Seasonal and Nocturnal Activity of Culicoides spp. (Diptera: Ceratopogonidae) Adapted to Different Environments in the Balearic Islands
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Species Composition, Sex Ratio, and Gonothrophic Condition
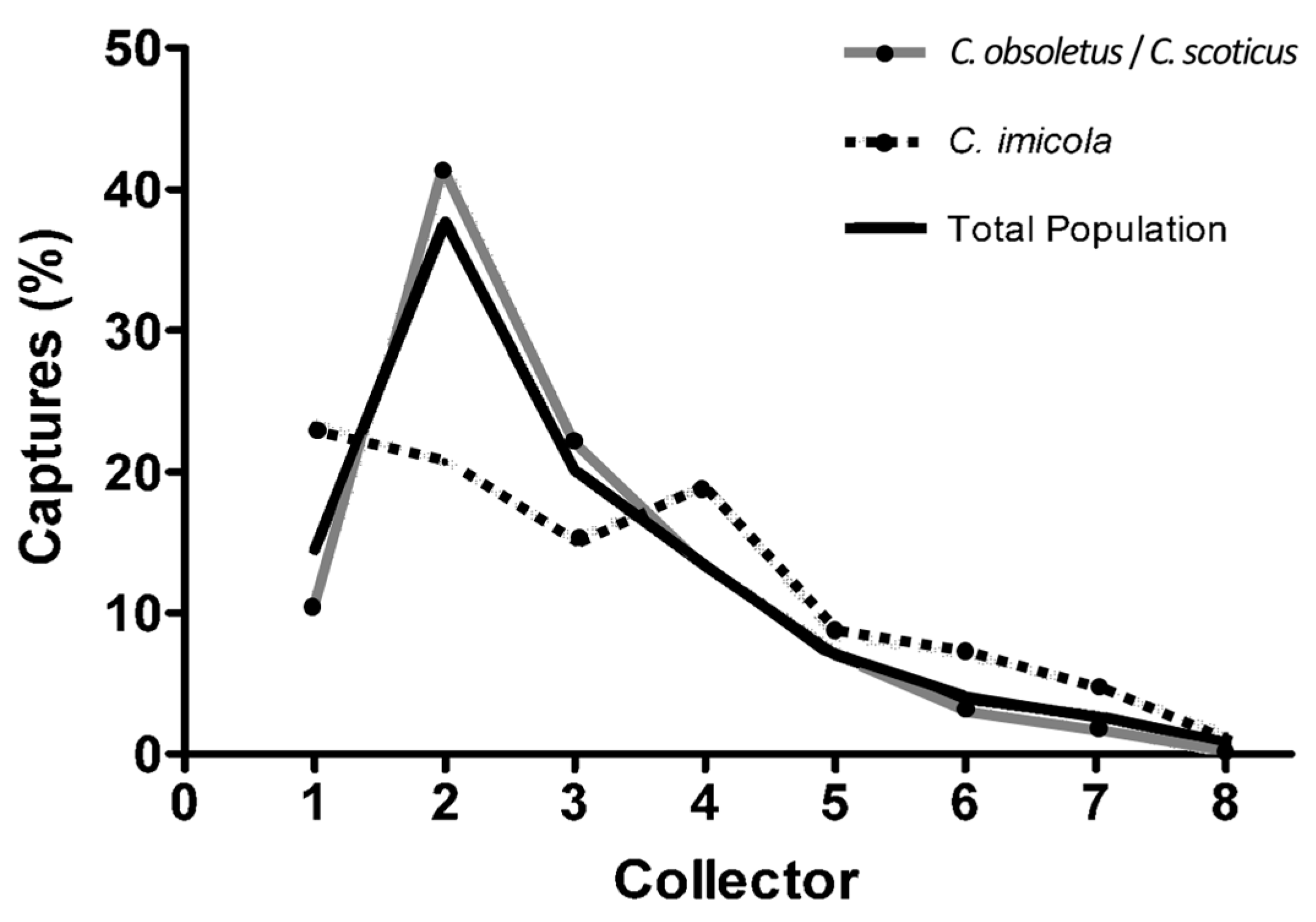
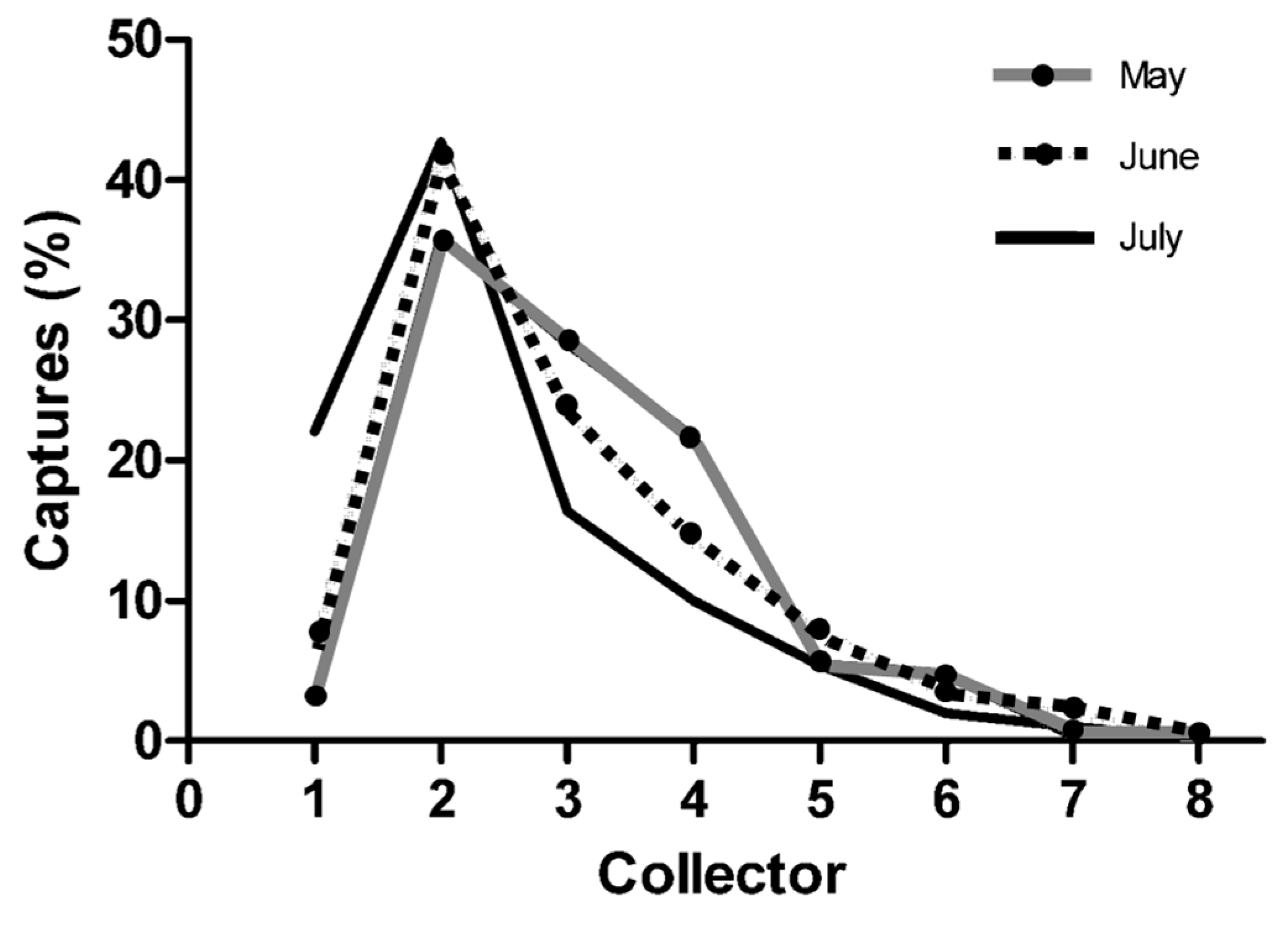
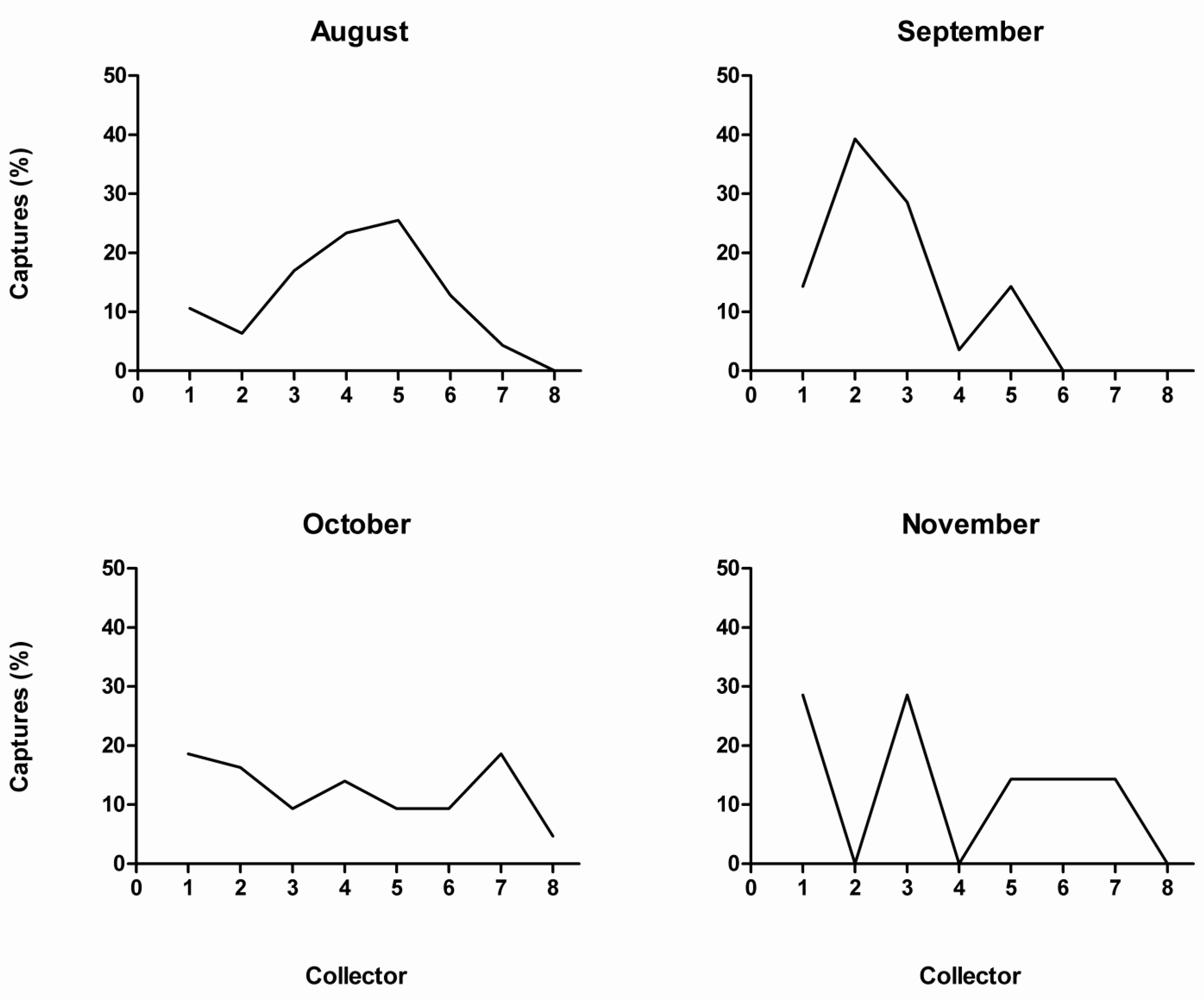
3.2. Seasonal and Nocturnal Activity Pattern
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du Toit, R.M. The transmission of blue-tongue and horse-Sickness by Culicoides. Onderst. J. Vet. Sci. An. Indust. 1944, 19, 7–16. [Google Scholar]
- Linley, J.R. Biting midges (Diptera: Ceratopogonidae) as vectors of non-viral animal pathogens. J. Med. Entomol. 