Rotifers (Rotifera: Monogononta) Associated with Littoral Macrophyte Habitats in Flooded Neotropical Ponds: A Qualitative Study
Abstract
1. Introduction
2. Methods
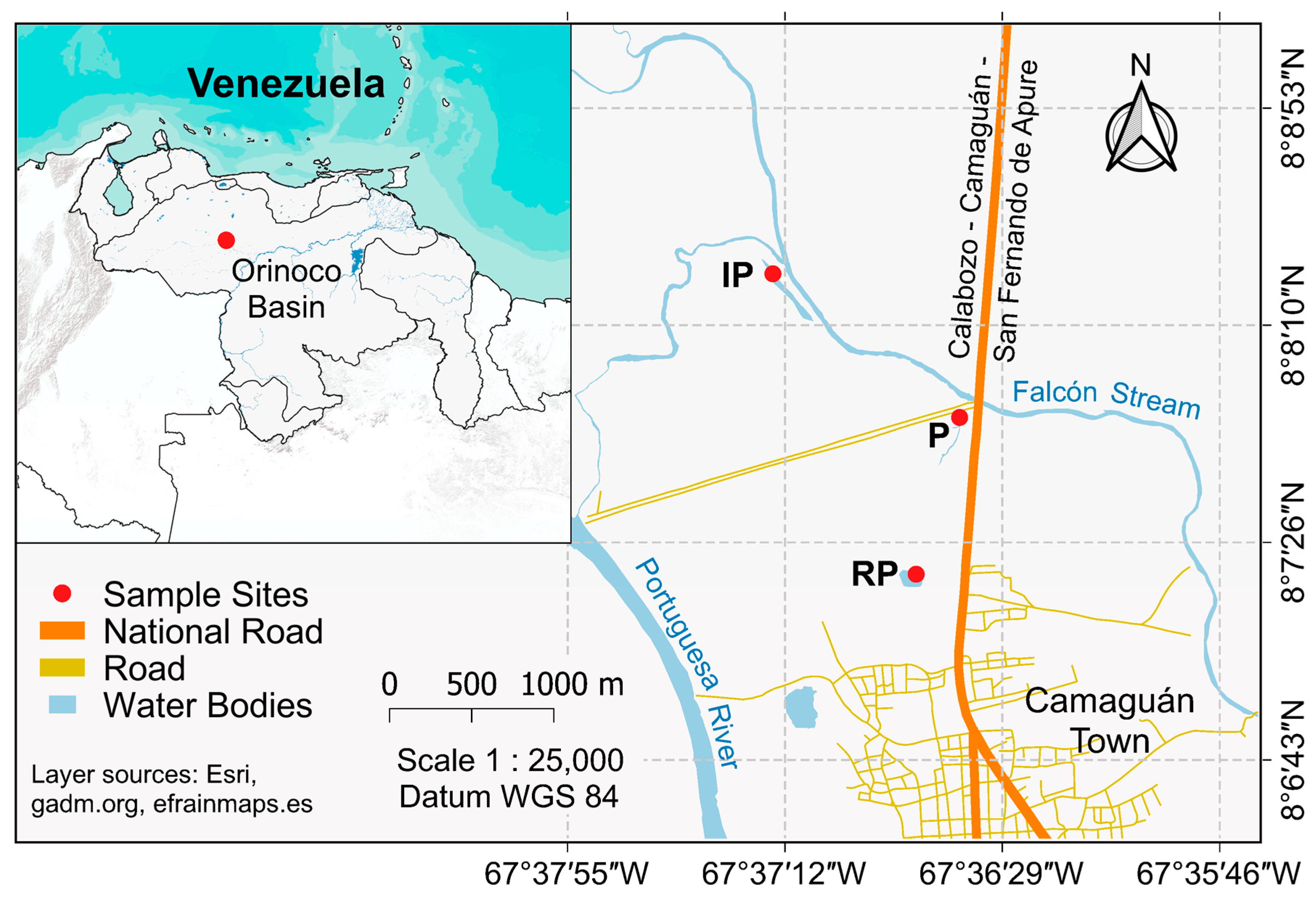
2.1. Sampling Sites
2.2. Field Samplings
2.3. Laboratory Analysis
2.4. Data Analysis
3. Results
3.1. Composition of Littoral Macrophytes
3.2. Composition and Taxa Richness of Rotifers
3.3. Frequency of Occurrence of Rotifers
3.4. Relative Numerical Frequency of Rotifers
3.5. The Richness of Taxa of Periphytic and Planktonic Rotifers
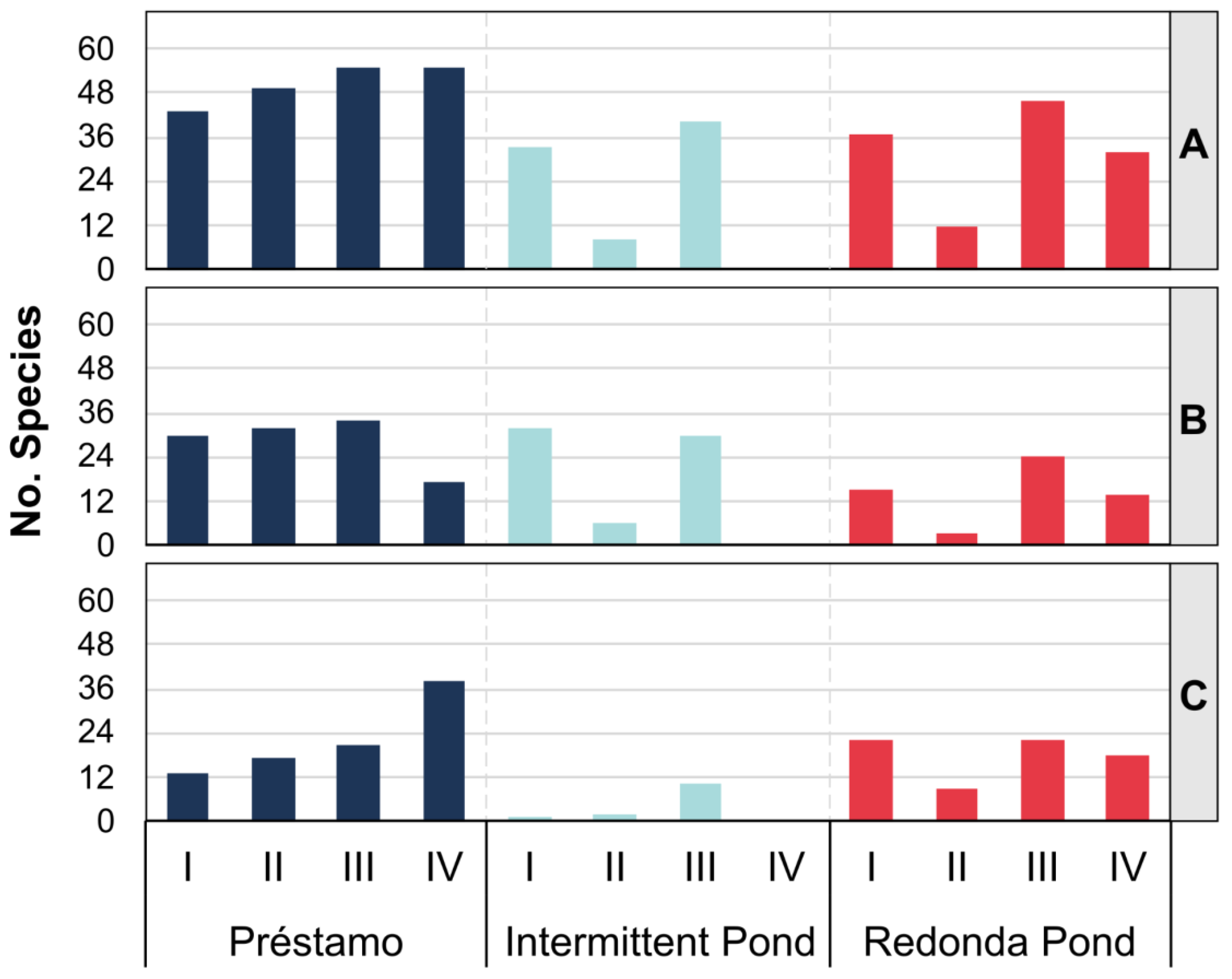
3.6. Rotifer Taxa Richness in Each Species of Aquatic Macrophyte
3.7. The Similarity between Strata
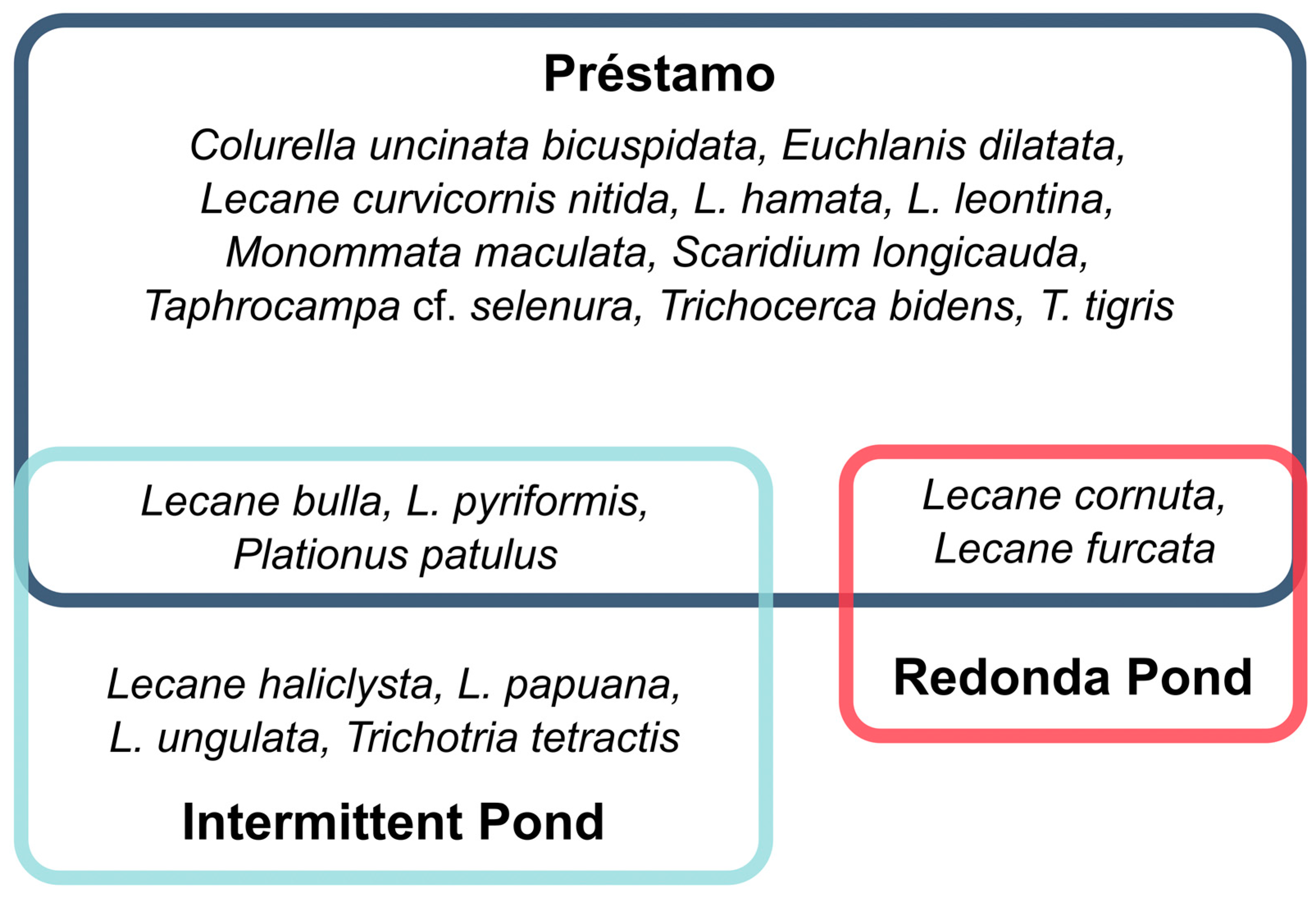
3.8. The Similarity of the Total Richness of Rotifers between Ponds and between Sampling Seasons
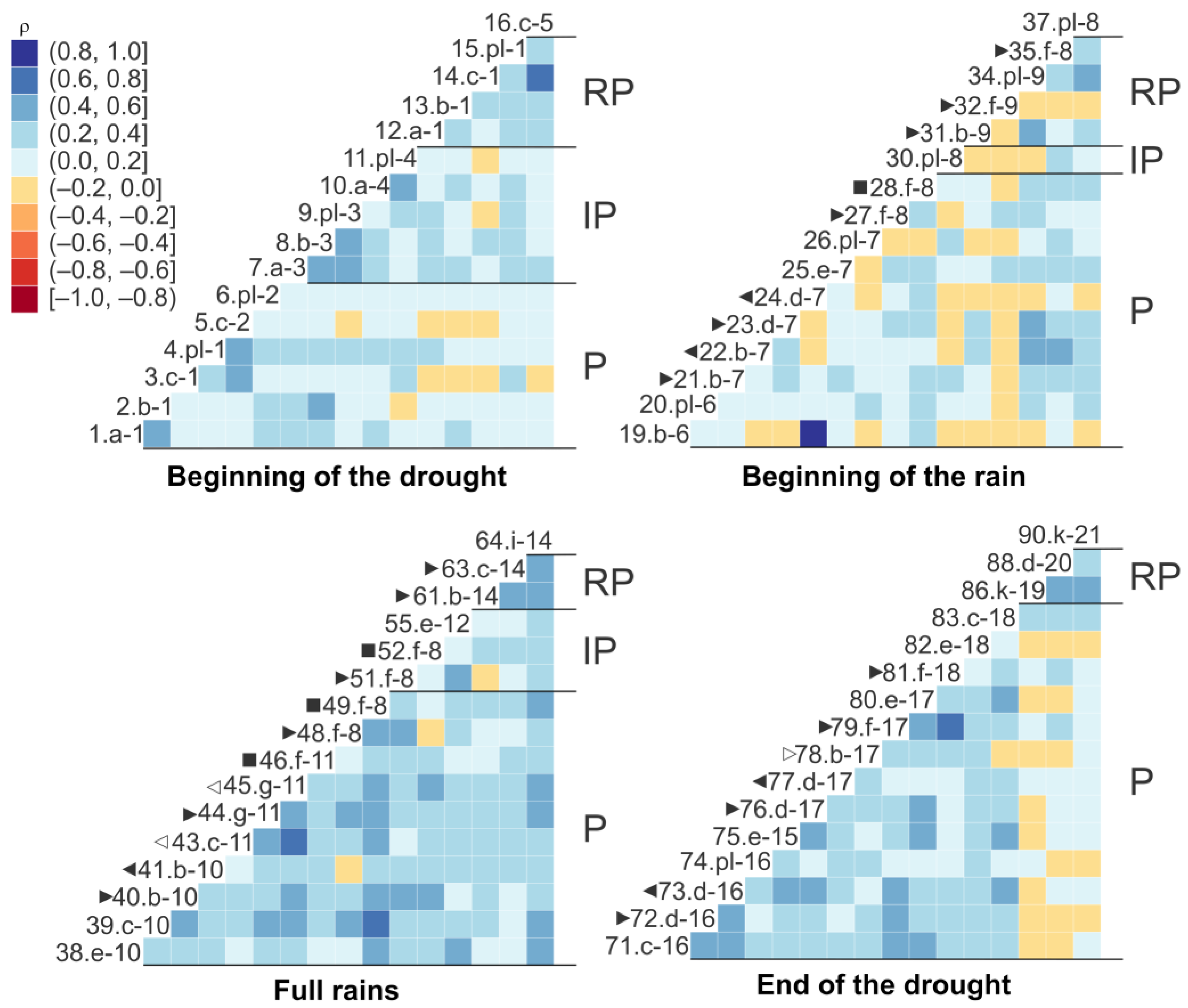
3.9. The Correlation of the Fauna of Littoral and Periphytic Planktonic Rotifers by Pond and Strata in Each Sampling Season
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, P.N.; Henry, R. Is the Littoral Zone Taxonomically and Functionally More Diverse? Investigating the Rotifer Community of a Tropical Shallow Lake. Limnology 2022, 23, 429–440. [Google Scholar] [CrossRef]
- Arndt, H. Rotifers as Predators on Components of the Microbial Web (Bacteria, Heterotrophic Flagellates, Ciliates)—A Review; Springer: Amsterdam, The Netherlands, 1993; pp. 231–246. [Google Scholar]
- Nogrady, T. Rotifera, Volume 1: Biology, Ecology and Systematics. In Guides to the Identification of the Microinvertebrates of the Continental Waters of the World; Backhuys: Leiden, The Netherlands, 1993. [Google Scholar]
- Wallace, R.L. Rotifers: Exquisite Metazoans. Integr. Comp. Biol. 2002, 42, 660–667. [Google Scholar] [CrossRef]
- De Meester, L.; Gómez, A.; Okamura, B.; Schwenk, K. The Monopolization Hypothesis and the Dispersal–Gene Flow Paradox in Aquatic Organisms. Acta Oecologica 2002, 23, 121–135. [Google Scholar] [CrossRef]
- Segers, H. Global Diversity of Rotifers (Rotifera) in Freshwater. Hydrobiologia 2008, 595, 49–59. [Google Scholar] [CrossRef]
- Wallace, R.L.; Snell, T.W. Rotifera. In Ecology and Classification of North American Freshwater Invertebrates; Elsevier: Amsterdam, The Netherlands, 2010; pp. 173–235. [Google Scholar]
- Schindler, D.E.; Scheuerell, M.D. Habitat Coupling in Lake Ecosystems. Oikos 2002, 98, 177–189. [Google Scholar] [CrossRef]
- Ejsmont-Karabin, J.; Karpowicz, M. Rotifera in Lake Subhabitats. Aquat. Ecol. 2021, 55, 1285–1296. [Google Scholar] [CrossRef]
- Cabral, C.R.; Diniz, L.P.; da Silva, A.J.; Fonseca, G.; Carneiro, L.S.; de Melo Júnior, M.; Caliman, A. Zooplankton Species Distribution, Richness and Composition across Tropical Shallow Lakes: A Large-Scale Assessment by Biome, Lake Origin, and Lake Habitat. EDP Sci. 2020, 56, 25. [Google Scholar] [CrossRef]
- Duggan, I.C. The Ecology of Periphytic Rotifers; Springer: Dordrecht, The Netherlands, 2001; pp. 139–148. [Google Scholar]
- Halabowski, D.; Bielańska-Grajner, I.; Lewin, I.; Sowa, A. Diversity of Rotifers in Small Rivers Affected by Human Activity. Diversity 2022, 14, 127. [Google Scholar] [CrossRef]
- Villabona-González, S.L.; Aguirre, N.J.; Estrada, A.L. Influencia de las Macrófitas sobre la Estructura Poblacional de Rotíferos y Microscrustáceos en un Plano de Inundación Tropical. Rev. Biol. Trop 2011, 59, 853–870. [Google Scholar] [CrossRef]
