Discovery of the Puparia of a Whitefly Species Found on Malvaceae in the Pliocene Rajdanda Formation, Jharkhand, Eastern India
Abstract
1. Introduction
2. Material and Methods
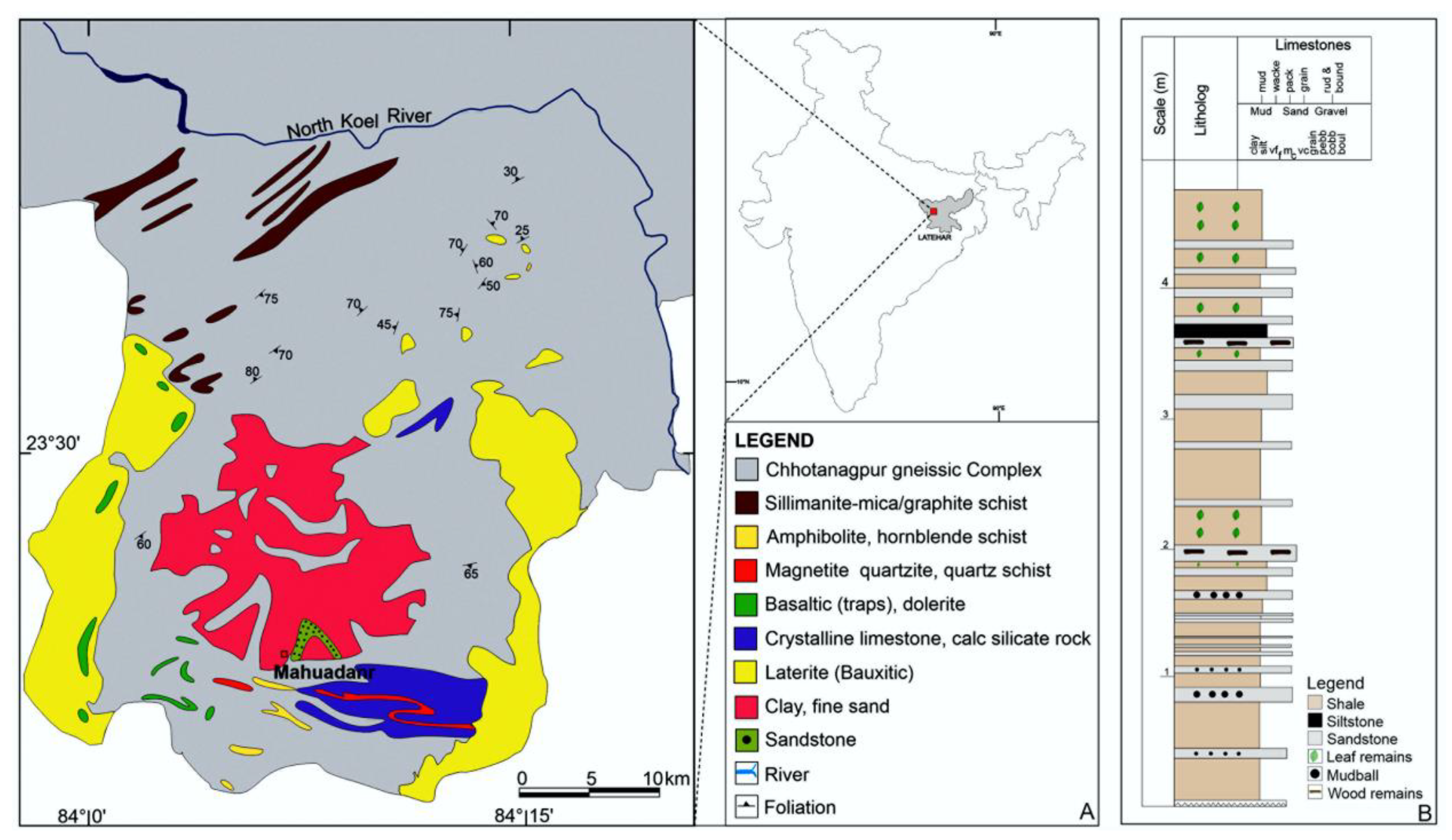
3. Geological Setting
4. Systematic Paleontology
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimaldi, D.A.; Engel, M.S. Evolution of the Insects; Cambridge University Press: Cambridge, UK; New York, NY, USA; Melbourne, Australia, 2005. [Google Scholar]
- Szwedo, J. The unity, diversity and conformity of bugs (Hemiptera) through time. Earth Environ. Sci. Trans. R. Soc. Edinb. 2018, 107, 109–128. [Google Scholar] [CrossRef]
- Henry, T.J. Biodiversity of Heteroptera. In Insect Biodiversity Science and Society, 2nd ed.; Foottit, A.G., Adler, P.H., Eds.; Wiley-Blackwell: Chichester, UK, 2017; Volume 1, pp. 279–336. [Google Scholar] [CrossRef]
- Bartlett, C.R.; Deitz, L.L.; Dmitriev, D.A.; Sanborn, A.F.; Soulier-Perkins, A.; Wallace, M.S. The diversity of the true hoppers (Hemiptera: Auchenorrhyncha). In Insect Biodiversity Science and Society; Foottit, A.G., Adler, P.H., Eds.; Wiley-Blackwell: Chichester, UK, 2018; Volume 2, pp. 501–590. [Google Scholar] [CrossRef]
- Drohojowska, J.; Szwedo, J.; Żyła, D.; Huang, D.Y.; Müller, P. Fossils reshape the Sternorrhyncha evolutionary tree (Insecta, Hemiptera). Sci. Rep. 2020, 10, 11390. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.; Azar, D.; Szwedo, J.; Drohojowska, J.; Huang, D.Y. Paraprotopsyllidiidae fam. nov., a new thrips-like protopsyllidioid family from mid-Cretaceous Burmese amber (Hemiptera; Sternorrhyncha). Cretac. Res. 2021, 120, 104726. [Google Scholar] [CrossRef]
- Hakim, M.; Azar, D.; Huang, D.Y. A new species of Protopsyllidioidea from Cretaceous amber. Palaeoentomology 2022, 5, 623–630. [Google Scholar] [CrossRef]
- Chou, I. Some viewpoints about insect taxonomy. Acta Entomol. Sinica 1963, 12, 586–596. [Google Scholar]
- Westwood, J.O. An Introduction to the Modern Classification of Insects Founded on the Natural Habits and Corresponding Organization of Different Families; Longman, Orme, Brown and Green: London, UK, 1840. [Google Scholar] [CrossRef]
- Shcherbakov, D.E. The most primitive whiteflies (Hemiptera; Aleyrodidae; Bernaeinae subfam. nov.) from the Mesozoic of Asia and Burmese amber, with an overview of Burmese amber hemipterans. Bull. Nat. Hist. Mus. Geol. 2000, 56, 29–37. [Google Scholar]
- Quaintance, A.L.; Baker, A.C. Classification of the Aleyrodidae. Part 1; Technical series (United States. Bureau of Entomology), no. 27; Government Printing Office: Washington, DC, USA, 1913; Volume 27, pp. 1–93. [Google Scholar] [CrossRef]
- Enderlein, G. Udamoselis, eine neue Aleurodiden-Gattung. Zool. Anz. 1909, 34, 230–233. [Google Scholar] [CrossRef]
