Microclimatic Fluctuation throughout the Day Influences Subtropical Fruit-Feeding Butterfly Assemblages between the Canopy and Understory
Abstract
1. Introduction
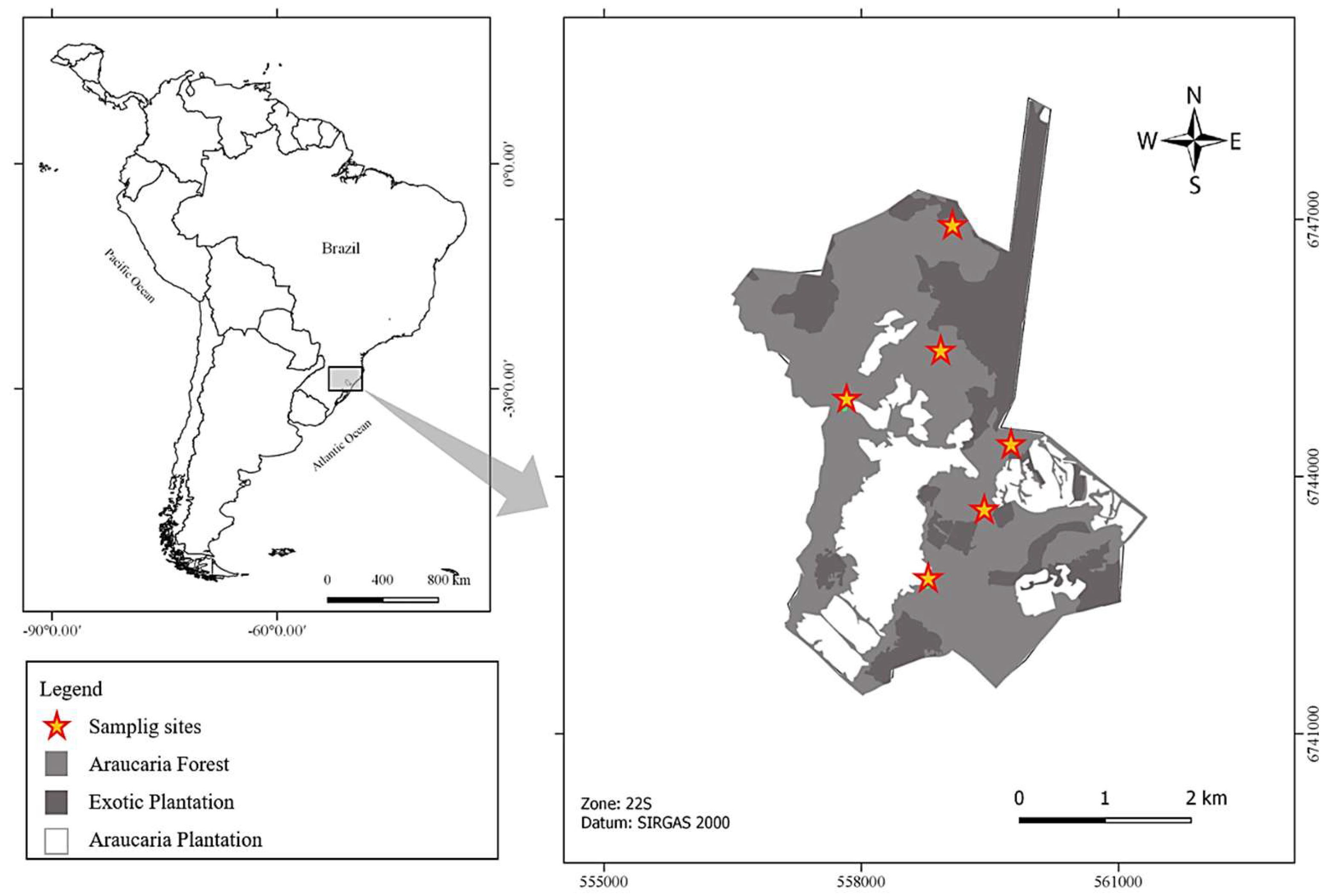
2. Materials and Methods
3. Results
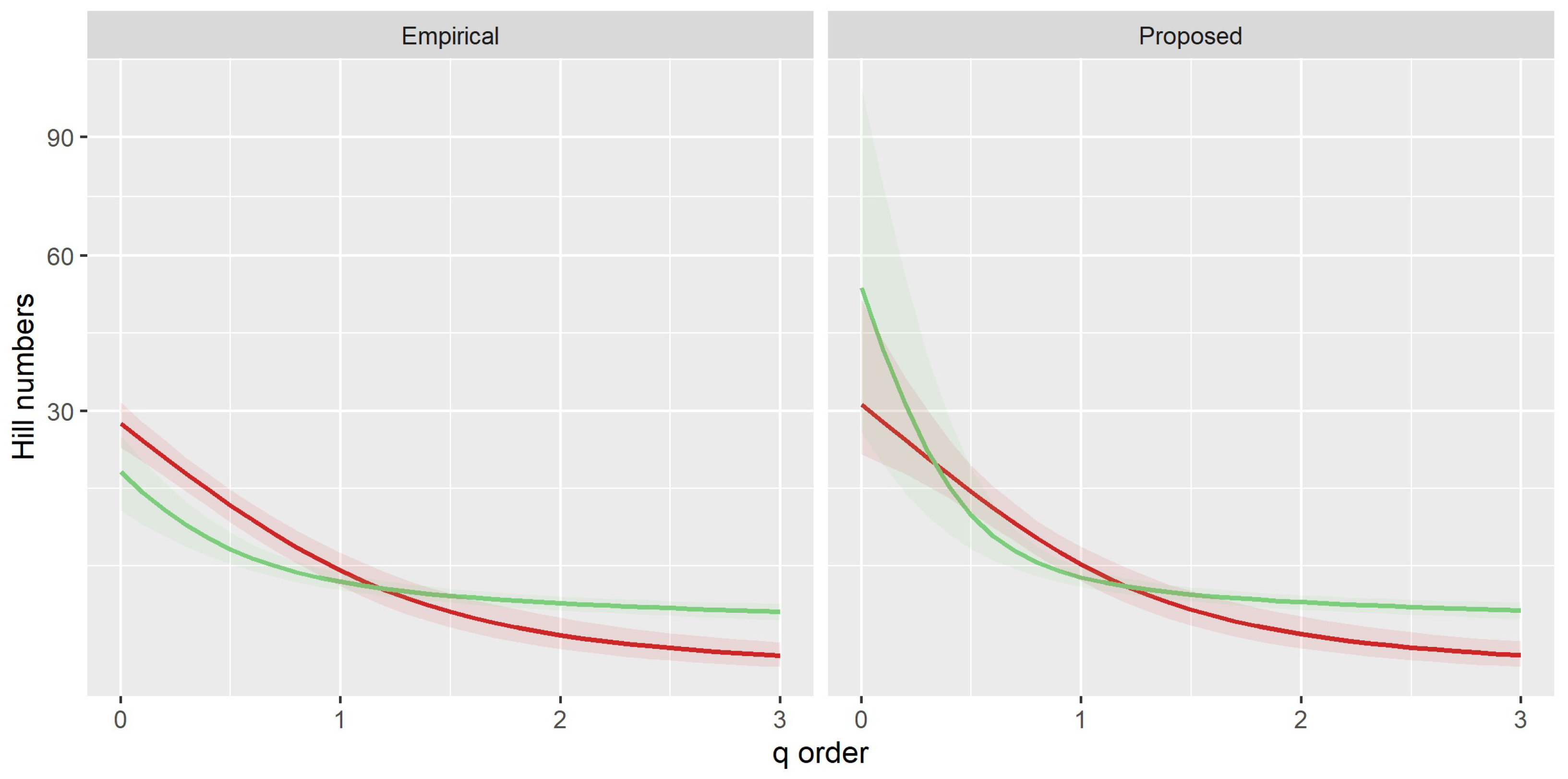
3.1. Environmental Effects on Richness and Abundance
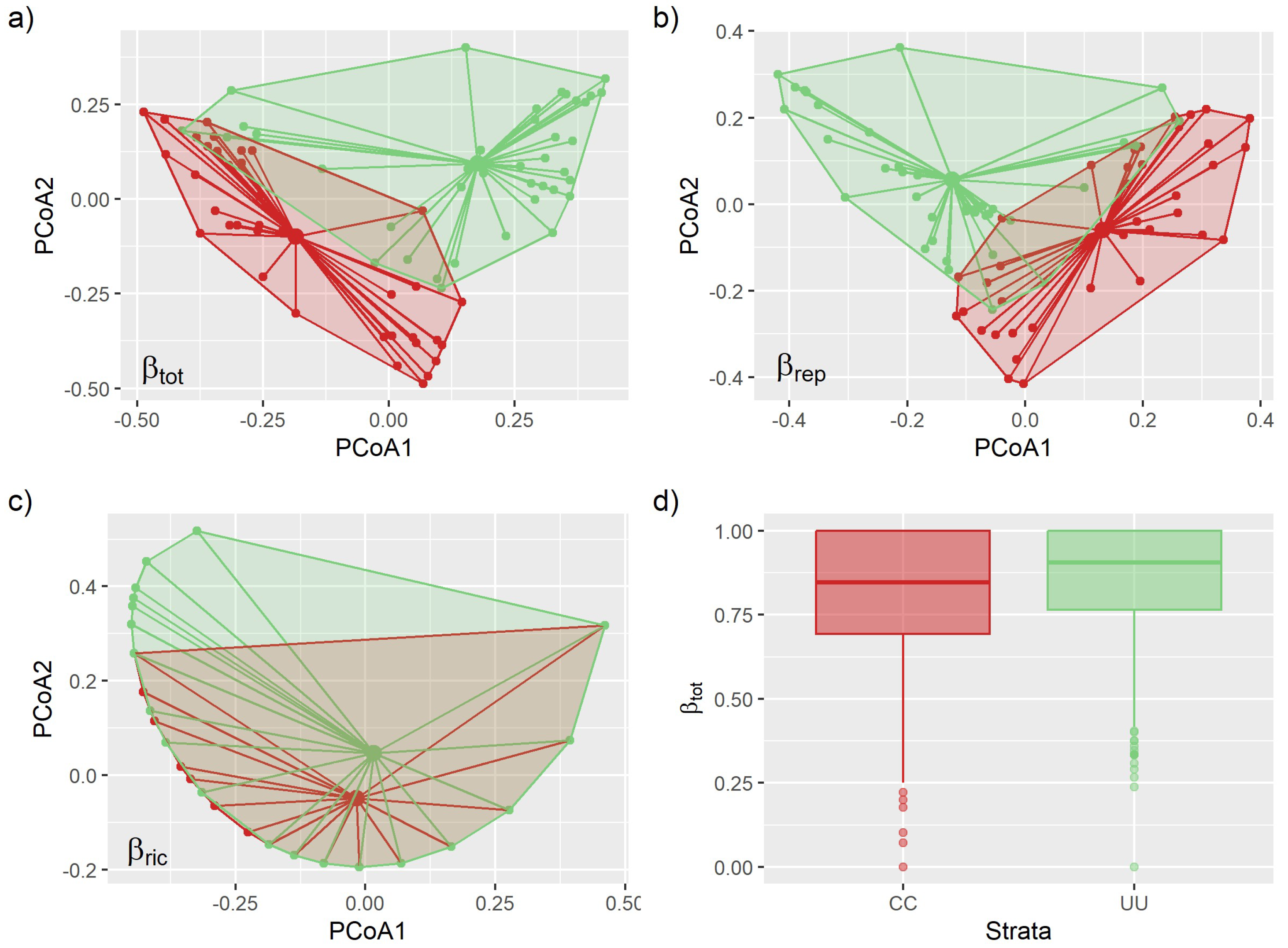
3.2. Environmental Effects on Beta Diversity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, K.S.; Freitas, A.V.L. Atlantic Forest Butterflies: Indicators for Landscape Conservation. Biotropica 2000, 32, 934–956. [Google Scholar] [CrossRef]
- Iserhard, C.A.; Romanowski, H.P.; Richter, A.; Mendonça, J.M.D.S. Monitoring Temporal Variation to Assess Changes in the Structure of Subtropical Atlantic Forest Butterfly Communities. Environ. Entomol. 2017, 46, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Basset, Y.; Hammond, P.M.; Barrios, H.; Holloway, J.D.; Miller, S.E. Vertical Stratification of Arthropod Assemblages. In Arthropods of Tropical Forests: Spatio-Temporal Dynamics and Resource Use in the Canopy; Cambridge University Press: Cambridge, UK, 2003; pp. 17–27. [Google Scholar]
- Schulze, C.H.; Linsenmair, K.E.; Fiedler, K. Understorey versus Canopy: Patterns of Vertical Stratification and Diversity among Lepidoptera in a Bornean Rain Forest. Plant Ecol. 2001, 153, 133–152. [Google Scholar] [CrossRef]
- Devries, P.J. Stratification of Fruit-Feeding Nymphalid Butterflies in a Costa Rican Rainforest. J. Res. Lepid. 1988, 26, 98–108. [Google Scholar]
- Araujo, P.F.; Freitas, A.V.L.; De Souza, G.A.; Ribeiro, D.B. Studies on Neotropical Fauna and Environment Vertical Stratification on a Small Scale: The Distribution of Fruit-Feeding Butterflies in a Semi- Deciduous Atlantic Forest in Brazil. Stud. Neotrop. Fauna. Environ. 2020, 56, 10–39. [Google Scholar] [CrossRef]
