Abstract
The genus Salvia has a worldwide distribution, and its contribution to traditional medicine and as an aromatic plant has been recognized since ancient times, with few documented species having a similar phytochemical composition. In this study, the effects of natural growth conditions (in situ) and ex situ cultivation and sampling locations on the phenolic compound contents and antioxidant activity of methanolic extracts of Salvia circinata from Oaxaca, Mexico, were investigated. Stem and young leaf samples were collected from plants growing in situ in two locations in Oaxaca and later from plants propagated clonally or vegetatively from propagules obtained in situ but grown ex situ. In both sets of samples, the contents of total polyphenols and flavonoids and the antioxidant activity were evaluated by spectrophotometry, and subsequently, the contents of phenols and specific flavonoids were identified and quantified by high-performance liquid chromatography with diode-array detection (HPLC–DAD). The growth conditions and locality of origin of the samples significantly influenced the contents of total polyphenols and flavonoids and antioxidant activity, with the in situ conditions in the locality of Reforma, Oaxaca, favoring higher levels. Two phenolic acids (chlorogenic and rosmarinic) and two flavonoids (isoquercitrin and rutin) were identified, and the concentrations of these compounds were influenced by the growth conditions and localities of origin of the samples.
1. Introduction
The genus Salvia (Lamiaceae) has a worldwide distribution and is widely used in traditional medicine. Of particular interest is the subgenus Calosphace, which contains 587 species with a large number endemic to North and South America (500 species). The countries with the highest diversity of Salvia are Mexico with 295 recognized species, Peru with 77, Colombia with 60, and Brazil with 58 [1,2,3,4]. Many studies have documented the ethnobotany, ethnomedicinal uses and chemical composition of the Salvia species distributed in the Mediterranean region, Europe, Central Asia and South Africa [1,4,5].
Evaluations of the chemical composition and biological activity of Salvia species indicate that they have pharmacological potential in treating gastrointestinal and respiratory disorders and preventing neurodegenerative disorders. In addition, their antimicrobial, antioxidant, antiinflammatory, cytotoxic and antispasmodic activities provide phytochemical evidence supporting their use in traditional medicine [6,7,8,9,10]. Decoctions and infusions of Salvia leaves and stems are used in the treatment of abdominal pain, vomiting, cardiac issues, cold, anxiety and gastrointestinal disorders and have preventive medicinal potential against diarrhea, diabetes, cancer, ulcers and helminthiasis [10,11,12]. The effectiveness of species Salvia circinata is partly due to its composition. For example, Moreno-Pérez et al. [13,14] report that the antinociceptive and antiinflammatory medicinal properties are conferred by the presence of the neo-clerodane terpene amarisolide A and the flavonoid pedalitin. Flores-Bocanegra et al. [11] indicate that the antihyperglycemic action in rat models is due to the inhibitory activity of the enzyme alpha-glucosidase. Additionally, Salinas-Arellano et al. [15] note that the presence of flavonoids and terpenoids, such as the flavonoids rutin, isoquercitrin and pedalitin, which have anxiolytic and/or antinociceptive properties, inhibits the activity of protein tyrosine phosphatase 1B (PTP-1B).
Nurzyńska-Wierdak [16] highlights that under natural growth conditions, the biosynthesis of phenolic compounds in plants and their antioxidant and/or biological properties are affected by ecological–environmental conditions, genotype (inherited ability to synthesize metabolites) and ontogenetic variation, which are determinants of the synthesis of a wide range of secondary metabolites. Changes in chemical profiles during ontogeny, e.g., between different ages, organ types and plant phenological or developmental stages, determine the chemical composition of a plant sample and help to determine the suitable harvesting or sampling period. In wild medicinal plants, Bautista et al. [17] and Li et al. [18] agree that internal genetic factors related to development (e.g., regulatory genes and enzymes) and external environmental factors such as light, temperature, salinity and water availability influence the synthesis, concentration and accumulation of antioxidant phenolic compounds, and the effect of developmental and environmental factors changes depending on the species.
Regarding the environmental effect on the phytochemical composition of Salvia species, Bettaieb et al. [19] evaluated the effect of medium to severe water deficit on Salvia officinalis and concluded that in general, drought increases the abundance of phenolic compounds, but the concentration of specific compounds differs, with both significant increases and decreases; for example, the concentrations of rosmarinic and chlorogenic acids increase, while those of the flavonoids quercetin and quercetin 3-D-galactoside decrease. A similar response was observed by Tavakoli et al. [20] when evaluating the effect of ecological factors and soil characteristics on the concentration of phenols and flavonoids in S. multicaulis. The authors concluded that the response differs by compound: with low temperatures, the concentration of total phenolic compounds increases, but cold stress reduces the concentration of phenolic acids. Similar responses were observed for flavonoids when plants were under prolonged stress caused by low rainfall, low relative humidity, low soil organic matter content and low soil pH. That is, the set of growth conditions and/or plant genotypes of Salvia species influence the contents of secondary metabolites. The objective of this study was to evaluate the effects of natural (in situ) and cultivated (ex situ) growing conditions on the contents of phenolic compounds and antioxidant activity in S. circinata, a medicinal plant used in the Mixtec communities of Oaxaca, Mexico.
