Abstract
We used a combination of morphological, molecular and biological data to characterize a novel Henneguya (Myxozoa) species infecting the Amazonian prochilodontid Semaprochilodus insignis or “kissing prochilodus”, a popular food fish and aquarium species in the Brazilian Amazon. Twenty-one S. insignis were caught live from the Tapajós river, Pará State, Brazil, then examined for myxozoan infections. Cysts of a novel Henneguya species were observed in the connective tissue of the fins. Myxospores measured 48 ± 4.9 (39.5–60.8) µm total length, of which caudal appendages were 33 ± 4.5 (26.4–45.2) µm and spore body was 15 ± 1.6 (12.4–20.5) µm. The spore body was 4.0 ± 0.6 (2.7–5.3) µm wide × 3.2 ± 0.4 (2.7–3.6) µm thick, with two unequal polar capsules (nematocysts) 7.2 ±0.8 (5.2–8.3) × 1.5 ± 0.3 (1.0–2.2) µm for the larger capsule and 5 ± 0.7 (4.0–6.3) × 1.4 ± 0.2 (1.0–1.8) µm for the smaller capsule. Polar tubules had 8–13 turns. Generative cells, immature and mature myxospores were observed within plasmodia. Ultrastructure showed plasmodia surrounded by collagen fibers, with the plasmodial membrane having pinocytotic channels. Phylogenetic analysis of small subunit ribosomal DNA sequences showed that the new Henneguya species clustered as a sister taxon to Henneguya tietensis, a parasite of the gills of the prochilodontid fish Prochilodus lineatus, from the geographically distant Paraná–Paraguai River basin.
1. Introduction
The Tapajós River is one of the largest tributaries in the Brazilian Amazon, and its drainage has an enormous fish diversity encompassing more than 490 species, of which about 17% are endemic [1]. The Tapajós River drainage is ~489,000 km2, being the fifth largest tributary of the Amazon basin [1]. Among the fish diversity in the Tapajós basin, Prochilodontidae are detritivorous freshwater fish popularly known as curimbatá and jaraqui in Portuguese, bocachicos in Spanish and flannel-mouth characiforms in English [2]. This fish family harbors three genera: Ichthyoelephas Posada, 1909, distributed from the rivers of Andes in Colombia and Ecuador; Prochilodus Agassiz in Spix and Agassiz, 1829, distributed along all major South American rivers on both sides of the Andes; and the Semaprochilodus Fowler, 1941, broadly distributed east of the Andes through the Amazon, Tocantins and Orinoco basins and some coastal rivers draining the Guiana shield [3]. Semaprochilodus insignis (Jardine, 1841) along Semaprochilodus taeniurus (Valenciennes, 1821) are important protein sources for people in the Central Amazon [4].
Myxosporeans are a diverse group of cnidarian endoparasites of aquatic animals (mostly) with more than 2600 described species [5]. The genus Henneguya Thélohan, 1892 harbors 254 species with worldwide distribution. They infect freshwater and marine fishes, and some species can cause important diseases in their host fish [6,7,8,9]. In neotropical, freshwater habitats of South America, species of Myxobolidae are the most commonly encountered and described myxozoans [8,9,10,11,12].
Three myxozoans are known to infect S. insignis: Myxobolus insignis Eiras, Malta, Varella and Pavanelli, 2005; Myxobolus maiai Müller, Naldoni, Corrêa and Adriano, 2022; and Myxobolus iarakiensis Müller, Naldoni, Corrêa and Adriano, 2022, all of which sporulate in gills [13,14]. Herein, we characterize a novel Henneguya species, which sporulates in plasmodia in the fins of S. insignis. We describe the parasite using morphological and ultrastructural characters, and small subunit ribosomal DNA (ssrDNA) sequence and phylogenetic analysis.
2. Material and Methods
2.1. Sampling
Twenty-one S. insignis were sampled from the Tapajós River, in the municipality of Santarém, in the state of Pará, Brazil, in October 2021. The fish were caught using seine nets, and sampling was authorized by the Brazilian Ministry of the Environment (SISBIO # 66053-1 and SisGen (Como, Italy) # A656D8E). Fish were transported alive to a field laboratory on the shore of the river. The methodology was approved by the Ethics Research Committee of the Federal University of São Paulo (CEUA # 6549290920), in accordance with Brazilian law (Federal Law No. 11794, 8 October 2008). Fish were killed, measured and necropsied, and the organs were examined in fresh mounts under stereo- and compound microscopes. Tissue fragments up to 10 mm containing plasmodia were fixed in 10% neutral-buffered formalin for morphological and histological analysis [15], buffered 2.5% glutaraldehyde for ultrastructural analysis and in 100% ethanol for DNA analysis.
