Where the Immatures of Triassic Diptera Developed
Abstract
1. Introduction
2. Hypothetical Ancestral Lifestyle of Dipteran Larvae
3. Larval Respiratory System and Mode of Life
4. Larval Respiratory System in Mesozoic Members of Extant Families
5. On the Absence of Dipteran Immatures in Some Triassic Lacustrine Konservat-Lagerstätten
6. Anisian Diptera
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adler, P.H.; Courtney, G.W. Ecological and societal services of aquatic Diptera. Insects 2019, 10, 70. [Google Scholar] [CrossRef]
- Hennig, W. Kritische Bemerkungen über den Bauder Flügelwurzel bei den Dipteren und die Frage nach der Monophylie der Nematocera. Stuttg. Beitr. Naturk. 1968, 193, 1–23. [Google Scholar]
- Hennig, W. Insect Phylogeny; John Wiley & Sons: Hoboken, NJ, USA, 1981; pp. 1–514. [Google Scholar]
- Greeney, H.F. The insects of plant-held waters: A review and bibliography. J. Trop. Ecol. 2001, 17, 241–260. [Google Scholar] [CrossRef]
- Wagner, R.; Barták, M.; Borkent, A.; Courtney, G.; Godddeeris, B.; Haenni, J.-P.; Knutson, L.; Pont, A.; Rotheray, G.E.; Rozkosny, R.; et al. Global diversity of dipteran families (Insecta Diptera) in freshwater (excluding Simulidae, Culicidae, Chironomidae, Tipulidae and Tabanidae). Hydrobiologia 2008, 595, 489–519. [Google Scholar] [CrossRef]
- Monchadsky, A.S. On seasonal dimorphism of larvae of Mochlonyx culiciformis De Geer (Diptera, Culicidae). Trudy ZIN AN SSSR 1953, 13, 363–372. (In Russian) [Google Scholar]
- de Jong, H.; Oosterbroek, P.; Gelhaus, J.; Reusch, H.; Young, C. Global diversity of craneflies (Insecta, Diptera: Tipulidea or Tipulidae sensu lato) in freshwater. Hydrobiologia 2008, 595, 457–467. [Google Scholar] [CrossRef]
- Drake, M. The importance of temporary waters for Diptera (true-flies). AquaDoc 2001, 17, 26–39. [Google Scholar]
- Duckhouse, D.A.; Duckhouse, S.R. Insecta: Diptera, Psychodidae. In Freshwater Invertebrates of the Malaysian Region; Yule, C.M., Sen, Y.H., Eds.; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2004; pp. 750–762. [Google Scholar]
- Edwards, F.W. The phylogeny of nematocerous Diptera: A critical review of some recent suggestions. Verh. III Int. Entomol.-Kongr. 1926, 2, 111–130. [Google Scholar]
- Hinton, H.E. On the reduction of functional spiracles in the aquatic larvae of the Holometabola, with notes on the moulting process of spiracles. Trans. R. Entomol. Soc. Lond. 1947, 98, 449–473. [Google Scholar] [CrossRef]