- Evans, G.A.; Martin, J.H.; Drohojowska, J.; Szwedo, J.; Dubey, A.K.; Dooley, J.W.; Stocks, I.C. Whiteflies of the World (Hemiptera: Sternorrhyncha, Aleyrodidae)—A catalogue of the taxonomy, distribution, hosts and natural enemies of whiteflies. Part 1–Subfamilies Aleurodicinae, Udamoselinae, Bernaeinae and other fossil whiteflies. Zootaxa, 2023; in press. [Google Scholar]
- Gerling, D. Whiteflies: Their Bionomics, Pest Status and Management; Intercept Ltd.: Andover, UK, 1990. [Google Scholar]
- Drohojowska, J.; Wegierek, P.; Evans, G.A.; Huang, D.Y. Are contemporary whiteflies “living fossils”? Morphology and systematic status of the oldest representatives of the Middle-Late Jurassic Aleyrodomorpha (Sternorrhyncha, Hemiptera) from Daohugou. Palaeoentomology 2019, 2, 171–182. [Google Scholar] [CrossRef]
- Schlee, D. Verwandtschaftsforschung an fossilien und rezenten Aleyrodina (Insecta, Hemiptera). Stuttg. Beitr. Naturkd. 1970, 213, 1–72. [Google Scholar]
- Rietschel, S. Aleurochiton petri n. sp., eine Mottenschildlaus (Homoptera, Aleyrodina) aus dem Pliozän von Neu-Isenburg, Hessen. Carolinea 1983, 41, 97–100. [Google Scholar]
- Poinar, G.O., Jr. Life in Amber; Stanford University Press: Palo Alto, CA, USA, 1992. [Google Scholar]
- Schmidt, A.R.; Perrichot, V.; Svojtka, M.; Anderson, K.B.; Belete, K.H.; Bussert, R.; Dorfelt, H.; Jancke, S.; Mohr, B.; Mohrmann, E.; et al. Cretaceous African life captured in amber. Proc. Natl. Acad. Sci. USA 2010, 107, 7329–7334. [Google Scholar] [CrossRef]
- Drohojowska, J.; Szwedo, J. A new whitefly from Lower Cretaceous Lebanese amber (Hemiptera: Sternorrhyncha: Aleyrodidae). Insect Syst. Evol. 2011, 42, 179–196. [Google Scholar] [CrossRef]
- Drohojowska, J.; Szwedo, J. New Aleyrodidae (Hemiptera: Sternorrhyncha: Aleyrodomorpha) from Eocene Baltic amber. Pol. J. Entomol. 2011, 80, 659–677. [Google Scholar] [CrossRef]
- Drohojowska, J.; Szwedo, J. Whiteflies (Hemiptera: Sternorrhyncha: Aleyrodidae) from the Lowermost Eocene Oise amber. Zootaxa 2013, 3636, 319–347. [Google Scholar] [CrossRef] [PubMed]
- Drohojowska, J.; Szwedo, J. Gapenus rhinariatus gen. sp. n. from the Lower Cretaceous amber of Lebanon (Hemiptera: Sternorrhyncha: Aleyrodidae). In Insect Evolution in An Amberiferous and Stone Alphabet. Proceedings of the 6th International Congress on Fossil Insects, Arthropods and Amber, Byblos, Lebanon, 14–18 April 2013; Azar, D., Engel, M.S., Jarzembowski, E., Krogmann, L., Nel, A., Santiago-Blay, J., Eds.; Brill: Leiden, The Netherlands, 2013; pp. 99–110. [Google Scholar]
- Drohojowska, J.; Perkovsky, E.E.; Szwedo, J. New genus and species of Aleyrodidae from the Eocene Baltic amber (Hemiptera: Sternorrhyncha: Aleyrodomorpha). Pol. J. Entomol. 2015, 84, 259–269. [Google Scholar] [CrossRef]