1985, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Meiswinkel, R.; Venter, G.J.; Nevill, E.M. Vectors: Culicoides spp. In Infectious Diseases of Livestock; Coetzer, J.A.W., Tustin, R.C., Eds.; Oxford University Press: Cape Town, South Africa, 2004; pp. 93–136. [Google Scholar]
- Gibbens, N. Schmallenberg virus: A novel viral disease in northern Europe. Vet. Rec. 2012, 170, 58. [Google Scholar] [CrossRef]
- Calvete, C.; Miranda, M.A.; Estrada, R.; Borràs, D.; Sarto i Monteys, V.; Collantes, F.; García de Francisco, J.M.; Moreno, N.; Lucientes, J. Spatial distribution of Culicoides imicola, the main vector of Bluetongue virus, in Spain. Vet. Rec. 2006, 158, 130–131. [Google Scholar] [CrossRef] [PubMed]
- Barros, S.C.; Ramos, F.; Luís, T.M.; Vaz, A.; Duarte, M.; Henriques, M.; Cruz, B.; Fevereiro, M. Molecular epidemiology of Bluetongue virus in Portugal during 2004-2006 outbreak. Vet. Microbiol. 2007, 124, 25–34. [Google Scholar] [CrossRef]
- Thiry, E.; Saegerman, C.; Guyot, H.; Kirten, P.; Losson, B.; Rollin, F.; Bodmer, M.; Czaplicki, G.; Toussaint, J.F.; De Clercq, K. Bluetongue in northern Europe. Vet. Rec. 2006, 159, 327. [Google Scholar] [CrossRef]
- Nolan, D.V.; Carpenter, S.; Barber, J.; Mellor, P.S.; Dallas, J.F.; Mordue, J.A.; Piertney, S.B. Rapid diagnostic PCR assays for members of the Culicoides obsoletus and Culicoides pulicaris species complexes, implicated vectors of bluetongue virus in Europe. Vet. Microbiol. 2007, 24, 82–94. [Google Scholar] [CrossRef]
- Versteirt, V.; Balenghien, T.; Tack, W.; Wint, W. A first estimation of Culicoides imicola and Culicoides obsoletus/Culicoides scoticus seasonality and abundance in Europe. EFSA Support. Publ. 2017, 14, 1182E. [Google Scholar] [CrossRef]
- Gómez-Tejedor, C. Brief overview of the Bluetongue situation in Mediterranean Europe, 1998–2004. Vet. Ital. 2004, 40, 57–60. [Google Scholar]
- Pérez de Diego, A.C.; Sánchez-Cordón, P.J.; Sánchez-Vizcaíno, J.M. Bluetongue in Spain: From the first outbreak to 2012. Transbound. Emerg. Dis. 2014, 61, e1–e11. [Google Scholar] [CrossRef]
- RASVE (Red de Alerta Sanitaria Veterinaria). Programa nacional de erradicación de lengua azul. Dirección General de Sanidad de la Producción Agraria. Ministerio de Medio Ambiente y Medio Rural y Marino. Available online: https://www.mapa.gob.es/es/ganaderia/temas/sanidad-animal-higiene-ganadera/sanidad-animal/ (accessed on 12 December 2018).