- Kovalev, V.G. New data on the initial stages of evolution of Diptera. In Diptera (Insecta), Their Systematics, Geographic Distribution and Ecology; Academy of Sciences USSR: Leningrad, Former Soviet Union, 1983; pp. 60–66. (In Russian) [Google Scholar]
- Colless, D.H.; McAlpine, D.K. Diptera. In The Insects of Australia; Melbourne University Press: Carlton, Australia, 1970; pp. 656–740. [Google Scholar]
- Lukashevich, E.D.; Shcherbakov, D.E. First description of Tanyderidae (Diptera) larvae from South America. Russ. Entomol. J. 2014, 23, 121–138. [Google Scholar] [CrossRef]
- Lukashevich, E.D.; Shcherbakov, D.E. On morphology of Tanyderus pictus (Diptera: Tanyderidae) pupa and adult from Chile. Russ. Entomol. J. 2016, 25, 79–95. [Google Scholar] [CrossRef]
- Keilin, D. Respiratory systems and respiratory adaptations in larvae and pupae of Diptera. Parasitology 1944, 36, 1–66. [Google Scholar] [CrossRef]
- Krivosheina, N.P.; Mamaev, B.M. A Key to Wood-Inhabiting Larvae of Diptera; Nauka: Moscow, Russia, 1967; pp. 1–367. (In Russian) [Google Scholar]
- Krivosheina, N.P.; Mamaev, B.M. Cramptonomyiidae (Diptera, Nematocera), a dipteran family new for the fauna of the USSR: Its morphology, ecology, and phylogenetic relationships. Entomol. Rev. 1970, 49, 886–898. [Google Scholar]
- Pilgrim, R.L.C. The aquatic larva and the pupa of Choristella philpotti Tillyard, 1917 (Mecoptera: Nannochoristidae). Pac. Insects 1972, 14, 151–168. [Google Scholar]
- Narchuk, E.P. Diptera. Generic part. In Key to Freshwater Invertebrates of Russia and Adjacent Lands; Tsalolikhin, S.J., Ed.; ZIN RAS: St. Petersburg, Russia, 1999; Volume 4, pp. 8–32. [Google Scholar]
- Bouchard, R.W. Guide to Aquatic Invertebrates of the Upper Midwest; Water Resources Center, University of Minnesota: St. Paul, MN, USA, 2004; pp. 1–215. [Google Scholar]
- Salmela, J.; Autio, O.; Ilmonen, J. A survey on the nematoceran (Diptera) communities of southern Finnish wetlands. Memo. Soc. Fauna Flora Fenn. 2007, 83, 33–47. [Google Scholar]
- Sota, T.; Mogi, M. Species richness and altitudinal variation in the aquatic metazoan community in bamboo phytotelmata from North Sulawesi. Res. Popul. Ecol. 1996, 38, 275–281. [Google Scholar] [CrossRef]