- Drohojowska, J.; Tomanek, N.; Gröhn, C.; Szwedo, J. A second aleurodicinae from the Eocene Baltic amber—Medocellodes blackmani gen. et sp. nov. (Hemiptera, Sternorrhyncha, Aleyrodidae). Zootaxa 2022, 5183, 245–253. [Google Scholar] [CrossRef]
- Szwedo, J.; Drohojowska, J.; Popov, Y.A.; Simon, E.; Węgierek, P. Aphids, true hoppers, jumping plant-lice, scale insects, true bugs and whiteflies (Insecta: Hemiptera) from the Insect Limestone (latest Eocene) of the Isle of Wight, UK. Earth Environ. Sci. Trans. R. Soc. Edinb. 2019, 110, 331–396. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Wang, B.; Zheng, Y. A new whitefly (Hemiptera, Sternorrhyncha, Aleyrodidae) in mid-Cretaceous Kachin amber, northern Myanmar. Cretac. Res. 2020, 106, 104256. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.C.; Zheng, Y.; Wang, X. The whitefly subfamily Aleurodicinae (Hemiptera, Sternorrhyncha, Aleyrodidae) in mid-Cretaceous Kachin amber, northern Myanmar. Cretac. Res. 2021, 118, 104668. [Google Scholar] [CrossRef]
- Chen, J.; Zhuo, D.; Yu, S.; Zheng, Y.; Yang, F.; An, B.; Ren, G. The discovery of a new aleurodicine whitefly in Cenomanian Burmese amber (Sternorrhyncha, Aleyrodidae). Cretac. Res. 2022, 134, 105163. [Google Scholar] [CrossRef]
- Weigelt, J. Der heutige Stand der Geiseltalforschung. Naturwissenschaften 1940, 22, 343–350. [Google Scholar] [CrossRef]
- Jarzembowski, E.A.; Ross, A.J. Time flies: The geological record of insects. Geol. Today 1993, 9, 218–223. [Google Scholar] [CrossRef]
- Jarzembowski, E.A.; Coram, R.A. New fossil insect records from the Purbeck of Dorset and the Wealden of the Weald. Proc. Dorset Nat. Hist. Archaeol. Soc. 1997, 118, 119–124. [Google Scholar]
- Puri, S.N.; Mishra, V.P. On the find of Upper Tertiary plant fish and bird fossils near Rajdanda Palamau district Bihar. Rec. Geol. Surv. India 1982, 112, 55–58. [Google Scholar]
- Hazra, T.; Spicer, R.A.; Hazra, M.; Mahato, S.; Spicer, T.E.; Bera, S.; Valdes, P.J.; Farnsworth, A.; Hughes, A.C.; Jian, Y.; et al. Latest Neogene monsoon of the Chotanagpur Plateau, eastern India, as revealed by fossil leaf architectural signatures. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 545, 109641. [Google Scholar] [CrossRef]
- Hazra, T.; Adroit, B.; Hazra, M.; Spicer, R.A.; Spicer, T.E.; Bera, S.; Khan, M.A. New discovery of rare insect damage in the Pliocene of India reinforces the biogeographic history of Eurasian ecosystems. Rev. Palaeobot. Palynol. 2022, 298, 104589. [Google Scholar] [CrossRef]
- Bande, M.B.; Srivastava, G.P. Late Cenozoic plant impressions from Mahuadanr Valley, Palamu District, Bihar. Palaeobotanist 1990, 37, 331–366. [Google Scholar] [CrossRef]
