Saproxylic Beetle Community in the Expansion Site of a Megaproject and in the Surrounding Area in the Western Italian Alps
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Design, Identification of Beetles and Plants
2.3. Forest Structural Parameters
2.4. Data Analysis
2.4.1. Characterization of Forested Sites and Beetle Communities
2.4.2. Relationship between Beetle Diversity and Forest Variables
3. Results
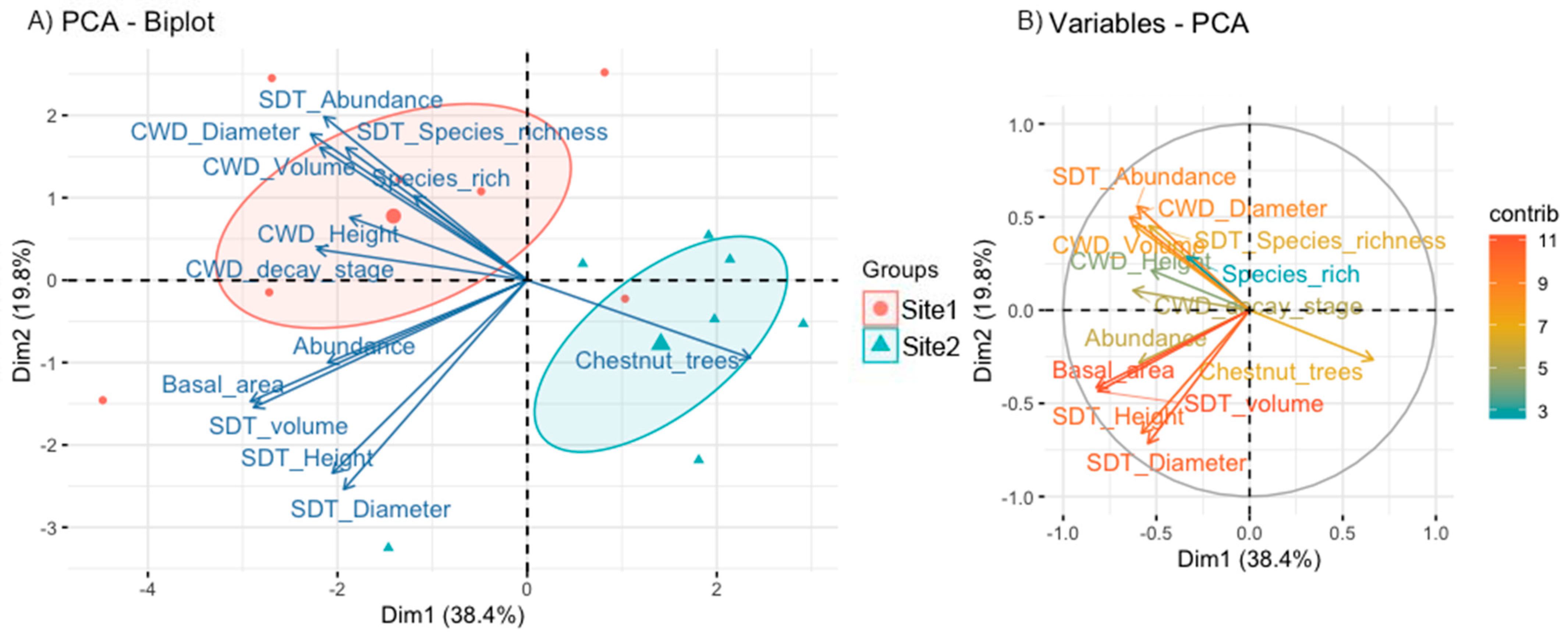
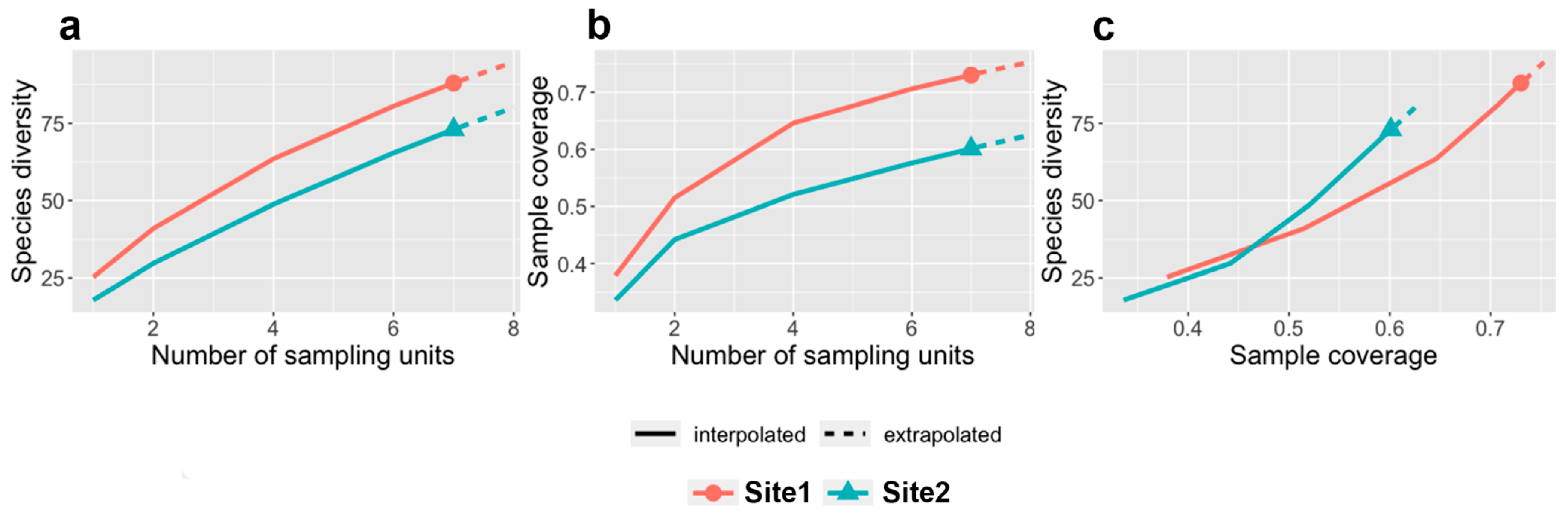
3.1. Characterization of Forested Sites
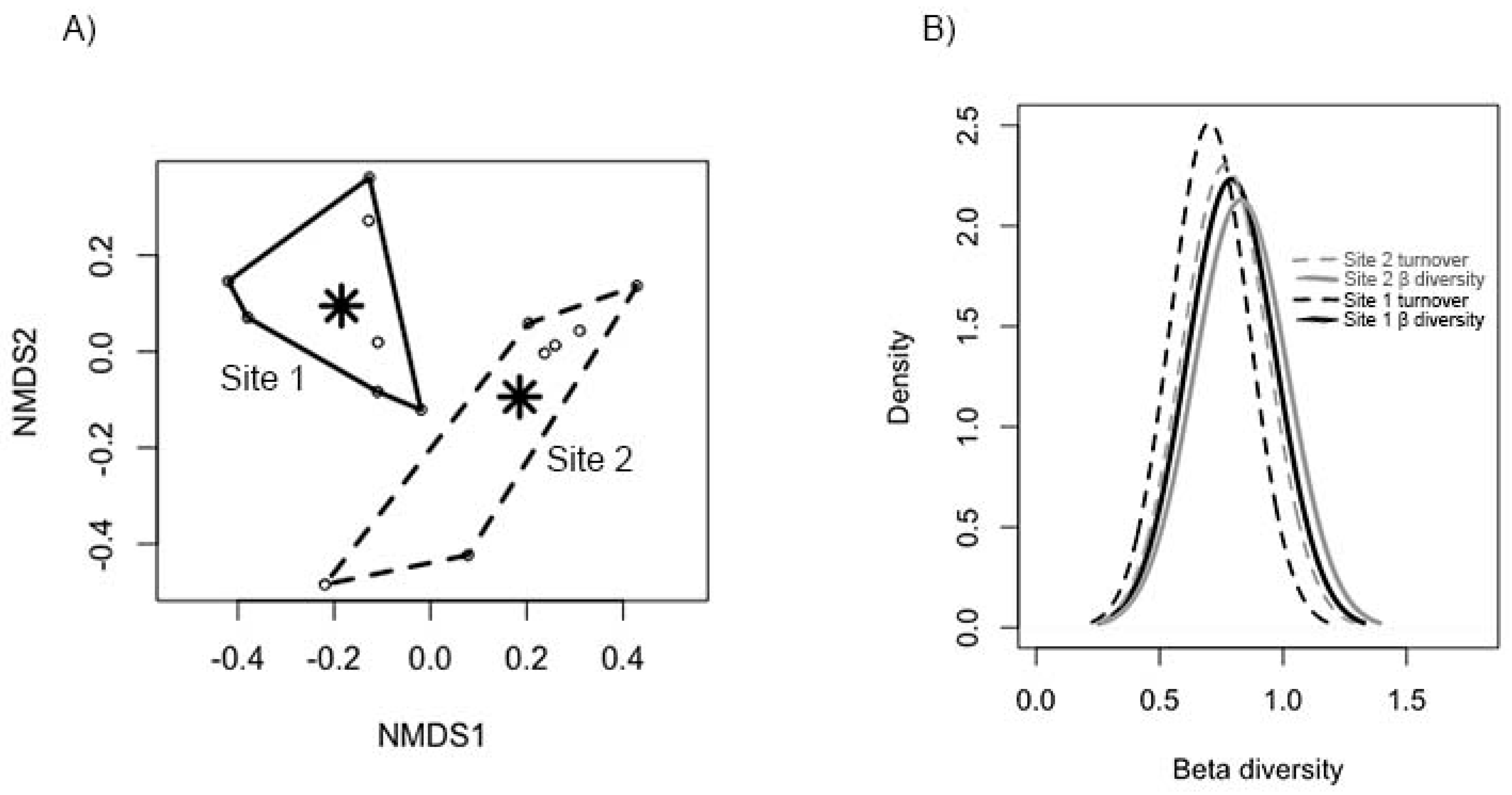
3.2. Characterization of Beetle Communities
3.3. Relationship between Beetle Diversity and Forest Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gellert, P.K.; Lynch, B.D. Mega-projects as displacements. Inter. Soc. Sci. J. 2003, 55, 15–25. [Google Scholar] [CrossRef]
- Flyvbjerg, B. What you should know about megaprojects and why: An overview. Proj. Manag. J. 2014, 45, 6–19. [Google Scholar] [CrossRef]
- Gürtler, K. Trees Versus Concrete: Deforestation in the North Bosphorus Region and Civil Society Responses. Heinrich Boell Stiftung-Turkey. 2016. Available online: https://tr.boell.org/de/2016/04/15/trees-versus-concrete-deforestation-north-bosphorus-region-and-civil-society-responses (accessed on 15 March 2023).