- Meksuwan, P.; Pholpunthin, P.E.; Walsh, J.; Segers, H.; Wallace, R.L. Nestedness in Sessile and Periphytic Rotifer Communities: A Meta-Analysis. Int. Rev. Hydrobiol. 2014, 99, 48–57. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N. Habitat Choice in Rotifera Communities of Three Shallow Lakes: Impact of Macrophyte Substratum and Season. Hydrobiologia 2007, 593, 27–37. [Google Scholar] [CrossRef]
- Serafim-Júnior, M.; Perbiche-Neves, G.; Lansac-Tôha, F.A. An Assessment of the Factors Determining Rotifer Assemblage in River-Lake Systems: The Effects of Seasonality and Habitat. Zool. Curitiba 2019, 36, 1–8. [Google Scholar] [CrossRef]
- Pontin, R.M.; Shiel, R. Periphytic Rotifer Communities of an Australian Seasonal Floodplain Pool. Hydrobiologia 1995, 313, 63–67. [Google Scholar] [CrossRef]
- Lucena-Moya, P.; Duggan, I.C. Macrophyte Architecture Affects the Abundance and Diversity of Littoral Microfauna. Aquat. Ecol. 2011, 45, 279–287. [Google Scholar] [CrossRef]
- Zoppi de Roa, E.; Gordon, E.; González, F.; Montiel, E. El Plancton y la Vegetación en una Sabana Inundable (Apure). Acta Biol. Venez. 2009, 29, 69–83. [Google Scholar]
- Andrade-Sossa, C.; García-Folleco, M.; Rodríguez-Munar, C.A.; Duque, S.R.; Realpe, E. Efectos de la Fluctuación del Nivel del Agua Sobre La Estructura del Ensamblaje de Rotíferos en el Lago Largo (Sistema Yahuarcaca-Llanura de Inundación del Río Amazonas-Colombia). Caldasia 2011, 33, 519–537. [Google Scholar]
- de Paggi, S.B.J.; Muñoz, S.; Frau, D.; Paggi, J.C.; Scarabotti, P.; Devercelli, M.; Meerhoff, M. Horizontal Distribution of Rotifers in a Subtropical Shallow Lake (Paraná Floodplain, Argentina). Fundam. Appl. Limnol. Arch. Furhydrobiol. 2012, 180, 321–333. [Google Scholar] [CrossRef]
- Kuczyńska-Kippen, N.; Špoljar, M.; Mleczek, M.; Zhang, C. Elodeids, but Not Helophytes, Increase Community Diversity and Reduce Trophic State: Case Study with Rotifer Indices in Field Ponds. Ecol. Indic. 2021, 128, 107829. [Google Scholar] [CrossRef]
- Michelangelli, F.; Zoopi de Roa, E.; Pourriot, R. Rotíferos de las Sabanas Inundables. Edo. Apure, Venezuela. Cah Orstom Sér Hydrobiol. 1980, 13, 47–59. [Google Scholar]
- Vásquez, E.; Rey, J.A. Longitudinal Study of Zooplankton along the Lower Orinoco River and Its Delta (Venezuela). Annls Limnol. 1989, 25, 107–120. [Google Scholar] [CrossRef]
- Zoppi de Roa, E.; Pardo, M.J.; Vásquez, W. Nuevas Adiciones a la Fauna de Rotíferos de Venezuela. Rev. Hydrobiol. Trop. 1993, 26, 165–173. [Google Scholar]
- Pardo, M.J.; Zoppi de Roa, E.; Vásquez, W. Estudio Preliminar sobre la Composición del Zooplancton de la Región Sureste del Estado Guárico, Venezuela. Mem. Soc. Cienc. Nat. Salle 1994, 54, 109–121. [Google Scholar]
- López, C.; Ochoa, E. Algunos Rotíferos de la Península de Paraguaná. Acta Científica Venez. 1994, 5, 214–217. [Google Scholar]
- Palacios-Cáceres, M.; de Roa Zoppi, E. Variations in Zooplankton Richness in a Flooding Savanna of Venezuela. Int. Ver. Für Theor. Angew. Limnol. Verh. 1998, 26, 1989–1993. [Google Scholar] [CrossRef]
- Reverol, Y.; Delgado, J.; López, C.; Sánchez, L. Zooplankton Community Composition in Floodplain Lakes of Caura River, Venezuela. Bol. Cent. Investig. Biol. 2008, 42, 53–72. [Google Scholar]
- Torres, R.; Zoppi de Roa, E.; Gordon, E.; González, F.; Delgado, L. Relaciones Plancton-Vegetación en Humedales Continentales de Venezuela. Acta Biol. Venez. 2014, 34, 75–98. [Google Scholar]