- Srivastava, G.P.; Bande, M.B. Fossil woods of Terminalia and Lagerstroemia from the Late Cenozoic beds of Mahuadanr, Palamu District. Bihar. Palaeobotanist 1992, 39, 333–337. [Google Scholar] [CrossRef]
- Srivastava, G.P.; Mishra, V.P.; Bande, M.B. Further contribution to the Late Cenozoic flora of Mahuadanr, Palamu District. Bihar. Geophytology 1992, 22, 229–234. [Google Scholar]
- Singh, S.K.; Prasad, M. Addition to the Upper Tertiary flora of Mahuadanr valley, district Latehar, Jharkhand, India. Proc. Nat. Acad. Sci. India. Sec. B. Biol. Sci. 2009, 79, 402–409. [Google Scholar]
- Singh, S.K.; Prasad, M. Some new fossil leaves from the Late Tertiary sediments of Mahuadanr Valley, Latehar District, Jharkhand, India. J. App. Biosci. 2009, 35, 35–42. [Google Scholar]
- Singh, S.K.; Prasad, M. Floral diversity and climate during Late Tertiary period in Mahuadanr Valley, Jharkhand, India. Phytomorphology 2009, 59, 19. [Google Scholar]
- Singh, S.K.; Prasad, M. Record of fossil leaves of Ziziphus and Lagerstroemia from Mahuadanr Valley, Jharkhand, India and their ecological implications. Paleobotanist 2010, 59, 55–61. [Google Scholar] [CrossRef]
- Hazra, M.; Hazra, T.; Spicer, R.A.; Sarkar, S.K.; Spicer, T.E.; Bera, S.; Khan, M.A. In situ occurrence of a gall midge (Insecta, Diptera, Cecidomyiidae) on fossilized angiosperm leaf cuticle fragments from the Pliocene sediments of eastern India. J. Asia-Pac. Entomol. 2020, 23, 762–771. [Google Scholar] [CrossRef]
- Hazra, M.; Hazra, T.; Spicer, R.A.; Sarkar, S.K.; Spicer, T.E.; Bera, S.; Khan, M.A. Galling: The prevalent form of insect folivory in the latest Neogene monsoon–influenced tropical forests of the Chotanagpur Plateau, Eastern India. Palaeoworld 2022, 31, 550–564. [Google Scholar] [CrossRef]
- Hazra, T.; Hazra, M.; Kumar, S.; Mahato, S.; Bera, M.; Bera, S.; Khan, M.A. First fossil evidence of Palaeocarya (Engelhardioideae: Juglandaceae) from India and its biogeographical implications. J. Syst. Evol. 2021, 59, 1307–1320. [Google Scholar] [CrossRef]
- Hazra, T.; Hazra, M.; Spicer, R.A.; Spicer, T.E.; Mahato, S.; Bera, S.; Kumar, S.; Khan, M.A. Pliocene Albizia (Fabaceae) from Jharkhand, eastern India: Reappraisal of its biogeography during the Cenozoic in Southeast Asia. Palaeoworld 2021, 31, 153–168. [Google Scholar] [CrossRef]
- Hazra, T.; Spicer, R.A.; Hazra, M.; Sarkar, S.K.; Spicer, T.E.V.; Bera, S.; Khan, M.A. First fossil evidence of leaf-feeding caterpillars from India and their feeding strategies. Lethaia 2021, 54, 891–905. [Google Scholar] [CrossRef]
- Hazra, T.; Mahato, S.; Bera, S.; Khan, M.A. First fossil evidence of Indian tulip tree. Bot. Lett. 2022, 169, 284–293. [Google Scholar] [CrossRef]
- Kumar, M.; Prakash, A.; Srivastava, G.P.; Shukla, M. Dispersed organic matter (DOM) types and depositional environment of Neogene sediments of Mahuadanr valley, Palamu, Bihar. J. Geol. Soc. India 2000, 55, 317–325. [Google Scholar]
