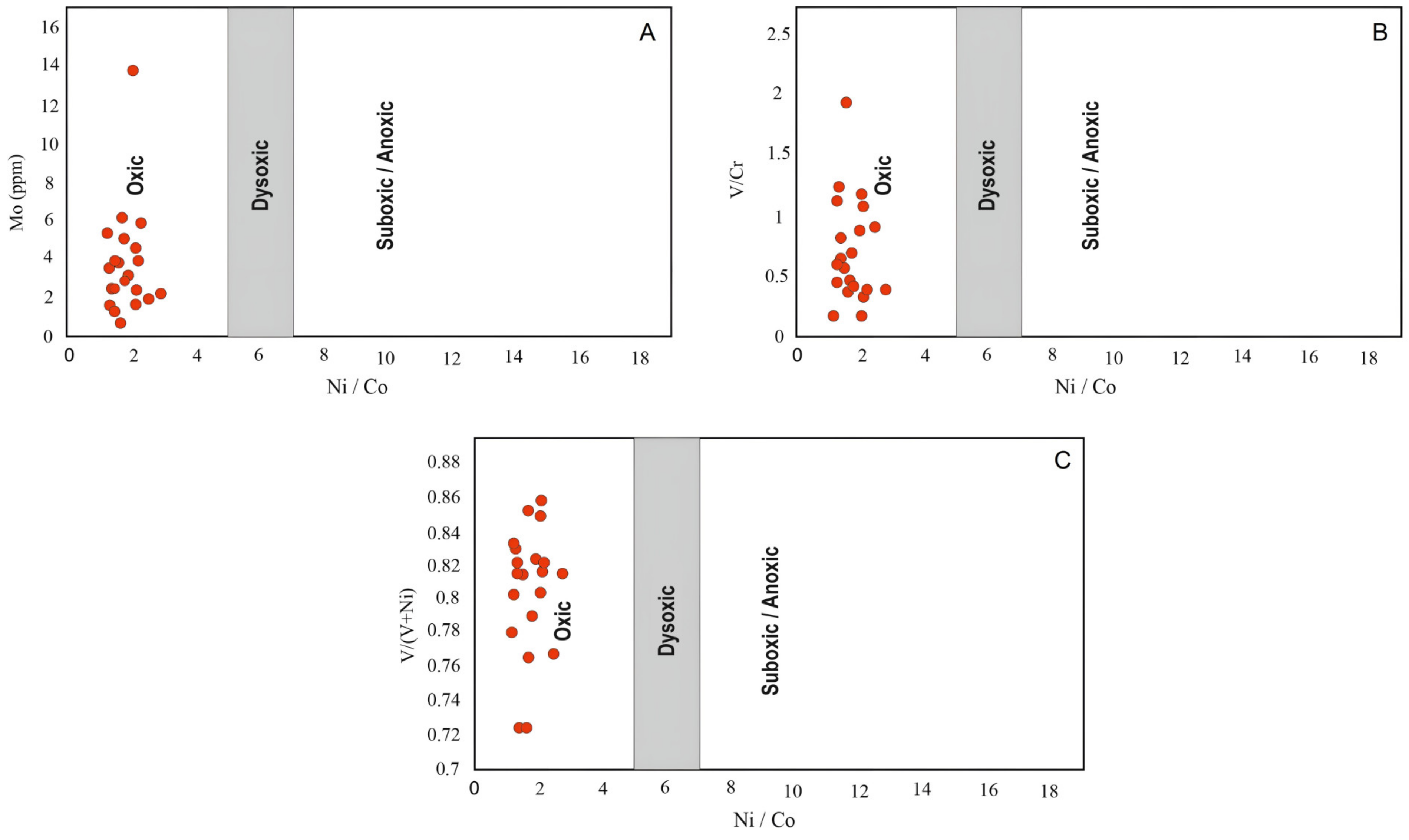
4.2.1. Paleo-Redox Proxies
4.2.2. Paleoclimate and Salinity Proxies
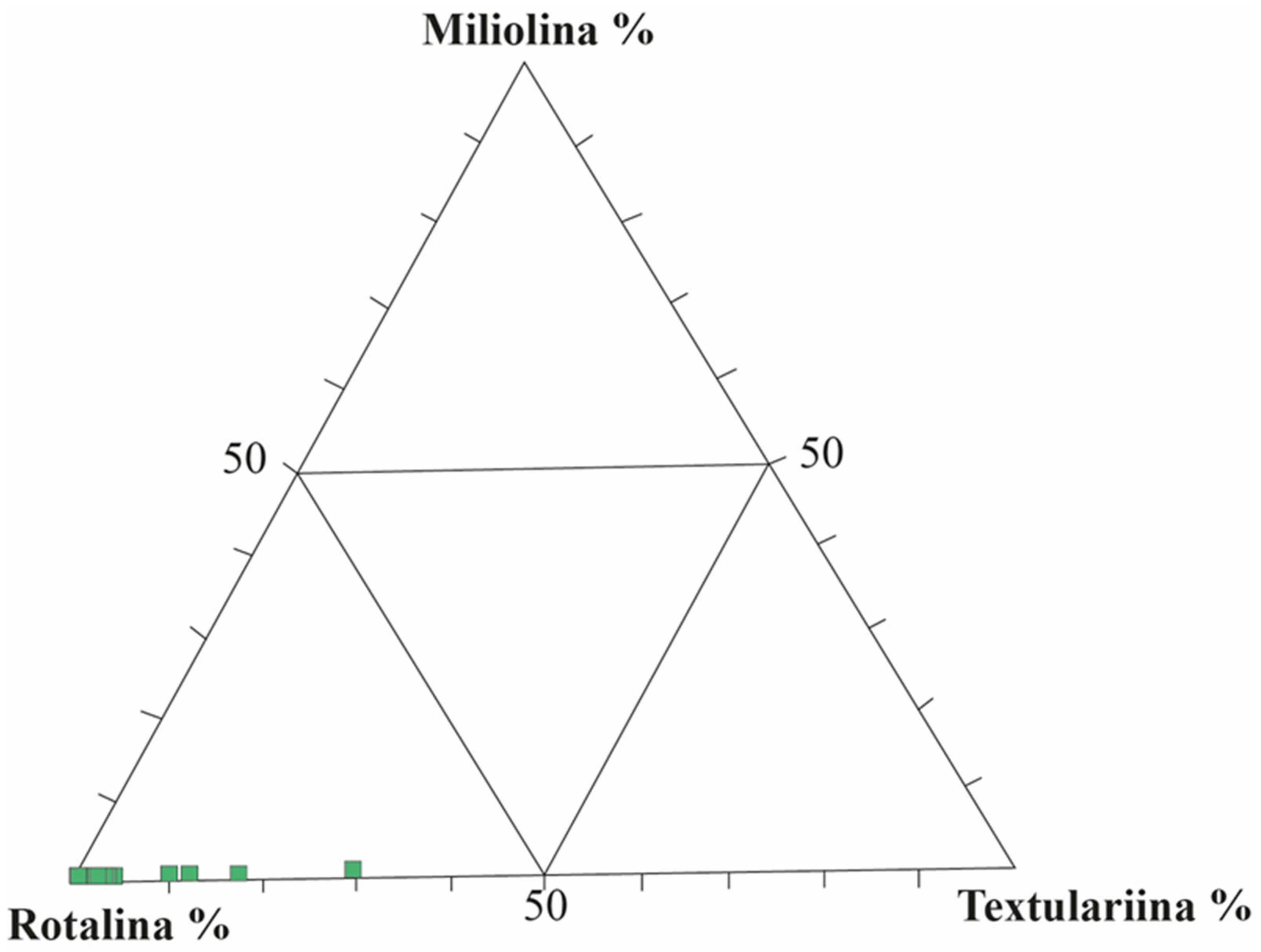
4.3. Foraminiferal Data
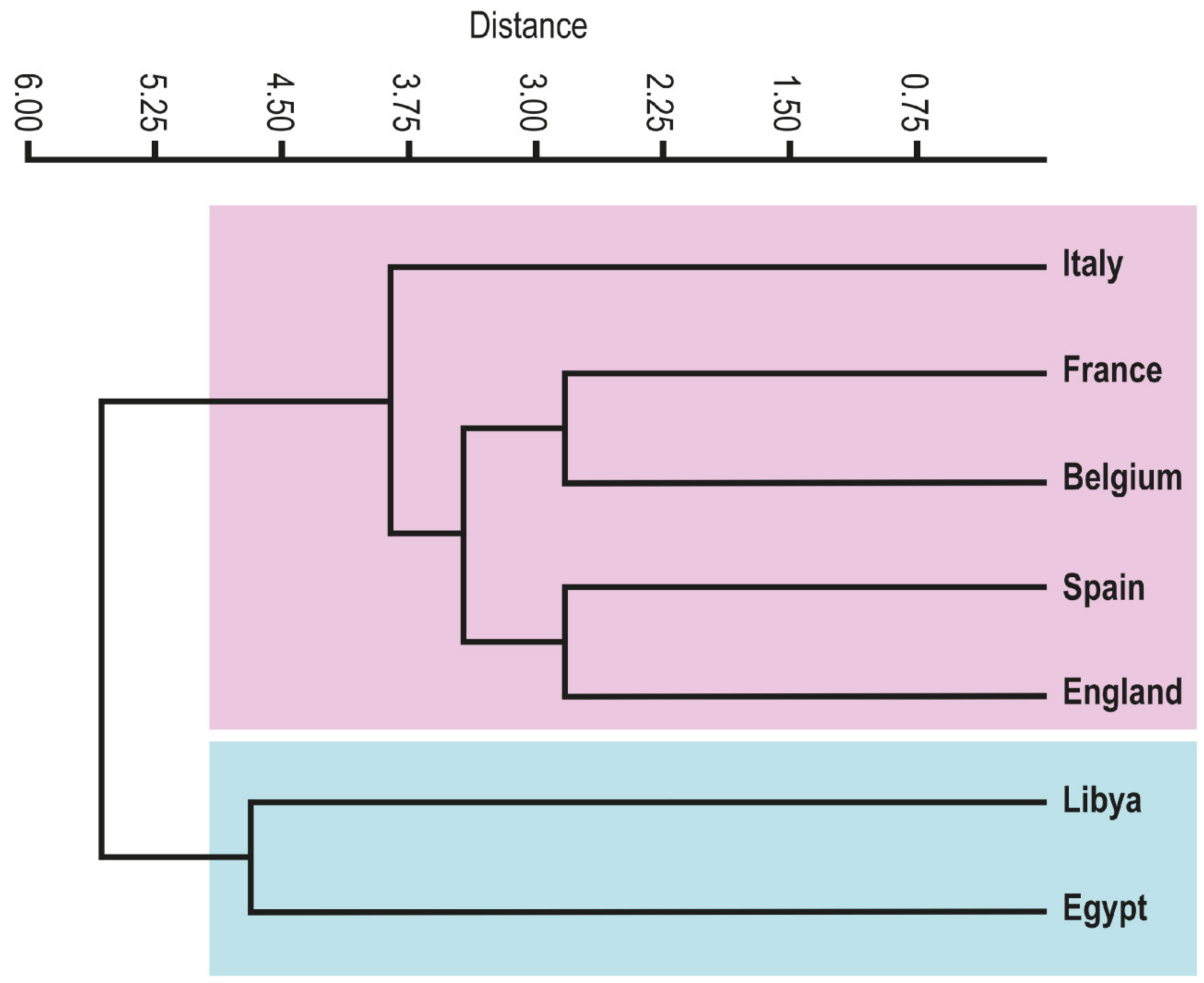
4.4. Paleobiogeography
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westerhold, T.; Marwan, N.; Drury, A.J.; Liebrand, D.; Agnini, C.; Anagnostou, E.; Barnet, J.S.; Bohaty, S.M.; De Vleeschouwer, D.; Florindo, F. An astronomically dated record of Earth’s climate and its predictability over the last 66 million years. Science 2020, 369, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Zachos, J.C.; Dickens, G.R.; Zeebe, R.E. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 2008, 451, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Bohaty, S.M.; Zachos, J.C. Significant Southern Ocean warming event in the late middle Eocene. Geology 2003, 31, 1017–1020. [Google Scholar] [CrossRef]
- Ganeshram, R.S.; Calvert, S.E.; Pedersen, T.F.; Cowie, G.L. Factors controlling the burial of organic carbon in laminated and bioturbated sediments off NW Mexico: Implications for hydrocarbon preservation. Geochim. Cosmochim. Acta 1999, 63, 1723–1734. [Google Scholar] [CrossRef]
- Böning, P.; Brumsack, H.-J.; Böttcher, M.E.; Schnetger, B.; Kriete, C.; Kallmeyer, J.; Borchers, S.L. Geochemistry of Peruvian near-surface sediments. Geochim. Cosmochim. Acta 2004, 68, 4429–4451. [Google Scholar] [CrossRef]
- Borchers, S.; Schnetger, B.; Böning, P.; Brumsack, H.J. Geochemical signatures of the Namibian diatom belt: Perennial upwelling and intermittent anoxia. Geochem. Geophys. Geosystems 2005, 6, 6. [Google Scholar] [CrossRef]
- Zhao, J.; Jin, Z.; Jin, Z.; Geng, Y.; Wen, X.; Yan, C. Applying sedimentary geochemical proxies for paleoenvironment interpretation of organic-rich shale deposition in the Sichuan Basin, China. Int. J. Coal Geol. 2016, 163, 52–71. [Google Scholar] [CrossRef]
- Hardenbol, J.; Thierry, J.; Farley, M.B.; Jacquin, T.; De Graciansky, P.-C.; Vail, P.R. Mesozoic and Cenozoic sequence chronostratigraphic framework of European basins. Soc. Sediment. Geol. 1998, 60, 3–13. [Google Scholar]
- Leckie, R.M.; Olson, H.C. Foraminifera as proxies for sea-level change on siliciclastic margins. SEPM Spec. Publ. 2003, 75, 5–19. [Google Scholar]
- Rowe, H.D.; Loucks, R.G.; Ruppel, S.C.; Rimmer, S.M. Mississippian Barnett Formation, Fort Worth Basin, Texas: Bulk geochemical inferences and Mo–TOC constraints on the severity of hydrographic restriction. Chem. Geol. 2008, 257, 16–25. [Google Scholar] [CrossRef]
- Haq, B.U.; Hardenbol, J.; Vail, P. Chronology of fluctuating sea levels since the Triassic. Science 1987, 235, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- von Zittel, K.A. Beitraege zur Geologie und Palaeontologie der Libyschen Wüste und der Angrenzenden Gebiete von Aegypten; Veralg Theodor Fischer: Cassel, France, 1883; p. 147. [Google Scholar]
- Hume, W.F. The effects of secular oscillation in Egypt during the Cretaceous and Eocene periods. Q. J. Geol. Soc. 1911, 67, 118–148. [Google Scholar] [CrossRef]
- Cuvillier, J. Contribution a ľEtude Géologique du Mokattam. Bull. Inst. ďEgypte 1924, 6, 93–102. [Google Scholar]
- Elewa, A.M. Quantitative analysis and palaeoecology of Eocene Ostracoda and benthonic foraminifera from Gebel Mokattam, Cairo, Egypt. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 211, 309–323. [Google Scholar] [CrossRef]
- Shahin, A.; Bassal, A.; El-Halaby, O.; El-Baz, S. Middle Eocene benthonic foraminiferal biostratigraphy and paleoenvironment at the Qattamia area, northern Eastern Desert, Egypt. Egypt. J. Paleontol. 2007, 7, 29. [Google Scholar]
- Aly, H.; Abd El-Aziz, S.; Abd El-Gaied, I. Middle and Upper Eocene benthic foraminifera from Wadi Bayad El Arab-Gebel Homret Shaibon area, Northeastern Beni Suef, Nile Valley, Egypt. Egypt. J. Paleontol. 2011, 11, 79–131. [Google Scholar]