- Torres, R.; Zoppi de Roa, E.; Montiel, E. Relación Espacial y Temporal del Zooplancton con la Vegetación Acuática en un Humedal Herbáceo (Península de Paria, Venezuela). Mem. De La Fund. La Salle De Cienc. Nat. 2015, 175, 5–22. [Google Scholar]
- Hortal, J.; Nabout, J.C.; Calatayud, J.; Carneiro, F.M.; Padial, A.; Santos, A.; Siqueira, T.; Bokma, F.; Mauricio Bini, L.; Ventura, M. Perspectives on the Use of Lakes and Ponds as Model Systems for Macroecological Research. J. Limnol. 2014, 73, 46–60. [Google Scholar] [CrossRef]
- Pardo, M.J.; Zoppi de Roa, E. Rotíferos Monogononta Planctónicos en Lagunas de La Reserva de Fauna “Esteros de Camaguán”, Estado Guárico, Venezuela. Acta Biol. Venez. 2014, 34, 165–173. [Google Scholar]
- Cochran, W.G. Sampling Techniques; John Wiley & Sons: Hoboken, NJ, USA, 1977; ISBN 81-265-1524-4. [Google Scholar]
- Matteucci, S.D.; Colma, A. Metodología para el Estudio de la Vegetación; Secretaria General de la Organización de los Estados Americanos: Washington, DC, USA, 1982; p. 22. [Google Scholar]
- Koste, W.; Voigt, M. Rotatoria: Die Radetiere Mitteleuropas. Ein Bestimmungswerk, Begrundet von Max Voigt; Borntraeger: Stuttgart, Germany, 1978. [Google Scholar]
- Koste, W.; Shiel, R. Rotifera from Australian Inland Waters. II. Epiphanidae and Brachionidae (Rotifera: Monogononta). Invertebr. Syst. 1987, 1, 949–1021. [Google Scholar] [CrossRef]
- Koste, W.; Shiel, R. Rotifera from Australian Inland Waters. III. Euchlanidae, Mytilinidae and Trichotriidae (Rotifera: Monogononta). Trans. R Soc. Aust. 1989, 113, 85–114. [Google Scholar]
- Koste, W.; Shiel, R. Rotifera from Australian Inland Waters. IV. Colurellidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. Inc. Inc. Rec. South Aust. Mus. 1989, 113, 119–143. [Google Scholar]
- Koste, W.; Robertson, B. Taxonomic Studies of the Rotifera from Shallow Waters on the Island of Maracá, Roraima, Brazil. Amaz. Limnol. Oecologia Reg. Syst. Fluminis Amazon. 1990, 11, 185–200. [Google Scholar]
- Shiel, R.; Koste, W. Rotifera from Australian Inland Waters. VIII. Trichocercidae (Rotifera: Monogononta). Trans. R. Soc. S. Aust. Inc. Inc. Rec. South Aust. Mus. 1992, 116, 1–27. [Google Scholar]
- López, C. Nuevos Rotíferos para Aguas Continentales de Venezuela. Rev. Hydrobiol. Trop 1993, 26, 65–70. [Google Scholar]
- Segers, H. Rotifera of Some Lakes in the Floodplain of the River Niger (Imo State, Nigeria) I. New Species and Other Taxonomic Considerations. Hydrobiologia 1993, 250, 39–61. [Google Scholar] [CrossRef]
- Segers, H. Guides to the Identification of the Microinvertebrates of the Continental Waters of the World: Volume 2. Rotifera: The Lecanidae (Monogononta); SPB Academic Publishing: Leiden, The Netherlands, 1995. [Google Scholar]
- Segers, H.; Ferrufino, N.L.; De Meester, L. Diversity and Zoogeography of Rotifera (Monogononta) in a Flood Plain Lake of the Ichilo River, Bolivia, with Notes on Little-Known Species. Int. Rev. Hydrobiol. 1998, 83, 439–448. [Google Scholar] [CrossRef]
- de Paggi, S.J.; Koste, W. Additions to the Checklist of Rotifers of the Superorder Monogononta Recorded from Neotropis. Int. Rev. Gesamten Hydrobiol. Hydrogr. 1995, 80, 133–140. [Google Scholar] [CrossRef]
- Jersabek, C.D.; Leitner, M. The Rotifer World Catalog; World Wide Web Electronic Publication: Washington, DC, USA, 2013. [Google Scholar]
- Shiel, R.; Merrick, C.; Ganf, G. The Rotifera of Impoundments in Southeastern Australia; Springer: Berlin/Heidelberg, Germany, 1987; pp. 23–29. [Google Scholar]
- Sneath, P.H.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification, 1st ed.; Donald Kennedy & Roderic B. Park: San Francisco, CA, USA, 1973. [Google Scholar]