2.2. Morphological and Ultrastructural Analysis
For morphological analysis, 30 fixed mature myxospores were measured using a Carl Zeiss Axio Imager A2 light microscope equipped with an Axio Cam, and AxioVision AxioVs 40V4.8.2 software, following the guidelines of Lom and Arthur [15] and Sellyei et al. [16]. All measurements are expressed in μm as mean ± standard deviation (SD) and range in parentheses. For ultrastructural analysis, tissue fragments containing plasmodia were fixed in 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer (pH 7.4) for at least 12 h, then washed in the same buffer, post-fixed in OsO4 for 1 h, and dehydrated in an acetone series. All of these processes were performed at 4 °C. The material was then embedded in EMbed 812 resin (Sigma-Aldrich, St. Louis, MO, USA). Semi-thin sections were stained with toluidine blue solution and examined using light microscopy. Ultrathin sections were double-stained with uranyl acetate and lead citrate, and examined in a LEO 906 electron microscope at 60 kV in the Electron Microscopy Laboratory of the University of Campinas (UNICAMP), São Paulo, Brazil.
2.3. DNA Extraction, PCR and Sequencing
Total genomic DNA was isolated using the DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions, and adjusted to a final volume of 50 µL. For PCR, 10–50 ng of genomic DNA, 12.5 μL of 2× Dream Taq Green PCR Master Mix (Thermo Scientific—Carlsbad, CA, USA), 0.2 μM of each specific primer, and nuclease-free water (Thermo Scientific—Carlsbad, CA, USA) were used in a final reaction volume of 25 μL. ssrDNA was amplified, using the primer pairs and conditions described by Capodifoglio et al. [17], in which ssrDNA was amplified in two parts: ~1000 bp using ERIB1 [18] and ACT1r [19]; and ~1200 bp using primers TEDf [17] and ERIB10 [18]. Identity of the fish host was confirmed by PCR and sequencing of the mitochondrial COI gene using published methods [20].
PCR products were run on a 1.5% agarose gel with TBE buffer (0.045 M Tris-borate, 0.001 M EDTA pH 8.0), stained with Sybr Safe DNA gel stain (Thermo Scientific—Carlsbad, CA, USA) and analyzed in a MiniBis Pro transilluminator. The sizes of the amplified fragments were estimated by comparison with 1 kb Plus DNA Ladder (Thermo Scientific—Carlsbad, CA, USA). Amplicons were purified using the QIAquick PCR Purification kit (Qiagen) and sequenced in both directions with PCR primers using the BigDye v.3.1 Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, CA, USA). Sequences were read with a Life Technologies—Applied Biosystems ABI 3730 DNA genetic analyzer, and were assembled and edited to obtain a single consensus sequence for each specimen using Sequencer v.5.2.4 (Gene Codes, Ann Arbor, MI, USA), then submitted to GenBank.
2.4. Sequence Alignment and Phylogenetic Analyses
Alignment was carried out using ssrDNA sequences of myxozoans (Myxobolus/Henneguya/Thelohanellus sequences) that were at least 80% similar to the novel Henneguya sequence, plus myxobolid parasites of South American Prochilodontidae. The outgroups chosen were Myxidium peruviensis Espinoza, Mertins, Gama, Pata and Mathews, 2014 (KY996746) and Myxidium turturibus Aguiar, Adriano and Mathews, 2017 (KX611144). Sequences were aligned using the default parameters of the Muscle algorithm (Edgar, 2004) performed in Geneious 7.1.3 [21]. To evaluate substitution saturation, the aligned matrix was tested and the Iss index estimated using DAMBE 5 [22].
The best-fit model of nucleotide evolution in the resulting matrixes was determined to be GTR + G + I using the Akaike information criterion in jModelTest [23]. Phylogenetic analyses were performed using Bayesian Inference (BI) and Maximum Likelihood inference (ML) using resources available in the CIPRES Science Gateway [24]. Bayesian analysis employed the following nucleotide substitution model settings for both datasets: lset nst = 6, rates = invariable, ncat = 4, shape = estimate, inferrates = yes and basefreq = empirical. For the Markov chain Monte Carlo (MCMC) search, chains were run with 10,000,000 generations, saving one tree every 1500 generations. The first 25% of generations were discarded as burn-in, and the consensus tree (majority rule) was estimated using the remaining topologies; only nodes with posterior probabilities >90% were considered well supported. Maximum Likelihood inference (ML) was implemented using RAxML [25], with bootstrap support values of 1000 repetitions, and only nodes with bootstrap values > 70% were considered well supported. The trees were visualized using FigTree v.1.3.1 [26].
3. Results
Of the 21 S. insignis adult specimens examined (average length 20.4 ± 1.8 (16–23) cm), 9 (43%) had plasmodia of a Henneguya species in the fins; this parasite is described herein. We also observed plasmodia and myxospores of a Myxobolus species (six fish were infected; 28%) in the fins; however, insufficient material was available to make a formal description at this time.
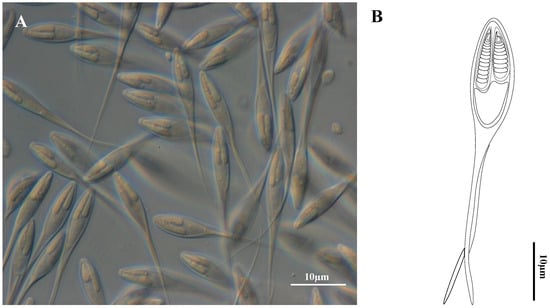
Figure 1.