- Hirao, T.; Murakami, M.; Kashizaki, A. Importance of the Understory Stratum to Entomofaunal Diversity in a Temperate Deciduous Forest. Ecol. Res. 2009, 24, 263–272. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Arthropod Vertical Stratification in Temperate Deciduous Forests: Implications for Conservation-Oriented Management. Ecol. Manag. 2011, 261, 1479–1489. [Google Scholar] [CrossRef]
- Maguire, D.Y.; Robert, K.; Brochu, K.; Larrivée, M.; Buddle, C.M.; Wheeler, T.A. Vertical Stratification of Beetles (Coleoptera) and Flies (Diptera) in Temperate Forest Canopies. Environ. Entomol. 2014, 43, 9–17. [Google Scholar] [CrossRef]
- Ashton, L.A.; Nakamura, A.; Basset, Y.; Burwell, C.J.; Cao, M.; Eastwood, R.; Odell, E.; de Oliveira, E.G.; Hurley, K.; Katabuchi, M.; et al. Vertical Stratification of Moths across Elevation and Latitude. J. Biogeogr. 2016, 43, 59–69. [Google Scholar] [CrossRef]
- Danks, H.V. Winter Habitats and Ecological Adaptations for Winter Survival. Insects Low Temp. 1991, 1, 231–259. [Google Scholar] [CrossRef]
- Tal, O.; Freiberg, M.; Morawetz, W. Microclimate Variability in the Canopy of a Temperate Forest. Canopy Arthropod Res. Eur. 2008, 448, 49–59. [Google Scholar]
- Hodkinson, I.D. Terrestrial Insects along Elevation Gradients: Species and Community Responses to Altitude. Biol. Rev. Camb. Philos. Soc. 2005, 80, 489–513. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.S. Conservation of Neotropical Environments: Insects as Indicators; The Royal Entomological Society of London: London, UK, 1991. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Tabarelli, M.; Aguiar, A.V.; Ribeiro, M.C.; Metzger, J.P.; Peres, C.A. Prospects for Biodiversity Conservation in the Atlantic Forest: Lessons from Aging Human-Modified Landscapes. Biol. Conserv. 2010, 143, 2328–2340. [Google Scholar] [CrossRef]