- González, E.; Pardo, M.J.; Torres, R.; Scott-Frías, J.; López, C. Studies on Freshwater Zooplankton of Venezuela: Present and Future Perspectives. Limnologica 2022, 126051. [Google Scholar] [CrossRef]
- Vásquez, E.; Pardo, M.J.; Zoppi de Roa, E.; López, C. Rotifer Fauna from Venezuela. Amazoniana 1998, 15, 11–24. [Google Scholar]
- Hauer, J. Rotatorien Aus Venezuela Und Kolumbien. Ergebn Dt Limnol Venez. Exp. 1952, 1, 277–312. [Google Scholar]
- Infante, A. Zooplankton of Lake Valencia (Venezuela): I. Species Composition and Abundance: With 4 Figures and 2 Tables in the Text. Int. Ver. Für Theor. Angew. Limnol. Verh. 1978, 20, 1186–1191. [Google Scholar] [CrossRef]
- Medina, M.; Vásquez, E. Estudio de los Rotíferos de una Laguna de Inundación de Aguas Negras del Bajo Caroni, Venezuela. Mem. Soc. Cienc. Nat. Salle 1988, 48, 105–119. [Google Scholar]
- Saunders, J.F.; Lewis, W.M. Zooplankton Abundance and Transport in a Tropical White-Water River. Hydrobiologia 1988, 162, 147–155. [Google Scholar] [CrossRef]
- Koste, W.; Robertson, B. Taxonomic Studies of the Rotifera (Phylum Aschelminthes) from a Central Amazonian Varzea Lake, Lago Camaleão (Ilha de Marchantaria, Rio Solimões, Amazonas, Brazil). Amaz. Limnol. Oecologia Reg. Syst. Fluminis Amazon. 1983, 8, 225–254. [Google Scholar]
- José de Paggi, S. Composition and Seasonality of Planktonic Rotifers in Limnetic and Littoral Regions of a Floodplain Lake. (Paraná River System). Rev. Hydrobiol. Trop. 1993, 26, 53–63. [Google Scholar]
- Segers, H.; Dumont, H. 102+ Rotifer Species (Rotifera: Monogononta) in Broa Reservoir (SP., Brazil) on 26 August 1994, with the Description of Three New Species. Hydrobiologia 1995, 316, 183–197. [Google Scholar] [CrossRef]
- Segers, H.; De Meester, L. Rotifera of Papua New Guinea, with the Description of a New Scaridium Ehrenberg, 1830. Arch. Für Hydrobiol. 1994, 131, 111–125. [Google Scholar] [CrossRef]
- de Paggi, S.J. Diversidad de Rotíferos Monogonta del Litoral Fluvial Argentino. Miscelánea 2004, 12, 186. [Google Scholar]
- Paggi, J.; José de Paggi, S. Zooplâncton de Ambientes Lóticos e Lênticos Do Rio Paraná Médio. Acta Limnol. Bras. 1990, 3, 685–719. [Google Scholar]
- Bonecker, C.; Lansac-Tôha, F.; Staub, A. Qualitative Study of Rotifers in Different Environments of the High Parana River Floodplain (Ms), Brazil. Rev. Unimar 1994, 16, 1–16. [Google Scholar]
- Rocha, O.; Sendacz, S.; Matsumura-Tundisi, T. Composition, Biomass and Productivity of Zooplankton in Natural Lakes and Reservoirs of Brazil. In Limnology in Brazil; ABC/SLB: Rio de Janeiro, Brazil, 1995; pp. 151–165. [Google Scholar]
- Lansac-Tôha, F.; Bonecker, C.C.; Velho, L.M.; Lima, A.F. Composição, Distribuição e Abundância Da Comunidade Zooplanctônica. Vazzoler Aeam Al 1997, 117–155. [Google Scholar]
- Samanez, I.; Zambrano, F. Observaciones sobre la Diversidad y Algunas Características Ecológicas del Plancton en el Departamento de Madre de Dios, Perú. Publ. Mus. Hist. Nat. UNMSM A 1995, 51, 1–10. [Google Scholar]
- Bonecker, C.; Lansac-Tôha, F. Community Structure of Rotifers in Two Environments of the Upper River Paraná Floodplain (MS)-Brazil. Hydrobiologia 1996, 325, 137–150. [Google Scholar] [CrossRef]
- Aoyagui, A.S.; Bonecker, C.C. Rotifers in Different Environments of the Upper Paraná River Floodplain (Brazil): Richness, Abundance and the Relationship with Connectivity. Hydrobiologia 2004, 522, 281–290. [Google Scholar] [CrossRef]
