Microcrustaceans (Cladocera and Copepoda) of the Boreal/Tropical Transition Zone in the Russian Far East: A Case Study of Species Associations in Three Large Lakes
Abstract
1. Introduction
2. Materials and Methods
- (1)
- WE, widely distributed Eurasian faunistic complex;
- (2)
- EAA, widely distributed in East Asia and could penetrate North America;
- (3)
- EA, endemics belonging to the Far Eastern zone of endemism;
- (4)
- ST, southern tropical;
- (5)
- WS, non-revised widely distributed species.
3. Results
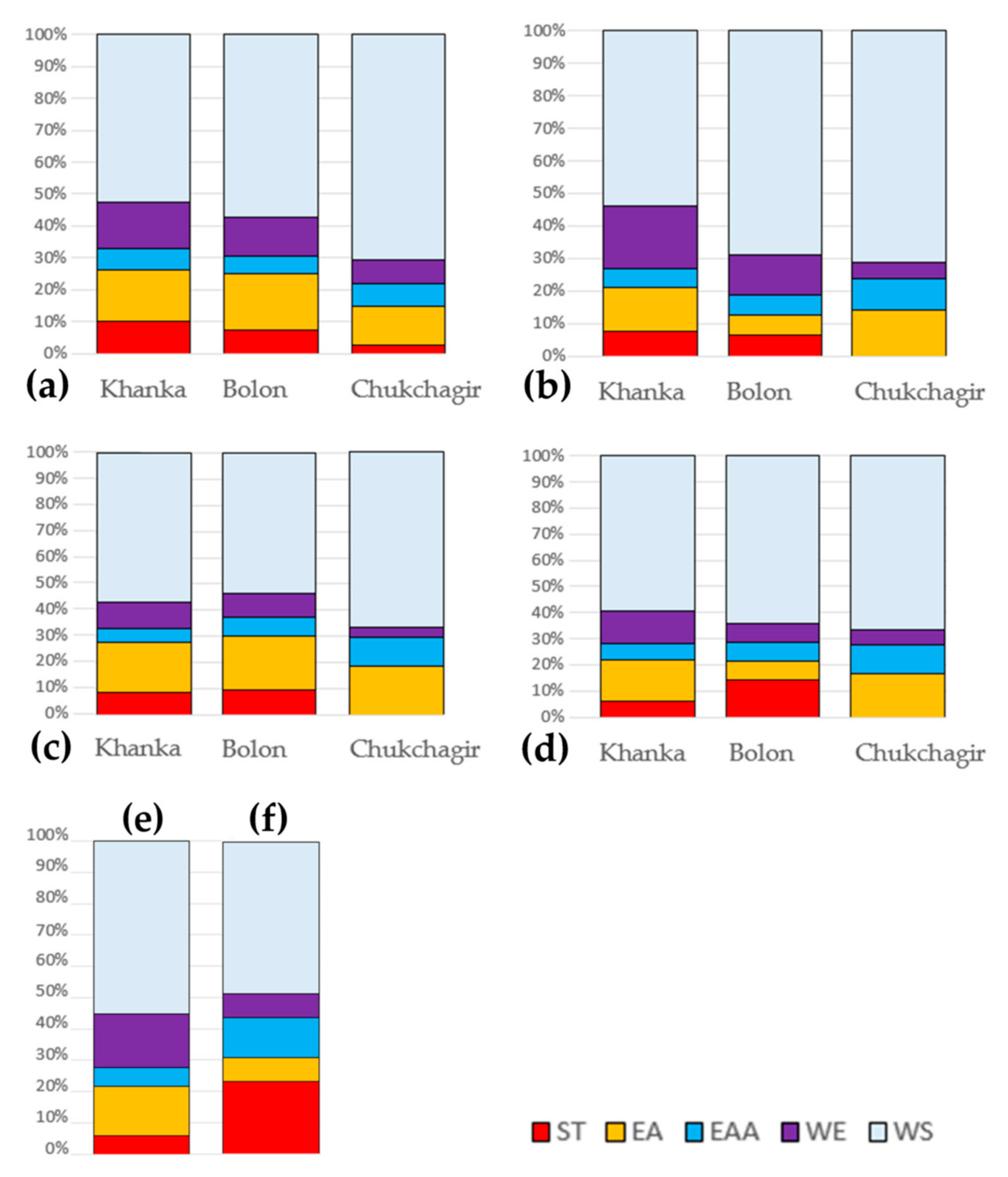
3.1. Species Richness
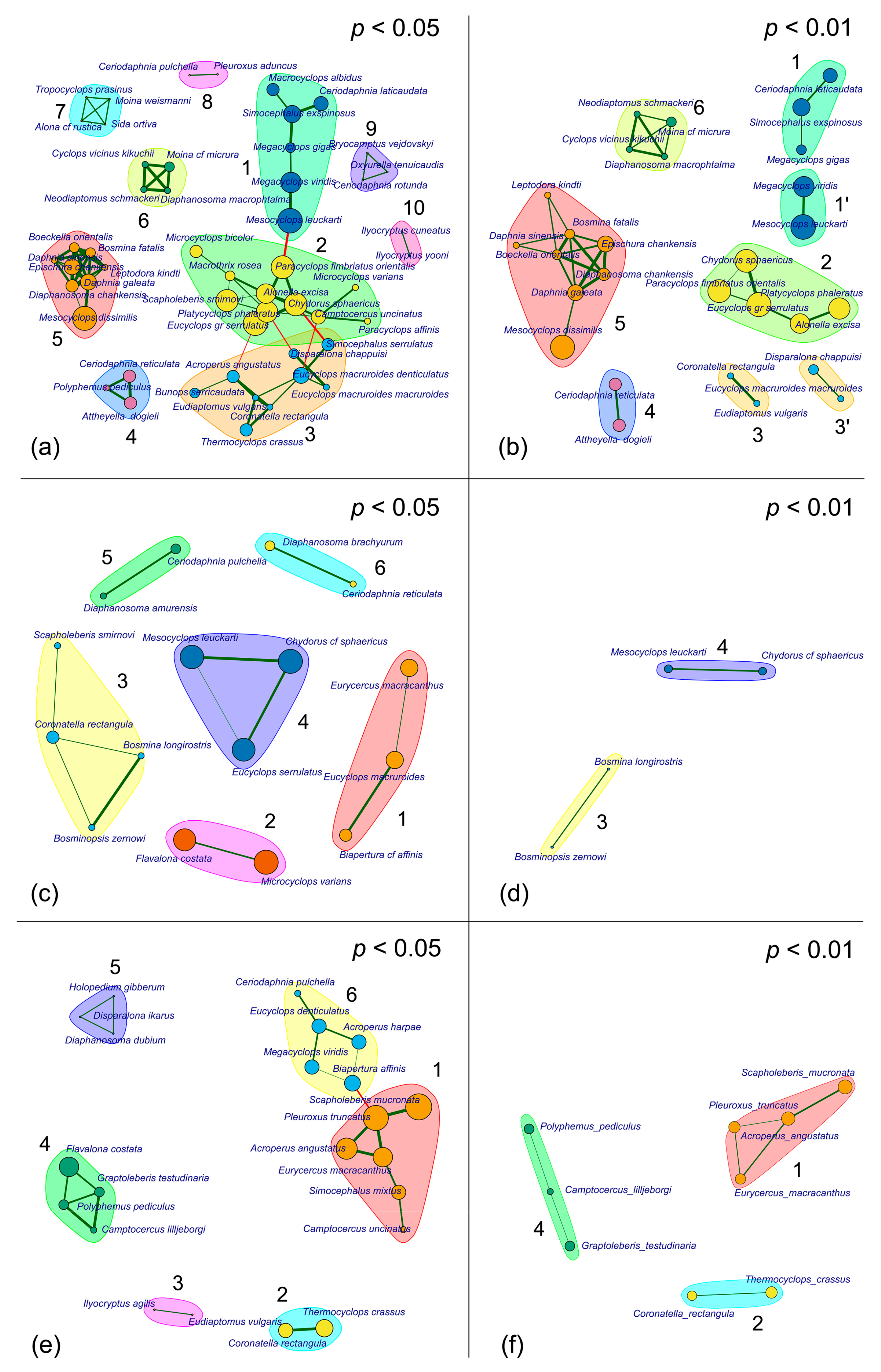
3.2. Association Structure
3.3. Coenophilous and Coenophobic Taxa
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallace, A.R. The Geographical Distribution of Animals; Harper and Brothers: New York, NY, USA, 1876. [Google Scholar]
- Dumont, H.J.; Negrea, Ș. Branchiopoda; Backhuys: Leiden, The Netherlands, 2002; ISBN 9789057821127. [Google Scholar]
- Boxshall, G.A.; Defaye, D. Global diversity of copepods (Crustacea: Copepoda) in freshwater. Hydrobiologia 2008, 595, 195–207. [Google Scholar] [CrossRef]
- Darwin, C. On the dispersal of freshwater Bivalves. Nature 1882, 25, 529–530. [Google Scholar] [CrossRef]
- Baas Becking, L. Geobiologie of Inleiding Tot de Milieukunde; W.P. Van Stockum & Zoon: Hague, The Netherlands, 1934. [Google Scholar]
- Frey, D.G. Questions concerning cosmopolitanism in Cladocera. Arch. Hydrobiol. 1982, 93, 484–502. [Google Scholar]
- Frey, D.G. The Non-Cosmopolitanism of Chydorid Cladocera: Implications for Biogeography and Evolution; A.A. Balkema: Rotterdam, The Netherlands, 1987; ISBN 90-6191-593-7. [Google Scholar]
- Frey, D.G. The taxonomy and biogeography of the Cladocera. Hydrobiologia 1987, 145, 5–17. [Google Scholar] [CrossRef]
- Frey, D.G. Separation of Pleuroxus laevis Sars, 1861 from two resembling species in North America: Pleuroxus straminius Birge, 1879 and Pleuroxus chiangi n.sp. (Cladocera, Chydoridae). Can. J. Zool. 1988, 66, 2534–2563. [Google Scholar] [CrossRef]