- Haenni, J.P.; Vaillant, F. Description of dendrolimnobiontic larvae of Scatopsidae (Diptera) with a review of our knowledge of the preimaginal stages of the family. Mitt. Schweiz. Entomol. Ges. 1994, 67, 43–59. [Google Scholar]
- Krivosheina, N.P. European larvae Bibionidae (Diptera, Nematocera), with key to some species. Pedobiologia 1962, 1, 210–227. (In Russian) [Google Scholar]
- Skartveit, J. The larvae of European Bibioninae (Diptera, Bibionidae). J. Nat. Hist. 2002, 36, 449–485. [Google Scholar] [CrossRef]
- Tan, J.; Hua, B. Morphology of immature stages of Bittacus choui (Mecoptera: Bittacidae) with notes on its biology. J. Nat. Hist. 2008, 42, 2127–2142. [Google Scholar] [CrossRef]
- Jiang, L.; Yue, C.; Hua, B. Larval morphology of Panorpoidea kuandianensis (Insecta, Mecoptera, Panorpodidae) and its evolutionary implication. Zookeys 2014, 398, 69–82. [Google Scholar]
- Kalugina, N.S.; Kovalev, V.G. Jurassic Diptera of Siberia; Nauka: Moscow, Russia, 1985; pp. 1–198. (In Russian) [Google Scholar]
- Lukashevich, E.D. Larvae—A key to evolution of Culicoidea (Diptera) in the Mesozoic. Alavesia 2008, 2, 59–72. [Google Scholar]
- Krzemiński, W.; Jarzembowski, E. Aenne triassica sp. n., the oldest representative of the family Chironomidae (Insecta: Diptera). Polish J. Entomol. 1999, 68, 445–449. [Google Scholar]
- Lukashevich, E.D. The oldest occurrence of Chaoboridae (Insecta: Diptera). Russ. Entomol. J. 2022, 31, 417–421. [Google Scholar] [CrossRef]
- Lukashevich, E.D.; Krzemiński, W. New Jurassic Tanyderidae (Diptera) from Asia with first find of larvae. Zoosymposia 2009, 3, 155–172. [Google Scholar] [CrossRef]
- Kalugina, N.S. New psychodomorph dipterans from the Mesozoic of Siberia (Diptera: Eoptychopteridae, Ptychopteridae). Paleontol. Zh. 1989, 1, 65–77. [Google Scholar]
- Jell, P.A.; Duncan, P.M. Invertebrates, mainly insects, from the freshwater, Lower Cretaceous, Koonwarra Fossil Bed (Korumburra Group), South Gippsland, Victoria. Mem. Ass. Austral. Palaeontol. 1986, 3, 111–205. [Google Scholar]
- Kalugina, N.S. True flies. Muscida (=Diptera). Infraorders Tipulomorpha and Culicomorpha. Insects in the Early Cretaceous ecosystems of the West Mongolia. Moscow: Nauka. Trans. Joint Sov.-Mongol. Palaeontol. Exped. 1986, 28, 112–125. (In Russian) [Google Scholar]
- Krzemińska, E.; Krzemiński, W.; Dahl, C. Monograph of Fossil Trichoceridae (Diptera): Over 180 Million Years of Evolution; Institute of Systematics and Evolution of Animals PAS: Krakow, Poland, 2009; pp. 1–172. [Google Scholar]