- Garcia, A.P.P.; Lansac-Tôha, F.A.; Bonecker, C.C. Species Composition and Abundance of Rotifers in Different Environments of the Floodplain of the Upper Paraná River, Brazil. Rev. Bras. Zool. 1998, 15, 327–343. [Google Scholar] [CrossRef]
- Vásquez, E. Estudio de las Comunidades de Rotiferos del Orinoco Medio, Bajo Caroni y Algunas Lagunas de Inundacion (Venezuela). Fund. Salle Cienc. Nat. 1984, 44, 95–108. [Google Scholar]
- Lansac-Tôha, F.; Bonecker, C.; Velho, L.; Simões, N.; Dias, J.; Alves, G.; Takahashi, E. Biodiversity of Zooplankton Communities in the Upper Paraná River Floodplain: Interannual Variation from Long-Term Studies. Braz. J. Biol. 2009, 69, 539–549. [Google Scholar] [CrossRef]
- Hardy, E.R.; Robertson, B.; Koste, W. About the Relationship between the Zooplankton and Fluctuating Water Levels of Lago Camaleão, a Central Amazonian Várzea Lake. Amaz. Limnol. Oecologia Reg. Syst. Fluminis Amazon. 1984, 9, 43–52. [Google Scholar]
- Astiz, S.; Álvarez, H. El Zooplancton en el Alto y Medio Río Orinoco, Venezuela. Acta Científica Venez. 1998, 49, 5–18. [Google Scholar]
- Myers, F.J. The Rotatorian Fauna of the Pocono Plateau and Environs. Proc. Acad. Nat. Sci. Phila. 1942, 94, 251–285. [Google Scholar]
- Pejler, B. Relation to Habitat in Rotifers. In Rotifera VII: Proceedings of the Seventh Rotifer Symposium; Springer: Dordrecht, The Netherlands, 1995; Volume 109, pp. 267–278. [Google Scholar]


| Taxa | GD | Secondary Strata | Season |
|---|---|---|---|
| Brachionus ahlstromi Lindeman, 1939 | PT | pl | IV |
| Brachionus bidentatus Anderson, 1889 | PT | pl | I |
| Brachionus dolabratus Harring, 1914 | NT | pl | IV |
| Brachionus mirus Daday, 1905 | NT | pl | I, IV |
| Brachionus quadridentatus Hermann, 1783 | C | pl, e, f, h | I, III, IV |
| Cephalodella forficula (Ehrenberg, 1838) | C | d, e, k | IV |
| Cephalodella gibba (Ehrenberg, 1830) | C | c, d, e, f, k | I, II, IV |
| Colurella colurus (Ehrenberg, 1830) | C | f | III |
| Colurella obtusa (Gosse, 1886) | C | b, c, f | III |
| Colurella uncinata (Müller, 1773) | C | d | IV |
| ** Colurella uncinata bicuspidata (Ehrenberg, 1830) | C | pl, b, c, d, e | I, II, III, IV |
| ** Dicranophorus forcipatus (Müller, 1786) | C | pl, a, b, f | I, II, III |
| Dicranophorus sp. | b, d | I, II, IV | |
| * Dipleuchlanis ornata Segers, 1993 | UD | pl, b, c | II |
| Dipleuchlanis propatula (Gosse, 1886) | C | pl, b, c, d, e, f | III, IV |
| Euchlanis dilatata Ehrenberg, 1830 | C | pl, b, f, k | I, II, III, IV |
| Euchlanis dilatata lucksiana Hauer, 1930 | C | pl, a, b | I, II, IV |
| Euchlanis incisa Carlin, 1939 | C | pl, a, b, e | I, II, III |
| Filinia opoliensis (Zacharias, 1898) | PT | pl, c | I, IV |
| Filinia terminalis (Plate, 1886) | C | pl, c, d | I |
| Hexarthra intermedia (Wiszniewski, 1929) | C | pl, c, d | I |
| Hexarthra intermedia brasiliensis (Hauer, 1953) | PT | pl | I, II |
| Keratella americana Carlin, 1943 | PT? | pl, f | I, IV |
| Keratella cochlearis (Gosse, 1851) | C | pl, c, e, f | I, II |
| Keratella lenzi Hauer, 1953 | PT | pl | II |
| Keratella procurva (Thorpe, 1891) | PT | pl, c | I, IV |
| Keratella tropica (Apstein, 1907) | C | pl | I, II, III |
| Lecane arcula Harring, 1914 | C | pl, b, c, d, f, g, j, k | III, IV |
| Lecane bulla (Gosse, 1851) | C | pl, a, b, c, d, e, f, g, i | I, II, III, IV |
| Lecane closterocerca (Schmarda, 1859) | C | pl, a, b, c, d, e, f, i, j, k | I, III, IV |