- Frey, D.G. Species of Pleuroxus (Anomopoda, Chydoridae) from the subantarctic islands and southernmost South America: A partial unravelling of the Pleuroxus aduncus problem. Hydrobiologia 1993, 262, 145–188. [Google Scholar] [CrossRef]
- Smirnov, N.N. Chydoridae Fauni Mira. Fauna SSSR. Rakoobraznie [Chydoridae of the World’s Fauna. Fauna of USSR. Crustacea]; Nauka: St. Petersburg, Russia, 1971. [Google Scholar]
- Smirnov, N.N. The Macrothricidae of the World; SPB Academic Publishing: Hague, The Netherlands, 1992. [Google Scholar]
- Smirnov, N.N. Cladocera: The Chydorinae and Sayciinae (Chydoridae) of the World; SPB Academic Publishing: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Taylor, D.J.; Finston, T.L.; Hebert, P.D.N. Biogeography of a widespread freshwater crustacean: Pseudocongruence and cryptic endemism in the North American Daphnia laevis complex. Evolution 1998, 52, 1648–1670. [Google Scholar] [CrossRef]
- De Melo, R.; Hebert, P.D.N. A taxonomic reevaluation of North American Bosminidae. Can. J. Zool. 1994, 72, 1808–1825. [Google Scholar] [CrossRef]
- Adamowicz, S.J.; Hebert, P.D.N.; Marinone, M.C. Species diversity and endemism in the Daphnia of Argentina: A genetic investigation. Zool. J. Linn. Soc. 2004, 140, 171–205. [Google Scholar] [CrossRef]
- Adamowicz, S.J.; Petrusek, A.; Colbourne, J.K.; Hebert, P.D.N.; Witt, J.D.S. The scale of divergence: A phylogenetic appraisal of intercontinental allopatric speciation in a passively dispersed freshwater zooplankton genus. Mol. Phylogenet. Evol. 2009, 50, 423–436. [Google Scholar] [CrossRef] [PubMed]
- Kotov, A.A.; Garibian, P.G.; Bekker, E.I.; Taylor, D.J.; Karabanov, D.P. A new species group from the Daphnia curvirostris species complex (Cladocera: Anomopoda) from the eastern Palaearctic: Taxonomy, phylogeny and phylogeography. Zool. J. Linn. Soc. 2021, 191, 772–822. [Google Scholar] [CrossRef]
- Ma, X.; Petrusek, A.; Wolinska, J.; Gieβler, S.; Zhong, Y.; Yang, Z.; Hu, W.; Yin, M. Diversity of the Daphnia longispina species complex in Chinese lakes: A DNA taxonomy approach. J. Plankton Res. 2015, 37, 56–65. [Google Scholar] [CrossRef]