- Kvifte, G.M.; Wagner, R. Psychodidae (sand flies, moth flies or owl flies). In Manual of Afrotropical Diptera. Volume 2. Nematocerous Diptera and Lower Brachycera; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; South African National Biodiversity Institute: Pretoria, South Africa, 2017; pp. 607–632. [Google Scholar]
- Cockerell, T.D.A. Fossil insects. Ann. Entomol. Soc. Am. 1917, 10, 1–22. [Google Scholar] [CrossRef]
- Zherikhin, V.V. Pattern of insect burial and conservation. In History of Insects; Rasnitsyn, A.P., Quicke, D.L.J., Eds.; Kluver Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2002; pp. 17–64. [Google Scholar]
- Bertone, M.A.; Courtney, G.W.; Wiegmann, B.M. Phylogenetics and temporal diversification of the earliest true flies (Insecta: Diptera) based on multiple nuclear genes. Syst. Entomol. 2008, 33, 668–687. [Google Scholar] [CrossRef]
- Krzemiński, W.; Krzemińska, E.; Papier, F. Grauvogelia arzvilleriana sp. n.—The oldest Diptera species (Lower/Middle Triassic of France). Acta Zool. Cracov. 1994, 37, 95–99. [Google Scholar]
- Krzemiński, W.; Krzemińska, E. Triassic Diptera: Descriptions, revisions and phylogenetic relations. Acta Zool. Cracov. 2003, 46, 153–184. [Google Scholar]
- Bashkuev, A.; Sell, J.; Aristov, D.; Ponomarenko, A.; Sinitshenkova, N.; Mahler, H. Insects from the Buntsandstein of Lower Franconia and Thuringia. Paläontol. Z. 2012, 86, 175–185. [Google Scholar] [CrossRef]
- Lukashevich, E.D. The oldest Diptera (Insecta) from the Upper Buntsandstein (early Middle Triassic) of Europe. Zootaxa 2021, 5067, 135–143. [Google Scholar] [CrossRef]
- Peñalver, E.; Matamales-Andreu, R.; Nel, A.; Pérez-de la Fuente, R. Early adaptation of true flies (Diptera) to moist and continental environments. Pap. Palaeontol. 2022, 8, e1472. [Google Scholar] [CrossRef]
- Lara, M.B.; Lukashevich, E.D. The first Triassic dipteran (Insecta) from South America, with review of Hennigmatidae. Zootaxa 2013, 3710, 81–92. [Google Scholar] [CrossRef]
- Shcherbakov, D.E.; Lukashevich, E.D.; Blagoderov, V.A. Triassic Diptera and initial radiation of the order. Int. J. Dipterol. Res. 1995, 6, 75–115. [Google Scholar]
- Blagoderov, V.; Grimaldi, D.A.; Fraser, N.C. How time flies for flies: Diverse Diptera from the Triassic of Virginia and early radiation of the order. Am. Mus. Novit. 2007, 3572, 1–39. [Google Scholar] [CrossRef]