- Hueck, K. As Florestas Da Ameérica Do Sul: Ecologia, Composição e Importancia Economica; Editora de Universidade de Brasilia: Brasilia, Brazil, 1972. [Google Scholar]
- dos Santos, J.P.; Iserhard, C.A.; Carreira, J.Y.O.; Freitas, A.V.L. Monitoring Fruit-Feeding Butterfly Assemblages in Two Vertical Strata in Seasonal Atlantic Forest: Temporal Species Turnover Is Lower in the Canopy. J. Trop. Ecol. 2017, 33, 345–355. [Google Scholar] [CrossRef]
- Checa, M.F.; Levy, E.; Rodriguez, J.; Willmott, K. Rainfall as a Significant Contributing Factor to Butterfly Seasonality along a Climatic Gradient in the Neotropics. BioRxiv 2019, 1, 1–32. [Google Scholar] [CrossRef]
- Checa, M.F.; Barragán, A.; Rodríguez, J.; Christman, M. Temporal Abundance Patterns of Butterfly Communities (Lepidoptera: Nymphalidae) in the Ecuadorian Amazonia and Their Relationship with Climate. Ann. Soc. Entomol. Fr. (N.S.) 2009, 45, 470–486. [Google Scholar] [CrossRef]
- Checa, M.F.; Rodriguez, J.; Willmott, K.R.; Liger, B. Microclimate Variability Significantly Affects the Composition, Abundance and Phenology of Butterfly Communities in a Highly Threatened Neotropical Dry Forest. Fla. Entomol. 2014, 97, 1–13. [Google Scholar] [CrossRef]
- Bonebrake, T.C.; Ponisio, L.C.; Boggs, C.L.; Ehrlich, P.R. More than Just Indicators: A Review of Tropical Butterfly Ecology and Conservation. Biol. Conserv. 2010, 143, 1831–1841. [Google Scholar] [CrossRef]