2. Materials and Methods
2.1. Plant Material
S. circinata Cav. (Syn. Salvia amarissima Ortega) is a perennial herbaceous plant that reaches 0.3 to 1.5 m in height, with hispid stems and blue/purple inflorescences. The species is endemic to Mexico, from San Luis Potosi to Oaxaca, at elevations ranging from 1650 to 2800 m. S. circinata grows preferentially in disturbed areas of temperate forests, shrubs and grasslands [21,22]. This species is used in traditional Mexican medicine in the treatment of gastrointestinal disorders (stomach pain, diarrhea, helminthiasis, ulcers) and diabetes [12]. In San Martin Huamelulpam, Oaxaca (sampling region), S. circinata is known as ‘yucucahua morada’. This species grows in places near forests and in disturbed sites, such as on the margins of farm plots or roads and in patches near streams and water sources during the rainy season (June–November). It is tolerated in backyards or orchards as an ornamental plant. Additionally, it is applied as a medicine in the form of infusions of leaves and stems that are fresh, dried or chewed to cure or prevent stomach pain (‘latido’) and diarrhea.
2.2. Experimental Design and Sampling
S. circinata was sampled based on a bifactorial design, where Factor A was the growth condition (growth environment, E): plants growing in natural conditions (in situ) in the municipality of San Martin Huamelulpam (Huamelulpam), Oaxaca, and plants grown in ex situ conditions in Santa Cruz Xoxocotlan, Oaxaca, using propagules of the same plants identified in situ that were assumed to be clones of the original plants. As part of the design, Factor B was the site or localitions of ecogeographic origin of the populations in their natural conditions: Reforma and La Union localities within the municipal territory of San Martin Huamelulpam (population, Po). Therefore, combining the two levels of Factor A with the two levels of Factor B, we designed an experiment with four experimental combinations (A × B or E × Po). Table 1 describes the geographic and environmental conditions of the collection sites of S. circinata plants recollected in situ and cultivated ex situ. Therefore, the in situ and ex situ sampling design was proposed to compare the populations of plants growing under natural conditions versus the same plants propagated clonally or vegetatively and grown under cultivation conditions as part of our statistical hypotheses.

Table 1.
Geographic and environmental conditions of S. circinata sampled from Oaxaca, Mexico.
For the experiment, the first sampling stage (in situ conditions) consisted of collecting three samples of leaves and young stems from 10 to 15 individuals of S. circinata in a state of flowering or incipient flowering (approximately 1 kg of fresh tissue per sample) at three different sides of the population or from a ‘patch’ of plants in the locality of Reforma and another similar sample at La Union, San Martin Huamelulpam, Oaxaca, between September and October 2020 (after the rainy season). The second sampling stage corresponded to ex situ cultivation of plants grown from sections or propagules of S. circinata plants collected from the same in situ sampling sites and cultivated inside a metal structure covered with shade cloth from September 2020 to May 2021. The plants were grown in 20 L pots in a substrate containing sand, soil and organic matter at a 1:1:1 ratio. Soil moisture was maintained at field capacity, and the daily temperature and relative humidity were recorded. Three pots were grown with plants from the Reforma locality and three pots from the La Union locality. Three plant tissue samples from each pot were collected in May 2021 based on the same criteria used for in situ sampling. In addition, soil and substrate samples were taken from each field sampling site (in situ) and ex situ cultivation pot for chemical analysis (Table 1).
2.3. Determination of Phenolic Compounds and Antioxidant Activity by Spectrophotometry
2.3.1. Sample Preparation
The in situ and ex situ plant material was washed, cut into small pieces, dried at 40 °C in a dehydrator (L’Equipe Model 528) and pulverized using a mill (Krups® model GX4100, Mexico). Subsequently, samples were stored at −20 °C in an airtight container until evaluation. For the spectrophotometric determinations of phenolic compounds and antioxidant activity, a methanolic extract was obtained from 0.1 g of dry milled sample and 25 mL of 60% (v/v) methanol, which was homogenized for 60 s in intervals of 30 s (Ultra Turrax T 25 Digital, IKA, Staufen, Germany) and centrifuged at 11,000 rpm (Eppendorf AG, Model 5811F, Hamburg, Germany) at 4 °C for 15 min, and the supernatant was used for the respective analyses. All determinations were carried out in triplicate.
2.3.2. Total Polyphenols
The analysis of total polyphenols was performed according to the Folin–Ciocalteu method described by Singleton and Rossi [25], where the absorption was measured at 750 nm using a spectrophotometer (VELAB, Model VE-5600UV PC, Pharr, TX, USA), and the concentration was calculated with reference to a calibration curve of a gallic acid standard (0.021 to 0.165 mg mL−1, r2 = 0.999). The results were expressed as milligrams of gallic acid equivalents per gram of dry weight (mg GAE g−1 dw).
2.3.3. Flavonoids
The flavonoid contents were determined using two colorimetric methods. The first method followed the methodology proposed by Lin and Tang [26], which uses the reaction of aluminum chloride (AlCl3) in the presence of flavonoids to estimate the contents of flavonols and flavones such as luteolin [27] as a function of absorbances at 415 nm in a spectrophotometer. The concentration was estimated with reference to a standard quercetin calibration curve (0.01 to 0.17 mg mL−1, r2 = 0.999) and was expressed as milligrams of quercetin equivalents per gram of dry weight (mg QE g−1 dw). The second estimation method involved the use of sodium nitrite (NaNO2) in an alkaline medium [28] to evaluate the total contents of rutin, luteolin and catechins [27]. The absorbance at 510 nm was recorded, and the estimation was quantified based on a standard catechin calibration curve (0.01 to 0.5 mg mL−1, r2 = 0.999). The results were expressed as milligrams of catechin equivalents per gram of dry weight (mg CE g−1 dw).
