Abstract
The transformation of forests into agricultural and livestock systems negatively affects the ecological dynamics and the ecosystem services provided by different groups of insects, including dung beetles, which stand out for their importance in recycling livestock dung. Since the 1980s, farmers in different regions of the world have been using Ivermectin to control parasites that affect cattle. The main route of elimination of this molecule and its metabolites is through manure, which affects the richness, abundance, and biomass of dung beetles when they use dung from treated animals. To quantify this effect, we carried out an experimental design in the field in the Colombian Caribbean, where nine cattle farms were evaluated, of which three were taken for each of the different cattle management practices most used in the region: (i) Ivermectin not applied, (ii) two doses of Ivermectin at 1% applied per year and (iii) two doses of Ivermectin at 3.15% applied per year. To assess the richness, abundance, biomass, and functional groups of dung beetles, during the dry and wet seasons, 30 pitfall traps were baited on each farm with fresh cattle manure with the same management doses described above. A total of 25,441 individuals belonging to 19 genera and 30 species were collected. The richness, abundance, and biomass of beetle assemblages decreased along the gradient represented by management without using Ivermectin and management where Ivermectin was used. Paracoprid beetles were the functional group that was most negatively affected in cattle farms with Ivermectin use. In cattle farms where Ivermectin was not used, there was a greater diversity and higher functional structure of dung beetle assemblages than in those where this veterinary medicinal product was used. Using Ivermectin generates short- and long-term effects on the richness, abundance, biomass, and functional groups of dung beetles in livestock systems in the Colombian Caribbean. Therefore, we suggest using integrated treatment management to prevent the recycling fauna from being affected.
Keywords:
Aphodiinae; ecosystem services; grassland; Ivermectin; livestock management; Scarabaeinae; seasons 1. Introduction
With the development of livestock systems, the rate of conversion of forest cover areas into large areas of monocultures (pastures) for livestock feeding has increased; although livestock production increased, biodiversity, habitat structure, and ecosystem services were negatively affected [1] Grasslands have a predominance of grasses that develop due to the interaction between climate, soil, and biota, and, often, these interactions are affected by anthropogenic activities, such as livestock overgrazing, the type of grazing, the use of agrochemicals for fertilization, insect control, pest control, and the use of veterinary medicinal products in livestock [2]. In addition, a process of soil degradation begins due to erosion, loss of organic matter, and surface compaction due to livestock trampling and agricultural mechanization.
According to the Colombian Federation of Livestock Farmers [3], cattle farming is the economic activity with the most significant presence in the Colombian countryside; it is found in all ecosystems and in different production systems, such as breeding, rearing, fattening, specialized dairy, and dual purposes. Livestock is the main agricultural activity in the country, and it generates approximately 810,000 direct jobs (6% of national employment) and contributes 1.4% of the country’s gross domestic product. By 2022, the livestock inventory was 28.2 million heads, ranking 7th worldwide, with 512,000 cattle farms dedicated to the bovine activity, and 67.1% of them had less than 25 animals [4]. According to the Instituto Geográfico Agustín Codazzi [5], only 2.4% (2.7 million hectares) of Colombian land is suitable for livestock, but there are currently more than 14 million hectares occupied by livestock, which is over 11 million hectares with livestock land use conflicts.
One of the by-products of livestock farming in pastures is manure, which is the primary food source and nesting site for dung beetles (Coleoptera: Scarabaeidae) [6]. Within the degradation of excrement in the soil, two processes are carried out: (i) one is biotic and accompanied by a rich fauna that includes beetles, ants, termites, flies, and micro-organisms such as bacteria and fungi [7], (ii) and the other process is abiotic, where climatic factors such as temperature, rainfall, and soil moisture play a role [8]. Within the coprophagous fauna, dung beetles are essential elements for the ecosystem functioning in grasslands [9,10]. The adult dung beetles feed on the liquid suspension of the excrement, while the larvae eat the solid remains of the plants that were not digested by the cows [11]. Thus, these beetles, in the process of digging tunnels in the soil, improve soil aeration (i.e., macroporosity) and fertility [12,13], which accelerates microbial metabolism where compounds such as phosphorus, potassium, ammonia, nitrogen, and carbon present in the recycled manure are rapidly released [13,14]. In this process, the infiltration of water into the soil is improved [12], which facilitates grass growth [15], and the structure and water retention capacity of the soil is preserved [16,17]. Dung beetle activity thus prevents the long-term accumulation of manure in pastures [18]. In addition, dung beetles control fly populations [19] and reduce the re-infection of livestock by gastrointestinal nematodes that have developed in dung [20].
Sanitary management has become one of the most limiting factors in livestock production worldwide [21]. Consequently, veterinary drugs may affect the regular interaction of soil-degrading fauna such as dung beetles, dipterans, edaphic mesofauna, and earthworms [22,23]. Depending on the chemical family to which they belong, their mode of administration, and their residual concentration in excreted dung, the veterinary drugs vary in their toxicity to dung beetles [24]. From the 1980s onwards, the development of macrocyclic lactones made it possible to control both internal and external parasites of livestock (endectocides) [25,26]. From among these inexpensive and broad-spectrum parasiticides, Ivermectin has become the most widely used antiparasitic by livestock farmers [27]. Ivermectin is administered in different forms, which include topically (pour-on), orally, or by subcutaneous injection. In all cases, the molecule is not completely metabolized by the animals, and the parent molecule remains intact in the feces for a long time, often for several months [28].
Once Ivermectin is applied to cattle, its residues begin to appear in the feces from the first days [29], and toxic dung affects dung beetles by impacting their oviposition, fertility [30,31], decreasing their olfactory and locomotor capacity [32], and modifying their attraction to dung [33,34]. Even low doses of Ivermectin can significantly affect them [32]. In addition, the regular use of Ivermectin in cattle leads to a significant reduction in the abundance and richness of beetles, which slows the degradation of manure in pastures [18,30,34,35] and disturbs the soil nutrient cycle [14,18]. The physiological and behavioral disorders produced by Ivermectin in the short-term in dung beetles may have a long-term impact on the structure of their assemblages and their functional efficiency at the ecosystem level [9,18]. Although studies on the unintended effects of Ivermectin on dung beetle assemblages have been conducted recently worldwide, such as in Europe [36], Canada, the United States [34,37,38], and Mexico [39,40,41], in South America these studies are still scarce (only in Colombia: [42] and Brazil: [35]).
The present study aims to assess the unintended effects of Ivermectin use on the richness, abundance, and biomass of dung beetles in Colombian Caribbean cattle farms by comparing farms where Ivermectin is not used or is used in different doses (i.e., 1% and 3.15%), during distinct periods of the year (dry and rainy seasons). The objective was to answer the following questions: (i) What is the effect of the use of Ivermectin on dung beetle assemblages (richness, abundance, and biomass) in cattle farms in the Colombian Caribbean? (ii) Do the functional groups of dung beetles present different responses for use of Ivermectin? (iii) Are there species associated with each use of Ivermectin (e.g., dung without IVM, with a low dose (IVM 1%), and with a high dose (IVM 3.15%)? As a hypothesis, we expect that the use of Ivermectin in Colombian Caribbean livestock production systems will generate adverse effects on the structure (composition, richness, abundance, functional groups, and biomass) of the dung beetle assemblages.
2. Material and Methods
2.1. Study Area
The study area covers three different municipalities: Los Palmitos, Morroa, and Sincelejo in the department of Sucre, Caribbean region of Colombia (Figure 1). The temperature in this region ranges between 24 and 32 °C, and rainfall varies between 858 and 1607 mm annually on average [43]. The rainy season runs from April to November, and the dry season runs from December to March. Due to its climatic characteristics, the region corresponds to the Dry Forest (Bs-T) life zone [44]. Livestock in this region is mainly made up of breeds of Bos primigenius L., 1758 (zebu cattle). Cattle are usually managed in extensively used pastures to produce milk and meat (dual-purpose farming), and animals are rotated in two or three paddocks. The selected cattle farms present a silvopastoral configuration, where grasses abound (Bothriochloa pertusa (L.) A. Camus, F. Poaceae) in association with trees and shrubs; the surface area of each farm is approximately 30 ha, and the cattle graze all year round. The average stocking rate is 1.5 animal units per hectare (1.5 AU/ha), considering one unit as 450 kg of average live weight.
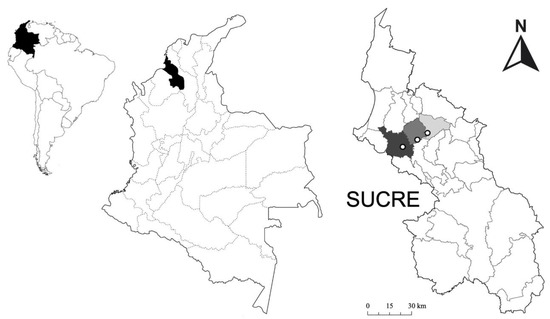
Figure 1.
Location of the study sites in the municipalities of Sincelejo (black), Morroa (dark gray), and Los Palmitos (light gray), Sucre—Colombia.
2.2. Livestock Management
To assess and compare the effect of the use of Ivermectin (IVM) on the diversity and structure of dung beetle assemblages, the study was carried out on nine livestock farms in the Colombian Caribbean, at least 3 to 5 km apart from each other. Selected farms represent the typical parasite control in the Colombian Caribbean region: (i) NO-IVM = Ivermectin was not applied (nor any other product for more than 30 years), (ii) TWO-IVM 1% = Two doses of Ivermectin at 1% concentration per year, and (iii) TWO-IVM 3.15% = Two doses of Ivermectin at 3.15% concentration per year. These two doses were usually applied in February (dry season) and in October (rainy season).