- De León, L.F.; Lopez, O.R. Biodiversity beyond Trees: Panama’s Canal Provides Limited Conservation Lessons for Nicaragua. Biodiv. Cons. 2016, 25, 2821–2825. [Google Scholar] [CrossRef]
- Piccini, I.; Pittarello, M.; Gili, F.; Dotta, A.; Lorizzo, R.; Magnani, C.; Grieco, P.; Lonati, M.; Bertolino, S.; Bonelli, S. Using Forest Compensation Funds to Reverse Biodiversity Loss: A Case Study of Turin–Lyon High-Speed Railway Line. Sustainability 2022, 14, 4411. [Google Scholar] [CrossRef]
- Jonsson, B.G.; Kruys, N.; Ranius, T. Ecology of species living on dead wood–lessons for dead wood management. Silva Fenn. 2005, 39, 289–309. [Google Scholar] [CrossRef]
- Ulyshen, M.D.; Wagner, T.L. Quantifying arthropod contributions to wood decay. Methods Ecol. Evol. 2013, 4, 345–352. [Google Scholar] [CrossRef]
- Larrieu, L.; Paillet, Y.; Winter, S.; Bütler, R.; Kraus, D.; Krumm, F.; Lachat, T.; Michel, A.K.; Ragnery, B.; Vandekerkhove, K. Tree related microhabitats in temperate and Mediterranean European forests: A hierarchical typology for inventory standardization. Ecol. Indic. 2018, 84, 194–207. [Google Scholar] [CrossRef]
- Ulyshen, M.D. Strengthening the case for saproxylic arthropod conservation: A call for ecosystem services research. Insect Conserv. Divers. 2013, 6, 393–395. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Margules, C.R.; Botkin, D.B. Indicators of biodiversity for ecologically sustainable forest management. Conserv. Biol. 2000, 14, 941–950. [Google Scholar] [CrossRef]
- Davies, Z.G.; Tyler, C.; Stewart, G.B.; Pullin, A.S. Are current management recommendations for saproxylic invertebrates effective? A systematic review. Biodivers. Conserv. 2008, 17, 209–234. [Google Scholar] [CrossRef]
- Zumr, V.; Remeš, J.; Pulkrab, K. How to Increase Biodiversity of Saproxylic Beetles in Commercial Stands through Integrated Forest Management in Central Europe. Forests 2021, 12, 814. [Google Scholar] [CrossRef]
- Paillet, Y.; Bergès, L.; Hjältén, J.; Ódor, P.; Avon, C.; Bernhardt-Römermann, M.; Bijlsma, R.J.; De Bruyn, L.; Fuhr, M.; Grandin, U.; et al. Biodiversity Differences between Managed and Unmanaged Forests: Meta-Analysis of Species Richness in Europe. Conserv. Biol. 2010, 24, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Parisi, F.; Di Febbraro, M.; Lombardi, F.; Biscaccianti, A.B.; Campanaro, A.; Tognetti, R.; Marchetti, M. Relationships between stand structural attributes and saproxylic beetle abundance in a Mediterranean broadleaved mixed forest. For. Ecol. Manag. 2019, 432, 957–966. [Google Scholar] [CrossRef]
- Parisi, F.; Frate, L.; Lombardi, F.; Tognetti, R.; Campanaro, A.; Biscaccianti, A.B.; Marchetti, M. Diversity patterns of Coleoptera and saproxylic communities in unmanaged forests of Mediterranean mountains. Ecol. Indic. 2020, 110, 105873. [Google Scholar] [CrossRef]
- Parisi, F.; Pioli, S.; Lombardi, F.; Fravolini, G.; Marchetti, M.; Tognetti, R. Linking deadwood traits with saproxylic invertebrates and fungi in European forests—A review. iForest 2018, 11, 423–436. [Google Scholar] [CrossRef]
- Bouget, C.; Larrieu, L.; Nusillard, B.; Parmain, G. In search of the best local habitat drivers for saproxylic beetle diversity in temperate deciduous forests. Biodivers. Conserv. 2013, 22, 2111–2130. [Google Scholar] [CrossRef]
- Persiani, A.M.; Audisio, P.; Lunghini, D.; Maggi, O.; Granito, V.M.; Biscaccianti, A.B.; Chiavetta, U.; Marchetti, M. Linking taxonomical and functional biodiversity of saproxylic fungi and beetles in broad-leaved forests in southern Italy with varying management histories. Plant Biosyst. 2010, 144, 250–261. [Google Scholar] [CrossRef]
- Jonsell, M.; Schroeder, M.; Weslien, J. Saproxylic beetles in high stumps of spruce: Fungal flora important for determining the species composition. Scand. J. For. Res. 2005, 20, 54–62. [Google Scholar] [CrossRef]
- Parisi, F.; Lombardi, F.; Marziliano, P.A.; Russo, D.; De Cristofaro, A.; Marchetti, M.; Tognetti, R. Diversity of saproxylic beetle communities in chestnut agroforestry systems. Iforest-F 2020, 13, 456–465. [Google Scholar] [CrossRef]
- Carpaneto, G.M.; Baviera, C.; Biscaccianti, A.B.; Brandmayr, P.; Mazzei, A.; Mason, F.; Battistoni, A.; Teofili, C.; Rondinini, C.; Fattorini, S.; et al. A Red List of Italian Saproxylic Beetles: Taxonomic overview, ecological features and conservation issues (Coleoptera). Fragm. Entomol. 2015, 47, 53–126. [Google Scholar] [CrossRef]
- Piccini, I.; Di Pietro, V.; Bonelli, S. Zerynthia polyxena locally monophagous on Aristolochia pallida in the Susa Valley. Environ. Entomol. 2021, 50, 1425–1431. [Google Scholar] [CrossRef] [PubMed]
- Piccini, I.; Pittarello, M.; Di Pietro, V.; Lonati, M.; Bonelli, S. New approach for butterfly conservation through local field-based vegetational and entomological data. Ecosphere 2022, 13, e4026. [Google Scholar] [CrossRef]
- Piccini, I.; Depetris, M.; Paradiso, F.; Cochis, F.; Audisio, M.; Artioli, P.; Smargiassi, S.; Bonifacino, M.; Giuliano, D.; La Cava, S.; et al. Macro-moth (Lepidoptera) Diversity of a Newly Shaped Ecological Corridor and the Surrounding Forest Area in the Western Italian Alps. Diversity 2023, 15, 95. [Google Scholar] [CrossRef]
- Manetti, M.C.; Amorini, E.; Becagli, C.; Conedera, M.; Giudici, F. Productive potential of chestnut (Castanea sativa Mill.) stands in Europe. For. Snow Landsc. Res. 2001, 76, 471–476. [Google Scholar]
- Regione Piemonte. Piano Territoriale Regionale (Ptr). Norme di Attuazione. Assessorato all’Urbanistica e Programmazione Territoriale. Beni Ambientali. Edilizia e Legale. Regione Piemonte. 2011. Available online: https://www.regione.piemonte.it/web/temi/ambiente-territorio/territorio/piano-territoriale-regionale-ptr (accessed on 7 February 2022).