- Saber, S.G.; Salama, Y.F. Facies analysis and sequence stratigraphy of the Eocene successions, east Beni Suef area, eastern Desert, Egypt. J. Afr. Earth Sci. 2017, 135, 173–185. [Google Scholar] [CrossRef]
- Abd El-Gaied, I.M.; Salama, Y.F.; Saber, S.G.; Sayed, M.M. Benthic foraminiferal communities of the Eocene platform, north Eastern Desert, Egypt. J. Afr. Earth Sci. 2019, 151, 121–135. [Google Scholar] [CrossRef]
- Abd El-Gaied, I.M.; Attia, G.M.; Mahmoud, A.E.-A.A.; Bakr, S.A. Foraminiferal biostratigraphy and paleoenvironment of the middle and Upper Eocene succession at Cairo—Helwan area, north Eastern Desert, Egypt. J. Afr. Earth Sci. 2019, 158, 103516. [Google Scholar] [CrossRef]
- Salama, Y.; Sayed, M.; Saber, S.; Abd El-Gaied, I. Eocene planktonic foraminifera from the north Eastern Desert, Egypt: Biostratigraphic, paleoenvironmental and sequence stratigraphy implications. Palaeontol. Electron. 2021, 24, 1–29. [Google Scholar] [CrossRef]
- Sayed, M.M.; Abd El-Gaied, I.M.; Abdelhady, A.A.; Abd El-Aziz, S.M.; Wagreich, M. Ostracods sensitivity to reconstructing water depths and oxygen levels: A case study from the Middle-Late Eocene of the Beni Suef area (Egypt). Mar. Micropaleontol. 2022, 175, 102155. [Google Scholar] [CrossRef]
- Sayed, M. Foraminiferal Study of the Eocene Successions at Beni Suef—El Zaafarana District, Northeastern Desert, Egypt. M.Sc. Thesis, Beni Suef University, Beni Suef, Egypt, 2019. [Google Scholar]
- Mansour, H.; Philobbos, E. Lithostratigraphic classification of the surface Eocene carbonates of the Nile Valley, Egypt: A review. Bull. Fac. Sci. Assiut Univ. 1983, 12, 129–151. [Google Scholar]
- Sallam, E.; Issawi, B.; Osman, R. Stratigraphy, facies, and depositional environments of the Paleogene sediments in Cairo–Suez district, Egypt. Arab. J. Geosci. 2015, 8, 1939–1964. [Google Scholar] [CrossRef]
- Sallam, E.; Wanas, H.; Osman, R. Stratigraphy, facies analysis and sequence stratigraphy of the Eocene succession in the Shabrawet area (north Eastern Desert, Egypt): An example for a tectonically influenced inner ramp carbonate platform. Arab. J. Geosci. 2015, 8, 10433–10458. [Google Scholar] [CrossRef]
- Tawfik, M.; El-Sorogy, A.S.; Moussa, M. Relationships between sequence stratigraphy and diagenesis of corals and foraminifers in the Middle Eocene, northern Egypt. Turk. J. Earth Sci. 2017, 26, 147–169. [Google Scholar] [CrossRef]
- King, C.; Dupuis, C.; Aubry, M.-P.; Berggren, W.A.; Robert, O.B.K.; Galal, W.F.; Baele, J.-M. Anatomy of a mountain: The Thebes limestone formation (lower Eocene) at Gebel Gurnah, Luxor, Nile valley, upper Egypt. J. Afr. Earth Sci. 2017, 136, 61–108. [Google Scholar] [CrossRef]
- Serra-Kiel, J.; Hottinger, L.; Caus, E.; Drobne, K.; Ferrandez, C.; Jauhri, A.K.; Less, G.; Pavlovec, R.; Pignatti, J.; Samso, J.M. Larger foraminiferal biostratigraphy of the Tethyan Paleocene and Eocene. Bull. Société Géologique Fr. 1998, 169, 281–299. [Google Scholar]
- Pipperr, M.; Reichenbacher, B. Biostratigraphy and paleoecology of benthic foraminifera from the Eggenburgian “Ortenburger Meeressande” of southeastern Germany (Early Miocene, Paratethys).(With 8 figures and 2 tables). Neues Jahrb. Fur Geol. Und Palaontol.-Abh. 2009, 254, 41. [Google Scholar] [CrossRef]
- Dinçer, F. Eocene benthic foraminiferal assemblages from Central Anatolia (Turkey): Biostratigraphy, stable isotope data, paleoenvironmental and paleontological interpretations. J. Afr. Earth Sci. 2016, 114, 143–157. [Google Scholar] [CrossRef]
- Roozpeykar, A.; Moghaddam, I.M. Benthic foraminifera as biostratigraphical and paleoecological indicators: An example from Oligo-Miocene deposits in the SW of Zagros basin, Iran. Geosci. Front. 2016, 7, 125–140. [Google Scholar] [CrossRef]