2.3. Sampling and Experimental Design
To collect dung beetles, pitfall traps baited with fresh manure from the cattle of each farm under study were used, where three groups of seven cattle were taken for each of the treatments used. The first group remained without treatment (control group), the second was given IVM at 1% (low dose Ivermectin; trade name Ivomec®), and the third group was given IVM at 3.15% (high dose Ivermectin; trade name Ivomec® Gold). One ml of IVM was used subcutaneously in the neck region for every 50 kg of body weight (each dose provides 200 µg and 630 µg of Ivermectin (1% and 3.15%) per kg of body weight, respectively). Using dung from treated and not controlled animals as baits helped determine whether the beetles were attracted to any of them.
As each season is characterized by distinct assemblages of dung beetles [45,46], the fieldwork was carried out in the selected cattle farms during the dry season (February) and the rainy season (September) of 2020. The experimental design included three factors: (i) livestock management related to the use of Ivermectin: NO-IVM, TWO-IVM 1%, and TWO-IVM 3.15%; (ii) season: dry and rainy; and (iii) treatments related to the presence of Ivermectin in the baits: without IVM, IVM 1 %, and IVM 3.15%. The pitfall traps consisted of a 3.5 L plastic container with a circular opening of 20 cm in diameter and a height of 15 cm, which was buried at ground level and filled with approximately 500 mL of a preservative solution (50% of water, 50% alcohol at 70%, and a few drops of neutral liquid detergent). A plastic net with a 5 cm diameter opening was placed over the trap, holding the bait (about 1000 g), enclosed in a gauze cloth.
In each farm, 30 sampling traps were installed [47], which were placed in the paddocks in grids 50 m apart [48] and were reviewed and collected after 48 h [40]. Of the 30 baits used in the traps, 10 had excrement without IVM, 10 had a low dose (IVM 1%), and 10 had a high dose (IVM 3.15%). The treatments were randomly located in the paddocks. The manure for the baits was obtained from the cattle treated on the study farms four days after the antiparasitic product was applied, which is the time necessary to ensure that the substance was present in the dung [49]. The manure was collected in the morning before beginning the activity of the beetles to avoid colonization [32]. A total of 540 pitfall traps (270 traps for each season, rainy and dry) were placed in the field to determine variations in the diversity and structure of the dung beetle assemblages.
2.4. Dung Beetles’ Diversity, Identification, and Biomass
For each pitfall trap, the number of species (richness), the number of individuals per species (abundance), and the biomass per species and total (g) were counted. Captured beetles were identified to the species level using different taxonomic identification keys for Scarabaeinae [50,51,52,53,54,55] and Aphodiinae [56,57,58,59]. Specimens were deposited in the senior last author’s reference collection (CJAN). Biomass per species was quantified using a precision balance (Ohaus Pioneer PA323 0.001 g, Ohaus Corporation, Sunnyvale, CA, USA).
2.5. Data Analysis
A list of the species collected in the samplings was compiled, which were grouped according to two criteria. The first took into account the way in which beetles use and relocate dung (paracoprids, telecoprids, and endocoprids) [60], and the second was recorded according to body size, with species divided into small (<10 mm), medium (10 to 18 mm), and large (>18 mm) [61], thus establishing nine functional groups [6]: small paracoprids (Pp), small telecoprids (Tp), small endocoprids (Ep), medium paracoprids (Pm), medium telecoprids (Tm), medium endocoprids (Em), large paracoprids (Pg), large telecoprids (Tg), and large endocoprids (Eg). Then, we estimated the sample completeness for each livestock management using a sample coverage analysis with iNEXT package (see [62,63]) in R version 3.2.1 [64]. In addition, we plotted species rank abundance distributions to visually compare patterns of species dominance in the dung beetle assemblage sampled in each livestock management type.
We used Generalized Linear Mixed Models (GLMMs) to verify the effect of different livestock management approaches (explanatory variable) on the abundance, species richness, and biomass (response variables) of dung beetles, with livestock management as a fixed factor and sampling season (dry and rainy) as a random factor. We classified the sampled species into three functional groups related to their nesting behavior: endocoprids (dwellers), telecoprids (rollers), and paracoprids (tunnelers) [60]. In addition to analyzing the total abundance, species richness, and biomass data, we also used GLMMs to analyze the effects of livestock management on the abundance, species richness, and biomass of each dung beetle functional group separately. We used the Poisson error for species richness, which is a negative binomial error distribution with log link function for abundance, as these data showed over-dispersion, and we used the Gaussian error for biomass [65]. These analyses were undertaken using the “glmer.nb” function in the LME4 package in R version 3.2.1 [64]. We used the indicator value method following Dufrêne and Legendre [66], to identify associations between dung beetle species and types of bait (see [67]). We used 5000 randomizations to determine the statistical significance of the observed indicator value (Monte Carlo test; p < 0.05). This analysis was performed with the ‘indicspecies’ package in R software [64,68].
3. Results
3.1. Seasonal Differences in Dung Beetle Assemblages
A total of 25,441 individuals of the Scarabaeidae family belonging to the subfamilies Aphodiinae and Scarabaeinae were collected and grouped into 30 species, 19 genera, and nine tribes (Table 1). The Scarabaeinae subfamily was represented by 25 species (n = 10,617, 41.7%), followed by Aphodiinae with five species (n = 14,824, 58.3%) (Table 1). The genera with the highest number of species were Canthon (n = 5), Onthophagus (n = 4), Ataenius, Uroxys, Dichotomius, and Eurysternus (n = 2; Table 1). For the first time, 10 new species, identified at the species-specific level, were reported for this region according to the list by Noriega et al., (2013): Ataenius complicatus Harold, 1869, Ataenius crenulatus Schmidt, 1910, Cartwrightia cartwrighti Cartwright, 1967, Coprophanaeus gamezi Arnaud, 2002, Dichotomius coenosus (Erichson, 1848), Nialaphodius nigrita (Fabricius, 1801), Onthophagus buculus Mannerheim, 1829, Onthophagus clypeatus Blanchard, 1846, Sylvicanthon aequinoctialis (Harold, 1868), and Xenoheptaulacus tricostatus (Harold, 1869). Cartwrightia is a new genus record for Colombia [69]. It is interesting to note the presence of two introduced species in the region: Digitonthophagus gazella (Fabricius, 1787) and N. nigrita. The sample coverage estimator revealed a high sampling efficiency (>99% in all livestock management) (Table 1). This indicates that we had conducted an adequate effort to represent the dung beetle assemblages in our sampling sites.

Table 1.
List of dung beetle species and number of individuals collected in the nine cattle farms of the Caribbean region (Colombia). Livestock management: No-IVM = Ivermectin is not applied per year; Two-IVM 1% = Two doses of Ivermectin 1% applied per year; and Two-IVM 3.15% = Two doses of Ivermectin 3.15% applied per year. Functional groups: small paracoprids (Pp), small telecoprids (Tp), small endocoprids (Ep), medium paracoprids (Pm), medium telecoprids (Tm), medium endocoprids (Em), large paracoprids (Pg), large telecoprids (Tg), and large endocoprids (Eg). Season (Ll = Rainy and S = Dry).
The rainy season registered a higher richness, abundance, and biomass of dung beetles (28 spp.; n = 23,318, 91.7%; 774.2 g, 97.1%). The most abundant species were A. crenulatus (n = 12,702, 49.9%), followed by Onthophagus marginicollis Harold, 1880 (n = 6849, 26.9%) and D. gazella (n = 2763, 10.9%) (Table 1). The paracoprids were the guild with the highest richness (14 spp.; n = 9851, 38.7%), followed by the endocoprids (7 spp.; n = 13,212, 51.9%), and finally the telecoprids (7 spp.; n = 255, 1.0%) (Table 1). The dry season registered a lower richness, abundance, and biomass of beetles (14 spp.; n = 2123, 8.3%; 23.0 g, 2.9%), and the most abundant species was N. nigrita (n = 1752, 6.9%), followed by A. crenulatus (n = 218, 0.9%) and D. gazella (n = 102, 0.4%) (Table 1). The paracoprids (6 spp.; n = 120, 0.5%) and the endocoprids (6 spp.; n = 2001, 7.9%) were the richest guilds, followed by the telecoprids (2 spp.; n = 2) (Table 1). In terms of functional groups, no large endocoprids were found in either of the two seasons and for the dry season, and large paracoprids and telecoprids were absent, as well as medium telecoprids. For both seasons, small beetles made up the dominant group of the assemblage (20 spp., n = 22235, 87.4%) (Table 1). Of the 30 species recorded, 12 species were continuously present during the two seasons, with the most abundant being A. crenulatus (n = 12920, 50.8%), followed by O. marginicollis (n = 6855, 26.9%) and D. gazella (n = 2865, 11.3%) (Table 1); a total of 16 exclusive species were present during the rainy season and 2 species were present for the dry season (Table 1).
3.2. Differences in Dung Beetle Assemblages between Livestock Treatments
The NO-IVM treatment presented the highest richness, abundance, and biomass of dung beetles (26 spp.; n = 11,334, 44.6%; 374.5 g, 47.0%), followed by Two-IVM 1% (17 spp.; n = 10,222, 40.2%; 262.9 g, 33.0%), and, finally, TWO-IVM 3.15% (17 spp.; n = 3885, 15.3%; 159.9 g, 20.1%) (Table 1). NO-IVM and TWO-IVM 1% treatments were the most similar (81.6%), followed by TWO-IVM 3.15% treatment, which was the most different among all the treatments (58.4%). The IVM 3.15% and NO-IVM treatments were more similar (94.0%), followed by the IVM 1% treatment, which was more different (84.9%). The identity of the dominant species changed over the cattle removal age.