- Molleman, F.; Kop, A.; Brakefield, P.M.; DeVries, P.J.; Zwaan, B.J. Vertical and Temporal Patterns of Biodiversity of Fruit-Feeding Butterflies in a Tropical Forest in Uganda. Biodivers. Conserv. 2006, 15, 107–121. [Google Scholar] [CrossRef]
- Graça, M.B.; Pequeno, P.A.C.L.; Franklin, E.; Morais, J.W. Coevolution between Flight Morphology, Vertical Stratification and Sexual Dimorphism: What Can We Learn from Tropical Butterflies? J. Evol. Biol. 2017, 30, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- DeVries, P.J.; Alexander, L.G.; Chacon, I.A.; Fordyce, J.A. Similarity and Difference among Rainforest Fruit-Feeding Butterfly Communities in Central and South America. J. Anim. Ecol. 2012, 81, 472–482. [Google Scholar] [CrossRef]
- Mena, S.; Kozak, K.M.; Cárdenas, R.E.; Checa, M.F. Forest Stratification Shapes Allometry and Flight Morphology of Tropical Butterflies. Proc. R. Soc. B Biol. Sci. 2020, 287, 20201071. [Google Scholar] [CrossRef]
- DeVries, P.J.; Penz, C.M.; Hill, R.I. Vertical Distribution, Flight Behaviour and Evolution of Wing Morphology in Morpho Butterflies. J. Anim. Ecol. 2010, 79, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, J.A.; DeVries, P.J. A Tale of Two Communities: Neotropical Butterfly Assemblages Show Higher Beta Diversity in the Canopy Compared to the Understory. Oecologia 2016, 181, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, G.M.; Soares, G.R.; Santos, T.P.; Dáttilo, W.; Freitas, A.V.L.; Ribeiro, S.P. Equal but Different: Natural Ecotones Are Dissimilar to Anthropic Edges. PLoS ONE 2019, 14, e0213008. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.B.; Freitas, A.V.L. The Effect of Reduced-Impact Logging on Fruit-Feeding Butterflies in Central Amazon, Brazil. J. Insect. Conserv. 2012, 16, 733–744. [Google Scholar] [CrossRef]