- WOAH (World Organization for Animal Health). Terrestrial Animal Health Code, 28th ed.; OIE: Paris, France, 2019; Volumes 1 and 2, 518p. [Google Scholar]
- Sellers, R.F.; Pedgley, D.E.; Tucker, M.R. Possible windborne spread of Bluetongue to Portugal, June–July 1956. J. Hyg. 1978, 81, 189–196. [Google Scholar] [CrossRef]
- Sellers, R.F.; Gibbs, E.P.J.; Herniman, K.A.J.; Pedgeley, D.E.; Tucker, M.R. Possible origin of the bluetongue epidemic in Cyprus, August 1977. J. Hyg. 1979, 83, 547–555. [Google Scholar] [CrossRef]
- Hendricks, G.; Gilbert, M.; Staubach, C.; Elbers, A.; Mintiens, K.; Gerbier, G.; Ducheyne, E. A wind density model to quantify the airborne spread of Culicoides species during north-western Europe bluetongue epidemic, 2006. Prev. Vet. Med. 2008, 87, 162–181. [Google Scholar] [CrossRef] [PubMed]
- Nelson, R.L.; Bellamy, R.E. Patterns of flight activity of Culicoides variipennis (Coquillett) (Diptera: Ceratopogonidae). J. Med. Entomol. 1971, 8, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Barnard, D.R.; Jones, R.H. Diel and seasonal patterns of flight activity of Ceratopogonidae in northeast Colorado: Culicoides. Environ. Entomol. 1980, 9, 446–451. [Google Scholar] [CrossRef]
- Sanders, C.J.; Shortall, C.R.; Gubbins, S.; Burgin, L.; Gloster, J.; Harrington, R.; Reynolds, D.R.; Mellor, P.S.; Carpenter, S. Influence of season and meteorological parameters on flight activity of Culicoides biting midges. J. Appl. Ecol. 2011, 48, 1355–1364. [Google Scholar] [CrossRef]
- Purse, B.V.; Carpenter, S.; Venter, G.J.; Bellis, G.; Mullens, B.A. Bionomics of temperate and tropical Culicoides midges: Knowledge gaps and consequences for transmission of Culicoides-borne viruses. Annu. Rev. Entomol. 2015, 60, 373–392. [Google Scholar] [CrossRef]
- Cuéllar, A.C.; Jung Kjær, L.; Baum, A.; Stockmarr, A.; Skovgard, H.; Nielsen, S.A.; Andersson, M.G.; Lindström, A.; Chirico, J.; Lühken, R.; et al. Monthly variation in the probability of presence of adult Culicoides populations in nine European countries and the implications for targeted surveillance. Parasites Vectors 2018, 11, 608. [Google Scholar] [CrossRef]
- Barceló, C.; Estrada, R.; Lucientes, J.; Miranda, M.A. A Mondrian matrix of seasonal patterns of Culicoides nulliparous and parous females at different latitudes in Spain. Res. Vet. Sci. 2020, 129, 154–163. [Google Scholar] [CrossRef]
- Barceló, C.; Purse, B.V.; Estrada, R.; Lucientes, J.; Miranda, M.A.; Searle, K.R. Environmental drivers of adult seasonality and abundance of biting midges Culicoides (Diptera: Ceratopogonidae), bluetongue vector species in Spain. J. Med. Entomol. 2021, 58, 350–364. [Google Scholar] [CrossRef]
- Rawlings, P. A key, based on wing patterns of biting midges (genus Culicoides Latreille-Diptera: Ceratopogonidae) in the Iberian Peninsula, for use in epidemiological studies. Graellsia 1996, 52, 57–71. [Google Scholar] [CrossRef]
- Dyce, A.L. The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. Aust. J. Entomol. 1969, 8, 11–15. [Google Scholar] [CrossRef]
- AEMET (Agencia Estatal de Meteorología) Ministry for the Ecological Transition and the Demographic Challenge. Available online: https://eportal.mapa.gob.es/websiar/SeleccionParametrosMap.aspx?dst=1 (accessed on 5 May 2023).