Henneguya correai n. sp. parasite of the fins of Semaprochilodus insignis. (A) Photomicrograph of mature myxospores (frontal view) under Differential Interference Contrast. (B) Schematic drawing of a myxospore in frontal view.
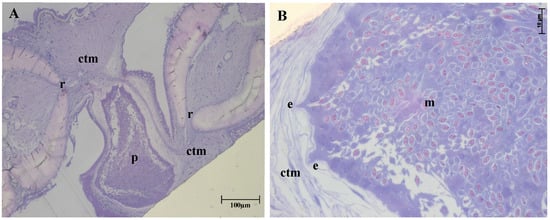
Figure 2.
Histological thin section of fin of Semaprochilodus insignis with plasmodium of Henneguya correai n. sp. (A): Parasite plasmodium (p) in host connective tissue (ctm) between the rays (r). (B): Enlarged portion of A showing host–parasite boundary plasmodial expansions (e) toward the layered host connective tissue (ctm) and maturing myxospores within (m). Toluidine blue stain.
Formalin-fixed, mature myxospores (n = 30) in frontal view measured 47.9 ± 4.9 (39.5–60.8) µm total length, 14.4 ± 1.6 (12.4–20.5) µm spore body length, 4.0 ± 0.6 (2.7–5.3) µm wide and 33.7 ± 4.5 (26.4–45.2) caudal appendages length. In lateral view, the valves were asymmetrical and myxospores were 3.1 ± 0.4 (2.7–3.6) (n = 4) µm thick. Polar capsules (nematocysts) were unequal: the larger was 7.2 ± 0.8 (5.2–8.3) µm long and 1.5 ± 0.3 (1.0–2.2) µm wide and contained a polar tubule with 11–13 turns; the smaller capsule was 5.6 ± 0.7 (4.0–6.3) µm long, 1.4 ± 0.2 (1.0–1.8) µm wide and contained a tubule with 8–9 turns. (Table 1, Figure 1A,B).
Host type: Semaprochilodus insignis (Jardine, 1841), Characiformes: Prochilodontidae (GenBank accession number OK413187, Müller et al. [14]).
Locality: Tapajós River, municipality of Santarém, Pará state, Brazil (2°26′22.53″ S; 54°53′34.72″ W).
Type material: Syntypes-air-dried, stained with Giemsa solution and mounted in medium on permanent slides (accession number ZUEC MYX 116).
Prevalence: 9/21 (43%).
Site of Infection: Connective tissue in fins.
Representative ssrDNA sequence: GenBank accession number OR036989.
Etymology: The specific epithet is in honor of Prof. Dr. Lincoln Lima Corrêa for his important support and contribution to myxozoan parasite research in the Amazon.
Histology of semi-thin sections (Figure 2) showed parasite plasmodia developing in the connective tissue of fins, between host fin rays. The plasmodia had pseudopodia extending into layered host tissue (Figure 2B) without compromising the adjacent epithelial layer. Ultrastructural analysis revealed that plasmodia of H. correai n. sp. had numerous pinocytotic channels in the plasmodial membrane linking the outside to the ectoplasm zone of the plasmodium, while generative cells, immature and mature myxospores were observed deeper in the plasmodium (Figure 3A–C).
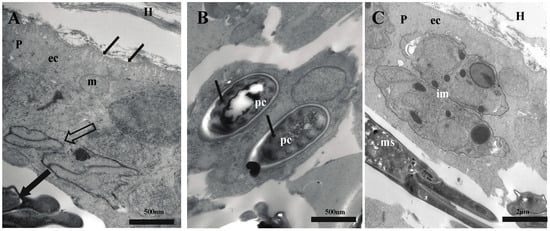
Figure 3.
Electron micrographs of sections of Henneguya correai n. sp. in fins of Semaprochilodus insignis. (A) Host–parasite interface showing the plasmodium (p) developing in host (H) connective tissue; large empty arrow indicates immature myxospores, and large black arrow shows mature myxospore. The parasite ectoplasm (ec) has pinocytic channels, thin black arrows, with a mitochondrion (m); (B) mature myxospore with polar capsules (pc) and its coiled polar tubule (thin black arrows); (C) plasmodium showing longitudinal sections of immature myxospores (im) in the periphery and mature myxospores (ms) within.
Sequencing of H. correai n. sp. ssrDNA yielded 1763 bp, and the BLASTn search of the NCBI database showed the closest sequence was Henneguya tietensis Vieira, Rangel, Tagliavini, Abdallah, Santos and Azevedo, 2020 (MN653131) with 88.2% similarity [12]. ML and BI phylogenetic analysis based on 46 taxa over 950 bp revealed similar topologies of South American myxobolids, recovering two primary clades well supported by posterior probability (PP) and bootstrap (B) (Figure 4). In the clade A, which comprised Myxobolus and Henneguya species from several fish families, the new species appears as a sister species of H. tietensis. Clade B comprises Myxobolus species parasites from different fish families and Thelohanellus marginatus Rocha, Casal, Velasco, Alves, Matos, Al-Quraishy and Azevedo, 2014, a parasite of a pimelodid (Figure 4) [27].