- Bouget, C.; Brustel, H.; Brin, A.; Valladares, L. Evaluation of window flight traps for effectiveness at monitoring dead wood-associated beetles: The effect of ethanol lure under contrasting environmental conditions. Agric. For. Entomol. 2009, 11, 143–152. [Google Scholar] [CrossRef]
- Montgomery, M.E.; Wargo, P.M. Ethanol and other host-derived volatiles as attractants to beetles that bore into hardwoods. J. Chem. Ecol. 1983, 9, 181–190. [Google Scholar] [CrossRef]
- Horchler, P.J.; Morawetz, W. Canopy structure and its effect on canopy organisms: A general introduction and some first finding of the Leipzig Canopy Crane Project with special reference to vertical stratification. In Canopy Arthropod Research in Europe; Floren, A., Schmidl, J., Eds.; Bioform: Nuremberg, Germany, 2008; pp. 31–48. [Google Scholar]
- Lawrence, J.F. 2. Classification (families & subfamilies). In Handbook of Zoology. Arthropoda, 2nd ed.; Beutel, R.G., Leschen, R.A.B., Eds.; De Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2016; Volume 1, pp. 13–22. [Google Scholar]
- Löbl, I.; Smetana, A. Catalogue of Palaearctic Coleoptera. Elateroidea, Derodontoidea, Bostrichoidea, Lymexyloidea, Cleroidea, and Cucujoidea; Apollo Books: Stenstrup, Denmark, 2007; Volume 4. [Google Scholar]
- Löbl, I.; Smetana, A. Catalogue of Palaearctic Coleoptera. Chrysomeloidea; Apollo Books: Stenstrup, Denmark, 2010; Volume 6. [Google Scholar]
- Löbl, I.; Löbl, D. Catalogue of Palaearctic Coleoptera. Hydrophiloidea, Staphylinoidea; Revised and Updated Edition; Brill: Leiden, The Netherlands, 2015; Volume 2/1. [Google Scholar]
- Löbl, I.; Löbl, D. Catalogue of Palaearctic Coleoptera. Scarabaeoidea, Scirtoidea, Dascilloidea, Buprestoidea, and Byrrhoidea; Revised and Updated Edition; Brill: Leiden, The Netherlands, 2016; Volume 3. [Google Scholar]
- Iwan, D.; Löbl, I. Catalogue of Palaearctic Coleoptera. Tenebrionoidea; Revised and Updated Second Edition; Brill: Leiden, The Netherlands, 2020; Volume 5. [Google Scholar]
- Alonso-Zarazaga, M.L. Cooperative Catalogue of Palaearctic Coleoptera Curculionoidea, 2nd ed.; Monografías electrónicas SEA 14; Sociedad Entomológica Aragonesa S.E.A.: Zaragoza, Spain, 2023. [Google Scholar]
- Audisio, P. Fauna Europaea: Coleoptera, Nitidulidae. Fauna Europaea Version. 2017. Available online: https://fauna-eu.org (accessed on 9 March 2023).
- Esser, J. Über die Identität von Cryptophilus integer (Heer, 1841) (Coleoptera: Erotylidae). Entomol. Nachr. Und Ber. 2016, 60, 213–218. [Google Scholar]
- Esser, J. On the identity of Cryptophagus dentatus (Herbst, 1793) (Coleoptera: Cryptophagidae). Entomol. Blätter Und Coleopt. 2017, 113, 99–103. [Google Scholar]
- Etzler, F.E. A revision of the genus Hemicrepidius Germar, 1839 (Coleoptera: Elateridae) of the New World, with comments on global classification. Coleopt. Soc. Monogr. 2020, 18, 1–126. [Google Scholar] [CrossRef]
- Calix, M.; Alexander, K.N.; Nieto, A.; Dodelin, B.; Soldati, F.; Telnov, D.; Vazquez-Albalate, X.; Aleksandrowicz, O.; Audisio, P.; Istrate, P.; et al. European Red List of Saproxylic Beetles; IUCN: Brussels, Belgium, 2018. [Google Scholar]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Albano, A.; Alessandrini, A.; Ardenghi, N.M.G.; Astuti, G.; Bacchetta, G.; Ballelli, S.; Banfi, E.; et al. An updated checklist of the vascular flora native to Italy. Plant Biosyst. 2018, 152, 179–303. [Google Scholar] [CrossRef]
- Motta, R.; Berretti, R.; Lingua, E.; Piussi, P. Coarse woody debris, forest structure and regeneration in the Valbona Forest Reserve, Paneveggio, Italian Alps. For. Ecol. Manag. 2006, 235, 155–163. [Google Scholar] [CrossRef]
- IPLA and Regione Piemonte. Alta Valle Susa, Piano Forestale Territoriale, Torino, Italy. 2000. Available online: http://www.sistemapiemonte.it/montagna/sifor/dwd/relazioni/af30_rel_p1.pdf (accessed on 20 February 2023).
- Tabacchi, G.; Di Cosmo, L.; Gasparini, P. Aboveground tree volume and phytomass prediction equations for forest species in Italy. Eur. J. For. Res. 2011, 130, 911–934. [Google Scholar] [CrossRef]
- Hunter, M.L. Wildlife, forests, and forestry. In Principles of Managing Forests for Biological Diversity; Prentice Hall: Englewood Cliffs, NJ, USA, 1990; p. 370. [Google Scholar]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed), R package version 0.2, Regression Models. 2019. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 20 November 2022).
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 November 2022).