- Kramer, W.L.; Greiner, E.C.; Gibbs, E.P.J. Seasonal variations in population size, fecundity, and parity rates of Culicoides insignis (Diptera: Ceratopogonidae) in Florida, USA. J. Med. Entomol. 1985, 22, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Del Río, R.; Barceló, C.; Lucientes, J.; Miranda, M.A. Detrimental effect of cypermethrin treated nets on Culicoides populations (Diptera; Ceratopogonidae) and non-targeted fauna in livestock farms. Vet. Parasitol. 2014, 199, 230–234. [Google Scholar] [CrossRef]
- Braverman, Y. Characteristics of Culicoides (Diptera, Ceratopogonidae) breeding places near Salisbury, Rhodesia. Ecol. Entomol. 1978, 3, 163–170. [Google Scholar] [CrossRef]
- Bishop, A.L.; McKenzie, H.J.; Barchia, I.M.; Spohr, L.J. Moon phase and other factors affecting light-trap catches of Culicoides brevitarsis Kieffer (Diptera: Ceratopogonidae). Aust. J. Entomol. 2000, 39, 29–32. [Google Scholar] [CrossRef]
- Miranda, M.A.; Borràs, D.; Rincón, C. Seasonal abundance of Culicoides imicola and C. obsoletus group in the Balearic Islands (Spain). Vet. Ital. 2004, 40, 292–295. [Google Scholar]
- Viennet, E.; Garros, C.; Rakotoarivony, I.; Allene, X.; Gardes, L.; Lhoir, J.; Fuentes, I.; Venail, R.; Crochet, D.; Lancelot, R.; et al. Host-seeking activity of bluetongue virus vectors: Endo/exophagy and circadian rhythm of Culicoides in Western Europe. PLoS ONE 2012, 7, e48120. [Google Scholar] [CrossRef]
- Lysyk, T.J.; Danyk, T. Effect of temperature on life history parameters of adult Culicoides sonorensis (Diptera: Ceratopogonidae) in relation to geographic origin and vectorial capacity for Bluetongue virus. J. Med. Entomol. 2007, 44, 741–751. [Google Scholar] [CrossRef]
- Rawlings, P.; Mellor, P.S. African horse sickness and the overwintering of Culicoides spp. in the Iberian peninsula. Rev. Sci. Tech. Off. Int. Epiz. 1994, 13, 753–761. [Google Scholar] [CrossRef]
- Anderson, J.R.; Linhares, A.X. Comparison of several different trapping methods for Culicoides variipennis (Diptera: Ceratopogonidae). J. Am. Mosq. Control. Assoc. 1989, 5, 325–334. [Google Scholar] [PubMed]
- Carpenter, S.; Szmaragd, C.; Barber, J.; Labuschagne, K.; Gubbins, S.; Mellor, P. An assessment of Culicoides surveillance techniques in northern Europe: Have we underestimated a potential bluetongue virus vector? J. Appl. Ecol. 2008, 45, 1237–1245. [Google Scholar] [CrossRef]
- Viennet, E.; Garros, C.; Lancelot, R.; Allène, X.; Gardès, L.; Rakotoarivony, I.; Crochet, D.; Delécolle, J.C.; Moulia, C.; Baldet, T.; et al. Assessment of vector/host contact: Comparison of animal-baited traps and UV-light/suction trap for collecting Culicoides biting midges (Diptera: Ceratopogonidae), vectors of Orbiviruses. Parasites Vectors 2011, 4, 119. [Google Scholar] [CrossRef] [PubMed]