| Lecane cornuta (Müller, 1786) | C | pl, a, b, c, d, e, f, g, i, j, k | I, II, III, IV |
| Lecane crepida Harring, 1914 | PT | pl, b, f, g | III |
| Lecane curvicornis nitida (Murray, 1913) | PT | pl, a, b, c, d, e, f, g, i, k | I, II, III, IV |
| Lecane decipiens (Murray, 1913) | C | pl, b, d, e, f | I, II, III, IV |
| Lecane doryssa Harring, 1914 | PT | c, f, g, i | II, III |
| ** Lecane elegans Harring, 1914 | PT | a, b, c, d, f, i | I, II, III, IV |
| Lecane elongata Harring & Myers, 1926 | PT | f | III |
| Lecane furcata (Murray, 1913) | C | a, b, c, d, f, i, k | I, II, III, IV |
| Lecane haliclysta Harring & Myers, 1926 | PT | pl, a, b, c, e, f, g, i | I, II, III |
| Lecane hamata (Stokes, 1896) | C | pl, a, b, c, d, e, f, g, i, k | I, II, III, IV |
| Lecane hastata (Murray, 1913) | C | pl, b, c, f, k | I, II, IV |
| Lecane hornemanni (Ehrenberg, 1834) | C | pl, b, f | I, III |
| Lecane inermis (Bryce, 1892) | C | pl, b, c, e, f, g, i, j, k | II, III, IV |
| Lecane leontina (Turner, 1892) | C | pl, a, b, c, d, e, f, g, h, i | I, II, III, IV |
| Lecane levistyla (Olofsson, 1917) | C | g | III |
| Lecane ludwigii (Eckstein, 1883) | C | pl, b, d, g | I, II, III, IV |
| Lecane lunaris (Ehrenberg, 1832) | C | pl, a, b, e, f, h | I, II, III |
| Lecane monostyla (Daday, 1897) | C | pl | III |
| Lecane papuana (Murray, 1913) | PT | pl, b, c, e, f, h, k | I, II, III, IV |
| Lecane proiecta Hauer, 1956 | NT | pl | IV |
| Lecane punctata (Murray, 1913) | C | d | IV |
| ** Lecane pusilla Harring, 1914 | C | b, c, f | III |
| Lecane pyriformis (Daday, 1905) | C | pl, a, b, c, d, f, g, k | I, II, III, IV |
| Lecane quadridentata (Ehrenberg, 1830) | C | pl, d, e, f, i | I, II, III, IV |
| Lecane rhytida Harring & Myers, 1926 | PT | b, c, d, e, f, i | I, III, IV |
| Lecane signifera ploenensis (Voigt, 1902) | C | pl, b, c, d, e, f, i | I, II, III |
| Lecane ungulata (Gosse, 1887) | C | pl, a, b, c, e, f, g, k | I, II, III, IV |
| Lepadella cf. heterodactyla Fadeev, 1925 | C | f | III |
| Lepadella acuminata (Ehrenberg, 1834) | C | e | IV |
| Lepadella dactyliseta (Stenroos, 1898) | C | h | I |
| ** Lepadella donneri Koste, 1972 | NT | pl, c, d, e, f, g | I, III, IV |
| Lepadella imbricata Harring, 1914 | C | c, d, f | III, IV |
| Lepadella latusinus (Hilgendorf, 1899) | PT | d, e, f | III, IV |
| Lepadella ovalis (Müller, 1786) | C | b, c, d | I, IV |
| Lepadella patella (Müller, 1773) | C | pl, c, d | I, II, III, IV |
| ** Lepadella quinquecostata (Lucks, 1912) | C | pl, c, k | III, IV |
| Lepadella rhomboides (Gosse, 1886) | C | pl, b, c, h | I, III, IV |
| Lepadella triptera (Ehrenberg, 1830) | C | pl, c | III |
| Macrochaetus collinsii (Gosse, 1867) | C | pl, b, e | I, III |
| Macrochaetus sericus (Thorpe, 1893) | C | pl, b, e, f | III |
| Monommata maculata Harring & Myers, 1930 | C | pl, b, c, e, f | I, II, III, IV |
| Mytilina bisulcata (Lucks, 1912) | C | pl | IV |
| Mytilina michelangellii Reid & Turner, 1988 | PT | pl, b, f | I, III |
| ** Mytilina unguipes (Lucks, 1912) | C | pl, d, f | III, IV |
| Mytilina ventralis (Ehrenberg, 1830) | C | pl, b, c, d, e, g | II, III, IV |
| Plationus patulus (Müller, 1786) | C | pl | I, II, III, IV |
| Plationus patulus macracanthus (Daday, 1905) | PT | pl, e, f | I, II, III |
| Platyias leloupi Gillard, 1957 | PT | pl, d | II, IV |
| Platyias quadricornis (Ehrenberg, 1832) | C | pl, b, c, d, e, f | II, IV |
| Polyarthra dolichoptera Idelson, 1925 | C | pl | I, IV |
| Polyarthra remata Skorikov, 1896 | C | pl | I, II, III, IV |