- Lakatos, C.; Urabe, J.; Makino, W. Cryptic diversity of Japanese Diaphanosoma (Crustacea: Cladocera) revealed by morphological and molecular assessments. Inland Waters 2015, 5, 253–262. [Google Scholar] [CrossRef]
- Schizas, N.V.; Street, G.T.; Coull, B.C.; Chandler, G.T.; Quattro, J.M. Molecular population structure of the marine benthic copepod Microarthridion littorale along the southeastern and Gulf coasts of the USA. Mar. Biol. 1999, 135, 399–405. [Google Scholar] [CrossRef]
- Rocha-Olivares, A.; Fleeger, J.W.; Foltz, D.W. Decoupling of molecular and morphological evolution in deep lineages of a meiobenthic harpacticoid copepod. Mol. Biol. Evol. 2001, 18, 1088–1102. [Google Scholar] [CrossRef] [PubMed]
- Garlitska, L.; Neretina, T.; Schepetov, D.; Mugue, N.; de Troch, M.; Baguley, J.G.; Azovsky, A. Cryptic diversity of the ‘cosmopolitan’ harpacticoid copepod Nannopus palustris: Genetic and morphological evidence. Mol. Ecol. 2012, 21, 5336–5347. [Google Scholar] [CrossRef]
- Marrone, F.; Lo Brutto, S.; Hundsdoerfer, A.K.; Arculeo, M. Overlooked cryptic endemism in copepods: Systematics and natural history of the calanoid subgenus Occidodiaptomus Borutzky 1991 (Copepoda, Calanoida, Diaptomidae). Mol. Phylogenet. Evol. 2013, 66, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Hołyńska, M.; Wyngaard, G.A. Towards a phylogeny of Cyclops (Copepoda): (in)congruences among morphology, molecules and zoogeography. Zool. Scripta 2019, 48, 376–398. [Google Scholar] [CrossRef]
- Kotov, A.A.; Korovchinsky, N.M.; Sinev, A.Y.; Smirnov, N.N. Cladocera (Crustacea, Branchiopoda) of the Zeya basin (Amurskaya Area, Russian Federation). 3. Systematic-faunistic and zoogeographic analysis. Zool. Zhurnal 2011, 90, 402–411. [Google Scholar]
- Garibian, P.G.; Neretina, A.N.; Korovchinsky, N.M.; Sinev, A.Y.; Tchabovsky, A.V.; Kotov, A.A.; Smirnov, N.N. The Southern part of Russian Far East and Korean Peninsula as a transition zone between the boreal and tropical faunas of the waterfleas (Cladocera, Crustacea). Zool. Zhurnal 2020, 99, 1094–1109. [Google Scholar] [CrossRef]