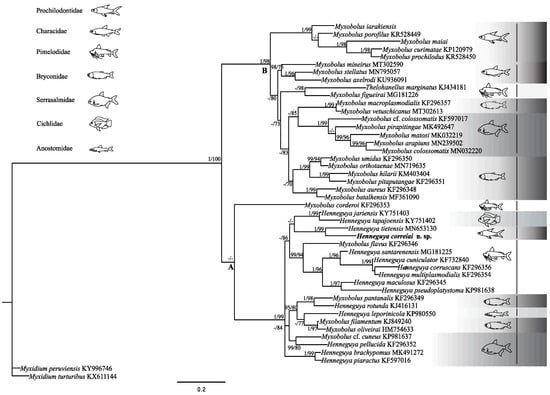
Figure 4.
Maximum Likelihood tree based on ssrDNA sequences of myxobolids most closely related to Henneguya correai n. sp. and selected myxobolids from South America. GenBank accession numbers are given after species names. Nodal support values are shown for posterior probability (PP) and bootstrap (B). Values for weakly supported nodes (<0.9 PP and <70 B) are not shown. Gray scale rectangles differentiate fish families and their schematic fish figures.

Table 1.
Morphometric comparison of Henneguya correai n. sp. with other species of Henneguya infecting Prochilodontidae.
Table 1.
Morphometric comparison of Henneguya correai n. sp. with other species of Henneguya infecting Prochilodontidae.
| Species | TL | BL | CA | BW | PCL | PCW | T | PT | Host | Site of Infection | Locality | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Henneguya correiai sp. nov. | 47.9 ± 4.9 (39.5–60.8) | 14.4 ± 1.6 (12.4–20.5) | 33.7 ± 4.5 ( 26.4–45.2) | 4.0 ± 0.59 (2.71–5.3) | 7.2 ± 0.8 (5.2–8.3) | 1.5 ± 0.3 (1.0–2.2) | 3.15 ± 0.4 (2.67–3.55) | 8–13 | Semaprochilodus insignis | Fins | Tapajós river, Amazon basin, PA, Brazil | This study |
| Henneguya tietensis | 55.5 ± 2.1 (53.5–57.0) | 16.2 ± 1.1 (15.0–16.9) | 39.0 ± 2.0 (35.2–39.7) | 5.5 ± 0.1 (5.4–5.6) | 7.3 ± 0.2 (7.1–7.5) | 1.7 ± 0.2 (1.3–1.7) | 3.8 ± 0.3 (3.7–4.2) | 11–13 | Prochilodus lineatus | Gills | Paraná river basin, Brazil | [12] |
| Henneguya caudalongula | 71.0 ± 1.4 | 16.6 ± 0.5 | 52.6 ± 1.5 | 4.6 ± 0.2 | 6.1 ± 0.2 | 1.6 ± 0.2 | _ | 10–11 | Prochilodus lineatus | Gills | Pirassununga, SP, Brazil | [28] |
| Henneguya paranaensis | 33.3 ± 1.5 | 11.4 ± 0.3 | 24.1 ± 1.5 | 4.1 ± 0.4 | 8.4 | 2.0 | _ | 10–12 | Prochilodus lineatus | Gills | Paraná river basin, Brazil | [29] |
TL—total length, BL—body length, CA—caudal appendages length, BW—body width, PCL—polar capsule length, PCW—polar capsule width, T—thickness, PT—polar tubule turns. All measurements are given as mean ± standard deviation in µm.
4. Discussions
Semaprochilodus insignis is an ecologically and economically important fish in the Amazon region, being harvested as a food source and traded as a tropical aquarium fish [4,30]. Juveniles provide around 45% of biomass consumed by piscivores cichlids [31]. Thus, the value of this species has stimulated research into its parasite fauna, and their potential disease impacts, with recent work identifying three novel myxosporean species: M. insignis; M. maiai; and M. iarakiensis [1,14].
Herein, we describe another novel myxozoan parasite of S. insignis, Henneguya correai n. sp. This taxon could be distinguished from all other described species on the basis of morphological and morphometric characters and ssrDNA sequence. Ultrastructural analysis revealed pinocytic channels at the host–parasite interface (Figure 3), which are common among Myxobolidae and considered to be related to nutrition and sporogonic development [10,11,19,28,32,33,34,35].
Comparison of parasite myxospores with other freshwater myxobolids [6,13,36,37], showed that H. correai n. sp. was morphologically and genetically most closely related to H. tietensis, a parasite of Prochilodus lineatus (Valenciennes, 1837), another South American prochilondontid. Target tissue differs between these species, with H. correai n. sp. sporulating in the fins, while H. tietensis matures in gills. Morphometric differences between the taxa include: different size polar capsules (nematocysts)—H. correai n. sp. has unequal capsules versus equal capsules in H. tietensis; smaller total length (47.9 µm for H. correai n. sp. versus 55.5 µm for H. tietensis), caudal appendages length (33.7 vs. 39.0 µm), spore body width (4.0 vs. 5.5 µm) and occasionally fewer polar tubule coils (8–13 vs. 11–13) (Table 1).