- Fanti, F. Guida alle Lucciole d‘Italia. Lampyridae; C&P Adver Effigi: Arcidosso, Italy, 2022. [Google Scholar]
- Piccini, I.; Cristiano, L.; Di Pietro, V.; Bonelli, S.; Biscaccianti, A.B. A revision of distribution, ecology and conservation issues of the threatened comb-claw beetle Gerandryus aetnensis (Coleoptera: Tenebrionidae, Alleculinae). Fragm. Entomol. 2021, 53, 13–20. [Google Scholar]
- Franc, N. Standing or downed dead trees—Does it matter for saproxylic beetles in temperate oak-rich forest? Can. J. For. Res. 2007, 37, 2494–2507. [Google Scholar] [CrossRef]
- Piętka, S.; Sotnik, A.; Damszel, M.; Sierota, Z. Coarse woody debris and wood-colonizing fungi differences between a reserve stand and a managed forest in the Taborz region of Poland. J. For. Res. 2019, 30, 1081–1091. [Google Scholar] [CrossRef]
- Lombardi, F.; Lasserre, B.; Tognetti, R.; Marchetti, M. Deadwood in relation to stand management and forest type in Central Apennines (Molise, Italy). Ecosystems 2008, 11, 882–894. [Google Scholar] [CrossRef]
- Johansson, T.; Hjältén, J.; de Jong, J.; von Stedingk, H. Environmental considerations from legislation and certification in managed forest stands: A review of their importance for biodiversity. For. Ecol. Manag. 2013, 303, 98–112. [Google Scholar] [CrossRef]
- Courbaud, B.; Larrieu, L.; Kozak, D.; Kraus, D.; Lachat, T.; Ladet, S.; Müller, J.; Paillet, Y.; Sagheb-Talebi, K.; Schuck, A.; et al. Factors influencing the rate of formation of tree-related microhabitats and implications for biodiversity conservation and forest management. J. Appl. Ecol. 2022, 59, 492–503. [Google Scholar] [CrossRef]
- Vítková, L.; Bače, R.; Kjučukov, P.; Svoboda, M. Deadwood management in Central European forests: Key considerations for practical implementation. For. Ecol. Manag. 2018, 429, 394–405. [Google Scholar] [CrossRef]
- Bouget, C.; Larrieu, L.; Brin, A. Key features for saproxylic beetle diversity derived from rapid habitat assessment in temperate forests. Ecol. Indic. 2014, 36, 656–664. [Google Scholar] [CrossRef]
- Similä, M.; Kouki, J.; Martikainen, P. Saproxylic beetles in managed and seminatural Scots pine forests: Quality of dead wood matters. For. Ecol. Manag. 2003, 174, 365–381. [Google Scholar] [CrossRef]
- Henneberg, B.; Bauer, S.; Birkenbach, M.; Mertl, V.; Steinbauer, M.J.; Feldhaar, H.; Obermaier, E. Influence of tree hollow characteristics and forest structure on saproxylic beetle diversity in tree hollows in managed forests in a regional comparison. Ecol. Evol. 2021, 11, 17973–17999. [Google Scholar] [CrossRef] [PubMed]
- De Zan, L.R.; Bellotti, F.; D’Amato, D.; Carpaneto, G.M. Saproxylic beetles in three relict beech forests of central Italy: Analysis of environmental parameters and implications for forest management. For. Ecol. Manag. 2014, 328, 229–244. [Google Scholar] [CrossRef]
- Micó, E.; García-López, A.; Brustel, H.; Padilla, A.; Galante, E. Explaining the saproxylic beetle diversity of a protected Mediterranean area. Biodivers. Conserv. 2013, 22, 889–904. [Google Scholar] [CrossRef]
- Schauer, B.; Steinbauer, M.J.; Vailshery, L.S.; Müller, J.; Feldhaar, H.; Obermaier, E. Influence of tree hollow characteristics on saproxylic beetle diversity in a managed forest. Biodivers. Conserv. 2018, 27, 853–869. [Google Scholar] [CrossRef]
- Jacobsen, R.M.; Sverdrup-Thygeson, A.; Birkemoe, T. Scale-specific responses of saproxylic beetles: Combining dead wood surveys with data from satellite imagery. J. Insect Conserv. 2015, 19, 1053–1062. [Google Scholar] [CrossRef]
| Trophic Categories | Red List IT | Red List EU | Site 1 | Site 2 | Total | |
|---|---|---|---|---|---|---|
| Scirtidae | 1 | 1 | 2 | |||
| Prionocyphon serricornis (Müller, 1821) | HW | NT | 1 | 1 | 2 | |
| Histeridae | 0 | 1 | 1 | |||
| Platysoma (Cylister) elongatum elongatum (Thunberg, 1787) | PR | LC | 0 | 1 | 1 | |
| Leiodidae | 4 | 2 | 6 | |||
| Agathidium (Neoceble) nigripenne (Fabricius, 1792) | MY | LC | 4 | 2 | 6 | |
| Staphylinidae | 6 | 0 | 6 | |||
| Aleochara (Xenochara) sparsa Heer, 1839 | 1 | 0 | 1 | |||
| Lordithon lunulatus (Linnaeus, 1760) | 1 | 0 | 1 | |||
| Omaliinae sp. 1 | 1 | 0 | 1 | |||
| Omaliinae sp. 2 | 3 | 0 | 3 | |||