2.3.4. Antioxidant Activity Evaluated by DPPH and FRAP
The DPPH (2,2-diphenyl-1-picrylhydrazyl, Sigma-Aldrich, St. Louis, MO, USA) method described by Brand-Williams et al. [29] was used. The absorbance was measured in a spectrophotometer at 517 nm, and the content was quantified with reference to a Trolox calibration curve (0.13 to 1.33 μmol mL−1, r2 = 0.999). Then, the antioxidant activity was evaluated by the FRAP (ferric reducing antioxidant power) method based on the description of Benzie and Strain [30]. In this case, the absorbance was recorded at 593 nm, and the concentration was calculated with reference to a Trolox calibration curve (0.05 to 1.0 μmol mL−1, r2 = 0.999). The results of both determinations were expressed as micromoles of Trolox equivalents per gram of dry weight (μmol TE g−1 dw).
2.4. Determination of Phenolic Acids and Flavonoids by HPLC–DAD
A methanolic extract was obtained from 0.2 g of dry milled sample and 10 mL of 60% (v/v) methanol, which was homogenized for 60 s in intervals of 30 s (Ultra Turrax T 25 Digital, IKA, Staufen, Germany) and sonicated in an ultrasonic bath (Cole-Parmer, Model 08895-43, Vernon Hills, IL, USA) for 1 h. Subsequently, the sample was centrifuged at 11,000 rpm (refrigerated Eppendorf 5810R centrifuge) at 4 °C for 15 min, and the supernatant was used for HPLC–DAD analysis. Then, a 2 mL aliquot of methanolic extract (60%) was filtered by a 0.2 μm PTFE syringe (Agilent Technologies®, Part. No. 5190-5086, Waldbronn, Germany) and placed in amber vials.
The analysis of phenolic acids and flavonoids was performed using HPLC–DAD following the method described by Pająk et al. [31] with some modifications described in a previous study [32]. The instrument was an HPLC (Agilent model Infinity II 1260 LC system) equipped with a solvent degasser, quaternary pump, temperature-controlled autosampler, column oven and DAD (Agilent Technologies, Santa Clara, CA, USA). A reversed-phase column (Agilent® Hypersil 5 ODS, 250 × 4.6 mm, 5 μm) was used. The sample analysis characteristics and conditions were described in Perez-Ochoa et al. [32], where the monitoring wavelengths for flavonoids and phenolic acids were 260 nm (rutin and isoquercitrin) and 320 nm (chlorogenic and rosmarinic acids), respectively. The identification of each compound was made with reference to the retention times and UV spectra of commercial standards (Phyproof®, Phytolab GmbH & Co. KG, Vestenbergsgreuth, Germany, and Sigma-Aldrich®, St. Louis, MO, USA) and quantification by calibration curves of reference standards: chlorogenic acid (1.27 to 203 µg mL−1, r2 = 0.998), rosmarinic acid (0.9 to 533 µg mL−1, r2 = 0.999), rutin (0.9 to 256 µg mL−1, r2 = 0.999) and isoquercitrin (0.5 to 72 µg mL−1, r2 = 0.999). The amount of each compound was expressed as micrograms per gram of dry weight (µg g−1 dw).
2.5. Statistical Analysis
Based on the information obtained from the phytochemical analysis of the samples, a database was established, and analysis of variance was performed using a completely random linear model. To evaluate the specific differences between localities of sample origin (Po, populations), between plant growth environments (E, in situ and ex situ) and among locality–environment interactions (Po × E), Tukey’s test (p < 0.05) was used for means comparisons. In addition, Pearson correlation analysis was performed between total polyphenols and flavonoids versus antioxidant activity, and principal component analysis was conducted using the average values per sample. All statistical analyses were performed with the SAS statistical package (SAS Institute Inc., Cary, NC, USA) [33].
3. Results
3.1. Total Phenolic Compounds and Antioxidant Activity
In the analysis of variance, significant differences in the contents of total polyphenols and flavonoids and antioxidant activity were observed (p < 0.01) between growth conditions (E) and between localities of origin of the samples or populations (Po). For the interaction between growth conditions and populations (E × Po), significant differences were recorded only for antioxidant activity evaluated by the FRAP method, indicating that for the concentration of polyphenols and flavonoids and antioxidant activity as determined by the DPPH method, the effect of growth conditions is independent of the effect of populations. The results of the analysis of variance showed that the variance due to the locality of origin of the samples or population (Po) was significantly greater than the effect of growth condition (E) and E × Po interactions on the content of phenolic compounds and antioxidant activity (Table 2).

Table 2.
Significance of the mean squares of the analysis of variance of phenolic compounds and antioxidant activity in S. circinata from San Martin Huamelulpam, Oaxaca, Mexico.
In the comparison of the means of the effect of the growth conditions (in situ vs. ex situ), the response pattern showed that in all the parameters evaluated, the plants grown in natural conditions had significantly higher concentrations of phenolic compounds and antioxidant activity than those grown ex situ. Analysis of the effect of the localities of origin of the samples (Po) showed that the leaves and stems collected in the locality of Reforma had significantly higher concentrations of polyphenols, flavonoids and antioxidant activity than those collected in La Union, Huamelulpam (Table 3).

Table 3.
Average content of phenolic compounds and antioxidant activity of S. circinata plants sampled in situ (natural conditions) and cultivated ex situ in Oaxaca, Mexico.
The interaction between localities of origin and growth conditions did not contribute significantly to differences in total polyphenols and flavonoids or antioxidant activity evaluated by DPPH; however, there was a significant difference in antioxidant activity as determined by FRAP (Table 3). This indicates independence of the study factors (Po and E) but with similar effect in magnitude and significantly greater than the effect of their interaction. These results suggest similar compound contents or antioxidant activity and reflect the fact that the magnitude of the effect of the growth conditions (ontogenetic and ecological factors) is similar and independent of the effect of the locality of origin of the samples.