- Papazzoni, C.A.; Fornaciari, E.; Giusberti, L.; Vescogni, A.; Fornaciari, B. Integrating shallow benthic and calcareous nannofossil zones: The Lower Eocene of the Monte Postale section (northern Italy). Palaios 2017, 32, 6–17. [Google Scholar] [CrossRef]
- Cotton, L.; Zakrevskaya, E.; van der Boon, A.; Asatryan, G.; Hayrapetyan, F.; Israyelyan, A.; Krijgsman, W.; Less, G.; Monechi, S.; Papazzoni, C.; et al. Integrated stratigraphy of the priabonian (upper Eocene) Urtsadzor section. Armen. Newsl. Stratigr. 2017, 50, 269–295. [Google Scholar] [CrossRef]
- Kaiho, K. Benthic foraminiferal dissolved-oxygen index and dissolved-oxygen levels in the modern ocean. Geology 1994, 22, 719–722. [Google Scholar] [CrossRef]
- Jorissen, F.J.; de Stigter, H.C.; Widmark, J.G. A conceptual model explaining benthic foraminiferal microhabitats. Mar. Micropaleontol. 1995, 26, 3–15. [Google Scholar] [CrossRef]
- Kaminski, M.A.; Boersma, E.; Tyszka, J.; Holbourn, A. Response of Deep-Water Agglutinated Foraminifera to Dysoxic Conditions in the California Borderland Basins; Grzbowski Foundation: Kraków, Poland, 1995. [Google Scholar]
- Farahani, T.; Yazdi, M.; Majidifard, M.R. Distribution and paleoecology of the middle Jurassic foraminifera from eastern Alborz (Goznawwi section). Geopersia 2017, 7, 219–236. [Google Scholar]
- Farag, I.; Ismail, M. Contribution to the stratigraphy of the Wadi Hof area (northeast of Helwan). Bull. Fac. Sci. Cairo Univ. 1959, 34, 147–168. [Google Scholar]
- Bishay, Y. Biostratigraphic Study of the Eocene in the Eastern Desert between Samalut and Assiut by the Large Foraminifera; Alexandria: Alexandria, Egypt, 1961. [Google Scholar]
- Bishay, Y. Studies on the Larger Foraminifera of the Eocene of the Nile Valley between Assiut, Cairo and SW Sinai. Ph.D. Thesis, Alexandria University, Alexandria, Egypt, 1966. [Google Scholar]
- Said, R. Planktonic foraminifera from the Thebes formation, Luxor, Egypt. Micropaleontology 1960, 6, 277–286. [Google Scholar] [CrossRef]
- Said, R. The Geology of Egypt; Elsevier: Amsterdam, The Netherlands; New York, NY, USA, 1962; Volume 377. [Google Scholar]
- Boukhary, M.; Abdelmalik, W. Revision of the stratigraphy of the Eocene deposits in Egypt. Neues Jahrb. Für Geol. Und Paläontologie-Mon. 1983, 6, 321–337. [Google Scholar] [CrossRef]
- Strougo, A.; Boukhary, M. The Middle Eocene-Upper Eocene boundary in Egypt: Present state of the problem. Rev. Micropaléontologie 1987, 30, 122–127. [Google Scholar]
- Elewa, A.M.T.; Omar, A.A.; Dakrory, A.M. Biostratigraphical and paleoenvironmental studies on some Eocene ostracodes and forminifers from the Fayum depression, western desert, Egypt. Egypt. J. Geol. 1998, 42, 439–469. [Google Scholar]
- Elewa, A.M.T. Paleoecology and paleogeography of Eocene ostracod faunas from the Nile Valley between Minia and Maghagha, Upper Egypt. In Migration of Organisms; Springer: Berlin/Heidelberg, Germany, 2005; pp. 25–69. [Google Scholar]
- Said, R. Explanatory Notes to Accompany the Geological Map of Egypt. 1:2.000.000; The Geological Survey of Egypt: Cairo, Egypt, 1971; Volume 56, p. 123. [Google Scholar]
- Said, R. The Geology of Egypt; Belkema: Rotterdam, The Netherlands; Berlin, Germany, 1990; Volume 734. [Google Scholar]
- Omara, S.; Mansour, H.; Youssef, M.; Khalifa, H. Stratigraphy, paleoenvironment and structural features of the area east of Beni Mazar, Upper Egypt. Bull. Fac. Sci. Assiut Univ. 1977, 6, 171–197. [Google Scholar]
- Boukhary; Abdulla, A.Y. Stratigraphy and petrology of the Eocene rocks at Maghagha (east and west), Nile Valley, Egypt. Qatar Univ. Sci. 1985, 5, 357–365. [Google Scholar]
- Kenawy, A.; Bassiouni, M.; Khalifa, H.; Aref, M. Stratigraphy of the Eocene outcrops between Assiut and Beni Suef, Nile Valley, Egypt. Bull. Fac. Sci. Assiut. Univ. 1988, 17, 161–193. [Google Scholar]