In comparison with other Henneguya species that parasitize prochilodontids, the novel species shares some morphological features with H. caudalonga Adriano, Arana and Cordeiro, 2005, and H. paranaensis Eiras, Pavanelli and Takemoto, 2004, such as body width and polar capsule width [28,29]. However, the new species differed morphometrically from H. caudalonga in multiple features, such as total length (47.9 vs. 71.0 µm), polar capsule length (7.2 vs. 6.1 µm) and polar tubule turns (8–13 vs. 10–11) (Table 1). Considering H. paranaensis, morphometric differences included total length (47.9 µm for H. correai n. sp. versus 33.3 µm for H. paranaensis), spore body length (14.4 vs. 11.4 µm), capsule length (7.2 vs. 8.4 µm) and number of polar tubule turns (8–13 vs. 10–12) (Table 1). Comparisons of H. correai n. sp. with species parasitizing non-prochilodontid hosts showed differences in at least one of the following characters: shape and/or size of the plasmodia and myxospores, number of polar tubule turns, host, site of infection or genetic differences [6,38].
Myxospore morphology, fish host family and tissue tropism often correlate strongly with ssrDNA phylogenetic patterns in Myxozoa [12,39,40,41,42]. The Myxobolidae, however, have many exceptions to these correlations, particularly in the case of myxospore morphology, where molecular data generally do not support the morphological distinctions of the genera Myxobolus and Henneguya based on the presence of caudal processes [40,41,42,43,44], or distinction of Myxobolus and Thelohanellus on the basis of polar capsule number [45]. Our phylogenetic analysis of South American Myxobolidae revealed clades with differing morphological heterogeneity: while clade A is a mixture of Henneguya and Myxobolus species, therein illustrating the polyphyletic nature of the group, Clade B consists of almost exclusively Myxobolus species, with one Thelohanellus species (Figure 4). This pattern of myxobolid sub-clades and lineages being morphologically similar has been observed before [41,45], and shows that some lineages can be exclusively one morphotype, while others have morphological plasticity (e.g., both with and without caudal projections—a mix of Myxobolus and Henneguya types). A deeper understanding of correlations between myxospore morphotypes and phylogeny might only be possible when the target alternate host annelids are known, to better characterize the evolutionary pressures driving myxospore morphology.
Regarding those myxobolid species that parasitize prochilodontid fish, our analysis showed clustering based on myxospore morphology. The Myxobolus species M. iarakiensis, M. porofilus Adriano, Arana, Ceccarelli and Cordeiro, 2002, M. maiai, M. curimatae Zatti, Naldoni, Silva, Maia and Adriano, 2015 and M. prochilodus Azevedo, Vieira, Vieira, Silva, Matos and Abdallah, 2014 comprise a lineage in clade B, while the two Henneguya parasites Henneguya correai n. sp. and H. tietensis cluster together in clade A [14,34,45,46]. These data reveal that despite overall polyphyly of myxobolid parasites of prochilodontids, there exist monophyletic sub-groups of the Henneguya and Myxobolus species. Furthermore, our analysis showed H. correai n. sp. clustered as a sister taxon to H. tietensis from Prochilodus lineatus, another prochilodontid fish from a sister genus to Semaprochilodus [3]. Despite being sister taxa that share a host family, these parasites have different tissue tropism: respectively, fins and gills. Thus, these two parasites lie within a broader clade of Henneguya species from gills and fins, but from cichlids and pimelodids. This illustrates that different host/parasite characters correlate with tree topology at different levels.
The Prochilodontidae are a characiform family comprising detritivorous migratory freshwater fishes that occur throughout South America [3]. Prochilodus lineatus occurs in the second largest South American watershed, the La Plata basin. It is hypothesized that in the geologic past there was connection between the Amazon and La Plata basins, either during marine incursions in the Miocene period, or as a result of episodic headwater connections between their upper tributaries during the Miocene and Pliocene [47,48,49,50]. We therefore postulate that the common ancestor of H. correai n. sp. and H. tietensis infected an ancestral prochilodontid in this connected system, with subsequent geographic separation driving speciation of host and parasite. Thus, it would be interesting in the future to collect more samples of Henneguya spp. in other rivers/basins and from other prochilondontids.
In summary, here we provide a taxonomic description of a novel Henneguya species from an economically and ecologically important prochilodontid fish from the Brazilian Amazon. Although no apparent disease was caused by Henneguya correiai n. sp., given that S. insignis is important in the ornamental fish trade, there is potential that if the parasite were to become established under aquarium conditions that this could impact fish health; however, knowledge of the parasite’s life cycle is needed to better assess these risk factors.