Pearson’s correlation analysis revealed a significant and positive relationship between total polyphenols and catechin and quercetin equivalent flavonoids with antioxidant activity evaluated by the DPPH and FRAP methods in S. circinata plants collected in situ (0.68 < r < 0.97; p < 0.001) and cultivated ex situ (0.74 < r < 0.95; p < 0.001). In both cases, the phenolic compounds evaluated contributed substantially to the antioxidant activity (as determined by the DPPH and FRAP methods) in methanolic extracts of S. circinata.
3.2. Profile of Phenolic Acids and Flavonoids by HPLC–DAD
In the HPLC–DAD analysis of phenolic compounds in methanolic extracts of leaves and young stems of S. circinata, two phenolic acids, chlorogenic and rosmarinic acids, and two glycosylated flavonoids derived from quercetin, rutin (quercetin-3-rutinoside) and isoquercitrin (quercetin-3-β-D-glucoside), were identified and quantified (Figure 1).
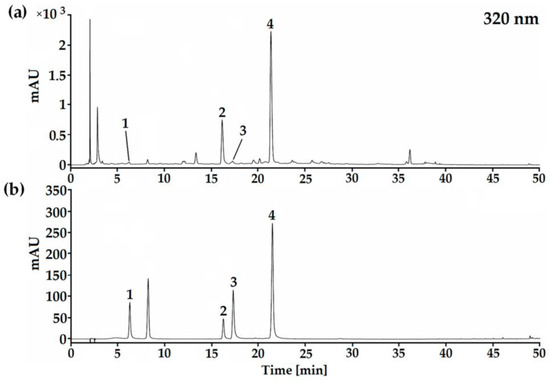
Figure 1.
Chromatographic profile based on HPLC–DAD analysis of phenolic compounds in methanolic extracts from leaves and young stems of S. circinata (a) and based on reference standards (b) (1) chlorogenic acid, (2) rutin, (3) isoquercitrin and (4) rosmarinic acid.
The analysis of variance showed significant differences (p < 0.05, 0.01) in isoquercitrin and rutin between growth environments (E), between localities of origin of the samples or populations (Po) and among the environment–locality interactions (E × Po). However, for chlorogenic acid, significant differences were recorded between populations and due to the E × Po interaction, and in rosmarinic acid, differences were observed between growth conditions (E) and due to the E × Po interaction. The results show that phenolic acids and flavonoids have differential response patterns depending on the study factors. For example, for chlorogenic acid and isoquercitrin, the variance due to the locality of origin of the samples was significantly greater than that due to the growth conditions or the E × Po interaction; for rosmarinic acid, the variance due to the effect of the E × Po interaction and growth conditions similarly influenced the concentration; and for rutin, the factor with the greatest influence on variance was the growth conditions (Table 4).

Table 4.
Significance of mean squares of the analysis of variance of phenolic acids and flavonoids identified in S. circinata by HPLC–DAD.
The S. circinata plants collected under natural growing conditions (in situ) presented significantly higher contents of rosmarinic acid, isoquercitrin and rutin than the plants cultivated ex situ, but the contents of chlorogenic acid did not differ significantly. The comparison of means between localities of origin of the samples (Po) showed two contrasting patterns. First, for the contents of chlorogenic acid and rutin, the samples from Reforma presented higher values than the samples from La Union. For the isoquercitrin content, the opposite pattern was observed, with significantly higher contents in samples from La Union than from Reforma (Table 5). That is, the growth conditions and localities of origin of the samples differentially influenced the composition of phenols and flavonoids in S. circinata.

Table 5.
Phenolic acid and flavonoid contents identified by HPLC–DAD in S. circinata sampled in situ (natural conditions) and cultivated ex situ in Oaxaca, Mexico.
The concentrations of chlorogenic acid, rosmarinic acid, isoquercitrin and rutin in methanolic extracts of S. circinata were influenced by the growth conditions or environments and populations or sample origins (E × Po), but the response pattern differed by compound. For example, for chlorogenic acid, the in situ and ex situ samples from the Reforma locality showed a higher concentration than the samples from La Union, indicating that the effect of locality was significant. For isoquercitrin content, the opposite effect of locality was observed. Additionally, the concentration patterns of rosmarinic acid and rutin differed, where the Reforma samples collected in situ (natural conditions) presented higher values than those cultivated ex situ and those originating from the La Union locality (in situ and ex situ) (Table 5).
In the principal component analysis (PCA), the S. circinata samples showed two dispersion patterns based on their composition of phenolic compounds and antioxidant activity (Figure 2). The samples of leaves and young stems of S. circinata collected in situ presented a pattern of high dispersion associated with the rosmarinic acid and rutin contents and antioxidant activity, and in contrast, the ex situ samples were less dispersed. In Figure 2, the natural conditions or in situ environment of origin locations influenced the dispersion and/or differentiation between Reforma and La Union. Therefore, it seems necessary to consider the effect of sites and locations on the multivariate differences between samples.
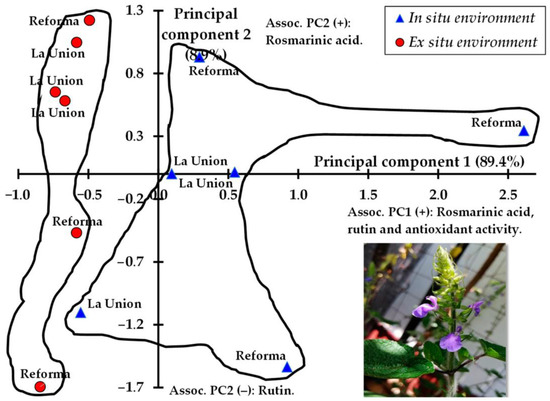
Figure 2.