- Moulina, B. La bordure occidentale du Gebel Qarara (Moyenne-Égypte): Éléments de géologie pour la compréhension du relief et de l’histoire de l’occupation humaine. Physio-Géo. Géographie Phys. Environ. 2011, 5, 11–44. [Google Scholar] [CrossRef]
- Boukhary, M.; Hussein, A.I.; Al-Sayigh, A.R. Lineage of Arxina schwageri (Silvestri, 1928) new genus (Nummulitacea) from Middle Eocene of Egypt and Sultanate of Oman. Hist. Biol. 2012, 24, 547–556. [Google Scholar] [CrossRef]
- Hegab, O.A.; Serry, M.A.; Anan, T.I.; Abd El-Wahed, A.G. Facies analysis, glauconite distribution and sequence stratigraphy of the middle Eocene Qarara Formation, El-Minya area, Egypt. Egypt. J. Basic Appl. Sci. 2016, 3, 71–84. [Google Scholar] [CrossRef]
- Loeblich, A.; Tappan, H. Foraminiferal Genera and Their Classification; Van Nosrand Reinhold Co.: New York, NY, USA, 1988; Volume 2, p. 970. [Google Scholar]
- Fatela, F.; Taborda, R. Con¢dence limits of species proportions in microfossil assemblages. Mar. Micropaleontol. 2002, 45, 169–174. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Murray, J. Distribution and Ecology of Living Benthic Foraminiferids; Crane and Russak: New York, NY, USA, 1973; p. 274. [Google Scholar]
- Kaiho, K. Global changes of Paleogene aerobic/anaerobic benthic foraminifera and deep-sea circulation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1991, 83, 65–85. [Google Scholar] [CrossRef]
- Le Calvez, Y. Revision des foraminiferes Lutetiens du Bassin de Paris. II. Rotaliidae et familles affines. Mem. Serv. Carte Geol. Detaill. Fr. 1949, 1–54. Available online: https://www.marinespecies.org/aphia.php?p=sourcedetails&id=259269 (accessed on 24 February 2023).
- Kaasschieter, J.P.H. Foraminifera of the Eocene of Belgium; Institut Royal des Sciences Naturelles de Belgique: Mémoires: Brussels, Belgium, 1961; Volume 147, pp. 1–271. [Google Scholar]
- Barbin, V.; Keller-Grünig, A. Benthic foraminiferal assemblages from the Brendola section (Priabonian stage stratotype area, northern Italy): Distribution, palaeoenvironment and palaeoecology. Mar. Micropaleontol. 1991, 17, 237–254. [Google Scholar] [CrossRef]
- Ortiz, S.; Thomas, E. Lower-middle Eocene benthic foraminifera from the Fortuna section (Betic Cordillera, southeastern Spain). Micropaleontology 2006, 52, 97–150. [Google Scholar] [CrossRef]
- Abd El-Gaied, I.M.; Abd El-Aziz, S.M. Stratigraphy and paleoenvironment of the Lower-Middle Eocene succession in the Darnah area, northeast Libya. J. Afr. Earth Sci. 2020, 169, 103774. [Google Scholar] [CrossRef]
- El Baz, S.M. Middle Eocene benthic foraminifera from Qattamiya area, Cairo–Suez district, Egypt: Biostratigraphy, paleoecology, and their relation to the Southern and Western Tethyan Provinces. Arab. J. Geosci. 2022, 15, 749. [Google Scholar] [CrossRef]
- Sami, M.; Mahdy, N.M.; Ntaflos, T.; Fathy, D. Composition and origin of Ti–Nb–Ta–Zr bearing minerals in the Abu Diab highly evolved granite from the Central Eastern Desert of Egypt. J. Afr. Earth Sci. 2020, 165, 103808. [Google Scholar] [CrossRef]
- Helal, S. Contribution to the Eocene benthic foraminifera and ostracoda of the Fayoum Depression, Egypt. Egypt. J. Paleontol. 2002, 2, 105–155. [Google Scholar]
- Abd El-Azeam, S. Stratigraphy and paleoenvironments of the Eocene rocks at wadi El hitan area, Fayoum depression, Egypt. Egypt. J. Paleontol. 2008, 8, 49–62. [Google Scholar]
- Mansour, H.; Philobbos, E.; Abdu, F. Contribution to the geology of the east and northeast of Beni Suef Nile Valley Egypt. Qatar Univ. Sci. 1982, 11, 52–65. [Google Scholar]
- El Dawy, M. Middle Eocene benthic foraminiferal biostratigraphy and paleoecology of east Beni Mazar area, Nile Valley, Egypt. Egypt. J. Geol. 1997, 41, 413–464. [Google Scholar]
- Abd El-Aziz, S. Stratigraphy of Eocene Sequences of Fayum—Minia District (New Road), Egypt. Ph.D. Thesis, Cairo University, Fayum branch, Egypt, 2002. [Google Scholar]