- Hill, J.; Hamer, K.; Tangah, J.; Dawood, M. Ecology of Tropical Butterflies in Rainforest Gaps. Oecologia 2001, 128, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Fermon, H.; Waltert, M.; Vane-Wright, R.I.; Mühlenberg, M. Forest Use and Vertical Stratification in Fruit-Feeding Butterflies of Sulawesi, Indonesia: Impacts for Conservation. Biodivers. Conserv. 2005, 14, 333–350. [Google Scholar] [CrossRef]
- Roche, K.N.; Piorkowski, J.M.; Sanyaolu, R.A.; Cordeiro, N.J. Vertical Distribution of Fruit-Feeding Butterflies with Evidence of Sex-Specific Differences in a Tanzanian Forest. Afr. J. Ecol. 2015, 53, 480–486. [Google Scholar] [CrossRef]
- Shaw, D.C. Vertical Organization of Canopy Biota. In Forest Canopies; Lowman, M.D., Rinker, H.B., Eds.; Elsevier Academic Press Burlington: Burlington, MA, USA, 2004; pp. 73–101. [Google Scholar]
- Xing, S.; Bonebrake, T.C.; Tang, C.C.; Pickett, E.J.; Cheng, W.; Greenspan, S.E.; Williams, S.E.; Scheffers, B.R. Cool Habitats Support Darker and Bigger Butterflies in Australian Tropical Forests. Ecol. Evol. 2016, 6, 8062–8074. [Google Scholar] [CrossRef] [PubMed]
- García-Robledo, C.; Kuprewicz, E.K.; Staines, C.L.; Erwin, T.L.; Kress, W.J. Limited tolerance by insects to high temperatures across tropical elevational gradients and the implications of global warming for extinction. Proc. Natl. Acad. Sci. USA 2016, 113, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, J.G.; Watt, W.B. Thermoregulatory Strategies in Colias Butterflies: Thermal Stress and the Limits to Adaptation in Temporally Varying Environments. Am. Nat. 1983, 121, 32–55. [Google Scholar] [CrossRef]
- ICMBio. Plano De Manejo Da Floresta Nacional De São Francisco De Paula; Technical Report; ICMBio: Brasília, Brazil, 2020; p. 54.