| Ca’s Boter | Rialema | ||||||
|---|---|---|---|---|---|---|---|
| Species | Month | Total Captures | Nights Sampled | Captures/Night | Total Captures | Nights Sampled | Captures/Night |
| C. obsoletus/C. scoticus | May | 1993 | 3 | 664.3 ± 11.2 a | |||
| June | 5514 | 7 | 853.2 ± 9.2 a | 6 | 1 | 6.0 ± 0.0 | |
| July | 272 | 3 | 90.7 ± 4.7 a | 12 | 5 | 2.4 ± 0.3 | |
| August | 47 | 3 | 15.7 ± 2.0 b | 2 | 2 | 1.0 ± 0.0 | |
| September | 28 | 6 | 4.7 ± 1.3 b | 6 | 4 | 1.5 ± 0.7 | |
| October | 43 | 10 | 4.3 ± 0.6 b | 6 | 4 | 1.5 ± 1.0 | |
| November | 7 | 3 | 2.3 ± 1.0 b | 2 | 5 | 0.4 ± 0.4 | |
| Total | 7904 | 35 | 225.8 ± 21.9 | 34 | 21 | 1.6 ± 0.3 | |
| C. imicola | May | 7 | 3 | 2.3 ± 0.2 b | |||
| June | 36 | 7 | 5.1 ± 0.9 b | 0 | 1 | 0.0 | |
| July | 9 | 3 | 3.0 ± 1.2 b | 2 | 5 | 0.4 ± 0.4 | |
| August | 7 | 3 | 2.3 ± 1.2 b | 1 | 2 | 0.5 ± 0.7 | |
| September | 56 | 6 | 9.7 ± 1.9 b | 3 | 4 | 0.8 ± 0.9 | |
| October | 259 | 10 | 28.8 ± 1.3 a | 5 | 4 | 1.3 ± 0,4 | |
| November | 41 | 3 | 13.7 ± 3.7 b | 1 | 5 | 0.2 ± 0,4 | |
| Total | 415 | 35 | 9.3 ± 0.5 | 12 | 21 | 1.0 ± 0.6 | |
| Site | Month | Av. T (C°) | Max. T (C°) | Min. T (C°) |
|---|---|---|---|---|
| Ca’s Boter | May | 18.8 | 33.1 | 6.0 |
| June | 24.4 | 39.7 | 13.2 | |
| July | 24.9 | 36.4 | 14.0 | |
| August | 27.0 | 39.4 | 16.8 | |
| September | 22.1 | 33.3 | 12.0 | |
| October | 18.8 | 30.1 | 3.9 | |
| November | 14.1 | 23.3 | 3.8 | |
| Rialema | May | 18.9 | 32.0 | 7.4 |
| June | 24.2 | 36.7 | 13.5 | |
| July | 25.3 | 37.5 | 14.9 | |
| August | 27.1 | 38.5 | 17.5 | |
| September | 22.5 | 32.6 | 12.2 | |
| October | 19.6 | 30.4 | 5.2 | |
| November | 15.0 | 23.6 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barceló, C.; del Río, R.; Miranda, M.A. Seasonal and Nocturnal Activity of Culicoides spp. (Diptera: Ceratopogonidae) Adapted to Different Environments in the Balearic Islands. Diversity 2023, 15, 690. https://doi.org/10.3390/d15050690
Barceló C, del Río R, Miranda MA. Seasonal and Nocturnal Activity of Culicoides spp. (Diptera: Ceratopogonidae) Adapted to Different Environments in the Balearic Islands. Diversity. 2023; 15(5):690. https://doi.org/10.3390/d15050690
Chicago/Turabian StyleBarceló, Carlos, Ricardo del Río, and Miguel A. Miranda. 2023. "Seasonal and Nocturnal Activity of Culicoides spp. (Diptera: Ceratopogonidae) Adapted to Different Environments in the Balearic Islands" Diversity 15, no. 5: 690. https://doi.org/10.3390/d15050690
APA StyleBarceló, C., del Río, R., & Miranda, M. A. (2023). Seasonal and Nocturnal Activity of Culicoides spp. (Diptera: Ceratopogonidae) Adapted to Different Environments in the Balearic Islands. Diversity, 15(5), 690. https://doi.org/10.3390/d15050690