- El-Dawy, M.; Dakrory, A. Biostratigraphy and paleoecology of the middle Eocene benthic foraminifers of east Beni Suef area, Nile Valley, Egypt. In Proceedings of the 4th International Conference on the Geology of Africa, Assiut, Egypt, 15–16 November 2005; pp. 623–656. [Google Scholar]
- Boukhary, M.; Hussein, A.I.; El-Morcey, I.A. Eocene larger foraminifera from Helwan, greater Cairo, Egypt. Rev. Micropaléontologie 2002, 45, 27–45. [Google Scholar] [CrossRef]
- Somayajulu, B.; Yadav, D.; Sarin, M. Recent sedimentary records from the Arabian Sea. Proc. Indian Acad. Sci.-Earth Planet. Sci. 1994, 103, 315–327. [Google Scholar] [CrossRef]
- Madhavaraju, J.; Ramasamy, S. Rare earth elements in limestones of Kallankurichchi formation of Ariyalur group, Tiruchirapalli Cretaceous, Tamil Nadu. Geol. Soc. India 1999, 54, 291–301. [Google Scholar]
- Armstrong-Altrin, J.; Machain-Castillo, M.; Rosales-Hos, L.; Carranza-Edwards, A.; Sanchez-Cabeza, J.; Ruiz-Ferdinandez, A.C. Provenance and depositional history of continental slope sediments in the Southwestern Gulf of Mexico unraveled by geochemical analysis. Cont. Shelf Res. 2015, 95, 15–26. [Google Scholar] [CrossRef]
- Ramos-Vázquez, M.A.; Armstrong-Altrin, J.S.; Rosales-Hoz, L.; Machain-Castillo, M.L.; Carranza-Edwards, A. Geochemistry of deep-sea sediments in two cores retrieved at the mouth of the Coatzacoalcos River delta, western Gulf of Mexico, Mexico. Arab. J. Geosci. 2017, 10, 148. [Google Scholar] [CrossRef]
- Hallberg, R. A geochemical method for investigation of palaeoredox conditions in sediments. Ambio Spec. Rep. 1976, 4, 139–147. [Google Scholar]
- Dypvik, H. Geochemical compositions and depositional conditions of Upper Jurassic and Lower Cretaceous Yorkshire clays, England. Geol. Mag. 1984, 121, 489–504. [Google Scholar] [CrossRef]
- Dill, H. Metallogenesis of early Paleozoic graptolite shales from the Graefenthal Horst (northern Bavaria-Federal Republic of Germany). Econ. Geol. 1986, 81, 889–903. [Google Scholar] [CrossRef]
- Jones, B.; Manning, D.A. Comparison of geochemical indices used for the interpretation of palaeoredox conditions in ancient mudstones. Chem. Geol. 1994, 111, 111–129. [Google Scholar] [CrossRef]
- Rimmer, S.M. Geochemical paleoredox indicators in Devonian–Mississippian black shales, central Appalachian Basin (USA). Chem. Geol. 2004, 206, 373–391. [Google Scholar] [CrossRef]
- Nagarajan, R.; Madhavaraju, J.; Nagendra, R.; Armstrong-Altrin, J.S.; Moutte, J. Geochemistry of Neoproterozoic shales of the Rabanpalli Formation, Bhima Basin, Northern Karnataka, southern India: Implications for provenance and paleoredox conditions. Rev. Mex. Cienc. Geológicas 2007, 24, 150–160. [Google Scholar]
- Mir, A.R. Rare earth element geochemistry of Post-to Neo-archean shales from Singhbhum mobile belt, Eastern India: Implications for tectonic setting and paleo-oxidation conditions. Chin. J. Geochem. 2015, 34, 401–409. [Google Scholar] [CrossRef]
- Xiong, X.; Xiao, J. Geochemical indicators of sedimentary environments-a summary. Earth Environ. 2011, 39, 405–414. [Google Scholar]
- Gogoi, M.; Mathur, N.; Kumar, T.S.; Walling, T.; Phukan, S. Geochemical characterization of shales of the Eocene Disang Group, Kohima Syncline, India: Inferences to hydrocarbon potential and depositional environment. Pet. Res. 2021, 6, 42–56. [Google Scholar] [CrossRef]
- Lewan, M.; Maynard, J. Factors controlling enrichment of vanadium and nickel in the bitumen of organic sedimentary rocks. Geochim. Cosmochim. Acta 1982, 46, 2547–2560. [Google Scholar] [CrossRef]
- Galarraga, F.; Reategui, K.; Martïnez, A.; Martínez, M.; Llamas, J.; Márquez, G. V/Ni ratio as a parameter in palaeoenvironmental characterisation of nonmature medium-crude oils from several Latin American basins. J. Pet. Sci. Eng. 2008, 61, 9–14. [Google Scholar] [CrossRef]