The identity of the dominant species changed over the cattle removal age. The identity of the dominant species changed among different treatments. However, A. crenulatus, O. marginicollis, and D. gazella were present among the three dominant species in the three treatments (Figure 2). Twelve species were recorded in all three treatments. The No-IVM had nine species recorded exclusively in this management, and it shared three species with TWO-IVM-1% and three species with TWO-IVM-3.15%. Two species were recorded exclusively in the TWO-IVM 1%; these treatments shared no species with the IVM 3.15%. Two species were exclusively collected in the TWO-IVM 3.15% treatment (Figure 3). The number of individuals was significantly lower in the TWO-IVM 3.15% than in the NO-IVM and TWO-IVM 1% treatment (χ22,4 = 10.35, p < 0.01; Figure 4A). The species richness was significantly higher in the NO-IVM than in the TWO-IVM 1% and TWO-IVM 3.15% (χ22,4 = 10.79, p < 0.01; Figure 4B). Regarding dung beetle biomass, the NO-IVM had higher biomass, followed by the TWO-IVM 1%, while the TWO-IVM 3.15% the treatment type had the lowest biomass (F2,4 = 6.79, p = 0.03; Figure 4C).
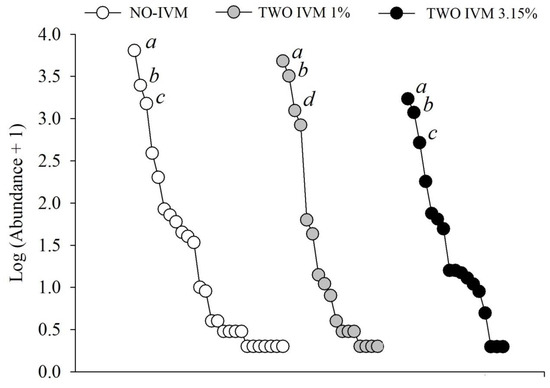
Figure 2.
Rank distribution of dung beetle species across cattle farms with different livestock management in Colombian Caribean. a, Ataenius crenulatus; b, Onthophagus marginicollis; c, Digitonthophagus gazella; d, Nialaphodius nigrita.
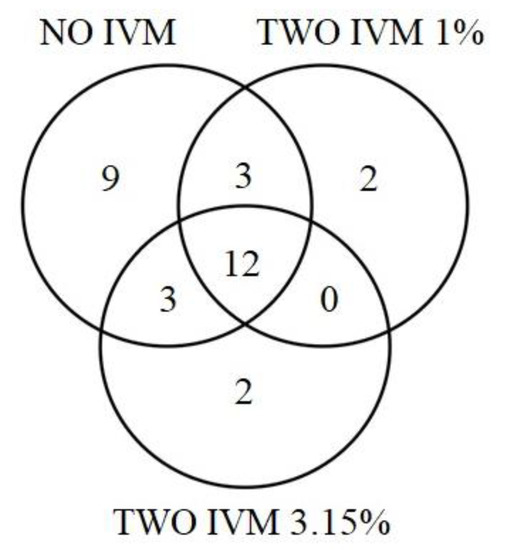
Figure 3.
Venn diagram showing proportion of shared dung beetle species among the assemblages sampled in cattle farms with different Ivermectin treatments. Diagram components represent species unique to NO-IVM (circle left), the number of species unique to TWO-IVM 1% (circle right) and to TWO-IVM 3.15% (circle below), and species shared by the different livestock management approaches (overlap).
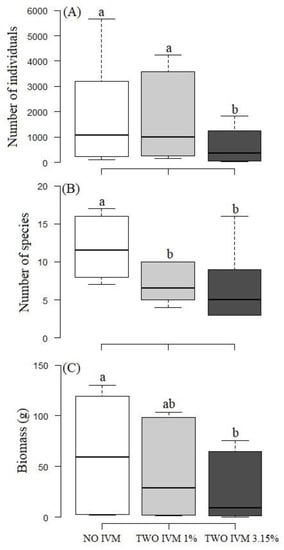
Figure 4.
Boxplots of the average abundance (A), species richness (B), and biomass (C) of dung beetles sampled in cattle farms with NO-IVM, TWO-IVM 1%, and TWO-IVM 3.15% livestock management approaches in the Colombian Caribbean. Different letters above the boxes indicate statistically significant differences (p < 0.05).
3.3. Differences in Dung Beetle Relocation Guilds between Livestock Treatments
The number of individuals of endocoprids (χ22,4 = 9.62, p < 0.01) and paracoprids (χ22,4 = 5.74, p = 0.05) were significantly higher in the NO-IVM. However, the number of individuals of telecoprids (χ22,4 = 2.21, p = 0.33) did not differ among treatments (Figure 5A–C). The species richness of the paracoprids (χ22,4 = 10.79, p < 0.01) was significantly higher in the NO-IVM than in the TWO-IVM 1% and TWO-IVM 3.15%, but no differences were found for any of the other functional groups: endocoprids (χ22,4 = 2.84, p = 0.24) and telecoprids (χ22,4 = 4.85, p = 0.08) (Figure 5D–F). Finally, the biomass of the paracoprids (F2,4 = 7.08, p = 0.03) was significantly higher in the NO-IVM than in the TWO-IVM 1% and TWO-IVM 3.15%. However, the biomass of the endocoprids (F2,4 = 3.56, p = 0.16) and telecoprids (F2,4 = 2.21, p = 0.33) did not significantly differ among treatments (Figure 5G–I). Of the 30 species evaluated for bait type preference, no one showed a preference for any bait type.
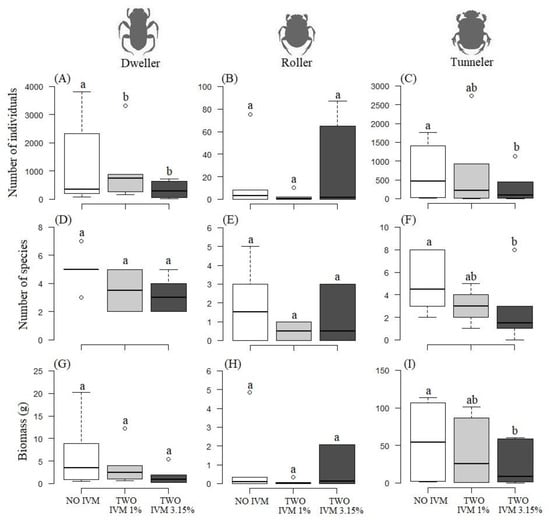
Figure 5.
Boxplots of the average abundance (A–C), species richness (D–F), and biomass (G–I) of dung beetle functional groups sampled in cattle farms with NO-IVM, TWO-IVM 1%, and TWO-IVM 3.15% livestock management approaches in the Colombian Caribbean. Different letters above the boxes indicate statistically significant differences (p < 0.05).
There was also a decreasing structure of beetle guilds in richness, abundance, and biomass, with a similar pattern in the NO-IVM (Paracoprids, 13 spp., n = 4126, 331.8 g > Endocoprids, 7 spp., n = 7119, 37.3 g > Telecoprids, 6 spp., n = 89, 5.4 g), in the most intervened TWO-IVM 1% (Paracoprids, 8 spp., n = 4101, 239.9 g > Endocoprids, 6 spp., n = 6108, 22.6 g > Telecoprids, 3 spp., n = 13, 0.4 g) and in the TWO-IVM 3.15% (Paracoprids, 8 spp., n = 1744, 136.9 g > Endocoprids, 5 spp., n = 1986, 9.6 g > Telecoprids, 4 spp., n = 155, 13.3 g) (Table 2). There was a tendency to lose species and complete functional groups as treatments without Ivermectin (NO-IVM) were passed towards the most intervened treatments (TWO-IVM 1% and TWO-IVM 3.15%) (Table 2). A very significant loss of species was observed between farms where Ivermectin was not used (NO-IVM) compared to farms where IVM was used: 11 species were lost between NO-IVM and TWO-IVM 1% (Table 1; Figure 6); 11 species were also lost between the group of farms without IVM and the group of farms with TWO-IVM 3.15 % (Table 1 and Figure 6). A total of 14 species were lost between the control farms without Ivermectin and the management with Ivermectin application.

Table 2.
Richness, abundance, and biomass of the guilds (paracoprid, endocoprid, and telecoprid) of coprophagous beetles collected in nine livestock farms in the Caribbean region (Colombia) in 2020, with information on livestock management approaches on the farms (No-IVM = No Ivermectin is applied per year; Two-IVM 1% = Two doses of Ivermectin 1% applied per year; Two-IVM 3.15% = Two doses of Ivermectin 3.15% applied per year), type of relocation (TR; P = Paracoprids, T = Telecoprids, and E = Endocoprids).