- Rambo, B. A Fisionomia Do Rio Grande Do Sul, 2nd ed.; Selbach: Porto Alegre, Brazil, 1956; ISBN 8585580119. [Google Scholar]
- Backes, A. Distribuição Geográfica Atual Da Floresta Com Araucária: Condicionamento Climático. In Floresta com Araucária: Ecologia, Conservação e Desenvolvimento Sustentável; Fonseca, C.R., Souza, A.F., Leal-Zanchet, A.M., Tânia, L., Dutra, A.B.G.G., Eds.; Holos: Ribeirão Preto, Brazil, 2009; p. 328. [Google Scholar]
- Freitas, A.V.L.; Iserhard, C.A.; dos Santos, J.P.; Carreira, J.Y.O.; Ribeiro, D.B.; Melo, D.H.A.; Rosa, A.H.; Marini-Filho, O.J.; Mattos Accacio, G.; Uehara-Prado, M. Studies with Butterfly Bait Traps: An Overview. Rev. Colomb. Entomol. 2014, 40, 203–212. [Google Scholar]
- Jost, L. Entropy and Diversity. Oikos 2006, 113, 363–375. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sande, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and Extrapolation with Hill Numbers: A Framework for Sampling and Estimation in Species Diversity Studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- DeVries, P.J. The Butterflies of Costa Rica and Their Natural History: Papilionidae, Pieridae, Nymphalidae; The Butterflies of Costa Rica and Their Natural History; Princeton University Press: Princeton, NJ, USA, 1987; ISBN 9780691024035. [Google Scholar]
- Legendre, P.; Legendre, L. Numerical Ecology. In Developments in Environmental Modelling; Elsevier: Amsterdam, The Netherlands, 2012; Volume 24, p. 1006. [Google Scholar]
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate Dispersion as a Measure of Beta Diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Dufrêne, M.; Legendre, P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- De Cáceres, M. How to Use the Indicspecies Package (Ver. 1.7.1). Available online: https://cran.r-project.org/web/packages/indicspecies/vignettes/IndicatorSpeciesAnalysis.html (accessed on 3 April 2023).
- R Core Team. R: A Language and Environment for Statistical Computing 2021. Available online: https://www.R-project.org (accessed on 3 April 2023).
- Chao, A.; Jost, L. Estimating Diversity and Entropy Profiles via Discovery Rates of New Species. Methods Ecol. Evol. 2015, 6, 873–882. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. INEXT: Interpolation and Extrapolation for Species Diversity. Methods in Ecology and Evolution 2020, 7, 1451–1456. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- De Caceres, M.; Legendre, P. Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 2009, 90, 3566–3574. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. 2020. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 3 April 2023).