- Criscione, J.; Grimaldi, D. The oldest predaceous water bugs (Insecta, Heteroptera, Belostomatidae), with implications for paleolimnology of the Triassic Cow Branch Formation. J. Paleontol. 2017, 91, 1166–1177. [Google Scholar] [CrossRef]
- Liutkus, C.M.; Beard, J.S.; Fraser, N.C.; Ragland, P.C. Use of fine-scale stratigraphy and chemostratigraphy to evaluate conditions of deposition and preservation of a Triassic Lagerstatte, south-central Virginia. J. Paleolimnol. 2010, 44, 645–666. [Google Scholar] [CrossRef]
- Sinitshenkova, N.D. A review of Triassic mayflies, with a description of new species from Western Siberia and Ukraine (Ephemerida=Ephemeroptera). Paleontol. J. 2000, 34, S275–S283. [Google Scholar]
- Shcherbakov, D.E. Madygen, Triassic Lagerstätte number one, before and after Sharov. Alavesia 2008, 2, 113–124. [Google Scholar]
- Sukacheva, I.D.; Sinitshenkova, N.D. A review of the Triassic caddisflies with a description of new species from the Middle–Upper Triassic of Kyrgyzstan. Paleontol. Zhurnal 2023, 1, 42–48. [Google Scholar]
- Ansorge, J. Insekten aus dem oberen Lias von Grimmen (Vorpommern, Norddeutschland). Neue Paläont. Abhandl. 1996, 2, 1–132. [Google Scholar]
- Lukashevich, E.D. Mesozoic Dixidae (Insecta: Diptera) and systematic position of Dixamima Rohdendorf, 1964 and Rhaetomyia Rohdendorf, 1962. Paleontol. Zhurnal 1996, 1, 48–53. [Google Scholar]
- Monchadsky, A.S. To the knowledge of mosquito larvae (Diptera, Culicidae). I. Larva Cryophila lapponica Mart. Parasitol. Sb. ZIN AN SSSR 1939, 7, 142–169. (In Russian) [Google Scholar]
- Voigt, S.; Buchwitz, M.; Fischer, J.; Kogan, I.; Moisan, P.; Schneider, J.W.; Spindler, F.; Brosig, A.; Preusse, M.; Scholze, F.; et al. Triassic life in an inland lake basin of the warm-temperate biome—The Madygen Lagerstätte (Southwest Kyrgyzstan, Central Asia). In Terrestrial Conservation Lagerstätten. Windows into the Evolution of Life on Land; Fraser, N.C., Sues, H.-D., Eds.; Dunedin: Edinburgh, UK, 2017; pp. 65–104. [Google Scholar]
- Ponomarenko, A.G. Composition and ecological characteristics of Mesozoic Coleoptera. In Mesozoic Coleoptera. Tr. Paleontol. Ins. AN SSSR 1977, 161, 1–16. (In Russian) [Google Scholar]