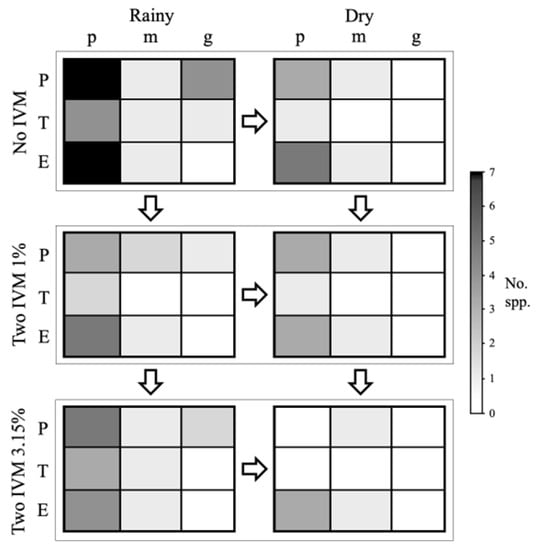
Figure 6.
Structure of functional groups (Guilds: P = Paracoprids; T = Telecoprids, and E = Endocoprids) and sizes of individuals (small, p < 10 mm; medium, m = 10 to 18 mm, and large, g > 18 mm), for livestock management (NO-IVM = Ivermectin is not applied per year, TWO-IVM 1% = Two doses of Ivermectin 1% applied per year, and TWO-IVM 3.15% = Two doses of Ivermectin 3.15% applied per year) in nine cattle farms in the municipalities of Los Palmitos, Sincelejo, and Morroa, department of Sucre (Colombia). The arrows indicate the possible directions in which the loss of groups can occur when increasing the concentrations or number of doses of Ivermectin.
4. Discussion
This study was carried out in nine cattle farms in the department of Sucre in the Caribbean region in a Bs-T life zone, which is an ecosystem with high diversity [70] and that is of importance in the provision of ecosystem services [71]. In the Neotropics, Bs-T is one of the most threatened habitats experiencing high transformation rates due to human activities [72,73]. Their transformation is mainly due to its proximity to large urban settlements that generate large agricultural and livestock areas [72,74]. By eliminating the forest, the vegetation cover is modified, and the land use changes, which modifies the environmental variables, producing changes in the composition and structure of the communities [75], which affects the functioning of ecosystems and the supply of services [76,77].
4.1. Dung Beetles’ Assemblage Analysis in Tropical Dry Forests
This study, although carried out in a transformed Bs-T area (livestock farms), recorded the highest richness of dung beetles for the department of Sucre (30 species and 19 genera), which represent 46.2% of the known species in the region (n = 65 species). Although most of the studies carried out in this region have been carried out in forest areas, those areas showed a lower richness of dung beetle species (e.g., [42,45,46,78,79,80,81,82]). If we compare the dung beetle richness in the present study with the results recorded for the rest of the Caribbean region of Colombia in the Bs-T, we find that the only values that exceed it are those of Solis et al. [83] and Rangel-Acosta and Martínez [84]; the other studies giving a lower richness (e.g., [61,85,86,87,88].
The higher species richness recorded here is partly due to the inclusion of cattle farms that were adjacent to forest fragments, which contributed to maintaining essential key parts of the beetle diversity. Some configurations of agricultural and livestock systems have been found to help maintain biodiversity and ecosystem services [89]. This was demonstrated by Gascon et al. [90] and Mendenhall et al. [91], who found that, within large areas of agricultural and livestock use where forest remnants, live fences, thickets, fruit, and timber trees are preserved, this landscape structure plays a positive role through elements contributing to maintaining species richness in the transformed matrix. In tropical regions, where local extinction rates are high due to habitat loss or fragmentation, secondary forests may, fortunately, support many forest species, while adjacent grasslands and clearings generally support fewer species, including few forest species [92]. These open areas in the forest matrix are mainly used by only a few generalist coprophagous species. Logging creates an extensive network of pathways within the forest, whose direct influence extends far beyond the pathway boundary [93]. Larger species, particularly paracoprids, are most affected and respond more than other functional groups of coprophagous beetles, but other groups are also influenced by changes in their microhabitat.
The genera with the highest number of species were Canthon and Onthophagus, and these results were similar to other studies from the Caribbean region of Colombia (e.g., [45,46,78,79,80,83,85,86,94,95]). The most abundant species in this ecosystem was A. crenulatus (Aphodiinae; see [96]), with more than 50% of the total individuals captured; followed by O. marginicollis and D. gazella which had been previously reported for the Bs-T in the department of Sucre (e.g., [45,78,80,82]). Despite not being an abundant species, it is important to highlight the presence of D. guildingii, as it is associated with well-preserved Bs-T ecosystems and has been proposed as a bioindicator for monitoring plans in the Caribbean region [72].
4.2. Dung Beetles’ Assemblage Structure according to Seasonality
The greatest richness, abundance, and biomass of dung beetles occurred in the rainy season, since there is a high degree of association with fresh resources, which is evidenced by a greater distribution, composition, and structure of the assemblages [97]. These changes occur in the tropical region according to pluviometric regimes (e.g., [98,99,100,101]) and the dominant seasonal pattern in the Neotropics (e.g., [83,85,95,97,102,103]) responding directly to precipitation [104]. With the arrival of the rainy season, the soils increase their water content, which moistens the subterranean pupation chambers of dung beetles and facilitates the emergence of the new generations [94], while the rains favor the development of new leaves, flowers, and seeds in the pastures. The increase in plant biomass provides a better availability of food resources to livestock [101,105], which in turn allows the production of more and better-quality dung used for feeding and nesting by dung beetles [106].
In the dry season, the richness, abundance, and biomass of dung beetles were lower (i.e., 14 spp. species less than during the rainy season), which reduces the functional activity of the species, which is restricted to the peaks of humidity and temperature that condition their physiology [107]. In the dry season, soils are dehydrated due to the lack of rain, which limits the activity of beetles, who dig a few nests and shallow galleries because of the hardness and compaction of soils [98]. In the specific case of areas used for livestock production, their soils are often compacted by the overload of cattle and further suffer from erosion due to trampling, high insolation, and loss of humidity due to low tree cover; all this makes the soils hard and difficult to dig to build galleries and incorporate manure into the soil [106,108]. Finally, the quality of the dung is poor for dung beetles, as livestock often ingest hard, fibrous, and low-protein plants. This results in a reduction in the assemblage structures, which is probably related to the breeding season of the Scarabaeinae species [109]. For this type of ecosystem, the activity of adult beetles is seasonal, as they present larval or diapausal stages during the dry season [60], as in the case of several Aphodiinae species in Mexico [110]. Soil temperature is another critical variable related to increasing or decreasing the richness and abundance of beetles; when the soil temperature reaches 45 °C, it can kill larvae in open areas such as cattle pastures [111]. Some studies (see [112]) show that human deforestation interventions for agriculture and livestock production have favored the more heliophilic coprophagous species to the detriment of the more forest-dwelling species and species dependent on the dung of large herbivores, which agrees with our results. In the humid tropics, the species typical of anthropized habitats are mostly opportunistic, while more specialized species tend to seek forest habitats for feeding and breeding [48].
In this study, 12 generalist species were present in the two seasons and represented more than 98% of the captured individuals, of which ten were small, and only two were medium-sized beetles. This pattern shows that large beetles were the most affected species and were probably the first to disappear in such affected ecosystems, as has already been demonstrated for other tropical forest areas [93]. In contrast, the elevated abundances of the introduced species (D. gazella and N. nigrita) during the dry season drew attention. It is possible that they are less competitive species that are displaced to these seasonal windows that present more hostile environmental conditions, or that their climatic niche is broader, since they are not restricted to their original distribution as has been proposed in some studies [113]. Of the 30 species of dung beetles found, 17 species are considered possible rare species (<10 individuals), which would suggest that the populations of these beetles are declining [85]. These results agree with Krebs [114], who explained that there were few abundant species and many rare species in the assemblages. Forest remnants contribute to the shelter and conservation of dung beetles; however, conservation strategies must be implemented to preserve and increase the areas of forest fragments and leave biological corridors between forest relicts, as well as agricultural and livestock areas, which preserve dung beetle assemblages.
The dung beetle assemblages, which were composed of members of all three food relocation guilds, were dominated by the paracoprids, which comprised the highest number of species, abundance, and biomass during the rainy season; this was determined by the type of soil (clay and loam-clay), which facilitated the construction of galleries to bury the excrements [115]. The most dominant species among the paracoprids were the small ones, as the large beetles were more sensitive to anthropogenic disturbances and, especially, to the reduction in the number and diversity of wild mammals [116]. The abundance of species in this guild could potentially maintain the functionality of the assemblage despite the absence of large species, provided that their high numbers are maintained [117,118]. The endocoprids, despite having the highest numbers, had lower richness and a biomass that was ten times lower than paracoprids. Telecoprids, which were even less numerous and featured a lower biomass, are considered good bioindicators of anthropogenic disturbances [19,92,119]. Due to its high species number, abundance, and biomass, the paracoprid guild provides essential ecosystem services on cattle farms by recycling the largest amount of cattle manure. Similar results were found by Noriega et al. [61] in three regions of Colombia. In the present study, the higher number of paracoprid species than species of other guilds is similar to the results reported by Barraza et al. [120] and Noriega et al. [88]. Forest fragmentation and deforestation affect the richness and abundance of dung beetles, thus reducing their functional efficiencies, such as nutrient recycling, seed dispersal, and pest control [17]. In livestock agroecosystems, many Scarabaeinae species of the telecoprid guild were disappearing and were replaced by species of the subfamily Aphodiinae. Similar results have been found in mountain forests [121,122].