- Garibian, P.G.; Chertoprud, E.S. First records of Cladocera and Copepoda from Chukchagir Lake and its basin (Khabarovsk Territory, Far East of Russia). Arthropoda Sel. 2022, 31, 10–18. [Google Scholar] [CrossRef]
- Kotov, A.A.; Sinev, A.Y.; Korovchinsky, N.M.; Smirnov, N.N.; Bekker, E.I.; Sheveleva, N.G. Cladocera (Crustacea, Branchiopoda) of the Zeya basin (Amurskaya Area, Russian Federation). 1. New taxa for fauna of Russia. Zool. Zhurnal 2011, 90, 131–142. [Google Scholar]
- Kotov, A.A.; Jeong, H.G.I.; Lee, W. Cladocera (Crustacea: Branchiopoda) of the south-east of the Korean Peninsula, with twenty new records for Korea. Zootaxa 2012, 3368, 50–90. [Google Scholar] [CrossRef]
- Kotov, A.A. Faunistic complexes of the Cladocera (Crustacea, Branchiopoda) of Eastern Siberia and the Far East of Russia. Zool. Zhurnal 2016, 95, 748–768. [Google Scholar] [CrossRef]
- Kotov, A.A.; Seleznev, D.G.; Garibian, P.G.; Korovchnsky, N.M.; Neretina, A.N.; Sinev, A.Y.; Jeong, H.-G.; Yang, H.-M.; Lee, W. History of Colonization of Jeju Island (Republic of Korea) by the Water Fleas (Crustacea: Cladocera) Is Reflected by the Seasonal Changes in Their Fauna and Species Associations. Water 2022, 14, 3394. [Google Scholar] [CrossRef]
- Barabanshchikov, E.I. Results of zooplankton studies in Khanka Lake in September 2020. V. Ya. Levanidov’s Mem. Meet. 2021, 9, 130–139. [Google Scholar] [CrossRef]
- Garibian, P.G.; Chertoprud, E.S.; Sinev, A.Y.; Korovchinsky, N.M.; Kotov, A.A. Cladocera and Copepoda (Crustacea: Branchiopoda) of the Lake Bolon and its basin (Far East of Russia). Arthropoda Sel. 2019, 28, 37–63. [Google Scholar] [CrossRef]
- Abell, R.; Thieme, M.L.; Revenga, C.; Bryer, M.; Kottelat, M.; Bogutskaya, N.; Coad, B.; Mandrak, N.; Balderas, S.C.; Bussing, W.; et al. Freshwater Ecoregions of the World: A New Map of Biogeographic Units for Freshwater Biodiversity Conservation. Bioscience 2008, 58, 403–414. [Google Scholar] [CrossRef]