- Ponomarenko, A.G.; Prokin, A.A. Review of the paleontological data on the evolution of aquatic beetles (Coleoptera). Paleontol. J. 2015, 49, 1383–1412. [Google Scholar] [CrossRef]
- Kirejtshuk, A.G.; Prokin, A.A. The position of the Paleozoic genus Tunguskagyrus Yan, Beutel et Lawrence in the family Triaplidae sensu n. (Coleoptera, Archostemata: Schizophoroidea). Entomol. Rev. 2018, 98, 872–882. [Google Scholar] [CrossRef]
- Gall, J.C. Faunes et paysages du Grès à Voltzia du Nord des Vosges. Essai paléoécologique sur le Buntsandstein supérieur. Mém. Serv. Carte Géol. Als. Lorr. 1971, 3, 1–318. [Google Scholar]
- Selden, P.; Nudds, J. Evolution of Fossil Ecosystems; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Brauckmann, C.; Schlüter, T. Neue Insekten aus der Trias von Unter-Franken. Geol. Palaeontol. 1993, 27, 181–199. [Google Scholar]
- Matamales-Andreu, R.; Peñalver, E.; Mujal, E.; Oms, O.; Scholze, F.; Juárez, J.; Galobart, A.; Fortuny, J. Early–Middle Triassic fluvial ecosystems of Mallorca (Balearic Islands): Biotic communities and environmental evolution in the equatorial western peri-Tethys. Earth Sci. Rev. 2021, 222, 103783. [Google Scholar] [CrossRef]
- Lukashevich, E.D.; Przhiboro, A.A.; Marchal-Papier, F.; Grauvogel-Stamm, L. The oldest occurrence of immature Diptera (Insecta), Middle Triassic, France. Ann. Soc. Entomol. Fr. 2010, 46, 4–22. [Google Scholar] [CrossRef]
- Rasnitsyn, A.P. Taxonomy and morphology of Dasyleptus Brongniart, 1885, with description of a new species (Insecta: Machilida: Dasyleptidae). Russ. Entomol. J. 1999, 8, 145–154. [Google Scholar]
- Gall, J.-C.; Grauvogel-Stamm, L. The early middle Triassic ‘Grès à Voltzia’ formation of Eastern France: A model of environmental refugium. C. R. Palevol. 2005, 4, 637–652. [Google Scholar] [CrossRef]
- Sinitshenkova, N.D.; Marchal-Papier, F.; Grauvogel-Stamm, L.; Gall, J.-C. The Ephemeridea (Insecta) from the Grès à Voltzia (early Middle Triassic) of the Vosges (NE France). Paläontol. Z. 2005, 79, 377–397. [Google Scholar] [CrossRef]
- Sinitshenkova, N.D. Ecological history of the aquatic insects. In History of Insects; Rasnitsyn, A.P., Quicke, D.L.J., Eds.; Kluver Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA; London, UK, 2002; pp. 388–417. [Google Scholar]
- Grauvogel, L. Contribution a l’étude du Grès à Voltzia. Bull. Soc. Géol. Fr. 1947, 5, 35–37. [Google Scholar]