4.3. Negative Effects of Livestock Management and Antiparasitic Use
The effect of Ivermectin on the diversity and structure of dung beetle assemblages showed significant differences in richness, abundance, and biomass between the farms where livestock parasite control did not involve the use of Ivermectin and where a greater diversity and higher numbers of dung beetles belonging to all guilds was observed when compared to those farms where Ivermectin is used routinely. The intensive and long-term use of Ivermectin in cattle farms in the Caribbean region has been detrimental to dung beetle populations. This difference in management to control livestock parasitism has resulted in the loss of 14 species of dung beetles. Different authors found similar results (e.g., [18,33,35,36,40]). For example, in the Czech Republic, Ivermectin-treated sites had ca. 35% lower species richness and 44% lower abundance per dung pat [36]. In SW England, species richness, diversity, and functional diversity were higher on farms with a history of using no parasiticides than on farms with parasiticides. Species of endocoprid (dung dwelling) beetles dominated the community on farms that used parasiticides, particularly macrocyclic lactones (e.g., Ivermectin), while paracoprid (dung burying) beetles were rare, possibly due to differential impacts depending on life history traits of the functional groups [20].
Ivermectin residues present in the manure of treated cattle are toxic to the dung beetles [123,124] for both larvae and adults [7,31,33,104,125], because they decrease the olfactory and locomotor capacity of the adults [32], thus altering the morphology of the ovaries and stopping vitellogenesis, which causes oocyte resorption and a decrease in fecundity [31,123], which results in the production of fewer larvae [39,126]. This substance can bioaccumulate and affect food chains [124]. As a result, these unintended effects of livestock treatments result in altered structure and diversity of dung beetle assemblages [18,35]. Most dung beetle species are susceptible to Ivermectin [9]. In this study, we found that some native species (A. crenulatus and O. marginicollis) and the introduced species D. gazella were potentially more resistant to Ivermectin use. It is possible that this potential resistance is related to the more saprophagous habits of the Eupariini species. It, therefore, would be important to continue to study D. gazella specifically, due to its status as an invasive species in Colombian livestock systems. However, it is also possible that, in the present case, the treatment of cattle did not coincide exactly with the emergence or oviposition period of these species, which appeared relatively unaffected. This situation should be compared to that documented in Mexico with some dung beetle species, whose emergence period coincided with the treatment of pastures with an herbicide [127]. The resilience of dung beetle assemblages to the frequent use of Ivermectin is unknown. Therefore, it is essential to continue studying the spatio-temporal dynamics of the assemblages in wider time windows [35]. At the ecosystem scale, species loss with decreased dung recycling functions was observed after a few years [18].
It is important to highlight that the effects of Ivermectin vary between functional groups [9,36]. The paracoprids and endocoprids were the functional groups who were the most strongly affected by Ivermectin in the Two-IVM 3.15% livestock management. In more general terms, large dung beetles, as well as paracoprid and telecoprid beetles, which are the most functionally efficient in terms of dung removal capacity, are the most vulnerable and the most prone to extinction [35,36,128]. The behavior of these functional groups may be necessary for determining how beetles respond physiologically to Ivermectin [41]. Ivermectin could have differential effects on species, thus affecting larger individuals more dramatically. However, the studies of Verdú et al. [32,124] and Martínez et al. [31] on species of different sizes showed no evident relation between size and vulnerability to ivermectin. The highest risk of extinction of large species is probably more related to their lower reproductive rate (K-strategists) than to lower resistance to Ivermectin. The larger dung beetles would be more exposed to extinction processes than small ones [48]. In our study, we did not find any large species of endocoprids for the three livestock management approaches, and there was only one large species of telecoprids for the No-IVM management (D. guildingii) and four large species of paracoprids in the No-IVM management. It is important to mention that there may be a potential bias in the analysis when including the Eupariini, since most of their species have more saprophagous habits; therefore, the abundances in the traps may be affected.
Finally, we did not find any significant preference on the part of the dung beetles for dung from untreated or ivermectin-treated animals (1% and 3.15%). Similar results have been reported by other authors (e.g., [18,42]), but results in the opposite direction have also been reported to show that dung beetles could be attracted to the dung of Ivermectin-treated animals (see [30,33,129,130]). This contradiction is probably only apparent. Ivermectin by itself does not attract dung beetles, but when animals are heavily parasitized, the antiparasitic treatment results in the lysis of the parasites and a strong discharge of several amino acids and volatile organic compounds that change the scent of the dung, which may increase its attractiveness to dung beetles, at least in the few days following the treatment [131].
5. Conclusions and Recommendations
In our study, we found that the use of Ivermectin for the control of livestock parasites had negative effects on dung beetle richness, abundance, and biomass in the short term, which can slow down the recycling processes of cattle manure and lead to an accumulation of dung on pastures, thus affecting soil fertility, structure, and aeration, as well as plant species diversity in paddocks, due to non-secondary seed dispersal [132] and less control of biting flies and parasites affecting livestock [133]. For future studies on the effects of Ivermectin on dung beetle assemblages, we suggest monitoring and controlling over time to determine the effects on the soil fauna of the farms. For future management and conservation strategies in the region’s livestock systems, the changes in diversity and ecosystem services provided by dung beetles must be considered. Livestock health management has become a major issue, as some veterinary drugs found in dung after treatment of animals can affect the regular interaction of the manure-degrading fauna. In order to reduce the effects of the most toxic molecules on the fauna involved in recycling, the frequency of treatments should be reduced, and the molecules and families of products should be diversified. In addition, older molecules that are still effective and safe for dung beetles should be used, such as benzimidazoles (albendazole) and imidazothiazole derivatives (levamisole) [24]. This would also mitigate the risk of parasite resistance in the case of indiscriminate and continuous use of the same molecule [134,135]. Finally, a simple measure to cut the parasite cycles would be the rotation of cattle, with short times of permanence of the animals in each division.
Author Contributions
H.L.T. and J.A.N. conceived the idea and designed the research; H.L.T., B.N. and V.T. gathered the data; H.L.T. and J.A.N. structured the manuscript; H.L.T., J.A.N. and C.M.A.C. analyzed the data and made tables and figures; J.A.N., C.M.A.C. and J.-P.L. corrected and improved different versions of the manuscript, and all authors contributed to the writing and approved the last version of the document. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data can be found within the article.
Acknowledgments
We thank the owners of the farms involved in this study. We thank Andrés Pérez and Eder Flores for their support in the fieldwork. We thank Jaime Mercado Ordóñez for his knowledge and support in the statistical analysis. We thank José Ramon Verdú for his valuable comments and suggestions to the manuscript. We thank Julián Clavijo Bustos for the taxonomic confirmation of the Aphodiinae and for valuable comments regarding the manuscript. We thank the El Piñal Technical Agricultural Educational Institution in Sucre (Colombia), for providing the necessary time slots to perform this work. PALB was supported by a Master’s scholarship from the Minas Gerais State Agency for Research and Development (FAPEMIG).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hernández, B.; Maes, J.; Harvey, C.; Vílches, S.; Medina, A.; Sánchez, D. Abundancia y diversidad de escarabajos coprófagos y mariposas diurnas en un paisaje ganadero en el departamento de Rivas, Nicaragua. Agrofor. Am. 2003, 10, 93–102. [Google Scholar]
- Flota-Bañuelos, C.; López-Collado, J.; Vargas-Mendoza, M.; Fajersson, P.; González-Hernández, H.; Martínez-Morales, I. Efecto de la Ivermectina en la dinámica espacio-temporal de escarabajos estercoleros en Veracruz, Mexico. Trop. Subtrop. Agroecosyst. 2012, 15, 227–239. [Google Scholar]
- FEDEGAN. Ganadería Colombiana Hoja de Ruta 2018–2022; FEDEGAN: Bogotá, Colombia, 2018; p. 126. [Google Scholar]
- ICA (Instituto Colombiano Agropecuario). Censos Pecuarios Nacional: Bogotá, Colombia. 2022. Available online: https://www.ica.gov.co/areas (accessed on 1 December 2022).
- IGAC (Instituto Geográfico Agustín Codazzi). XVIII Congreso Nacional de la Ciencia del Suelo; IGAC (Instituto Geográfico Agustín Codazzi): Villa de Leyva, Colombia, 2016. [Google Scholar]
- Doube, B.M. A functional classification for analysis of the structure of dung beetle assemblages. Ecol. Èntomol. 1990, 15, 371–383. [Google Scholar] [CrossRef]
- O´Hea, N.; Kirwan, L.; Finn, J. Experimental mixtures of dung fauna effect dung decomposition through complex effects of species interactions. Oikos 2010, 119, 1081–1088. [Google Scholar] [CrossRef]
- Lumaret, J.-P.; Kadiri, N. The influence of the first wave of colonizing insects on cattle dung dispersal. Pedobiologia 1995, 39, 506–517. [Google Scholar]
- Beynon, S.A.; Mann, D.J.; Slade, E.M.; Lewis, O.T. Species-rich dung beetle communities buffer ecosystem services in perturbed agro-ecosystems. J. Appl. Ecol. 2012, 49, 1365–1372. [Google Scholar] [CrossRef]
- Correa, C.M.A.; Braga, R.F.; Louzada, J.; Menéndez, R. Dung beetle diversity and functions suggest no major impacts of cattle grazing in the Brazilian Pantanal wetlands. Ecol. Èntomol. 2019, 44, 524–533. [Google Scholar] [CrossRef]
- Martínez, I.; Cruz, M.; Montes de Oca, E.; Suárez, T. La Función de los Escarabajos del Estiércol en los Pastizales Ganaderos, 1st ed.; Secretaría de Educación de Veracruz: México City, México, 2011; p. 49.