Dispersion of S. circinata samples by locality of origin and growth condition, based on the first two principal components of the analysis of phenolic compounds.
4. Discussion
The spectrophotometric analysis of methanolic extracts of S. circinata consistently showed that the samples of plants growing in their natural conditions (in situ) presented higher contents of polyphenols and flavonoids and higher antioxidant activity than plants grown ex situ (Table 2). This differential response to in situ and ex situ conditions reflects part of the factors that induce stress in S. circinata plants and suggests that the biosynthesis of metabolites is the product of a complex of ecophysiological and metabolic responses to stress conditions. For example, in ex situ conditions, the plants grew without moisture restrictions, and substrates had higher contents of organic matter and micro- and macronutrients than the plants that grew in situ, which were subject to the rainy season, possible stress due to water deficit or drought, lower contents of soil nutrients and lower temperatures (Table 1). The elements and factors that cause abiotic stress were noted by Arbona et al. [34] and Verma and Shukla [35] as responsible for inducing fluctuations in the concentration of secondary metabolites in plants.
Arbona et al. [34] and Yang et al. [36] emphasized that drought or water stress is one of the main limiting factors for the growth, reproductive development and survival of plants because it affects the photosynthetic capacity, nutrient absorption, osmotic regulation and other metabolic processes in plants that require water. In Salvia multicaulis, Tavakoli et al. [20] estimated a direct relationship between the low temperatures of the sampled regions and increases in total polyphenols and a positive relationship between flavonoid contents and sampling regions where there was limited rainfall and low relative humidity, soil organic matter content and soil pH. Bettaieb et al. [19] experimentally showed that severe drought induced a lower concentration of flavonoids and antioxidant activity in S. officinalis plants than in controls for which there were no moisture restrictions. This indicates that, under natural or experimental conditions (i.e., in cultivation), responses differ by species, and changes in the synthesis of phenolic compounds and antioxidant activity depend on the abiotic factor and severity or duration of stress.
Under natural conditions, S. circinata grows in forests and disturbed sites of San Martin Huamelulpam (sampling region) and in patches near streams and water sources during the rainy season (June–November) and sprouts and flowers every year from December to May. In April or May, there are low temperatures in this region (<18 °C), with frosts from November to January, and because there is little or no precipitation, there are no new shoots at this time. The localities sampled within the target municipality for this work were Reforma and La Union, and the distance between sampling sites was less than 3 km. Therefore, there is no reason to believe that the sampled plants were from independent populations but rather were subpopulations growing in different ecogeographic conditions or patches because this region is highly disturbed. In this context of disturbances and anthropogenic influences, the higher polyphenol and flavonoids concentrations and antioxidant activity in the samples collected in Reforma than in the samples collected in La Union is due, to a large extent, to differences in ecological, edaphic and microenvironmental or niche conditions between sampling localities (Table 3).
The differences in polyphenols, flavonoids and antioxidant activity between S. circinata collection sites are similar to the responses estimated by Farhat et al. [37] for the total phenol content and antioxidant activity in S. officinalis, S. verbenaca, S. aegyptiaca and S. argentea collected from sites with contrasting bioclimatic characteristics in Tunisia. Sarrou et al. [38] recorded similar significant differences in phenol and total flavonoid contents between two cultivated populations of S. fruticosa evaluated monthly from April to October (in a warm environment with high relative humidity), emphasizing that the differences between populations suggest genotypic differences and the influence of the harvest season. In this work, pH, P, Mn, and inorganic N were lower in Reforma than in La Union (Table 1). In addition to these differences, possible genetic differences generated by genetic drift due to natural selection pressures and anthropic disturbances cannot be excluded. The suggestion of genetic differences is based on the phenotypic model proposed by Lynch and Walsh [39], where plant phenotype is a result of genotypic, environmental and genotype–environment interaction effects (P = G + E + G × E), which is similar to the analysis model used in this work.
The absence of significant interaction effects between localities of origin and growth conditions (in situ and ex situ) on the content of total polyphenols, flavonoids (QE and CE) and antioxidant activity as evaluated by DPPH suggests that both factors act or influence each other independently. However, phenolic compounds showed a significant and positive correlation with antioxidant activity in the samples collected in situ and those from ex situ cultivation (0.68 < r > 0.97; p < 0.001). These results indicate that the samples consist of complex matrices of compounds; for example, in Salvia fruticosa, Sarrou et al. [38] identified 28 mono- and sesquiterpenes and 38 phenolic compounds, all with greater or lesser influence on antioxidant activity. Therefore, the medicinal use of S. circinata in Mexico and its positive effect against gastrointestinal disorders through decoctions or infusions is based on the complex of ingested compounds.
In extracts of S. circinata, compounds such as pedalitin, apigenin-7-O-β-D-glucoside, flavone 2-(3,4-dimethoxyphenyl)-5,6-dihydroxy-7-methoxy-4H-chromen-4-one, apigenin, 5,6-dihydroxy-7,3′,4′-trimethoxy-flavone, 5,6,4′-trihydroxy-7,3′-dimethoxyflavone, 6-hydroxyluteolin, rutin, isoquercitrin and rosmarinic acid have been identified [10,11,13,15]. In this study, the contents of chlorogenic acid, rosmarinic acid, rutin and isoquercitrin were identified and quantified in methanolic extracts of leaves and young stems of S. circinata, and the effects of sample locality of origin (Po, populations), growth environment (E) and locality–environment interaction (Po × E) and variations in the response pattern between compounds were identified (Table 4).