- Marchal-Papier, F. Les Insectes du Buntsandstein des Vosges (NE de la France). Biodiversité et Contributions aux Modalités de la Crise Biologique du Permo-Trias. Ph.D. Dissertation, Université Louis Pasteur, Strasbourg, France, 1998. [Google Scholar]
- Brundin, L. Transantarctic relationships and their significance, as evidenced by chironomid midges with a monograph of the subfamilies Podonominae and Aphroteniinae and the Austral Heptagyiae. Kungl. Sven. Vetensk. Handle Fjärde S. 1966, 11, 1–472. [Google Scholar]
- Cranston, P.S.; Edward, D. Afrochlus Freeman: An African gondwanan midge and the phylogeny of the Podonominae (Diptera: Chironomidae). Syst. Entomol. 1998, 23, 77–90. [Google Scholar] [CrossRef]
- Cranston, P.S.; Edward, D.H.D.; Colless, D.H. Archaeochlus Brundin: A midge out of time (Diptera: Chironomidae). Syst. Entomol. 1987, 12, 313–334. [Google Scholar] [CrossRef]
- Ash, S.R. Growth habit and systematics of the Upper Triassic plant Pelourdea poleoensis, Southwestern USA. Rev.Palaeobot. Palynol. 1987, 5, 37–49. [Google Scholar] [CrossRef]
- Lukashevich, E.D. First pupae of the Eoptychopteridae and Ptychopteridae from the Mesozoic of Siberia (Insecta: Diptera). Paleontol. J. 1995, 29, 164–170. [Google Scholar]
- Lukashevich, E.D. Limoniidae (Diptera) in the Upper Jurassic of Shar Teg, Mongolia. Zoosymposia 2009, 3, 131–154. [Google Scholar] [CrossRef]
- Lukashevich, E.D.; Arillo, A. New Eoptychoptera (Insecta: Diptera, Ptychopteridae) from the Lower Cretaceous of Spain. Cret. Res. 2016, 58, 254–264. [Google Scholar] [CrossRef]
- Wiegmann, B.M.; Yeates, D.K. Phylogeny of Diptera. In Manual of Afrotropical Diptera. Volume 1. Introductory Chapters and Keys to Diptera Families; Kirk-Spriggs, A.H., Sinclair, B.J., Eds.; South African National Biodiversity Institute: Pretoria, South Africa, 2017; pp. 253–265. [Google Scholar]
- Wood, D.M.; Borkent, A. Phylogeny and classification of the Nematocera. In Manual of Nearctic Diptera; McAlpine, J.F., Wood, D.M., Eds.; Biosystematics Research Centre: Ottawa, ON, Canada, 1989; Volume 3, pp. 1333–1370. [Google Scholar]
- Lukashevich, E.D.; Ribeiro, G.C. Mesozoic fossils and phylogeny of Tipulomorpha (Insecta: Diptera). J. Syst. Palaeontol. 2019, 17, 635–652. [Google Scholar] [CrossRef]
- Prokin, A.A.; Bashkuev, A.S. Trialarva coburgensis gen et sp. nov., a remarkable fossil holometabolan larva (Insecta: Coleoptera) from the Triassic of Germany. PalZ 2021, 95, 55–60. [Google Scholar] [CrossRef]