| Geotrupidae | 15 | 0 | 15 | |||
| Anoplotrupes stercorosus (Scriba, 1791) | 15 | 0 | 15 | |||
| Lucanidae | 0 | 2 | 2 | |||
| Sinodendron cylindricum (Linnaeus, 1758) | SX | LC | LC | 0 | 2 | 2 |
| Scarabaeidae | 5 | 8 | 13 | |||
| Cetonia (Cetonia) aurata pisana Heer, 1841 | SX (SP) | LC | 0 | 1 | 1 | |
| Gnorimus nobilis nobilis (Linnaeus, 1758) | SX | NT | LC | 1 | 2 | 3 |
| Gnorimus variabilis (Linnaeus, 1758) | SX | VU | NT | 3 | 1 | 4 |
| Onthophagus (Palaeonthophagus) fracticornis (Preyssler, 1790) | 1 | 0 | 1 | |||
| Onthophagus (Palaeonthophagus) verticicornis (Laicharting, 1781) | 0 | 1 | 1 | |||
| Protaetia (Cetonischema) speciosissima (Scopoli, 1786) | SX | LC | NT | 0 | 1 | 1 |
| Protaetia (Netocia) morio morio (Fabricius, 1781) | 0 | 2 | 2 | |||
| Throscidae | 2 | 1 | 3 | |||
| Aulonothroscus brevicollis (Bonvouloir, 1859) | SX | DD | 1 | 0 | 1 | |
| Trixagus carinifrons (Bonvouloir, 1859) | SX | DD | 1 | 1 | 2 | |
| Elateridae | 6 | 20 | 26 | |||
| Athous (Athous) haemorrhoidalis (Fabricius, 1801) | 0 | 1 | 1 | |||
| Brachygonus megerlei (Lacordaire, 1835) | PR | VU | NT | 1 | 2 | 3 |
| Brachygonus ruficeps (Mulsant & Guillebeau, 1855) | PR | EN | NT | 0 | 2 | 2 |
| Cardiophorus (Cardiophorus) anticus Erichson, 1840 | PR | NT | 1 | 0 | 1 | |
| Dicronychus cinereus (Herbst, 1784) | 0 | 3 | 3 | |||
| Elater ferrugineus ferrugineus Linnaeus, 1758 | PR | VU | NT | 3 | 0 | 3 |
| Hemicrepidius nigerrimus (Desbrochers des Loges, 1869) * | PR | EN | 1 | 1 | 2 | |
| Nothodes parvulus (Panzer, 1799) | 0 | 11 | 11 | |||
| Lampyridae | 0 | 3 | 3 | |||
| Lamprohiza boieldieui Jacquelin du Val, 1859 | 0 | 3 | 3 | |||
| Dermestidae | 1 | 2 | 3 | |||
| Anthrenus (Helocerus) fuscus Olivier, 1790 | 1 | 1 | 2 | |||
| Globicornis (Globicornis) nigripes (Fabricius, 1792) | SX | LC | 0 | 1 | 1 | |
| Ptinidae | 11 | 11 | 22 | |||
| Anobium punctatum (DeGeer, 1774) | XY | LC | 1 | 0 | 1 | |
| Hadrobregmus denticollis (Creutzer, 1796) | XY | LC | 4 | 2 | 6 | |
| Hadrobregmus pertinax (Linnaeus, 1758) | XY | LC | 0 | 1 | 1 | |
| Hemicoelus fulvicornis (Sturm, 1837) | XY | LC | 0 | 2 | 2 | |
| Mesocoelopus niger (Müller, 1821) | XY | LC | 4 | 0 | 4 | |
| Ptilinus pectinicornis (Linnaeus, 1758) | XY | LC | 1 | 5 | 6 | |
| Ptinus (Pseudoptinus) rufolimbatus Pic, 1908 | 0 | 1 | 1 | |||
| Ptinus (Ptinus) subpillosus Sturm, 1837 | 1 | 0 | 1 | |||
| Teredidae | 2 | 0 | 2 | |||
| Teredus cylindricus (Olivier, 1790) | PR | LC | 2 | 0 | 2 | |
| Latridiidae | 15 | 17 | 32 | |||
| Cartodere (Aridius) nodifer (Westwood, 1839) | MY | LC | 1 | 0 | 1 | |
| Enicmus atriceps Hansen, 1962 | MY | DD | 0 | 1 | 1 | |
| Enicmus brevicornis (Mannerheim, 1844) | MY | LC | 5 | 2 | 7 | |
| Enicmus rugosus (Herbst, 1793) | MY | LC | 7 | 14 | 21 | |
| Enicmus testaceus (Stephens, 1830) | MY | LC | 2 | 0 | 2 | |
| Anamorphidae | 2 | 1 | 3 | |||
| Symbiotes gibberosus (Lucas, 1846) | MB | LC | 2 | 1 | 3 | |
| Corylophidae | 2 | 0 | 2 | |||
| Arthrolips obscura (Sahlberg, 1833) | MY | DD | 2 | 0 | 2 | |
| Mycetophagidae | 686 | 27 | 713 | |||
| Litargus (Alitargus) balteatus LeConte, 1856 | MY | NA | 49 | 1 | 50 | |
| Litargus (Litargus) connexus (Geoffroy, 1785) | MY | LC | LC | 632 | 26 | 658 |
| Mycetophagus (Mycetophagus) quadripustulatus (Linnaeus, 1760) | MY | LC | LC | 2 | 0 | 2 |
| Mycetophagus (Ulolendus) atomarius (Fabricius, 1787) | MY | LC | LC | 1 | 0 | 1 |
| Mycetophagus (Ulolendus) piceus (Fabricius, 1777) | MY | NT | LC | 1 | 0 | 1 |
| Triphyllus bicolor (Fabricius, 1777) | MY | LC | LC | 1 | 0 | 1 |
| Tetratomidae | 0 | 1 | 1 | |||
| Hallomenus (Hallomenus) binotatus (Quensel, 1790) | MB | NT | 0 | 1 | 1 | |
| Melandryidae | 1 | 0 | 1 | |||
| Phloiotrya (Phloiotrya) rufipes (Gyllenhal, 1810) | MY | NT | 1 | 0 | 1 | |
| Mordellidae | 5 | 0 | 5 | |||
| Mordellaria aurofasciata (Comolli, 1837) | 5 | 0 | 5 | |||
| Zopheridae | 3 | 0 | 3 | |||
| Colydium filiforme Fabricius, 1792 | PR | NT | 1 | 0 | 1 | |
| Synchita undata Guérin-Méneville, 1844 | SX | NT | 2 | 0 | 2 | |
| Tenebrionidae | 10 | 18 | 28 | |||
| Allecula (Allecula) morio (Fabricius, 1787) | SX | LC | 4 | 2 | 6 | |
| Cteniopus (Cteniopus) sulphureus (Linnaeus, 1758) | SP (SX) | LC | 1 | 1 | 2 | |