Chlorogenic acid is common in tissues of the genus Salvia, as previously reported in S. officinalis by Bettaieb et al. [19], in S. fruticosa by Sarrou et al. [38] and in S. multicaulis by Tavakoli et al. [20]. In this study, the concentration of chlorogenic acid in S. circinata was significantly influenced by the locality of origin (Po) of the sample and the interaction between growth environments and the locality of origin (E × Po). The plants collected in Reforma had higher concentrations than their counterparts from La Union. Based on the E × Po interaction, the plants grown in situ or ex situ in Reforma had higher concentrations than those grown in La Union. These results suggest that in Reforma, there are populations of S. circinata with chlorogenic acid biosynthetic characteristics that differ (e.g., chemotype) from those in the La Union samples, and the greater effect of site or locality than of growth conditions on this compound is evident among the plants grown in situ and ex situ (Table 4 and Table 5). The results of Sarrou et al. [38] show that the sampling site and genotype in cultivated S. fruticosa exert a strong effect on the chlorogenic acid content, but Bettaieb et al. [19] noted that severe drought exerts an increasing effect on the chlorogenic acid content in S. officinalis. This indicates that environmental or seasonal and genetic or populational factors induce changes in chlorogenic acid biosynthesis, and the effects differ by species.
Rosmarinic acid is characteristic of the family Lamiaceae and one of the main constituents of the Salvia genus, and its potential antioxidant characteristics are associated with anticancer, antibacterial, antiviral and antidiabetic properties [5,40,41]. In this study, the rosmarinic acid content in S. circinata was influenced by the effect of growth conditions (E) and the interaction between the growth environment and locality of origin of the samples (E × Po). The results show that the natural conditions (in situ) have a significant effect on the biosynthesis and accumulation of this compound since the concentration in situ exceeded the concentration in plants cultivated ex situ by 20%; this pattern is repeated for the E × Po interaction, with the concentration of rosmarinic acid in the samples collected in situ in Reforma exceeding that estimated in ex situ samples from Reforma and in all samples originating from La Union by 26 to 43% (Table 5). Zengin et al. [42] noted that the rosmarinic acid content differed between the Salvia species S. euphratica, S. blepharochlaena and S. verticillata, and sampling site influences these differences, as shown by Farhat et al. [37] in four species of sage. For Sarrou et al. [38], the sampling site was the main determining factor of the concentration of rosmarinic acid in S. fruticosa, which contrasts with the results of Bettaieb et al. [19], who noted that drought or moderate water stress favors a higher concentration of this compound since severe drought induces growth. Therefore, the results suggest that growth conditions and moderate stress favor the biosynthesis and accumulation of rosmarinic acid in the leaves and young stems of different species of sage.
Rutin and isoquercitrin are antioxidant glycosylated flavonoids present in some species of Salvia, such as S. fruticosa [38], Salvia miltiorrhiza and S. amarissima from Oaxaca, Mexico [15]. The previously identified patterns of chlorogenic and rosmarinic acid contents are confirmed, in some way or in part. First, the concentrations of rutin and isoquercitrin were significantly higher in samples collected in situ than ex situ, but the contents differed by locality of origin. A higher concentration of rutin was estimated in samples from Reforma than in samples from La Union, and for isoquercitrin, the opposite pattern was recorded (La Union > Reforma). These results were confirmed by analyzing the interaction between growth environments and localities of origin. The isoquercitrin concentration was higher in samples collected in situ in La Union, while for rutin, the highest concentration was recorded in in situ samples from Reforma (Table 5). Therefore, the results show that plants growing in natural conditions (in situ) produce higher contents of isoquercitrin and rutin, but this also depends on the locality or site of origin of the samples.
The variation in the pattern of isoquercitrin and rutin concentrations in S. circinata shows that the ecological–environmental and geographic or site conditions, including probable interpopulation genetic differences, generated some stress and a greater increase in the biosynthesis and accumulation of these flavonoids in leaves and stems. For example, severe water stress or drought leads to the excessive production of reactive oxygen species, followed by oxidative stress and decreases in photosynthesis, and induces an increase in flavonoid contents as part of the responses of plants to counteract metabolic damage [19,34,43].
5. Conclusions
The spectrophotometric analysis of total polyphenols, flavonoids and antioxidant activity in methanolic extracts of leaves and young stems of S. circinata collected under natural conditions (in situ) and cultivated ex situ showed that the ecological–environmental and edaphic conditions of in situ growth and possible stress conditions significantly influenced the biosynthesis and accumulation of greater amounts of phenolic compounds and led to higher antioxidant activity than that observed without water restriction and with the provision of fertile substrate. In addition, the locality of origin of the samples significantly influenced the concentrations analyzed, even when the distance between the collection sites (Reforma and La Union) was less than 3 km. In the HPLC–DAD analysis, two phenolic acids (chlorogenic acid and rosmarinic acid) and two flavonoids (rutin and isoquercitrin) were identified with differential response patterns to the evaluated factors (growth environment and locality of origin of the sampled population) and their interaction. In this study, the concentration of chlorogenic acid was fundamentally influenced by the origin of the sample (Reforma > La Union). For rosmarinic acid, rutin and isoquercitrin, the in situ growth conditions were very influential in inducing a higher concentration than that in the ex situ plants. Rutin and isoquercitrin had higher concentrations under in situ conditions and showed opposite responses according to the locality of origin. The different patterns in the synthesis and concentration of specific phenolic compounds suggest the presence of different chemotypes or populations of S. circinata and help to partly explain the medicinal properties of the species for the treatment of gastrointestinal disorders.