- Brown, J.; Scholtz, C.H.; Janeau, J.-L.; Grellier, S.; Podwojewski, P. Dung beetles (Coleoptera: Scarabaeidae) can improve soil hydrological properties. Appl. Soil Ecol. 2010, 46, 9–16. [Google Scholar] [CrossRef]
- Irshad, M.; E Eneji, A.; Hussain, Z.; Ashraf, M. Chemical characterization of fresh and composted livestock manures. J. Soil Sci. Plant Nutr. 2013, 13, 115–121. [Google Scholar] [CrossRef]
- Sommer, C.; Bibby, B.M. The influence of veterinary medicines on the decomposition of dung organic matter in soil. Eur. J. Soil Biol. 2002, 38, 155–159. [Google Scholar] [CrossRef]
- Badenhorst, J.; Dabrowski, J.; Scholtz, C.H.; Truter, W.F. Dung beetle activity improves herbaceous plant growth and soil properties on confinements simulating reclaimed mined land in South Africa. Appl. Soil Ecol. 2018, 132, 53–59. [Google Scholar] [CrossRef]
- Lobo, J.; Veiga, C. Interés ecológico de la fauna coprófaga en pastos de uso ganadero. Ecología 1990, 4, 313. [Google Scholar]
- Nichols, E.; Spector, S.; Louzada, J.; Larsen, T.; Amezquita, S.; Favila, M.; The Scarabaeinae Research Network. Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biol. Conserv. 2008, 141, 1461–1474. [Google Scholar] [CrossRef]
- Verdú, J.R.; Lobo, J.M.; Sánchez-Piñero, F.; Gallego, B.; Numa, C.; Lumaret, J.-P.; Cortez, V.; Ortiz, A.J.; Tonelli, M.; García-Teba, J.P.; et al. Ivermectin residues disrupt dung beetle diversity, soil properties and ecosystem functioning: An interdisciplinary field study. Sci. Total Environ. 2018, 618, 219–228. [Google Scholar] [CrossRef]
- Braga, R.F.; Korasaki, V.; Andresen, E.; Louzada, J. Dung beetle community and functions along a habitat-disturbance gradient in the amazon: A rapid assessment of ecological functions associated to biodiversity. PLoS ONE 2013, 8, e57786. [Google Scholar] [CrossRef] [PubMed]
- Sands, B.; Wall, R. Sustained parasiticide use in cattle farming affects dung beetle functional assemblages. Agric. Ecosyst. Environ. 2018, 265, 226–235. [Google Scholar] [CrossRef]
- Coop, R.; Kyriazakis, I. Nutrition-parasite interaction. Vet. Parasitol. 1999, 84, 187–204. [Google Scholar] [CrossRef]
- Lumaret, J.-P.; Martínez, I. El impacto de productos veterinarios sobre insectos coprófagos: Consecuencias sobre la degradación del estiércol en pastizales. Acta Zoológica Mex. 2005, 21, 137–148. [Google Scholar] [CrossRef]
- Floate, K.D. Endectocide use in cattle and fecal residues: Environmental effects in Canada. Can. J. Vet.-Res. 2006, 70, 1–10. [Google Scholar]
- Lumaret, J.-P.; Errouissi, F. Use of anthemilthicides in herbivores and of risks for the non target fauna of pastures. Vet. Res. 2002, 33, 547–562. [Google Scholar] [CrossRef]
- Campbell, W. Ivermectin and Abamectin; Springer Science Business Media: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Jackson, H. Ivermectin as a systemic insecticide. Parasitol. Today 1989, 5, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Molinari, G. Ivermectinas: Evaluación de su Efecto Deletéreo Mediante Ensayos de Genotoxicidad. Ph.D. Thesis, Facultad de Ciencias Naturales y Museo, Universidad Nacional de la Plata, La Plata, Argentina, 2010. [Google Scholar]
- Wohde, M.; Blanckenhorn, W.U.; Floate, K.D.; Lahr, J.; Lumaret, J.-P.; Römbke, J.; Scheffczyk, A.; Tixier, T.; Düring, R.-A. Analysis and dissipation of the antiparasitic agent ivermectin in cattle dung under different field conditions. Environ. Toxicol. Chem. 2016, 35, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- González Canga, A.; Sahagún Prieto, A.M.; José Diez Liébana, M.; Martínez, N.F.; Vega, M.S.; Vieitez, J.J.G. The pharmacokinetics and metabolism of ivermectin in domestic animal species. Vet. J. 2009, 179, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Floate, K.D. Endectocide residues affect insect attraction to dung from treated cattle: Implications for toxicity tests. Med. Veter-Èntomol. 2007, 21, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Martínez, I.M.; Lumaret, J.-P.; Zayas, R.O.; Kadiri, N. The effects of sublethal and lethal doses of ivermectin on the reproductive physiology and larval development of the dung beetle Euoniticellus intermedius (Coleoptera: Scarabaeidae). Can. Èntomol. 2017, 149, 461–472. [Google Scholar] [CrossRef]
- Verdú, J.R.; Cortez, V.; Ortiz, A.J.; González-Rodríguez, E.; Martínez-Pinna, J.; Lumaret, J.-P.; Lobo, J.M.; Numa, C.; Sánchez-Piñero, F. Low doses of ivermectin cause sensory and locomotor disorders in dung beetles. Sci. Rep. 2015, 5, 13912. [Google Scholar] [CrossRef]
- Lumaret, J.-P.; Galante, E.; Lumbreras, C.; Mena, J.; Bertrand, M.; Bernal, J.L.; Cooper, J.F.; Kadiri, N.; Crowe, D. Field effects of Ivermectin residues on dung beetles. J. Appl. Ecol. 1993, 30, 428. [Google Scholar] [CrossRef]
- Floate, K. Does a repellent effect contribute to reduced levels of insect activity in dung from cattle treated with Ivermectin? Bull. Entomol. Res. 1998, 88, 291–297. [Google Scholar] [CrossRef]
- Correa, C.M.A.; Ferreira, K.R.; Abot, A.R.; Louzada, J.; Vaz-De-Mello, F.Z. Ivermectin impacts on dung beetle diversity and their ecological functions in two distinct Brazilian ecosystems. Ecol. Èntomol. 2022, 47, 736–748. [Google Scholar] [CrossRef]
- Ambrožová, L.; Sládeček, F.X.J.; Zítek, T.; Perlík, M.; Kozel, P.; Jirků, M.; Čížek, L. Lasting decrease in functionality and richness: Effects of ivermectin use on dung beetle communities. Agric. Ecosyst. Environ. 2021, 321, 107634. [Google Scholar] [CrossRef]
- Floate, K.D.; Wardhaugh, K.G.; Boxall, A.B.; Sherratt, T.N. Fecal residues of veterinary parasiticides: Nontarget effects in the pasture environment. Annu. Rev. Èntomol. 2005, 50, 153–179. [Google Scholar] [CrossRef]
- Pecenka, J.R.; Lundgren, J.G. Effects of herd management and the use of ivermectin on dung arthropod communities in grasslands. Basic Appl. Ecol. 2019, 40, 19–29. [Google Scholar] [CrossRef]
- Rosales, M.C.; Martinez, I.; López-Collado, J.; Vargas-Mendoza, M.; González-Hernández, H.; Fajersson, P. Effect of ivermectin on the survival and fecundity of Euoniticellus intermedius (Coleoptera: Scarabaeidae). Rev. Biol. Trop. 2012, 60, 333–345. [Google Scholar]
- Basto-Estrella, G.S.; Rodríguez-Vivas, R.I.; Delfín-González, H.; Reyes-Novelo, E. Dung beetle (Coleoptera: Scarabaeinae) diversity and seasonality in response to use of macrocyclic lactones at cattle ranches in the mexican neotropics. Insect Conserv. Divers. 2013, 7, 73–81. [Google Scholar] [CrossRef]
- Villada-Bedoya, S.; Chávez-Ríos, J.R.; Montoya, B.; Castelán, F.; Córdoba-Aguilar, A.; Escobar, F.; González-Tokman, D. Heat shock proteins and antioxidants as mechanisms of response to ivermectin in the dung beetle Euoniticellus intermedius. Chemosphere 2020, 269, 128707. [Google Scholar] [CrossRef] [PubMed]
- Tovar, H.; Noriega, J.A.; Caraballo, P. Efecto de la ivermectina sobre la estructura del ensamblaje de escarabajos coprófagos (Coleoptera: Scarabaidae: Aphodiinae-Scarabaeinae) en las sabanas colombianas de la región Caribe. Actual. Biol. 2016, 38, 157–166. [Google Scholar]
- Clavijo, J.; Barrera, R. Geología de las Planchas 44 Sincelejo y 52 Sahagún; Ministerio de Minas y Energía, Instituto de Investigación e Información Geocientífica, Minero-Ambiental y Nuclear, INGEOMINAS: Cartagena, Colombia, 2001; 63p.