- Flenniken, J.M.; Stuglik, S.; Iannone, B.V. Quantum GIS (QGIS): An introduction to a free alternative to more costly GIS platforms. EDIS 2020, 2020, 7. [Google Scholar] [CrossRef]
- Borutsky, E.V. Crustaceans Freshwater Harpacticoids. In Fauna of USSR, Crustacea 3; AN USSR Publishing: Moscow, Russia, 1952; pp. 1–425. [Google Scholar]
- Dussart, B.H.; Defaye, D. Répertoire mondial des Crustacés Copépodes des eaux Intérieures. In Calanoïdes; CNRS: Bordeaux, France, 1983. [Google Scholar]
- Krupa, E.G.; Dobrochotova, O.V.; Struge, T.C. Fauna of Calanoida (Crustacea: Copepoda) of Kazakhstan and Adjacent Territories; Etalon Print: Almaty, Kazakhstan, 2016. [Google Scholar]
- Borutsky, E.V.; Stepanova, L.A.; Kos, M.S. Key to Identification of Calanoida from Fresh Waters; Nauka: St. Petersburg, Russia, 1991. [Google Scholar]
- Alekseev, V.R.; Barabanshchikov, E.I. A species of the genus Mesocyclops (Cyclopoida, Copepoda) from Lake Khanka new for the Russian Fauna. Zool. Zhurnal 2006, 85, 1257–1260. [Google Scholar]
- Alekseev, V.R.; Chaban, O.A. New records of continental cyclopids (Crustacea: Copepoda: Cyclopiformes) from Eastern Siberia and Russian Far East. Arthropoda Sel. 2021, 30, 503–520. [Google Scholar] [CrossRef]
- Alexeev, V.R.; Tsalolokhin, S.Y. (Eds.) Key Book for Zooplankton and Zoobenthos of Fresh Waters of European Russia; KMK Press: Moscow, Russia, 2010; Volume 1, ISBN 9785873176847. [Google Scholar]
- Dussart, B.; Defaye, D. World Directory of Crustacea Copepoda of Inland Waters. In II Cyclopiformes; Backhuys Publishers: Leiden, The Netherlands, 2006. [Google Scholar]
- Fefilova, E.B. Fauna Evropeiskogo Severo-Vostoka Rossii; KMK. Tovarishchestvo Nauchnykh Izdaniy: Moscow, Russia, 2015; ISBN 9785990757226. [Google Scholar]
- Rylov, V.M. Cyclopoida of the fresh-water. In Fauna of USSR Crustacea; AN USSR Press: Moscow, Russia; St. Petersburg, Russia, 1948; Volume 2. [Google Scholar]
- Korovchinsky, N.M.; Kotov, A.A. (Eds.) Water Fleas (Crustacea: Cladocera) of North Eurasia. In Two Volumes; KMK Press: Moscow, Russia, 2021. [Google Scholar]