| Polyarthra vulgaris Carlin, 1943 | C | pl | III |
| Scaridium longicauda (Müller, 1786) | C | pl, b, c, e | I, II, III, IV |
| Synchaeta stylata Wierzejski, 1893 | C | pl | IV |
| ** Taphrocampa cf. annulosa Gosse, 1851 | C | d, e | IV |
| ** Taphrocampa cf. selenura Gosse, 1887 | C | pl, b, c, d, e | I, II, III, IV |
| Testudinella mucronata (Gosse, 1886) | C | pl, b, c | III |
| Testudinella mucronata haueriensis Gillard, 1967 | PT | pl, b | I, II, III |
| Testudinella patina (Hermann, 1783) | C | pl, a, b, c, e, f, g, k | I, II, III, IV |
| Testudinella patina dendradena Beauchamp, 1955 | C | pl, a, b, c, d | I, III, IV |
| Trichocerca bicristata (Gosse, 1887) | C | pl, b, e | I |
| Trichocerca bidens (Lucks, 1912) | C | pl, b, d, e, f | I, III, IV |
| Trichocerca braziliensis (Murray, 1913) | PT | pl, b, c, e, g, k | II, III, IV |
| Trichocerca insignis (Herrick, 1885) | C | pl | I |
| ** Trichocerca cf. kostei Segers, 1993 | PT | d, e | IV |
| Trichocerca mucosa (Stokes, 1896) | pl, b, c, d, f, k | I, II, III, IV | |
| Trichocerca myersi (Hauer, 1931) | C | pl | III |
| Trichocerca pusilla (Jennings, 1903) | C | pl, e | III, IV |
| Trichocerca similis grandis Hauer, 1965 | NT | pl | I, III, IV |
| Trichocerca tenuior (Gosse, 1886) | C | pl, a, e, f | I, II, III |
| Trichocerca tigris (Müller, 1786) | C | pl, b, c, d, f, h | I, II, III, IV |
| Trichotria tetractis (Ehrenberg, 1830) | C | pl, a, b, e, f | I, II, III, IV |
| Sessile org. | I, II, III, IV |
| Taxa | Beginning of the Drought | Beginning of the Rain | Full Rains | End of the Drought |
|---|---|---|---|---|
| Euchlanis incisa | 44 | 5 | 9 | 0 |
| Lecane bulla | 64 | 26 | 61 | 19 |
| Lecane cornuta | 78 | 42 | 33 | 67 * |
| Lecane haliclysta | 33 | 16 | 82 | 0 |
| Lecane hastata | 44 | 21 | 49 | 38 |
| Lecane inermis | 0 | 16 | 49 | 19 |
| Lecane leontina | 83 | 63 | 36 | 48 * |
| Lecane papuana | 33 | 42 | 3 | 10 |
| Lecane rhytida | 11 | 0 | 18 | 43 * |
| Taphrocampa cf. selenura | 6 | 5 | 3 | 43 * |
| Season | Taxa | Habitats | P | IP | RP |
|---|---|---|---|---|---|
| Beginning of the drought | Lecane curvicornis nitida | M. polycarpa | 50 | ||
| Lecane bulla | C. blepharoleptos | 44 | |||
| Plationus patulus | Plankton | 41 | |||
| Lecane cornuta | C. blepharoleptos | 46 | |||
| Beginning of the rain | Lecane cornuta | P. crassipes | 67 | ||
| P. cordata | 50 | ||||
| Lecane leontina | P. crassipes | 50 | |||
| P. crassipes | 50 | ||||
| Plankton | 75 | ||||
| Polyarthra remata | Plankton | 84 | |||
| Keratella cochlearis | H. amplexicaulis | 50 | |||
| Lecane haliclysta | H. amplexicaulis | 50 | |||
| Lecane inermis | H. amplexicaulis | 100 | |||
| Lecane papuana | Plankton | 40 | |||
| Full rains | Lecane cornuta | S. auriculata | 53 | ||
| Lecane closterocerca | Aeschynomene sp. | 33 | |||
| Lecane haliclysta | P. crassipes | 100 | |||
| Lecane inermis | M. polycarpa | 38 | |||
| H. amplexicaulis | 70 | 61 | 38 | ||
| M. polycarpa | 39 | ||||
| Testudinella patina | H. amplexicaulis | 43 | |||
| Plationus patulus macracanthus | Plankton | 34 | 32 | ||
| End of the drought | Lecane arcula | P. cordata | 33 | ||
| Lecane cornuta | S. auriculata | 33 | |||
| Lecane leontina | S. auriculata | 33 | |||
| Lecane proiecta | Plankton | 55 | |||
| Lecane quadridentata | S. auriculata | 30 | |||
| Lecane rhytida | S. auriculata | 33 | |||
| M. polycarpa | 30 | ||||
| Synchaeta stylata | Plankton | 41 |
| Taxa | V | PL |
|---|---|---|
| Brachionus ahlstromi | X | |