- Bechtel, A.; Gratzer, R.; Sachsenhofer, R. Chemical characteristics of Upper Cretaceous (Turonian) jet of the gosau group of gams/hieflau (styria, Austria). Int. J. Coal Geol. 2001, 46, 27–49. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Z.; Liu, J.; Xu, N.; Li, H. The selectively extractive Sr/Ba ratio and discrimination between marine and terrestrial sedimentary environments in terrigenous clastic sediments. Acta Geol. Sin. (Engl. Ed.) 2019, 93, 412–413. [Google Scholar]
- Qiu, X.; Liu, C.; Mao, G.; Deng, Y.; Wang, F.; Wang, J. Major, trace and platinum-group element geochemistry of the Upper Triassic nonmarine hot shales in the Ordos basin, Central China. Appl. Geochem. 2015, 53, 42–52. [Google Scholar] [CrossRef]
- Deng, H.W.; Qian, K. Sedimentary Geochemistry and Environment Analysis; Gansu Technology Publishing House: Lanzhou, China, 1993; pp. 1–150. [Google Scholar]
- Xu, J.; Liu, Z.; Bechtel, A.; Meng, Q.; Sun, P.; Jia, J.; Cheng, L.; Song, Y. Basin evolution and oil shale deposition during Upper Cretaceous in the Songliao Basin (NE China): Implications from sequence stratigraphy and geochemistry. Int. J. Coal Geol. 2015, 149, 9–23. [Google Scholar] [CrossRef]
- Wilson, J. Carbonate Facies in Geologic History; Springer: New York, NY, USA, 1975; p. 471. [Google Scholar]
- Rathburn, A.E.; Corliss, B.H. The ecology of living (stained) deep-sea benthic foraminifera from the Sulu Sea. Paleoceanography 1994, 9, 87–150. [Google Scholar] [CrossRef]
- Farouk, S.; Jain, S.; Belal, N.; Omran, M.; Al-Kahtany, K. Quantitative Middle Eocene benthic foraminiferal biofacies from west-central Sinai, Egypt: Implications to paleobathymetry and sequence stratigraphy. Mar. Micropaleontol. 2020, 155, 101823. [Google Scholar] [CrossRef]
- Sayed, D.M.; El-Shazly, S.H.; Salama, Y.F.; Badawy, H.S.; Abd El-Gaied, I.M. Macropaleontological and paleobiogeographical study of the Upper Eocene succession at Beni-Suef-Zaafrana road, north Eastern Desert, Egypt. J. Afr. Earth Sci. 2021, 174, 104046. [Google Scholar] [CrossRef]
- Murray, J.W.; Curry, D.; Haynes, J.; King, C. Paleogene. In Stratigraphical Atlas of Fossil Foraminifera, 2nd ed.; Jenkins, D.G., Murray, J.W., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1981; Volume 10, pp. 490–536. [Google Scholar]
- Aref, M.; Madkour, H. Recent benthic foraminifera of the Egyptian Red Sea coast, their taxonomy, ecology and cooperative studies with that of the Eastern Mediterranean Sea. J. Geol. 2000, 44, 257–286. [Google Scholar]
- Zohdi, A.; Mousavi-Harami, R.; Moallemi, S.A.; Mahboubi, A.; Immenhauser, A. Evolution, paleoecology and sequence architecture of an Eocene carbonate ramp, southeast Zagros Basin, Iran. GeoArabia 2013, 18, 49–80. [Google Scholar] [CrossRef]
- Oladimeji, A.; Adekeye, O.A.; Adeyinka, S.A.; Olatinpo, O.A.; Faseki, O.E. Foraminifera Biostratigraphy and Depositional Environment of Sediments in SILE Well, Offshore Dahomey Basin. MAYFEB J. Environ. Sci. 2017, 1, 18–33. [Google Scholar]
- Flügel, E. Microfacies of Carbonate Rocks: Analysis, Interpretation and Application; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2004; Volume 976. [Google Scholar]
- Bindiu-Haitonic, R.; Bălc, R.; Kövecsi, S.-A.; Pleș, G.; Silye, L. In the shadow of giants: Calcareous nannoplankton and smaller benthic foraminifera from an Eocene nummulitic accumulation (Transylvanian Basin, Romania). Mar. Micropaleontol. 2021, 165, 101988. [Google Scholar] [CrossRef]
- Boersema, A. Foraminifera. In Introduction to Marine Micropaleontology; Haq, B.U., Boersma, A., Eds.; Elsevier: New York, NY, USA, 1980; pp. 19–77. [Google Scholar]
- Jain, S.; Abdelhady, A.A.; Alhussein, M. Responses of benthic foraminifera to environmental variability: A case from the Middle Jurassic of the Kachchh Basin (Western India). Mar. Micropaleontol. 2019, 151, 101749. [Google Scholar] [CrossRef]