- Holdridge, L. Ecología Basada en Zonas de Vida, 1st ed.; Jiménez, H., Translator; Centro Científico Tropical IICA: San José, Costa Rica, 1987; p. 216. [Google Scholar]
- Navarro, I.; Roman, K.; Gomez, H.; Peréz, A. Listado de escarabajos coprófagos (Coleoptera: Scarabaeidae: Scarabaeinae) de La Serrania de Coraza, Sucre (Colombia). Rev. Colomb. Cienc. Anim. 2011, 3, 262–268. [Google Scholar] [CrossRef]
- Navarro, I.; Roman, K.; Gomez, H.; Peréz, A. Variación estacional en escarabajos coprófagos (Coleoptera: Scarabaeidae: Scarabaeinae) de la serranía de Coraza, Sucre (Colombia). Rev. Colomb. Cienc. Anim. 2011, 3, 102–110. [Google Scholar] [CrossRef]
- Mora-Aguilar, E.F.; Arriaga-Jiménez, A.; Correa, C.M.A.; da Silva, P.G.; Korasaki, V.; López-Bedoya, P.A.; Hernández, M.I.M.; Pablo-Cea, J.D.; Salomão, R.P.; Valencia, G.; et al. Toward a standardized methodology for sampling dung beetles (Coleoptera: Scarabaeinae) in the Neotropics: A critical review. Front. Ecol. Evol. 2023, 11, 1096208. [Google Scholar] [CrossRef]
- Larsen, T.H.; Forsyth, A. Trap spacing and transect design for dung beetle biodiversity studies. Biotropica 2005, 37, 322–325. [Google Scholar] [CrossRef]
- Pérez-Cogollo, L.; Rodríguez-Vivas, R.; Reyes-Novelo, E.; Delfín-González, H.; Muñoz-Rodríguez, D. Survival and reproduction of Onthophagus landolti (Coleoptera: Scarabaeidae) exposed to ivermectin residues in cattle dung. Bull. Èntomol. Res. 2016, 107, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, W.D. Revision of Phanaeus Macleay, a New World genus of scarabaeine dung beetles (Coleoptera: Scarabaeidae, Scarabaeinae). Contrib. Sci. 1994, 443, 1–105. [Google Scholar] [CrossRef]
- Genier, F. A revision of the Neotropical genus Ontherus Erichson (Coleoptera: Scarabaeidae, Scarabaeinae). Mem. Entomol. Soc. Can. 1996, 128, 3–170. [Google Scholar] [CrossRef]
- González, F.A.; Molano, F.; Medina, C. Los subgéneros Calhyboma, Hybomidium y Telhyboma (Coleoptera: Scarabaeidae: Scarabaeinae: Deltochilum) en Colombia. Rev. Colomb. Entomol. 2009, 35, 253–274. [Google Scholar] [CrossRef]
- Edmonds, W.; Zídek, J. A taxonomic review of the Neotropical genus Coprophanaeus Olsoufieff, 1924 (Coleoptera: Scarabaeidae, Scarabaeinae). Insecta Mundi 2010, 129, 1–111. [Google Scholar]
- Camero, E. Los escarabajos del género Eurysternus Dalman, 1824 (Coleoptera: Scarabaeidae) de Colombia. Bol. Soc. Entomol. Aragonesa 2010, 46, 147–179. [Google Scholar]
- Vaz-De-Mello, F.; Edmonds, W.D.; Ocampo, F.C.; Schoolmeesters, P. A multilingual key to the genera and subgenera of the subfamily Scarabaeinae of the New World (Coleoptera: Scarabaeidae). Zootaxa 2011, 2854, 1–73. [Google Scholar] [CrossRef]
- Cartwright, O.L. Two new species of Cartwrightia from Central and South America (Coleoptera: Scarabaeidae: Aphodiinae). Proc. U. S. Natl. Mus. 1967, 124, 1–8. [Google Scholar] [CrossRef]
- Dellacasa, G.; Bordat, P.; Dellacasa, M. A revisional essay of world genus-group taxa of Aphodiinae (Coleoptera: Aphodiidae). Mem. Della Soc. Entomol. Ital. Genova 2001, 79, 1–482. [Google Scholar]
- Dellacasa, M.; Gordon, R.D.; Dellacasa, G. Aphodiinae described or recorded by Bates in Biologia Centrali-Americana (Coleoptera Scarabaeoidea: Aphodiidae). Acta Zool. Mex. 2002, 86, 155–223. [Google Scholar] [CrossRef]
- Stebnicka, Z.T. The Genus Ataenius Harold, 1867 (Coleoptera: Scarabaeidae) of New World. Iconography; Institute of Systematics and Evolution of Animals: Kraków, Poland; Polish Academy of Sciences: Warsaw, Poland, 2007. [Google Scholar]
- Halffter, G.; Edmonds, W. The Nesting Behavior of Dung Beetles (Scarabaeinae): An Ecological and Evolutive Approach; Instituto de Ecología: Mexico City, Mexico, 1982; p. 176. [Google Scholar]
- Noriega, J.A.; March-Salas, M.; Castillo, S.; García-Q, H.; Hortal, J.; Santos, A.M.C. Human perturbations reduce dung beetle diversity and dung removal ecosystem function. Biotropica 2021, 53, 753–766. [Google Scholar] [CrossRef]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 1 December 2022).
- Crawley, M.J. Statistical Computing–An Introduction to Data Analysis Using S-Plus; John Wiley Sons: London, UK, 2013. [Google Scholar]
- Dufrene, M.; Legendre, P. Species assemblages and indicators species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar] [CrossRef]
- Correa, C.M.A.; Peres, N.D.; Holdbrook, R. Patterns of alimentary resource use by dung beetles in introduced Brazilian pastures: Cattle versus sheep dung. Èntomol. Sci. 2020, 23, 271–279. [Google Scholar] [CrossRef]
- Cáceres, M.D.; Jansen, F.; Dell, N. Package ‘Indicspecies’. 2022. Available online: https://cran.r-project.org/web/packages/indicspecies/indicspecies.pdf (accessed on 15 December 2022).
- Locarno, L.C.P.; Schoolmeesters, P. Small dung beetles of Colombia (Coleoptera Scarabaeoidea Aphodiinae) I: Preliminary catalog and key for registered species. Bol. Cient. Mus. Hist. Nat. Univ. Caldas 2019, 23, 279–302. [Google Scholar] [CrossRef]
- Miles, L.; Newton, A.C.; DeFries, R.S.; Ravilious, C.; May, I.; Blyth, S.; Kapos, V.; Gordon, J.E. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 2006, 33, 491–505. [Google Scholar] [CrossRef]
- Portillo-Quintero, C.; Sanchez-Azofeifa, A.; Calvo-Alvarado, J.; Quesada, M.; Santo, M.M.D.E. The role of tropical dry forests for biodiversity, carbon and water conservation in the neotropics: Lessons learned and opportunities for its sustainable management. Reg. Environ. Chang. 2014, 15, 1039–1049. [Google Scholar] [CrossRef]
- IAVH (Instituto de Investigación en Recursos Biológicos Alexander Von Humboldt). El Bosque Seco Tropical (Bs-T) en Colombia; Informe GEMA Programa de Inventario de la Biodiversidad, Instituto Alexander von Humboldt: Bogotá, Colombia, 1998. [Google Scholar]
- Pizano, C.; García, H. El Bosque Seco Tropical en Colombia; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH): Bogotá, Colombia, 2014; 353p. [Google Scholar]
- Whitmore, T. Tropical forest disturbance, disappearances, and species loss. In Tropical Forest Remnants: Ecology, Management, and Conservation of Fragmented Communities; Laurance, W.F., Bierregaard, R.O., Eds.; The University of Chicago Press: Chicago, IL, USA, 1997; pp. 3–12. [Google Scholar]
- Harvey, C.; Sáenz, J. Evaluación y Conservación de Biodiversidad en Paisajes Fragmentados de Mesoamérica; Harvey, C.A., Sáenz-Méndez, J.C., Eds.; INBio, CATIE: San José, Costa Rica, 2008; pp. 9–11. [Google Scholar]
- Slade, E.M.; Mann, D.J.; Lewis, O.T. Biodiversity and ecosystem functions of tropical forest dung beetles under contrasting logging regimes. Biol. Conserv. 2011, 144, 166–174. [Google Scholar] [CrossRef]
- López-Bedoya, P.A.; Bohada-Murillo, M.; Ángel-Vallejo, M.C.; Audino, L.D.; Davis, A.L.V.; Gurr, G.; Noriega, J.A. Primary forest loss and degradation reduces biodiversity and ecosystem functioning: A global meta-analysis using dung beetles as an indicator taxon. J. Appl. Ecol. 2022, 59, 1572–1585. [Google Scholar] [CrossRef]
- Amell-Caez, Y.; Decastro-Arrazola, I.; Garcia, I. Spatial diversity of dung beetle assemblages (Coleoptera: Scarabaeidae: Scarabaeinae) in five ecoregions from Sucre, Colombian Caribbean coast. Rev. Colomb. Entomol. 2019, 45, 1–10. [Google Scholar]
- Noriega, J.A.; Solis, C.; García, H.; Murillo-Ramos, L.; Renjifo, J.; Olarte, J. Sinopsis de los escarabajos coprófagos (Coleoptera: Scarabaeinae) del caribe colombiano. Caldasia 2013, 35, 465–477. [Google Scholar]
- Taboada-Verona, C.; Sermeño-Correa, C.; Sierra-Serrano, O.; Noriega, J.A. Checklist of the superfamily Scarabaeoidea (Insecta, Coleoptera) in an urban area of the Caribbean Colombia. Check List 2019, 15, 579–594. [Google Scholar] [CrossRef]