Author Contributions
Conceptualization M.I.M. and E.A.A., Methodology M.I.M., R.T.A.F. and E.A.A., formal analysis M.I.M., E.A.A. and S.D.A., resources E.A.A., S.D.A. and J.L.B., data curation E.A.A. and S.D.A., writing—original draft preparation M.I.M., writing review M.I.M., E.A.A., S.D.A., J.L.B. and R.T.A.F., supervision E.A.A., project administration E.A.A., funding acquisition E.A.A., S.D.A. and J.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was partially supported by regular project from São Paulo Research Foundation (FAPESP) (grants # 2018/24980-8 and 2019/17427-3) and by the Coordination for the Improvement of Higher Education Personnel (CAPES), Brazil, Finance Code 001. M.I. Muller was supported by postdoctoral and BEPE scholarships from the São Paulo Research Foundation (FAPESP) (grants # 2017/16546–3 and 2021/06692-8, respectively). E.A. Adriano received a research productivity grant from the Brazilian Fostering Agency CNPq (grant # 304687/2020-0).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional of Ethics Research Committee of the Federal University of São Paulo (CEUA # 6549290920—17/12/2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Nadayca Mateussi for help identifying the fish host and Juliana Naldoni for assisting in Transmission Eletron Microscopy methodology. The authors thank the fishermen Fernando Dias de Souza, Francisco dos Santos Pinto and Arlindo Teixeira Guimarães for their local knowledge of fish and providing study material from the Amazon and Tapajós rivers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buckup, P.A.; Santos, G.M. Ictiofauna da ecorregião Tapajós-Xingu: Fatos e perspectivas. Bol. Soc. Bras. Ictiol. 2010, 98, 3–9. [Google Scholar]
- Castro, R.M.C.; Vari, R.P. Check List of the Freshwater Fishes of South and Central America. Edipucrs 2003, 2004, 65–70. [Google Scholar]
- Melo, B.F.; Sidlauskas, B.L.; Hoekzema, K.; Frable, B.W.; Vari, R.P.; Oliveira, C. Molecular phylogenetics of the Neotropical fish family Prochilodontidae (Teleostei: Characiformes). Mol. Phylo. Evol. 2016, 102, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.M.; Ferreira, E.J.G.; Zuanon, J.A.S. Peixes comerciais de Manaus. Manaus: Ibama/AM. ProVárzea 2006, 1, 144. [Google Scholar]
- Okamura, B.; Hartigan, A.; Naldoni, J. Extensive uncharted biodiversity: The parasite dimension. Integr. Comp. Biol. 2018, 58, 1132–1145. [Google Scholar] [CrossRef]
- Eiras, J.C.; Adriano, E.A. A checklist of new species of Henneguya Thelohan, 1892 (Myxozoa: Myxosporea, Myxobolidae) described between 2002 and 2012. Syst. Parasitol. 2012, 83, 95–104. [Google Scholar] [CrossRef]
- Lom, J.; Dyková, I. Myxozoan genera: Definition and notes on taxonomy, lifecycle terminology and pathogenic species. Folia. Parasitol. 2006, 53, 1–36. [Google Scholar] [CrossRef]
- Eiras, J.C.; Cruz, C.F.; Saraiva, A.; Adriano, E.A. Synopsis of the species of Myxobolus (Cnidaria, Myxozoa, Myxosporea) described between 2014 and 2020. Folia. Parasitol. 2021, 68, 12. [Google Scholar] [CrossRef]
- Rangel, L.F.; Santos, M.J.; Rocha, S. Synopsis of the species of Henneguya Thelohan, 1892 (Cnidaria: Myxosporea: Myxobolidae) described since 2012. Syst. Parasitol. 2023, 100, 291–305. [Google Scholar] [CrossRef]
- Capodifoglio, K.R.; Adriano, E.A.; Naldoni, J.; Meira, C.M.; da Silva, M.R.; Maia, A.A. Novel myxosporean species parasitizing an economically important fish from the Amazon basin. Parasitol. Res. 2020, 119, 1209–1220. [Google Scholar] [CrossRef]
- Naldoni, J.; Carriero, M.M.; Moreira, G.S.; Silva, M.R.M.; Maia, A.A.M.; Adriano, E.A. Increasing the known biodiversity of cnidarian parasites of bryconid fishes from South America: Two novel Myxobolus species with ultrastructure and ssrDNA-based phylogeny. Parasitol. Res. 2020, 113, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.H.M.D.; Rangel, L.F.; Tagliavini, V.P.; Abdallah, V.D.; Santos, M.J.; Azevedo, R.K. Morphological and molecular analysis of Henneguya tietensis n.sp. (Cnidaria: Myxosporea), parasitizing the gills filaments of Prochilodus lineatus (Valenciennes, 1837) from Brazil. Parasitol. Res. 2021, 120, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Eiras, J.C.; Malta, J.C.O.; Varella, A.M.B.; Pavanelli, G.C. Myxobolus insignis sp. n. (Myxozoa, Myxosporea, Myxobolidae), a parasite of the Amazonian teleost fish Semaprochilodus insignis (Osteichthyes, Prochilodontidae). Mem. Inst. Osw. Cruz. 2005, 100, 245–247. [Google Scholar] [CrossRef]
- Müller, M.I.; Naldoni, J.; Corrêa, L.L.; Adriano, E.A. Two new species of Myxobolus parasitizing the gills of Semaprochilodus insignis in the Brazilian Amazon. Mic. Patho. 2022, 165, 105464. [Google Scholar] [CrossRef]
- Lom, J.; Arthur, J.R. A guideline for the preparation of species description in Myxosporea. J. Fish. Dis. 1989, 12, 151–156. [Google Scholar] [CrossRef]