- Lutze, G.; Coulbourn, W. Recent benthic foraminifera from the continental margin of northwest Africa: Community structure and distribution. Mar. Micropaleontol. 1984, 8, 361–401. [Google Scholar] [CrossRef]
- Sen Gupta, B.K.; Machain-Castillo, M.L. Benthic foraminifera in oxygen-poor habitats. Mar. Micropaleontol. 1993, 20, 183–201. [Google Scholar] [CrossRef]
- Den Dulk, M.; Reichart, G.-J.; Van Heyst, S.; Zachariasse, W.; Van der Zwaan, G. Benthic foraminifera as proxies of organic matter flux and bottom water oxygenation? A case history from the northern Arabian Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 161, 337–359. [Google Scholar] [CrossRef]
- Živkovic, S.; Glumac, B. Paleoenvironmental reconstruction of the Middle Eocene Trieste-Pazin basin (Croatia) from benthic foraminiferal assemblages. Micropaleontology 2007, 53, 285–310. [Google Scholar] [CrossRef]
- Bohaty, S.M.; Zachos, J.C.; Florindo, F.; Delaney, M.L. Coupled greenhouse warming and deep-sea acidification in the middle Eocene. Paleoceanography 2009, 24, PA2207. [Google Scholar] [CrossRef]
- Huyghe, D.; Castelltort, S.; Mouthereau, F.; Emmanuel, L.; Serra-Kiel, J.; Renard, M. Disappearance of a carbonate ramp at the Lutetian-Bartonian boundary in the Pyrenees (Spain): Evidences for the first glaciations of the Cenozoic? In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 19–24 April 2009; p. 5411. [Google Scholar]
- D’Onofrio, R.; Zaky, A.S.; Frontalini, F.; Luciani, V.; Catanzariti, R.; Francescangeli, F.; Giorgioni, M.; Coccioni, R.; Özcan, E.; Jovane, L. Impact of the Middle Eocene Climatic Optimum (MECO) on Foraminiferal and Calcareous Nannofossil Assemblages in the Neo-Tethyan Baskil Section (Eastern Turkey): Paleoenvironmental and Paleoclimatic Reconstructions. Appl. Sci. 2021, 11, 11339. [Google Scholar] [CrossRef]



| Age/Locality | Fayoum Area | Nile Valley | North Eastern Desert | Helwan Area | Present Study | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Elewa et al. [46] | Helal [68] | Abd EL Azeam [69] | Mansour et al. [70] | El Dawy [71] | Abd El-Aziz [72] | El Dawy and Dakrory [73] | Aly et al. [17] | Abd El-Gaied et al. [19] | Abd El-Gaied et al. [20] | ||
| late Middle Eocene (Bartonian) | Lenticulina alatolimbata | Bulimina jacksonensis/Uvigerina jacksonensis | Lenticulina cosata | Nummulites beaumonti | Nonion scaphum/ Pararotalia audouini | Palmula ansaryi | Bolivina jacksonensis/ Pararotalia audouini | Palmula ansaryi | Palmula ansaryi | Nonion scaphum | Brizalina cookei/ Nonionella insecta |
| Brizalina cookei | Nonion scaphum | ||||||||||
| Brizalina cookei | Brizalina cookei | ||||||||||
| Cibicidoides truncanus | Orbitolites complanatus | Uvigerina coaensis/ Uvigerina continusa | Nonionella africana | Cibicides mabahethi/Cancris a. primitivus | |||||||
| Middle Eocene (Lutetian) | Uvigerina nakadyi/Anomalinoides fayoumensis | Haplophragmoides emaclatus/ Ammobaculites cubensis | Anomalinoides fayoumensis | Quinqueloculina seminula | Bolivina carinata | ||||||
| Bulimina jacksonensis/Uvigerina jacksonensis | Nummulites gizehensis | ||||||||||
| Sample No. | Weight (g) | Organic Carbon TOC (%) | Inorganic Carbon % | Total Carbon % |
|---|---|---|---|---|
| 0 Q | 0.1000 | 0.3800 | 0.5098 | 0.8898 |
| 1Q | 0.1002 | 0.3724 | 0.5267 | 0.8991 |
| 2Q | 0.1001 | 0.2179 | 0.1101 | 0.3281 |
| 3Q | 0.1001 | 0.1969 | 0.1821 | 0.3789 |
| 4Q | 0.1000 | 0.1407 | 0.1599 | 0.3007 |
| 5Q | 0.1000 | 0.3625 | 3.6795 | 4.0420 |
| Species/Country | Egypt | Libya | Italy | France | Spain | England | Belgium |
|---|---|---|---|---|---|---|---|
| Bolivina anglica | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Bolivina carinata | 1 | 1 | 0 | 1 | 1 | 0 | 1 |