Author Contributions
Conceptualization and methodology, M.L.P.-O., A.M.V.-G., D.M.M.-C., S.S.-T., J.C.C.-R. and J.L.C.-S.; investigation and writing, M.L.P.-O., A.M.V.-G., S.H.-D. and J.L.C.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Instituto Politecnico Nacional-Mexico under projects no. SIP-20231194 and SIP-20230580.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank all families from the municipalities of San Martin Huamelulpam, Oaxaca, Mexico, who shared their experiences with medicinal plants. In addition, we appreciate the correction of the taxonomic classification of the plant species made by Rosalinda Medina-Lemos of the Institute of Biology, Universidad Nacional Autonoma de Mexico.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jenks, A.A.; Kim, S.-C. Medicinal plant complexes of Salvia subgenus Calosphace: An ethnobotanical study of new world sages. J. Ethnopharmacol. 2013, 146, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gordillo, M.; Bedolla-García, B.; Cornejo-Tenorio, G.; Fragoso-Martínez, I.; García-Peña, M.R.; González-Gallegos, J.G.; Lara-Cabrera, S.I.; Zamudio, S. Lamiaceae de México. Bot. Sci. 2017, 95, 780–806. [Google Scholar] [CrossRef]
- González-Gallegos, J.G.; Bedolla-García, B.Y.; Cornejo-Tenorio, G.; Fernández-Alonso, J.L.; Fragoso-Martínez, I.; García-Peña, M.D.R.; Harley, R.M.; Klitgaard, B.; Martínez-Gordillo, M.J.; Wood, J.R.I.; et al. Richness and distribution of Salvia subg. Calosphace (Lamiaceae). Int. J. Plant Sci. 2020, 181, 831–856. [Google Scholar] [CrossRef]
- Ortiz-Mendoza, N.; Aguirre-Hernández, E.; Fragoso-Martínez, I.; González-Trujano, M.E.; Basurto-Peña, F.A.; Martínez-Gordillo, M.J. A review on the ethnopharmacology and phytochemistry of the Neotropical sages (Salvia subgenus Calosphace; Lamiaceae) emphasizing Mexican species. Front. Pharmacol. 2022, 13, 867892. [Google Scholar] [CrossRef] [PubMed]
- Hernández-León, A.; Moreno-Pérez, G.F.; Martínez-Gordillo, M.; Aguirre-Hernández, E.; Valle-Dorado, M.G.; Díaz-Reval, M.I.; González-Trujano, M.E.; Pellicer, F. Lamiaceae in Mexican species, a great but scarcely explored source of secondary metabolites with potential pharmacological effects in pain relief. Molecules 2021, 26, 7632. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoen, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef]
- Mrabti, H.N.; El-Menyiy, N.; Charfi, S.; Saber, M.; Bakrim, S.; Alyamani, R.A.; Rauf, A.; Ali, A.M.H.; Abdallah, E.M.; El-Omari, N.; et al. Phytochemistry and biological properties of Salvia verbenaca L.: A comprehensive review. Biomed Res. Int. 2022, 2022, 3787818. [Google Scholar] [CrossRef] [PubMed]
- Jedidi, S.; Selmi, H.; Aloui, F.; Rtibi, K.; Sammari, H.; Abbes, C.; Sebai, H. Antioxidant properties, phytoactive compounds and potential protective action of Salvia officinalis flowers against combined gastro-intestinal ulcer and diarrhea experimentally induced in rat. Dose-Response Int. J. 2022, 20, 15593258221102313. [Google Scholar] [CrossRef]
- Randjelović, M.; Branković, S.; Miladinović, B.; Milutinović, M.; Živanović, S.; Mihajilov-Krstev, T.; Kitić, D. The benefits of Salvia sclarea L. ethanolic extracts on gastrointestinal and respiratory spasms. S. Afr. J. Bot. 2022, 150, 621–632. [Google Scholar] [CrossRef]
- Calzada, F.; Bautista, E.; Barbosa, E.; Salazar-Olivo, L.A.; Alvidrez-Armendáriz, E.; Yepez-Mulia, L. Antiprotozoal activity of secondary metabolites from Salvia circinata. Rev. Bras. Farmacogn. 2020, 30, 593–596. [Google Scholar] [CrossRef]
- Flores-Bocanegra, L.; González-Andrade, M.; Bye, R.; Linares, E.; Mata, R. α Glucosidase inhibitors from Salvia circinata. J. Nat. Prod. 2017, 80, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Figueroa, M.; Navarrete, A.; Rivero-Cruz, I. Chemistry and biology of selected Mexican medicinal plants. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A., Falk, H., Gibbons, S., Kobayashi, J., Asakawa, Y., Liu, J.K., Eds.; Springer: Cham, Switzerland, 2019; Volume 108. [Google Scholar] [CrossRef]
- Moreno-Pérez, G.F.; González-Trujano, M.E.; Martínez-Gordillo, M.J.; Miguel-Chávez, R.S.; Basurto-Peña, F.A.; Dorazco-González, A.; Aguirre-Hernández, E. Amarisolide A and pedalitin as bioactive compounds in the antinociceptive effects of Salvia circinata (Lamiaceae). Bot. Sci. 2019, 97, 355–365. [Google Scholar] [CrossRef]
- Moreno-Pérez, G.F.; Hernández-León, A.; Valle-Dorado, M.G.; Cano-Martínez, A.; Narváez-González, F.; Aguirre-Hernández, E.; Salgado-Ceballos, H.; González-Trujano, M.E. Neo-clerodane diterpenic influence in the antinociceptive and anti-inflammatory properties of Salvia circinnata Cav. J. Ethnopharmacol. 2021, 268, 113550. [Google Scholar] [CrossRef]