- Sellyei, B.; Molnár, K.; Czeglédi, I.; Preiszner, B.; Székely, C. Effect of 80% ethanol or 10% formalin fixation, freezing at −20 °C and staining on Myxobolus (Myxosporea) spores to be deposited in parasitological collections. Int. J. Parasitol. Parasites Wildl. 2022, 19, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Capodifoglio, K.R.H.; Adriano, E.A.; Silva, M.R.M.; Maia, A.A.M. Supplementary data of Henneguya leporinicola (Myxozoa, Myxosporea) a parasite of Leporinus macrocephalus from fish farms in the state of São Paulo, Brazil. Acta Parasitol. 2015, 60, 451–458. [Google Scholar] [CrossRef]
- Barta, J.R.; Martin, D.S.; Liberator, P.A.; Dashkevicz, M.; Anderson, J.W.; Feighner, S.D.; Elbrecht, A.; Perkinsbarrow, A.; Jenkins, M.C.; Danforth, H.D.; et al. Phylogenetic relation- ships among eight Eimeria species infecting domestic fowl inferred using complete small subunit ribosomal DNA sequences. J. Parasitol. 1997, 83, 262–271. [Google Scholar] [CrossRef]
- Hallet, S.L.; Diamant, A. Ultrastructure and small- subunit ribosomal DNA sequence of Henneguya lesteri n. sp. (Myxosporea), a parasite of sand whiting Sillago analis (Sillaginidae) from the coast of Queensland, Australia. Dis. Aquat. Org. 2001, 46, 197–212. [Google Scholar] [CrossRef]
- Ward, R.D.; Zemlak, T.S.; Innes, B.H.; Last, P.R.; Hebert, P.D.N. DNA barcoding Australia’s fish species. Philos. Trans. R Soc. B 2005, 360, 1847–1857. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software plat-form for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Xia, X. DAMBE5: A comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 2013, 30, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar]
- Guindon, S.; Gascuel, O. A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef]
- Rambaut, A. Molecular Evolution, Phylogenetics and Epidemiology: Fig-Tree. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 10 May 2021).
- Rocha, S.; Casal, G.; Velasco, M.; Alves, Â.; Matos, E.; Al-Quraishy, S.; Azevedo, C. Morphology and phylogeny of Thelohanellus marginatus n. sp. (Myxozoa: Myxosporea), a parasite infecting the gills of the fish Hypophthalmus marginatus (Teleostei: Pimelodidae) in the Amazon River. J. Eukaryot. Microbiol. 2014, 61, 586–593. [Google Scholar] [CrossRef]
- Adriano, E.A.; Arana, S.; Cordeiro, N.S. Histopathology and ultrastructure of Henneguya caudalongula sp. n. infecting Prochilodus lineatus (Pisces: Prochilodontidae) cultivated in the state of São Paulo, Brazil. Mem. Inst. Oswaldo. Cruz. 2005, 100, 177–181. [Google Scholar] [CrossRef]
- Eiras, J.C.; Pavanelli, G.C.; Takemoto, R.M. Henneguya paranaensis sp. n. (Myxozoa, Myxobolidae), a parasite of the teleost fish Prochilodus lineatus (Characiformes, Prochilodontidae) from the Paraná River, Brazil. Bull. Eur. Ass. Fish Pathol. 2004, 24, 308–311. [Google Scholar]
- Froese, R.; Pauly, R.D. FishBase. 2019. Available online: http://www.fishbase.org (accessed on 12 May 2021).
- Guerreiro, A.I.C.; Amadio, S.A.; Fabré, N.N.; Batista, V.S. Exploring the effect of strong hydrological droughts and floods on populational parameters of Semaprochilodus insignis (Actinopterygii: Prochilodontidae) from the Central Amazonia. Environ. Dev. Sustain. 2020, 23, 3338–3348. [Google Scholar] [CrossRef]
- El-Mansy, A.; Bashtar, A.R. Histopathological and ultrastrucutural studies of Henneguya suprabranchiae Landsberg, 1987 (Myxosporea: Myxobolidae) parasitizing the suprabranchial organ of the freshwater catfish Clarias gariepinus Burchell, 1822 in Egypt. Parasitol. Res. 2002, 88, 617–626. [Google Scholar]
- Zatti, S.A.; Naldoni, J.; Silva, M.R.; Maia, A.A.; Adriano, E.A. Morphology, ultrastructure and phylogeny of Myxobolus curimatae n. sp. (Myxozoa: Myxosporea) a parasite of Prochilodus costatus (Teleostei: Prochilodontidae) from the São Francisco River, Brazil. Parasitol. Int. 2015, 64, 362–368. [Google Scholar] [CrossRef]
- Zatti, S.A.; Arana, S.; Maia, A.A.M.; Adriano, E.A. Ultrastructural, ssrDNA sequencing of Myxobolus prochilodus and Myxobolus porofilus and details of the interaction with the host Prochilodus lineatus. Parasitol. Res. 2016, 115, 4573–4585. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.M.A.; Adriano, E.A.; Vieira, L.J.; Naldoni, J.; Santos, F.G.A. The Amazonian fish Colossoma macropomum harbors high myxosporean diversity: A description of the three novel species from a fish farm. Microb. Pathog. 2021, 153, 104808. [Google Scholar] [CrossRef] [PubMed]
- Madden, T. The Blast Sequence Analysis Tool. In The NCBI Handbook; McEntyre, J., Ostell., J., Eds.; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2002. Available online: https://blast.ncbi.nlm.nih.gov (accessed on 18 March 2022).