- Osborn, J.M.; Taylor, T.N.; White, J.A. Palaeofibulus gen. nov., a clamp-bearing fungus from the Triassic of Antarctica. Mycologia 1989, 81, 622–626. [Google Scholar] [CrossRef]
- Halbwachs, H.; Harper, C.J.; Krings, M. Fossil Ascomycota and Basidiomycota, with notes on fossil lichens and Nematophytes. Encycl. Mycol. 2021, 1, 378–395. [Google Scholar]
- Edwards, F.W.; Keilin, D. Diptera. Fam. Protorhyphidae, Anisopodidae, Pachyneuridae, Trichoceridae. Genera Insectorum 1928, 190, 1–41. [Google Scholar]
- Derraik, J.G.; Heath, A.C. Immature Diptera (excluding Culicidae) inhabiting phytotelmata in the Auckland and Wellington regions. N. Z. J. Mar. Freshw. Res. 2005, 39, 981–987. [Google Scholar] [CrossRef]
- Rohdendorf, B.B. Order Diptera. In Osnovy Paleontologii. Chlenistonogie: Trakheinye, Khelitserovye (Fundamentals of Paleontology: Arthropoda: Tracheata, Chelicerata), Akad; Nauk SSSR: Moscow, Russia, 1962; pp. 307–345. (In Russian) [Google Scholar]
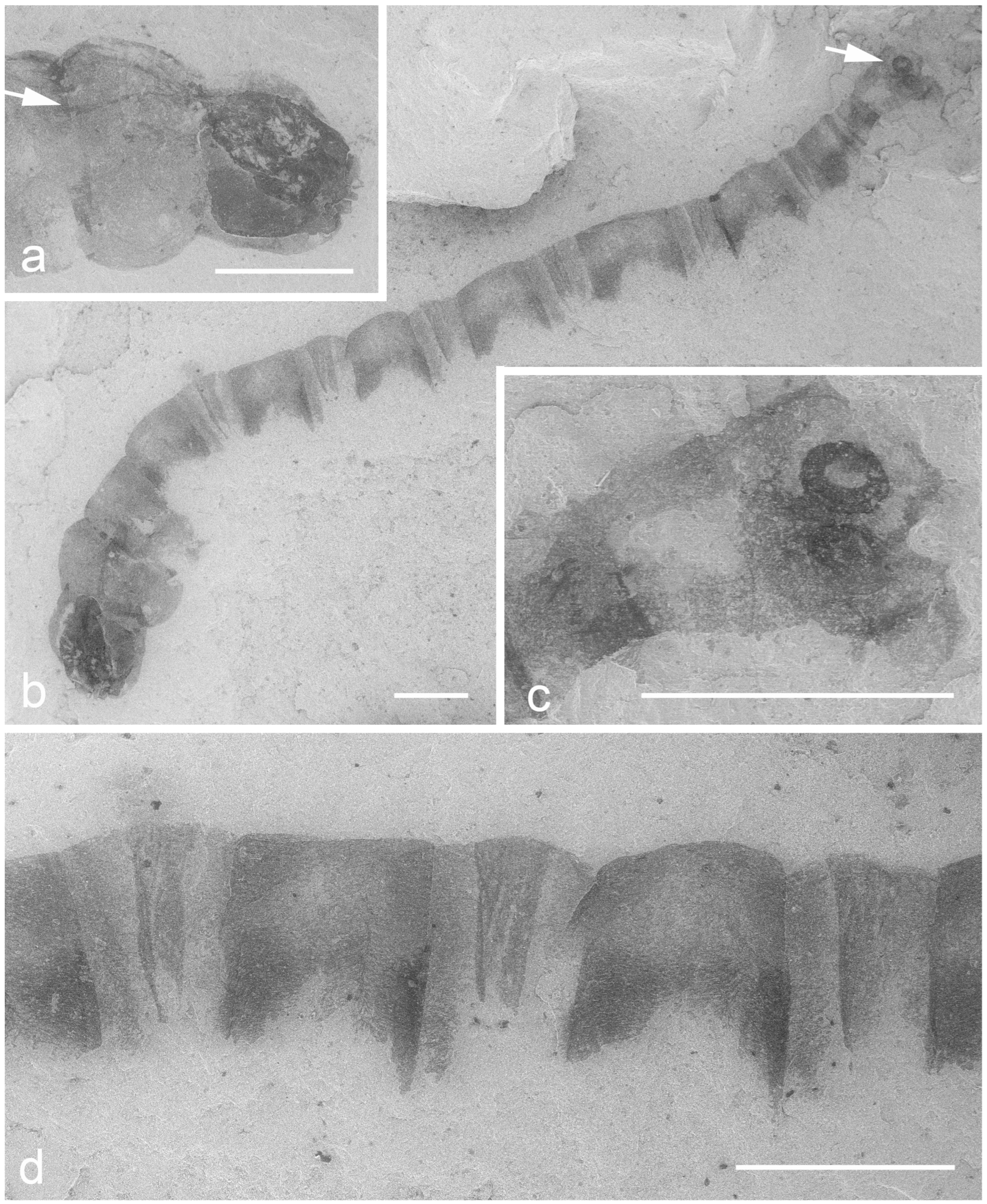
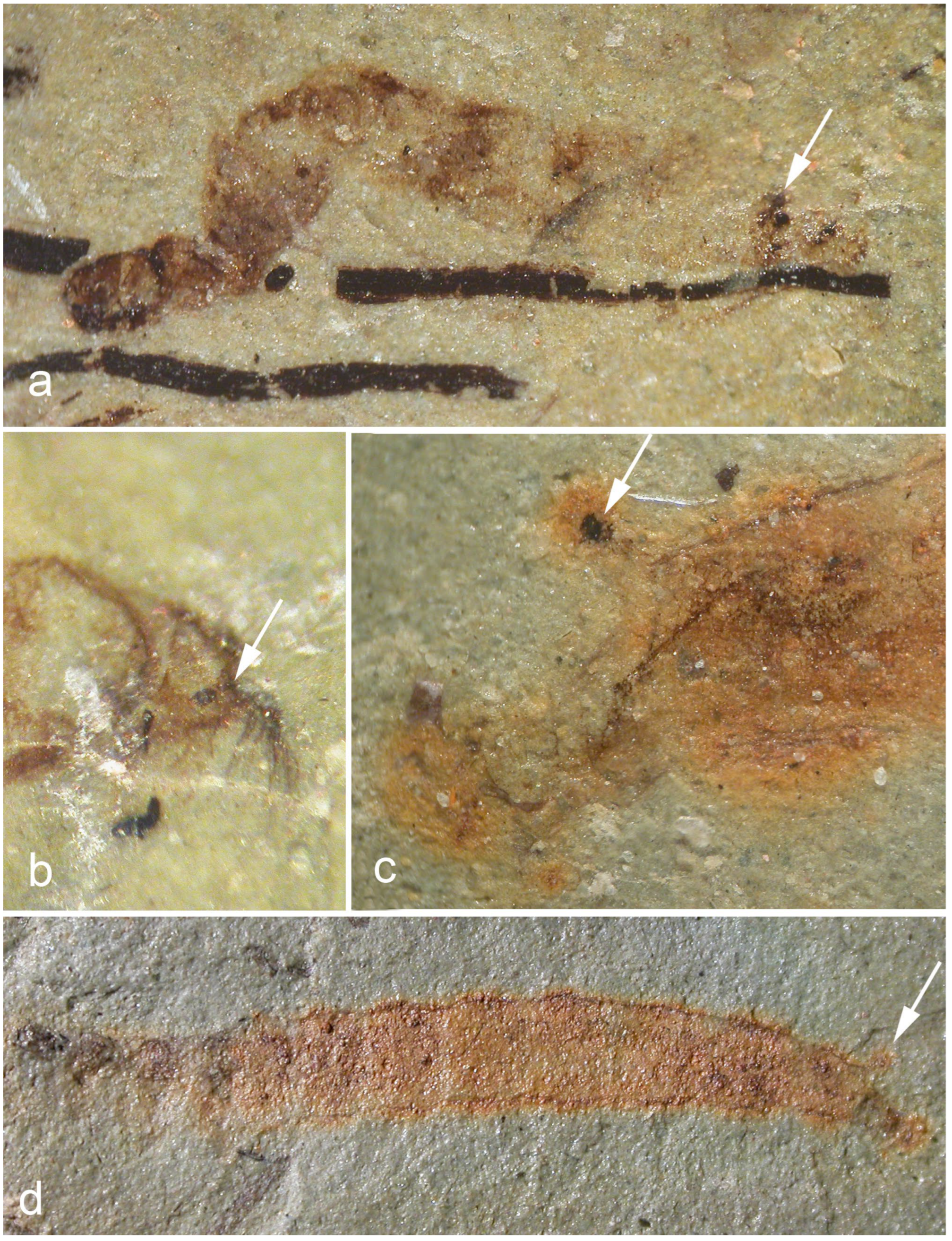
| Family | Amphipneustic | Metapneustic | Apneustic | Other Types | Mesozoic Larvae | Mesozoic Pupae |
|---|---|---|---|---|---|---|
| Limoniidae | + | + | pp | |||
| Pediciidae | + | |||||
| Tipulidae | + | |||||
| Cylindrotomidae | + | |||||
| Ptychopteridae | + | ll | pp | |||
| Tanyderidae | + | + | l | |||
| Psychodidae | + | + | l | |||
| Blephariceridae | + | |||||
| Deuterophlebiidae | + | |||||
| Nymphomyiidae | + | |||||
| Dixidae | + | |||||
| Corethrellidae | + | |||||
| Chaoboridae | + | + | lll | ppp | ||
| Culicidae | + | |||||
| Chironomidae | + | + | lll | ppp | ||
| Ceratopogonidae | + | |||||
| Simuliidae | + | ll | p | |||
| Thaumaleidae | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukashevich, E.D. Where the Immatures of Triassic Diptera Developed. Diversity 2023, 15, 582. https://doi.org/10.3390/d15040582
Lukashevich ED. Where the Immatures of Triassic Diptera Developed. Diversity. 2023; 15(4):582. https://doi.org/10.3390/d15040582
Chicago/Turabian StyleLukashevich, Elena D. 2023. "Where the Immatures of Triassic Diptera Developed" Diversity 15, no. 4: 582. https://doi.org/10.3390/d15040582
APA StyleLukashevich, E. D. (2023). Where the Immatures of Triassic Diptera Developed. Diversity, 15(4), 582. https://doi.org/10.3390/d15040582