- Salinas-Arellano, E.; Pérez-Vásquez, A.; Rivero-Cruz, I.; Torres-Colin, R.; González-Andrade, M.; Rangel-Grimaldo, M.; Mata, R. Flavonoids and terpenoids with PTP-1B inhibitory properties from the infusion of Salvia amarissima Ortega. Molecules 2020, 25, 3530. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Phenolic compounds from new natural sources–plant genotype and ontogenetic variation. Molecules 2023, 28, 1731. [Google Scholar] [CrossRef] [PubMed]
- Bautista, I.; Boscaiu, M.; Lidón, A.; Llinares, J.V.; Lull, C.; Donat, M.P.; Vicente, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 2016, 38, 9. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- Bettaieb, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Drought effects on polyphenol composition and antioxidant activities in aerial parts of Salvia officinalis L. Acta Physiol. Plant. 2011, 33, 1103–1111. [Google Scholar] [CrossRef]
- Tavakoli, M.; Esfahani, M.T.; Soltani, S.; Karamian, R.; Aliarabi, H. Effects of ecological factors on phenolic compounds in Salvia multicaulis Vahl (Lamiaceae). Biochem. Syst. Ecol. 2022, 104, 104484. [Google Scholar] [CrossRef]
- de Rzedowski, G.C.; Rzedowski, J. Flora Fanerogámica del Valle de México; Instituto de Ecología, A.C. y Comisión Nacional Para el Conocimiento y Uso de la Biodiversidad: Pátzcuaro, Mexico, 2005. [Google Scholar] [CrossRef]
- Lara-Cabrera, S.I.; Bedolla-García, B.Y.; Zamudio, S.; Domínguez-Vázquez, G. Diversidad de Lamiaceae en el estado de Michoacán, México. Acta Bot. Mex. 2016, 116, 107–149. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística y Geografía (INEGI). Compendio de Información Geográfica Municipal 2010; San Martín Huamelulpam: Oaxaca, Mexico; INEGI: Aguascalientes, Mexico, 2010; Available online: https://www.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/20/20239.pdf (accessed on 1 April 2023).
- Norma Oficial Mexicana (NOM). NOM-021-RECNAT-2000; Especificaciones de Fertilidad, Salinidad y Clasificación de Suelos. Estudios, Muestreo y Análisis; Diario Oficial de la Federación: Ciudad de Mexico, Mexico, 31 December 2002; Volume 73, p. 2000. Available online: https://catalogonacional.gob.mx/FichaRegulacion?regulacionId=22947 (accessed on 1 April 2023).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminum complexation reaction for flavonoid content assay. Food Anal. Meth. 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Pająk, P.; Socha, R.; Broniek, J.; Królikowska, K.; Fortuna, T. Antioxidant properties, phenolic and mineral composition of germinated chia, golden flax, evening primrose, phacelia and fenugreek. Food Chem. 2019, 275, 69–76. [Google Scholar] [CrossRef]
- Pérez-Ochoa, M.L.; Vera-Guzmán, A.M.; Mondragón-Chaparro, D.M.; Sandoval-Torres, S.; Carrillo-Rodríguez, J.C.; Chavez-Servia, J.L. Effects of growth conditions on phenolic composition and antioxidant activity in the medicinal plant Ageratina petiolaris (Asteraceae). Diversity 2022, 14, 595. [Google Scholar] [CrossRef]
- SAS Institute Inc. (SAS) Base SAS® 9.1.3 Procedures Guide, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006; Volume 1. [Google Scholar]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of various factors responsible for fluctuations in plant secondary metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Farhat, M.B.; Landoulsi, A.; Chaouch-Hamada, R.; Sotomayor, J.A.; Jordán, M.J. Characterization and quantification of phenolic compounds and antioxidant properties of Salvia species growing in different habitats. Ind. Crop. Prod. 2013, 49, 904–914. [Google Scholar] [CrossRef]
- Sarrou, E.; Martens, S.; Chatzopoulou, P. Metabolite profiling and antioxidative activity of sage (Salvia fruticosa Mill.) under the influence of genotype and harvesting period. Ind. Crop. Prod. 2016, 94, 240–250. [Google Scholar] [CrossRef]
- Linch, M.; Walsh, B. Genetics and Analysis of Quantitative Traits; Sinauer Associates, Inc.: Sunderland, MA, USA, 1998; pp. 107–129. [Google Scholar]
- Trivellini, A.; Lucchesini, M.; Maggini, R.; Mosadegh, H.; Villamarin, T.S.S.; Vernieri, P.; Mensuali-Sodi, A.; Pardossi, A. Lamiaceae phenols as multifaceted compounds: Bioactivity, industrial prospects and role of “positive-stress”. Ind. Crop. Prod. 2016, 83, 241–254. [Google Scholar] [CrossRef]
- Shojaeifard, Z.; Hemmatinejad, B.; Jassbi, A.R. Chemometrics-based LC-UV-ESIMS analyses of 50 Salvia species for detecting their antioxidant constituents. J. Pharm. Biomed. Anal. 2021, 193, 113745. [Google Scholar] [CrossRef]
- Zengin, G.; Llorent-Martínez, E.J.; Fernández-de Córdova, M.L.; Bahadori, M.B.; Mocan, A.; Locatelli, M.; Aktumsek, A. Chemical composition and biological activities of extracts from three Salvia species: S. blepharochlaena, S. uphratica var. leiocalycina, and S. veticillata subsp. amasica. Ind. Crop. Prod. 2018, 111, 11–21. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.D.; Mattanzion, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).