| Brachionus bidentatus | X | |
| Brachionus dolabratus | X | |
| Brachionus mirus | X | |
| Cephalodella forficula | X | |
| Cephalodella gibba | X | |
| Colurella colurus | X | |
| Colurella obtusa | X | |
| Colurella uncinata | X | |
| Dicranophorus sp. | X | |
| Dipleuchlanis ornata | X | |
| Hexarthra intermedia brasiliensis | X | |
| Keratella lenzi | X | |
| Keratella tropica | X | |
| Lecane doryssa | X | |
| Lecane elegans | X | |
| Lecane elongata | X | |
| Lecane levistyla | X | |
| Lecane monostyla | X | |
| Lecane proiecta | X | |
| Lecane punctata | X | |
| Lecane pusilla | X | |
| Lecane rhytida | X | |
| Lepadella acuminata | X | |
| Lepadella dactyliseta | X | |
| Lepadella heterodactyla | X | |
| Lepadella imbricata | X | |
| Lepadella latusinus | X | |
| Lepadella ovalis | X | |
| Lepadella rhomboides | X | |
| Lepadella triptera | X | |
| Mytilina bisulcata | X | |
| Plationus patulus | X | |
| Platyias quadricornis | X | |
| Polyarthra dolichoptera | X | |
| Polyarthra remata | X | |
| Polyarthra vulgaris | X | |
| Synchaeta stylata | X | |
| Taphrocampa cf.annulosa | X | |
| Trichocerca insignis | X | |
| Trichocerca cf. kostei | X | |
| Trichocerca myersi | X | |
| Trichocerca similis grandis | X |
| Number of Taxa | |||
|---|---|---|---|
| Macrophyte | All Plant | Roots | Stems/Leaves |
| Aeschynomene sp. | 5 | ||
| Cyperus sp. | 19 | 12 | 13 |
| Pontederia crassipes | 56 | 38 | 13 |
| Echinochloa crus-galli | 18 | ||
| Hymenachne amplexicaulis | 52 | 40 | 38 |
| Ludwigia helminthorrhiza | 7 | ||
| Marsilea polycarpa | 51 | 25 | 18 |
| Cyperus blepharoleptos | 21 | ||
| Pontederia cordata | 45 | 30 | 33 |
| Paspalum repens | 15 | ||
| Salvinia auriculata | 46 | ||
| All Plants | Plankton | P. crassipes | M. polycarpa | P. cordata | S. auriculata | H. amplexicaulis | |
|---|---|---|---|---|---|---|---|
| All plants | 1.00 | ||||||
| Plankton | 0.57 | 1.00 | |||||
| P. crassipes | 0.67 | 0.50 | 1.00 | ||||
| M. polycarpa | 0.61 | 0.42 | 0.52 | 1.00 | |||
| P. cordata | 0.54 | 0.30 | 0.36 | 0.47 | 1.00 | ||
| S. auriculata | 0.55 | 0.38 | 0.44 | 0.36 | 0.40 | 1.00 | |
| H. amplexicaulis | 0.62 | 0.41 | 0.54 | 0.44 | 0.38 | 0.44 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, M.J.; Scott-Frías, J.; Soto, L.M.; Stamou, G.; Michaloudi, E.; Torres, R.; González, E.; López, C. Rotifers (Rotifera: Monogononta) Associated with Littoral Macrophyte Habitats in Flooded Neotropical Ponds: A Qualitative Study. Diversity 2023, 15, 590. https://doi.org/10.3390/d15050590
Pardo MJ, Scott-Frías J, Soto LM, Stamou G, Michaloudi E, Torres R, González E, López C. Rotifers (Rotifera: Monogononta) Associated with Littoral Macrophyte Habitats in Flooded Neotropical Ponds: A Qualitative Study. Diversity. 2023; 15(5):590. https://doi.org/10.3390/d15050590
Chicago/Turabian StylePardo, María José, Joxmer Scott-Frías, Luz Marina Soto, Georgia Stamou, Evangelia Michaloudi, Rubén Torres, Ernesto González, and Carlos López. 2023. "Rotifers (Rotifera: Monogononta) Associated with Littoral Macrophyte Habitats in Flooded Neotropical Ponds: A Qualitative Study" Diversity 15, no. 5: 590. https://doi.org/10.3390/d15050590
APA StylePardo, M. J., Scott-Frías, J., Soto, L. M., Stamou, G., Michaloudi, E., Torres, R., González, E., & López, C. (2023). Rotifers (Rotifera: Monogononta) Associated with Littoral Macrophyte Habitats in Flooded Neotropical Ponds: A Qualitative Study. Diversity, 15(5), 590. https://doi.org/10.3390/d15050590