- Eiras, J.C.; Zhang, J.; Molnár, K. Synopsis of the species of Myxobolus Bütschli, 1882 (Myxozoa: Myxosporea, Myxobolidae) described between 2005 and 2013. Syst. Parasitol. 2014, 88, 11–36. [Google Scholar] [CrossRef] [PubMed]
- Eiras, J.C. Synopsis of the species of the genus Henneguya Thelohan, 1892 (Myxozoa: Myxosporea: Myxobolidae). Syst. Parasitol. 2002, 52, 43–54. [Google Scholar] [CrossRef]
- Molnár, K. Comments on the host, organ and tissue specificity of fish myxosporeans and on the types of their intrapiscine development. Parasitol. Hung 1994, 27, 5–20. [Google Scholar]
- Fiala, I. The phylogeny of Myxosporea (Myxozoa) based on small subunit ribosomal RNA gene analysis. Int. J. Parasitol. 2006, 36, 1521–1534. [Google Scholar] [CrossRef]
- Carriero, M.M.; Adriano, E.A.; Silva, M.R.M.; Ceccarelli, P.S.; Maia, A.A.M. Molecular phylogeny of the Myxobolus and Henneguya genera with several new South American species. PLoS ONE 2013, 8, e73713. [Google Scholar] [CrossRef]
- Liu, Y.; Whipps, C.M.; Gu, Z.M.; Zeng, L.B. Myxobolus turpisrotundus (Myxosporea: Bivalvulida) spores with caudal appendages: Investigating the validity of the genus Henneguya with morphological and molecular evidence. Parasitol. Res. 2010, 107, 699–706. [Google Scholar] [CrossRef]
- Kent, M.L.; Andree, K.B.; Bartholomew, J.L.; El-Matbouli, M.; Desser, S.S.; Devlin, R.H.; Feist, S.W.; Hedrick, R.P.; Hoffmann, R.W.; Khattra, J.; et al. Recent advances in our knowledge of the Myxozoa. J. Euk. Microb. 2001, 48, 395–413. [Google Scholar] [CrossRef]
- Ferguson, J.A.; Atkinson, S.D.; Whipps, C.M.; Kent, M.L. Molecular and morphological analysis of Myxobolus spp. of salmonid fishes with the description of a new Myxobolus species. J. Parasitol. 2008, 94, 1322–1334. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Whipps, C.M.; Guo, Q.; Gua, Z. Multiple evolutionary routes of the single polar capsule in Thelohanellus species (Myxozoa; Myxobolidae). Int. J. Parasitol. Parasites Wildl. 2019, 8, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Adriano, E.A.; Arana, S.; Ceccarelli, P.S.; Cordeiro, N.S. Light and scanning electron microscopy of Myxobolus porofilus sp. n. (Myxosporea: Myxobolidae) infecting the visceral cavity of Prochilodus lineatus (Pisces: Characiformes: Prochilodontidae) cultivated in Brazil. Folia Parasit. 2002, 49, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Ramos, V.A. Northern Andes. Tect. Evol. S. Am. 2000, 31, 453–480. [Google Scholar]
- Wesselingh, F.P.; Hoorn, C. Geological development of Amazon and Orinoco Basins. In Historical Bio-Geography of Neotropical Freshwater Fishes; Albert, J.S., Reis, R.E., Eds.; ResearchGate: Berlin, Germany, 2011; pp. 59–68. [Google Scholar]
- Brea, M.; Zucol, A.F. The Parana-Paraguay basin: Geology and paleoenvironments. In Historical Biogeography of Neotropical Freshwater Fishes; Albert, J.S., Reis, R.E., Eds.; ResearchGate: Berlin, Germany, 2011; pp. 69–87. [Google Scholar]
- Fontenelle, J.P.; Marques, F.P.L.; Kolmann, M.A.; Lovejoy, N.R. Biogeography of the neotropical freshwater stingrays (Myliobatiformes: Potamotrygoninae) reveals effects of continent-scale paleogeographic change and drainage evolution. J. Biog. 2021, 48, 1406–1419. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).