| Gerandryus aetnensis (Rottenberg, 1871) | SX | EN | 0 | 1 | 1 | |
| Gonodera luperus luperus (Herbst, 1783) | 1 | 0 | 1 | |||
| Hymenalia (Hymenalia) rufipes (Fabricius, 1792) | SX | LC | 2 | 1 | 3 | |
| Isomira (Isomira) hypocrita Mulsant, 1856 | 1 | 0 | 1 | |||
| Isomira (Isomira) marcida Kiesenwetter, 1863 | 0 | 2 | 2 | |||
| Isomira (Isomira) murina murina (Linnaeus, 1758) | 0 | 1 | 1 | |||
| Lagria (Lagria) hirta (Linnaeus, 1758) | 0 | 1 | 1 | |||
| Mycetochara (Ernocharis) thoracica (Gredler, 1854) | SX | NT | 0 | 5 | 5 | |
| Pentaphyllus testaceus (Hellwig, 1792) | SX | EN | 1 | 1 | 2 | |
| Prionychus ater (Fabricius, 1775) | SX | NT | 0 | 2 | 2 | |
| Pseudocistela ceramboides (Linnaeus, 1758) | SX | NT | 0 | 1 | 1 | |
| Oedemeridae | 0 | 1 | 1 | |||
| Nacerdes (Xanthochroa) carniolica carniolica (Gistel, 1834) | SX | LC | 0 | 1 | 1 | |
| Salpingidae | 17 | 7 | 24 | |||
| Salpingus planirostris (Fabricius, 1787) | SX | LC | 6 | 6 | 12 | |
| Salpingus ruficollis (Linnaeus, 1760) | SX | NT | 11 | 1 | 12 | |
| Anthicidae | 0 | 1 | 1 | |||
| Microhoria fasciata fasciata (Chevrolat, 1834) | 0 | 1 | 1 | |||
| Scraptiidae | 3 | 0 | 3 | |||
| Anaspis (Anaspis) lurida Stephens, 1832 | SX | LC | 2 | 0 | 2 | |
| Anaspis (Silaria) brunnipes (Mulsant, 1856) | 1 | 0 | 1 | |||
| Biphyllidae | 90 | 22 | 112 | |||
| Biphyllus frater (Aubé, 1850) | SX (MY, PR) | LC | 3 | 0 | 3 | |
| Diplocoelus fagi (Chevrolat, 1837) | SX (MY, PR) | LC | 87 | 22 | 109 | |
| Cleridae | 2 | 2 | 4 | |||
| Clerus mutillarius mutillarius Fabricius, 1775 | PR | NT | 1 | 1 | 2 | |
| Opilo mollis (Linnaeus, 1758) | PR | LC | 1 | 0 | 1 | |
| Thanasimus formicarius formicarius (Linnaeus, 1758) | PR | LC | 0 | 1 | 1 | |
| Melyridae | 4 | 4 | 8 | |||
| Clanoptilus (Clanoptilus) emarginatus (Krauss, 1902) | 0 | 1 | 1 | |||
| Danacea (Danacea) nigritarsis alpina Pic, 1894 | 2 | 0 | 2 | |||
| Danacea (Danacea) pallipes (Panzer, 1793) | 1 | 0 | 1 | |||
| Dasytes (Mesodasytes) plumbeus (Müller, 1776) | PR | LC | 1 | 3 | 4 | |
| Monotomidae | 0 | 1 | 1 | |||
| Rhizophagus (Rhizophagus) ferrugineus (Paykull, 1800) | MY (PR) | LC | 0 | 1 | 1 | |
| Erotylidae | 4 | 0 | 4 | |||
| Cryptophilus propinquus Reitter, 1874 * | MY | [LC] | 2 | 0 | 2 | |
| Dacne (Dacne) bipustulata (Thunberg, 1781) | MB | LC | LC | 1 | 0 | 1 |
| Triplax russica (Linnaeus, 1758) | MB | LC | LC | 1 | 0 | 1 |
| Cryptophagidae | 377 | 1232 | 1609 | |||
| Caenoscelis sibirica Reitter, 1889 | MY | [DD] | 1 | 0 | 1 | |
| Cryptophagus dentatus (Herbst, 1793) | MY | LC | 5 | 0 | 5 | |
| Cryptophagus micaceus Rey, 1889 | MB | DD | 2 | 2 | 4 | |
| Cryptophagus quadridentatus Mannerheim, 1843 * | 10 | 0 | 10 | |||
| Cryptophagus reflexus Rey, 1889 | 120 | 199 | 319 | |||
| Cryptophagus scanicus (Linnaeus, 1758) | MY | LC | 239 | 1031 | 1270 | |
| Laemophloeidae | 89 | 2 | 91 | |||
| Cryptolestes duplicatus (Waltl, 1839) | MY | NT | 1 | 0 | 1 | |
| Cryptolestes ferrugineus (Stephens, 1831) | SX | LC | 9 | 0 | 9 | |
| Laemophloeus monilis (Fabricius, 1787) | MY | LC | 7 | 2 | 9 | |
| Leptophloeus hypobori (Perris, 1855) | CO | LC | 1 | 0 | 1 | |
| Notolaemus unifasciatus (Latreille, 1804) | MY | NT | 4 | 0 | 4 | |
| Placonotus testaceus (Fabricius, 1787) | SX | LC | 67 | 0 | 67 | |
| Nitidulidae | 168 | 133 | 301 | |||
| Carpophilus (Ecnomorphus) sexpustulatus (Fabricius, 1792) | MY | NT | 3 | 0 | 3 | |
| Epuraea (Epuraea) fuscicollis (Stephens, 1835) | SF | LC | 131 | 131 | 262 | |
| Epuraea (Epuraea) marseuli Reitter, 1873 | MY | LC | 3 | 0 | 3 | |
| Haptoncus ocularis (Fairmaire, 1849) * | SF (SP) | NA | 25 | 2 | 27 | |
| Stelidota geminata (Say, 1825) | SF (SP) | NA | 6 | 0 | 6 | |
| Cerambycidae | 12 | 4 | 16 | |||
| Chlorophorus glabromaculatus (Goeze, 1777) | XY | LC | 1 | 0 | 1 | |
| Leiopus nebulosus nebulosus (Linnaeus, 1758) | XY | LC | 1 | 1 | 2 | |
| Morimus asper (Sulzer, 1776) * | XY | LC | 2 | 0 | 2 | |
| Pachytodes cerambyciformis (Schrank, 1781) | SX | LC | 1 | 0 | 1 | |
| Pachytodes erraticus erraticus (Dalman, 1817) | SX | LC | 1 | 0 | 1 | |
| Parmena balteus (Linnaeus, 1767) | XY | LC | 0 | 1 | 1 | |
| Phymatodes (Phymatodes) testaceus (Linnaeus, 1758) | XY | LC | LC | 0 | 1 | 1 |