- Bajpai, U.; Kumar, M.; Shukla, M.; Srivastava, A.P.; Srivastava, G.P. Nature and composition of pyrite framboids and organic substrate from degraded leaf cuticles of late Tertiary sediments, Mahuadanr Valley, Palamu, Bihar. Curr. Sci. 2001, 81, 102–106. Available online: https://www.jstor.org/stable/24105012 (accessed on 8 March 2023).
- Prakash, U.; Mishra, V.P.; Srivastava, G.P.S. Fossil wood resembling Sindora from the Tertiary of Palamau District, Bihar. Rec. Geol. Surv. India 1987, 118, 69–73. [Google Scholar]
- Singh, S.K.; Chauhan, M.S. Fungal remains from the Neogene sediments of Mahuadanr Valley, Latehar District, Jharkhand. India and their palaeoclimatic significance. J. Palaeontol. Soc. India 2008, 53, 73–81. [Google Scholar]
- Guleria, J.S. Neogene vegetation of peninsular India. Palaeobotanist 1992, 40, 285–311. [Google Scholar] [CrossRef]
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis; Editio decima, reformata. Tomus 1, Impensis Direct; Laurentii Salvii: Holmiae, Turkey, 1758; p. 702. [Google Scholar] [CrossRef]
- Amyot, C.J.-B.; Audinet-Serville, J.G. Deuxième Partie. Homoptères. Homoptera Latr. Histoire Naturelle des Insectes. Hemiptères; Librairie encyclopédique de Roret: Paris, France, 1843; pp. 1–676. [Google Scholar] [CrossRef]
- Takahashi, R. Key to the tribes and genera of Aleyrodidae of Japan, with descriptions of three new genera and one new species (Homoptera). Insecta Matsumurana 1954, 18, 47–53. [Google Scholar]
- Binu, A.; Palaniswami, M.S.; Henneberry, T.J. Encarsia transvena (Hymenoptera: Aphelinidae) development on different Bemisia tabaci Gennadius (Homoptera: Aleyrodidae) instars. Environ. Entomol. 2003, 32, 584–591. [Google Scholar] [CrossRef]
- Liu, T.-X.; Stansly, P.A.; Gerling, D. Whitefly parasitoids: Distribution, life history, bionomics, and utilization. Annu. Rev. Entomol. 2015, 60, 273–292. [Google Scholar] [CrossRef]
- Polaszek, A.; Vilhelmsen, L. Biodiversity of hymenopteran parasitoids. Curr. Opin. Insect. Sci. 2023, 1–7, in press. [Google Scholar] [CrossRef]
- Quaintance, A.L.; Baker, A.C. Classification of the Aleyrodidae. Part 2; Technical series (United States. Bureau of Entomology), no. 27; Government Printing Office: Washington, DC, USA, 1914; Volume 27, pp. 95–109. [Google Scholar]
- Regu, K.; David, B.V. Taxonomic studies on Indian aleyrodids of the tribe Aleurolobini (Aleyrodinae: Aleyrodidae: Homoptera). Fredrick Inst. Plant Prot. Toxicol. FIPPAT Entomol. Ser. 1993, 4, 1–79. [Google Scholar]
- Dubey, A.K.; Sundararaj, R. Key to whiteflies of the tribe Aleurolobini (Hemiptera: Aleyrodidae) of India with description of five new species and host records. Orient. Insects 2006, 40, 33–60. [Google Scholar] [CrossRef]