- Cardoso, P.; Rigal, F.; Carvalho, J.C. BAT—Biodiversity Assessment Tools, an R Package for the Measurement and Estimation of Alpha and Beta Taxon, Phylogenetic and Functional Diversity. Methods Ecol. Evol. 2015, 6, 232–236. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Madigosky, S.R. CHAPTER 2—Tropical Microclimatic Considerations. In Physiological Ecology; Lowman, M.D., Rinker, H.B.B.T.-F.C., Second, E., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 24–48. ISBN 978-0-12-457553-0. [Google Scholar]
- Wolda, H. Insect Seasonality: Why? Ann. Rev. Ecol. Sysi. 1988, 19, 1–18. [Google Scholar] [CrossRef]
- Menéndez, R.; González-Megías, A.; Collingham, Y.; Fox, R.; Roy, D.B.; Ohlemüller, R.; Thomas, C.D. Direct And Indirect Effects Of Climate And Habitat Factors On Butterfly Diversity. Ecology 2007, 88, 605–611. [Google Scholar] [CrossRef]
- Kingsolver, J.G.; Huey, R.B. Size, Temperature, and Fitness: Three Rules. Evol. Ecol. Res. 2008, 10, 251–268. [Google Scholar]
- Ribeiro, D.B.; Freitas, A.V.L. Large-Sized Insects Show Stronger Seasonality than Small-Sized Ones: A Case Study of Fruit-Feeding Butterflies. Biol. J. Linn. Soc. 2011, 104, 820–827. [Google Scholar] [CrossRef]
- Queiroz, J.M. Host Plant Use among Closely Related Anaea Butterfly Species (Lepidoptera, Nymphalidae, Charaxinae). Braz. J. Biol. 2002, 62, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Perez Rocha, M.; Bini, L.M.; Domisch, S.; Tolonen, K.T.; Jyrkänkallio-Mikkola, J.; Soininen, J.; Hjort, J.; Heino, J. Local Environment and Space Drive Multiple Facets of Stream Macroinvertebrate Beta Diversity. J. Biogeogr. 2018, 45, 2744–2754. [Google Scholar] [CrossRef]
- McGill, B.J.; Enquist, B.J.; Weiher, E.; Westoby, M. Rebuilding Community Ecology from Functional Traits. Trends Ecol. Evol. 2006, 21, 178–185. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Cavender-Bares, J.; Tilman, D.; Oakley, T.H. Using Phylogenetic, Functional and Trait Diversity to Understand Patterns of Plant Community Productivity. PLoS ONE 2009, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.; Wahlberg, N. Prehistorical Climate Change Increased Diversification of a Group of Butterflies. Biol. Lett. 2008, 4, 274–278. [Google Scholar] [CrossRef] [PubMed]
- van Asch, M.; Visser, M.E. Phenology of Forest Caterpillars and Their Host Trees: The Importance of Synchrony. Annu. Rev. Entomol. 2007, 52, 37–55. [Google Scholar] [CrossRef]
- van Dyck, H.; Matthysen, E. Thermoregulatory Differences between Phenotypes in the Speckled Wood Butterfly: Hot Perchers and Cold Patrollers? Oecologia 1998, 114, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, P. Canopy Surface Topography in a French Guiana Forest and the Folded Forest Theory. Plant Ecol. 2001, 153, 293–300. [Google Scholar] [CrossRef]

| df | logLik | AICc | Delta | Weight | Pseudo-R2 | |
|---|---|---|---|---|---|---|
| Model for richness | ||||||
| Null (Poisson) | 2 | −163.125 | 330.400 | 0.000 | 0.985 | |
| Full model (Poisson) | 8 | −160.424 | 338.800 | 8.370 | 0.015 | 0.417 1 |
| Model for abundance | ||||||
| Full model (negative binomial) | 9 | −258.573 | 537.600 | 0.000 | 0.908 | 0.472 1 |
| Null (negative binomial) | 3 | −267.928 | 542.200 | 4.580 | 0.092 |
| Estimate | SE | z-Value | Pr(>|z|) | |
|---|---|---|---|---|
| Intercept | 2.120 | 0.333 | 6.372 | 0.000 |
| Mean temperature (day) | 0.913 | 0.429 | 2.127 | 0.033 |