- Frey, D.G. Cladocera analysis. In Handbook of the Holocene Palaeoecology and Palaeohydrology; Berglund, B.E., Ralska-Jasiewiczowa, M., Eds.; John Wiley & Sons Ltd.: Caldwell, NJ, USA, 1986; pp. 667–692. [Google Scholar]
- Rumiantsev, V.A.; Drabkova, V.G.; Izmaylov, A.V. Great Lakes of the World; Lema Press: St. Petersburg, Russia, 2012; ISBN 978-5-98709-536-2. [Google Scholar]
- Einsle, U. Cyclops kikuchii Smirnov, 1932 (Copepoda,Cyclopoida), eine selbständige Art aus süddeutschen Gewäsern. Crustaceana 1994, 66, 240–246. [Google Scholar] [CrossRef]
- Zhikharev, V.S.; Gavrilko, D.E.; Shurganova, G.V. A Record of the Tropical Species Thermocyclops taihokuensis Harada, 1931 (Copepoda: Cyclopoida) in the European Russia. Povolzhskiy J. Ecol. 2019, 264–270. [Google Scholar] [CrossRef]
- Flössner, D. Die Haplopoda und Cladocera (Ohne Bosminidae) Mitteleuropas; Backhuys: Leiden, The Netherlands, 2000. [Google Scholar]
- Kotov, A.A. Adaptations of the Anomopoda (Cladocera) for benthic mode of life. Zool. Zhurnal 2006, 85, 1043–1059. [Google Scholar] [CrossRef]
- Bledzki, L.A.; Rybak, J.I. Freshwater Crustacean Zooplankton of Europe; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-29870-2. [Google Scholar]
- Rizo, E.Z.C.; Gu, Y.; Papa, R.D.S.; Dumont, H.J.; Han, B.-P. Identifying functional groups and ecological roles of tropical and subtropical freshwater Cladocera in Asia. Hydrobiologia 2017, 799, 83–99. [Google Scholar] [CrossRef]
- Johnson, N.L.; Kotz, S.; Kemp, A.W. Univariate Discrete Distributions, 2nd ed.; Wiley: New York, NY, USA, 1992; ISBN 978-0471548973. [Google Scholar]
- Duan, X.G. Better understanding of the multivariate hypergeometric distribution with implications in design-based survey sampling. arXiv 2021, arXiv:2101.00548. [Google Scholar]
- Brandes, U.; Delling, D.; Gaertler, M.; Gorke, R.; Hoefer, M.; Nikoloski, Z.; Wagner, D. On Modularity Clustering. IEEE Trans. Knowl. Data Eng. 2008, 20, 172–188. [Google Scholar] [CrossRef]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. InterJournal Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 30 November 2022).