- Manzari, S.; Quicke, D.L.J. A cladistic analysis of whiteflies, subfamily Aleyrodinae (Hemiptera: Sternorrhyncha: Aleyrodidae). J. Nat. Hist. 2006, 40, 2423–2554. [Google Scholar] [CrossRef]
- Santos-Garcia, D.; Mestre-Rincon, N.; Ouvrard, D.; Zchori-Fein, E.; Morin, S. Portiera gets wild: Genome instability provides insights into the evolution of both whiteflies and their endosymbionts. Genome Biol. Evol. 2020, 12, 2107–2124. [Google Scholar] [CrossRef]
- Förster, A. Kleine Monographien parasitischer Hymenopteren. Verh. Natur. Ver. Preuss. Rheinl. Westfalen 1878, 35, 42–82. [Google Scholar]
- Haldeman, S.S. Art. XVI.—On four new species of Hemiptera of the genera Ploiaria, Chermes, and Aleurodes, and two new Hymenoptera (Aleurodes aleurodinus, Eretmocerus corni), parasitic in the last named genus. Amer. J. Sci. Arts (Ser. 2) 1850, 9, 108–111. [Google Scholar]
- Lasalle, J.; Schauff, M.E. Systematics of the tribe Euderomphalini (Hymenoptera: Eulophidae): Parasitoids of whiteflies (Homoptera: Aleyrodidae). Syst. Entomol. 1994, 19, 235–258. [Google Scholar] [CrossRef]
- Mercet, R.G. Especies españolas del género Aphycus. Bol. R. Soc. Esp. Hist. Nat. 1917, 17, 138–139. [Google Scholar]
- Myartseva, S.N. Species of the genus Metaphycus Mercet (Hymenoptera: Encyrtidae) parasitizing whiteflies (Homoptera: Aleyrodidae). Zoosyst. Ross. 2006, 14, 266. [Google Scholar] [CrossRef]
- Chapin, E.A. The genera of the Chilocorini (Coleoptera, Coccinellidae). Bull. Mus. Comp. Zool. 1965, 133, 229–271. [Google Scholar]
- Michaud, J.P. Coccinellids in biological control. In Ecology and Behaviour of the Ladybird Beetles (Coccinellidae), 1st ed.; Hodek, I., van Emden, H.F., Honěk, A., Eds.; Blackwell Publishing Ltd.: Chichester, UK, 2012; pp. 488–519. [Google Scholar]
- Omkar. Parasitoids in Pest Management, 1st ed.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Hochberg, M.E.; Hawkins, B.A. Refuges as a predictor of parasitoid diversity. Science 1992, 255, 973–976. [Google Scholar] [CrossRef]
- Labandeira, C.C.; Li, L.F. The history of insect parasitism and the Mid-Mesozoic Parasitoid Revolution. In The Evolution and Fossil Record of Parasitism; Topics in Geobiology, 49; De Baets, K., Huntley, J.W., Eds.; Springer: Cham, Switzerland, 2021; pp. 377–533. [Google Scholar] [CrossRef]
- Wang, B.; Xu, C.P.; Jarzembowski, E.A. Ecological radiations of insects in the Mesozoic. Trends Ecol. Evol. 2022, 37, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Moore, M.J.; Wang, H.; Zhuy, Z.X.; Wang, H.F. Plastome evolution and phylogenetic relationships among Malvaceae subfamilies. Gene 2021, 765, 145103. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, T.; Areces-Berazain, F.; Hinsinger, D.D.; Thomas, D.C.; Wieringa, J.J.; Ganesan, S.K.; Strijk, J.S. Phylogenomics resolves deep subfamilial relationships in Malvaceae sl. G3 2021, 11, jkab136. [Google Scholar] [CrossRef]