| Temperature variation (night) | 1.221 | 0.457 | 2.674 | 0.007 |
| Mean temperature (day): understory | −0.183 | 0.388 | −0.471 | 0.638 |
| Temperature variation (night): understory | −0.979 | 0.416 | −2.352 | 0.019 |
| Canopy: mean humidity (day) | 1.400 | 0.464 | 3.016 | 0.003 |
| Understory: mean humidity (day) | 0.564 | 0.420 | 1.343 | 0.179 |
| Df | SumOfSqs | R2 | F | p | |
|---|---|---|---|---|---|
| βtot | |||||
| Mean temperature (day) | 1 | 0.562 | 0.018 | 1.625 | 0.081 |
| Temperature variation (night) | 1 | 0.378 | 0.012 | 1.094 | 0.970 |
| Mean humidity (day) | 1 | 0.561 | 0.018 | 1.623 | 0.161 |
| Strata | 1 | 1.165 | 0.037 | 3.367 | 0.001 |
| Residual | 73 | 25.246 | 0.811 | ||
| Total | 77 | 31.144 | 1.000 | ||
| βrep | |||||
| Mean temperature (day) | 1 | −0.493 | −0.050 | −4.265 | 0.983 |
| Temperature variation (night) | 1 | −0.072 | −0.007 | −0.619 | 0.999 |
| Mean humidity (day) | 1 | 0.074 | 0.008 | 0.639 | 0.779 |
| Strata | 1 | 0.339 | 0.035 | 2.937 | 0.060 |
| Residual | 73 | 8.435 | 0.864 | ||
| Total | 77 | 9.766 | 1.000 | ||
| βric | |||||
| Mean temperature (day) | 1 | 1.035 | 0.093 | 8.357 | 0.143 |
| Temperature variation (night) | 1 | 0.143 | 0.013 | 1.156 | 0.214 |
| Mean humidity (day) | 1 | 0.156 | 0.014 | 1.262 | 0.228 |
| Strata | 1 | 0.558 | 0.050 | 4.509 | 0.485 |
| Residual | 73 | 9.040 | 0.810 | ||
| Total | 77 | 11.155 | 1.000 |
| A | B | Stat | p | |
|---|---|---|---|---|
| Canopy | ||||
| Carminda paeon (Sat, Satyrini) | 0.906 | 0.632 | 0.756 | 0.001 |
| Zaretis strigosus (Cha, Anaeini) | 1.000 | 0.368 | 0.607 | 0.001 |
| Epiphile orea (Bib, Epiphilini) | 0.944 | 0.290 | 0.523 | 0.001 |
| Memphis moruus (Cha, Anaeini) | 1.000 | 0.263 | 0.513 | 0.001 |
| Smyrna blomfildia (Nym, Coeini) | 1.000 | 0.105 | 0.324 | 0.050 |
| Understory | ||||
| Pseudodebis ypthima (Sat, Satyrini) | 1.000 | 0.575 | 0.758 | 0.001 |
| Eryphanis reevesii (Sat, Brassolini) | 0.985 | 0.500 | 0.702 | 0.001 |
| Forsterinaria quantius (Sat, Satyrini) | 0.988 | 0.325 | 0.567 | 0.001 |
| Morpho epistrophus (Sat, Morphini) | 0.979 | 0.250 | 0.495 | 0.003 |
| Opoptera fruhstorferi (Sat, Brassolini) | 1.000 | 0.225 | 0.474 | 0.007 |
| Caligo martia (Sat, Brassolini) | 1.000 | 0.150 | 0.387 | 0.023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Richter, A.; de Souza Mendonça, M., Jr.; Gawlinski, K.; Iserhard, C.A. Microclimatic Fluctuation throughout the Day Influences Subtropical Fruit-Feeding Butterfly Assemblages between the Canopy and Understory. Diversity 2023, 15, 560. https://doi.org/10.3390/d15040560
Richter A, de Souza Mendonça M Jr., Gawlinski K, Iserhard CA. Microclimatic Fluctuation throughout the Day Influences Subtropical Fruit-Feeding Butterfly Assemblages between the Canopy and Understory. Diversity. 2023; 15(4):560. https://doi.org/10.3390/d15040560
Chicago/Turabian StyleRichter, Aline, Milton de Souza Mendonça, Jr., Karine Gawlinski, and Cristiano Agra Iserhard. 2023. "Microclimatic Fluctuation throughout the Day Influences Subtropical Fruit-Feeding Butterfly Assemblages between the Canopy and Understory" Diversity 15, no. 4: 560. https://doi.org/10.3390/d15040560
APA StyleRichter, A., de Souza Mendonça, M., Jr., Gawlinski, K., & Iserhard, C. A. (2023). Microclimatic Fluctuation throughout the Day Influences Subtropical Fruit-Feeding Butterfly Assemblages between the Canopy and Understory. Diversity, 15(4), 560. https://doi.org/10.3390/d15040560