- Razumovsky, S.M. Regularities in the Biocenosis Dynamics; Nauka Press: Moscow, Russia, 1981. [Google Scholar]
- Khapugin, A.A.; Kuzmin, I.V. Data for Distribution of Vascular Plants (Tracheophytes) of Urban Forests and Floodplains in Tyumen City (Western Siberia). Data 2022, 7, 180. [Google Scholar] [CrossRef]
- Grime, J.P. Vegetation classification by reference to strategies. Nature 1974, 250, 26–31. [Google Scholar] [CrossRef]
- Schartau, A.K.; Mariash, H.L.; Christoffersen, K.S.; Bogan, D.; Dubovskaya, O.P.; Fefilova, E.B.; Hayden, B.; Ingvason, H.R.; Ivanova, E.A.; Kononova, O.N.; et al. First circumpolar assessment of Arctic freshwater phytoplankton and zooplankton diversity: Spatial patterns and environmental factors. Freshw. Biol. 2022, 67, 141–158. [Google Scholar] [CrossRef]
- Shabalin, S.D. Far East Amur., Surface Water Resources of the USSR: Hydrological Study; Gidrometeoizdat: St. Petersburg, Russia, 1966. [Google Scholar]
- Rivier, I.K. Specific features of lacustrine planktic communities in differing ecological periods (the “ice-cover” and the “open-water” periods). Tr. Inst. Biol. Vnutr. Vod. 2016, 74, 59–76. [Google Scholar]
- De Meester, L.; Gómez, A.; Okamura, B.; Schwenk, K. The Monopolization Hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecologica 2002, 23, 121–135. [Google Scholar] [CrossRef]
- Aarnio, K.; Bonsdorff, E. Colonization rates and community structure of benthic meiofauna in shallow Baltic archipelago waters. Aqua Fenn. 1992, 22, 71–80. [Google Scholar]
- Chertoprood, E.S.; Azovsky, A.I.; Sapozhnikov, F.V. Colonization of sediments of different grain size by littoral harpacticoid copepods. Oceanology 2005, 45, 637–646. [Google Scholar]
- Novichkova, A.A.; Chertoprud, E.S. Crustaceans of Wrangel Island (Russia): Species composition, community structure and variability. Nat. Conserv. Res. 2020, 5, 37–50. [Google Scholar] [CrossRef]
- Kotov, A.A.; Karabanov, D.P.; Bekker, E.I.; Neretina, T.V.; Taylor, D.J. Phylogeography of the Chydorus sphaericus group (Cladocera: Chydoridae) in the Northern Palearctic. PLoS ONE 2016, 11, e0168711. [Google Scholar] [CrossRef] [PubMed]

| Lake | River Basin | Square, km2 | Maximum Depth, m | Latitude, m.a.s.l. | Date of Collection | Studied by | Number of Samples Studied Here | Number of Taxa Found Here |
|---|---|---|---|---|---|---|---|---|
| Khanka | Amur | 4070 | 10.6 | 64 | Sept 2009 | [27] (Cladocera only) | 31 | 91 |
| Bolon | Amur | 338 | 4 | 16 | Sept 2007, Aug 2016 | [34] | 18 | 56 |
| Chukchagir | Amgun | 350 | 6 | 68 | Aug 2017 | [28] | 29 | 41 |
| Geographic Faunistic Complex | Present in: | Assigned to Associations at p < 0.05 in: | ||||
|---|---|---|---|---|---|---|
| Khanka | Bolon | Chukchagir | Khanka | Bolon | Chukchagir | |
| ST | 9 | 4 | 1 | 4 | 1 | 0 |
| EA | 15 | 10 | 5 | 7 | 1 | 3 |
| EAA | 6 | 3 | 3 | 3 | 1 | 2 |
| WE | 13 | 7 | 3 | 10 | 2 | 1 |
| WS | 48 | 32 | 29 | 28 | 11 | 15 |
| Total | 91 | 56 | 41 | 52 | 16 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chertoptud, E.S.; Seleznev, D.G.; Garibian, P.G.; Kotov, A.A. Microcrustaceans (Cladocera and Copepoda) of the Boreal/Tropical Transition Zone in the Russian Far East: A Case Study of Species Associations in Three Large Lakes. Diversity 2023, 15, 338. https://doi.org/10.3390/d15030338
Chertoptud ES, Seleznev DG, Garibian PG, Kotov AA. Microcrustaceans (Cladocera and Copepoda) of the Boreal/Tropical Transition Zone in the Russian Far East: A Case Study of Species Associations in Three Large Lakes. Diversity. 2023; 15(3):338. https://doi.org/10.3390/d15030338
Chicago/Turabian StyleChertoptud, Elena S., Dmitry G. Seleznev, Petr G. Garibian, and Alexey A. Kotov. 2023. "Microcrustaceans (Cladocera and Copepoda) of the Boreal/Tropical Transition Zone in the Russian Far East: A Case Study of Species Associations in Three Large Lakes" Diversity 15, no. 3: 338. https://doi.org/10.3390/d15030338
APA StyleChertoptud, E. S., Seleznev, D. G., Garibian, P. G., & Kotov, A. A. (2023). Microcrustaceans (Cladocera and Copepoda) of the Boreal/Tropical Transition Zone in the Russian Far East: A Case Study of Species Associations in Three Large Lakes. Diversity, 15(3), 338. https://doi.org/10.3390/d15030338