- Bayer, C.; Kubitzki, K. Malvaceae. In Dicotyledons: The Families and Genera of Vascular Plants, Malvales, Capparales and Non-Betalain Caryophyllales; Kubitzki, K., Bayer, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; Volume 5, pp. 225–312. [Google Scholar]
- Labandeira, C.C.; Wilf, P.; Johnson, K.R.; Marsh, F. Guide to Insect (and Other) Damage Types on Compressed Plant Fossils, Version 3.0; Smithsonian Institution: Washington, DC, USA, 2007; pp. 1–25. [Google Scholar]
- Schachat, S.R.; Payne, J.L.; Boyce, C.K. Linking host plants to damage types in the fossil record of insect herbivory. Paleobiology 2023, 1–27. [Google Scholar] [CrossRef]
- Martínez-Delclòs, X.; Briggs, D.E.G.; Peñalver, E. Taphonomy of insects in carbonates and amber. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2004, 203, 19–64. [Google Scholar] [CrossRef]
- Drohojowska, J.; Zmarzły, M.; Szwedo, J. Evolutionary implications of new Postopsyllidiidae from mid-Cretaceous amber from Myanmar and sternorrhynchan nymphal conservatism. Sci. Rep. 2022, 12, 16446. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.E.G.; McMahon, D. The role of experiments in investigating the taphonomy of exceptional preservation. Palaeontology 2016, 59, 1–11. [Google Scholar] [CrossRef]
- Heingård, M.; Sjövall, P.; Schultz, B.P.; Sylvestersen, R.L.; Lindgren, J. Preservation and taphonomy of fossil insects from the earliest Eocene of Denmark. Biology 2022, 11, 395. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drohojowska, J.; Evans, G.A.; Khan, M.A.; Hazra, T.; Szwedo, J. Discovery of the Puparia of a Whitefly Species Found on Malvaceae in the Pliocene Rajdanda Formation, Jharkhand, Eastern India. Diversity 2023, 15, 564. https://doi.org/10.3390/d15040564
Drohojowska J, Evans GA, Khan MA, Hazra T, Szwedo J. Discovery of the Puparia of a Whitefly Species Found on Malvaceae in the Pliocene Rajdanda Formation, Jharkhand, Eastern India. Diversity. 2023; 15(4):564. https://doi.org/10.3390/d15040564
Chicago/Turabian StyleDrohojowska, Jowita, Gregory A. Evans, Mahasin Ali Khan, Taposhi Hazra, and Jacek Szwedo. 2023. "Discovery of the Puparia of a Whitefly Species Found on Malvaceae in the Pliocene Rajdanda Formation, Jharkhand, Eastern India" Diversity 15, no. 4: 564. https://doi.org/10.3390/d15040564
APA StyleDrohojowska, J., Evans, G. A., Khan, M. A., Hazra, T., & Szwedo, J. (2023). Discovery of the Puparia of a Whitefly Species Found on Malvaceae in the Pliocene Rajdanda Formation, Jharkhand, Eastern India. Diversity, 15(4), 564. https://doi.org/10.3390/d15040564