| Rutpela maculata maculata (Poda von Neuhaus, 1761) | XY | LC | 4 | 1 | 5 | |
| Stenurella bifasciata bifasciata (Müller, 1776) | SX | LC | 1 | 0 | 1 | |
| Stenurella melanura (Linnaeus, 1758) | SX | LC | 1 | 0 | 1 | |
| Chrysomelidae | 0 | 2 | 2 | |||
| Gonioctena (Goniomena) quinquepunctata quinquepunctata (Fabricius, 1787) | 0 | 1 | 1 | |||
| Luperini sp. | 0 | 1 | 1 | |||
| Anthribidae | 1 | 1 | 2 | |||
| Noxius curtirostris (Mulsant & Rey, 1861) | XY | LC | 1 | 0 | 1 | |
| Tropideres albirostris (Schaller, 1783) | XY | LC | 0 | 1 | 1 | |
| Curculionidae | 1008 | 210 | 1218 | |||
| Acalles (Acalles) parvulus parvulus Boheman, 1837 | SX | LC | 1 | 0 | 1 | |
| Anisandrus dispar (Fabricius, 1792) | MY | LC | 20 | 19 | 39 | |
| Hylastinus fankhauseri Reitter, 1895 | XY | LC | 2 | 2 | 4 | |
| Hylesinus toranio (Danthoine, 1788) | XY | LC | 1 | 2 | 3 | |
| Magdalis (Magdalis) phlegmatica (Herbst, 1797) | 0 | 1 | 1 | |||
| Phyllobius (Dieletus) argentatus argentatus (Linnaeus, 1758) | 0 | 1 | 1 | |||
| Scolytus intricatus (Ratzeburg, 1837) | XY | LC | 0 | 1 | 1 | |
| Xyleborinus saxesenii (Ratzeburg, 1837) | MY | LC | 984 | 183 | 1167 | |
| Xyleborus monographus (Fabricius, 1792) | MY | LC | 0 | 1 | 1 | |
| Total | 2552 | 1737 | 4289 |
| Forest Variables | Description | Site 1 | Site 2 | Wilcoxon p-Value | Size Effect (R) | ||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Basal area | Sum of the cross-sectional area at breast height (1.3 m aboveground) of standing dead trees or snags (m2/ha) | 1112.04 | 1406.77 | 323.17 | 591.38 | 0.219 | 0 |
| CWD volume | Volume of coarse woody debris and logs (CWD) (m3/ha) | 46.308 | 43.280 | 2.658 | 3.752 | 0.047 * | −0.224 |
| SDT volume | Volume of standing dead trees or snags and stumps (m3/ha) | 374.238 | 505.998 | 112.630 | 221.915 | 0.219 | 0 |
| SDT Height | Mean tree height of Standing dead trees or snags (m) | 15.78 | 8.201 | 13.28 | 11.488 | 0.578 | 0.704 |
| SDT Diameter | Mean tree diameter of standing dead trees or snags and stumps (cm) | 91.272 | 83.506 | 92.488 | 142.996 | 0.688 | 0.704 |
| CWD Height | Mean tree height of CWD (m) | 4.60 | 1.918 | 3.31 | 3.154 | 0.687 | −0.224 |
| CWD Diameter | Mean of max-diameter of CWD (cm) | 80.178 | 19.163 | 28.531 | 28.152 | 0.016 * | −0.67 |
| CWD decay stage | Assigned using the five-class scale used by [29]. | 2.13 | 0.395 | 1.33 | 1.414 | 0.310 | 0.224 |
| Species rich | Species richness of all living trees | 5.43 | 2.225 | 3.57 | 1.512 | 0.142 | −0.388 |
| Abundance | Abundance of all living trees | 36.43 | 16.672 | 34.57 | 10.753 | 0.937 | 0 |
| SDT Species richness | Species richness of SDT | 2 | 1 | 0.86 | 0.690 | 0.066 | −0.204 |
| SDT Abundance | Abundance of SDT | 6.57 | 4.685 | 1.29 | 1.113 | 0.042 * | −0.389 |
| Chestnut trees | Proportion of sweet chestnut trees | 0.324 | 0.249 | 0.670 | 0.118 | 0.016 | 0.707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccini, I.; Bellone, D.; Di Pietro, V.; Berretti, R.; Cristiano, L.; Caprio, E.; Biscaccianti, A.B.; Bonelli, S. Saproxylic Beetle Community in the Expansion Site of a Megaproject and in the Surrounding Area in the Western Italian Alps. Diversity 2023, 15, 556. https://doi.org/10.3390/d15040556
Piccini I, Bellone D, Di Pietro V, Berretti R, Cristiano L, Caprio E, Biscaccianti AB, Bonelli S. Saproxylic Beetle Community in the Expansion Site of a Megaproject and in the Surrounding Area in the Western Italian Alps. Diversity. 2023; 15(4):556. https://doi.org/10.3390/d15040556
Chicago/Turabian StylePiccini, Irene, Davide Bellone, Viviana Di Pietro, Roberta Berretti, Luca Cristiano, Enrico Caprio, Alessandro Bruno Biscaccianti, and Simona Bonelli. 2023. "Saproxylic Beetle Community in the Expansion Site of a Megaproject and in the Surrounding Area in the Western Italian Alps" Diversity 15, no. 4: 556. https://doi.org/10.3390/d15040556
APA StylePiccini, I., Bellone, D., Di Pietro, V., Berretti, R., Cristiano, L., Caprio, E., Biscaccianti, A. B., & Bonelli, S. (2023). Saproxylic Beetle Community in the Expansion Site of a Megaproject and in the Surrounding Area in the Western Italian Alps. Diversity, 15(4), 556. https://doi.org/10.3390/d15040556