| Bolivina jacksonensis | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Brizalina cookei | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Bulimina jacksonensis | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cancris auriculus primitivus | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| Cancris subconicus | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| Cibicides mabahethi | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Cibicidoides laurisae | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Fursenkoina dibolensis | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| Globulina gibba | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| Halkyardia minima | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Laevidentalina soluta | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Lagena apiculata | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Lagena hexagona | 1 | 0 | 1 | 1 | 0 | 1 | 1 |
| Lagena laevis | 1 | 0 | 0 | 1 | 0 | 1 | 1 |
| Lagena striata | 1 | 0 | 1 | 1 | 0 | 0 | 1 |
| Lagena sulcata | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Lenticulina alatolimbata | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Lenticulina clericii | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Lenticulina cultrata | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Lenticulina isidis | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Lenticulina limbata | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Lenticulina williamsoni | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Lenticulina yaguatensis | 1 | 1 | 0 | 0 | 1 | 0 | 1 |
| Lobatula lobatula | 1 | 0 | 1 | 1 | 1 | 0 | 1 |
| Neoeponides schreibersi | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Planulina cocoaensis | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Quinqueloculina seminula | 1 | 0 | 1 | 1 | 0 | 1 | 1 |
| Saracenaria triangularis | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Spiroplectammina adamsi | 1 | 0 | 1 | 0 | 0 | 0 | 1 |
| Spiroplectinella carinata | 1 | 1 | 1 | 0 | 1 | 0 | 1 |
| Textularia adalta | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Textularia adamsi | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Textularia agglutinans | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Uvigerina cocoaensis | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
| Uvigerina cookei | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| Uvigerina hispida | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Uvigerina mexicana | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| Uvigerina rippensis | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| Uvigerina seiata | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayed, M.M.; Heinz, P.; Abd El-Gaied, I.M.; Wagreich, M. Paleoclimate and Paleoenvironment Reconstructions from Middle Eocene Successions at Beni-Suef, Egypt: Foraminiferal Assemblages and Geochemical Approaches. Diversity 2023, 15, 695. https://doi.org/10.3390/d15060695
Sayed MM, Heinz P, Abd El-Gaied IM, Wagreich M. Paleoclimate and Paleoenvironment Reconstructions from Middle Eocene Successions at Beni-Suef, Egypt: Foraminiferal Assemblages and Geochemical Approaches. Diversity. 2023; 15(6):695. https://doi.org/10.3390/d15060695
Chicago/Turabian StyleSayed, Mostafa Mohamed, Petra Heinz, Ibrahim Mohamed Abd El-Gaied, and Michael Wagreich. 2023. "Paleoclimate and Paleoenvironment Reconstructions from Middle Eocene Successions at Beni-Suef, Egypt: Foraminiferal Assemblages and Geochemical Approaches" Diversity 15, no. 6: 695. https://doi.org/10.3390/d15060695
APA StyleSayed, M. M., Heinz, P., Abd El-Gaied, I. M., & Wagreich, M. (2023). Paleoclimate and Paleoenvironment Reconstructions from Middle Eocene Successions at Beni-Suef, Egypt: Foraminiferal Assemblages and Geochemical Approaches. Diversity, 15(6), 695. https://doi.org/10.3390/d15060695