- Bohórquez, J.; Montoya, J. Abundancia y preferencia trófica de Dichotomius belus (Coleoptera: Scarabaeidae) en la reserva forestal de Colosó, Sucre. Bol. Mus. Entomol. Univ. Val. 2009, 10, 1–7. [Google Scholar]
- Navarro, L.; Roman, K.; Gomez, H.; Pérez, A. Primer registro de Digitonthophagus gazella (Fabricius, 1787) para el departamento de Sucre, Colombia. Rev. Colomb. Cienc. Anim.-RECIA 2009, 1, 60–64. [Google Scholar] [CrossRef]
- Solis, C.; Noriega, J.A.; Herrera, G. Escarabajos coprófagos (Coleoptera: Scarabaeinae) en tres bosques seco del departamento del Atlántico-Colombia. Bol. Mus. Entomol. Univ. Val. 2011, 12, 33–41. [Google Scholar]
- Rangel-Acosta, J.; Martínez-Hernandez, N. Comparación de los ensamblajes de escarabajos copronecrófagos (Scarabaeidae: Scarabaeinae) entre fragmentos de bosque seco tropical y la matriz adyacente en el departamento del Atlántico-Colombia. Rev. Mex. Biodivers. 2017, 88, 389–401. [Google Scholar] [CrossRef]
- Rangel-Acosta, J.; Solano-Torres, J.; Martínez-Hernández, N. Variación temporal y vertical de los escarabajos coprófagos (Scarabaeidae: Scarabaeinae) en dos fragmentos de bosque seco tropical en el departamento del Atlántico-Colombia. Bol. Cient. Mus. Hist. Nat. 2018, 22, 179–198. [Google Scholar] [CrossRef]
- Ortega-Echeverría, C.; Navas, G.; Noriega, J. Estacionalidad del ensamblaje de escarabajos coprófagos (Coleoptera: Scarabaeinae) del jardín botánico de Cartagena “Guillermo Piñeres” Bolívar-Colombia. Caldasia 2019, 41, 124–138. [Google Scholar] [CrossRef]
- Rangel-Acosta, J.; Martínez-Hernández, N.; Yonoff-Zapat. Respuesta de los escarabajos coprófagos (Scarabaeidae: Scarabaeinae) a la modificación del hábitat causada por un incendio forestal en la Reserva Bijibana, Atlántico-Colombia. Rev. Mex. Biodivers. Rev. Mex. Biodivers. 2020, 91, e912879. [Google Scholar] [CrossRef]
- Noriega, J.A.; Zapata-Prisco, C.; García, H.; Hernández, E.; Hernández, J.; Martínez, R.; Santos-Santos, J.; Pablo-Cea, J.; Calatayud, J. Does ecotourism impact biodiversity? An assessment using dung beetles (Coleoptera: Scarabaeinae) as bioindicators in a tropical dry forest natural park. Ecol. Indic. 2020, 117, 106580. [Google Scholar] [CrossRef]
- Tscharntke, T.; Tylianakis, J.M.; Rand, T.A.; Didham, R.K.; Fahrig, L.; Batáry, P.; Bengtsson, J.; Clough, Y.; Crist, T.O.; Dormann, C.F.; et al. Landscape moderation of biodiversity patterns and processes—Eight hypotheses. Biol. Rev. 2012, 87, 661–685. [Google Scholar] [CrossRef] [PubMed]
- Gascon, C.; E Lovejoy, T.; Bierregaard, R.O., Jr.; Malcolm, J.R.; Stouffer, P.C.; Vasconcelos, H.L.; Laurance, W.F.; Zimmerman, B.; Tocher, M.; Borges, S. Matrix habitat and species richness in tropical forest remnants. Biol. Conserv. 1999, 91, 223–229. [Google Scholar] [CrossRef]
- Mendenhall, C.D.; Sekercioglu, C.H.; Brenes, F.O.; Ehrlich, P.R.; Daily, G.C. Predictive model for sustaining biodiversity in tropical countryside. Proc. Natl. Acad. Sci. USA 2011, 108, 16313–16316. [Google Scholar] [CrossRef] [PubMed]
- Nichols, E.; Larsen, T.; Spector, S.; Davis, A.; Escobar, F.; Favila, M.; Vulinec, K.; The Scarabaeinae Research Network. Global dung beetle response to tropical forest modification and fragmentation: A quantitative literature review and meta-analysis. Biol. Conserv. 2007, 137, 1–19. [Google Scholar] [CrossRef]
- Edwards, F.A.; Finan, J.; Graham, L.K.; Larsen, T.H.; Wilcove, D.S.; Hsu, W.W.; Chey, V.; Hamer, K.C. The impact of logging roads on dung beetle assemblages in a tropical rainforest reserve. Biol. Conserv. 2017, 205, 85–92. [Google Scholar] [CrossRef]
- Martínez-Hernández, N.; Atencia, S.; Cerpa, M.; Murillo, T.; Mejía, C. Composición y estructura de la fauna de escarabajos (Insecta: Coleoptera) atraídos por trampas de luz en la reserva ecológica de Luriza, Atlántico, Colombia. Bol. Soc. Entomol. Aragonesa (S.E.A.) 2010, 47, 373–381. [Google Scholar]
- Delgado-Gómez, P.; Lopera, A.; Rangel Ch, J.O. Variación Espacial del Ensamblaje de Escarabajos Coprófagos (Scarabaeidae: Scarabaeinae) en Remanentes de Bosque Seco en Chimichagua (Cesar, Colombia); Colombia diversidad biótica XII: La región Caribe de Colombia; En Rangel, J.O., Ed.; Universidad Nacional de Colombia: Bogotá, Colombia, 2012; pp. 833–849. [Google Scholar]
- Clavijo-Bustos, J.; Lopera-Toro, A.; Noriega, J.A. On the presence of Ataenius crenulatus Schmidt, 1910 in Colombia. Coleopt. Bull. 2023, in press. [Google Scholar]
- Noriega, J.A.; Solis, C.; Escobar, F.; Realpe, E. Escarabajos coprófagos (Coleoptera: Scarabaeidae) de la provincia de la Sierra Nevada de Santa Marta. Biota Colomb. 2007, 8, 77–86. [Google Scholar]
- Janzen, D.H. Seasonal change in abundance of large nocturnal dung beetles (Scarabaeidae) in a Costa Rican deciduous forest and adjacent horse pasture. Oikos 1983, 41, 274–283. [Google Scholar] [CrossRef]
- Breytenbach, W.; Breytenbach, G. Seasonal patterns in dung feeding Scarabaeidae in the Southern Cape. J. Entomol. Soc. South. Afr. 1986, 49, 359–366. [Google Scholar]
- Rougon, D.; Rougon, C. Dung beetles of the Sahel Region: 230–254. In Dung Beetle Ecology; Hanski, I.Y., Cambefort, Eds.; Princeton University: Princeton, NJ, USA, 1991. [Google Scholar]
- Escobar, F. Estudio de la comunidad de coleópteros coprófagos (Scarabaeidae) en un remante de bosque seco al norte del Tolima, Colombia. Caldasia 1997, 19, 419–430. [Google Scholar]
- Otavo, S.E.; Parrado-Rosselli, A.; Noriega, J.A. Superfamilia Scarabaeoidea (Insecta: Coleoptera) como elemento bio-indicador de perturbación antropogénica en un Parque Nacional amazónico. Rev. Biol. Trop. 2013, 61, 735–752. [Google Scholar] [CrossRef] [PubMed]
- Casas, C.; Pineda, N.; Monro, D.; Realpe, E.; Noriega, J. Variación estacional de la biomasa de un ensamble de escarabajos coprófagos (Coleoptera: Scarabaeinae) en un pastizal altoandino. Acta Biol. Colomb. 2021, 26, 318–326. [Google Scholar] [CrossRef]
- Andresen, E.; Feer, F. The role of dung beetles as secondary seed dispersers and their effect on plant regeneration in tropical rainforests. In Seed Fate: Predation, Dispersal and Seedling Establishment; Forget, P.-M., Lambert, J., Hulme, P., Wall, S.V., Eds.; CABI: Wallingford, UK, 2005; pp. 431–449. [Google Scholar]
- Horgan, F.G. Dung beetle assemblages in forests and pastures of El Salvador: A functional comparison. Biodivers. Conserv. 2008, 17, 2961–2978. [Google Scholar] [CrossRef]
- Escobar, F.; Chacón, P. Distribución espacial y temporal en un gradiente de sucesión de la fauna de coleopteros coprófagos (Scarabaeinae, Aphodiinae) en un bosque tropical montano, Nariño—Colombia. Rev. Biol. Trop. 2000, 48, 961–975. [Google Scholar]
- Halffter, G.; Montes de Oca, T. Daily and seasonal activities of a guild of the coprofagus, burrowing beetle (Coleoptera Scarabainae) in tropical grassland. Trop. Zool. 1995, 8, 159–180. [Google Scholar]
- Anduaga, S.; Huerta, C. Importance of dung incorporation activity by three species of coprophaneus beetles (Coleoptera: Scarabaeidae: Scarabaeinae) macrofauna in pastureland on “La Michilía” Biosphere Reserve in Durango, México. Environ. Entomol. 2007, 36, 555–559. [Google Scholar]
- Edwards, P. Seasonal variation in the dung of African mammals and consequences for coprophagous insects. Funct. Ecol. 1991, 5, 617–628. [Google Scholar] [CrossRef]
- Martínez, I.M.; Dellacasa, M.; Lumaret, J.-P.; Dellacasa, G. Phenology and reproductive cycles in Mexican aphodiine dung beetles (Coleoptera: Scarabaeidae: Aphodiinae: Aphodiini). Ann. Soc. Entomol. Fr. 2022, 58, 173–185. [Google Scholar] [CrossRef]
- Chown, S.; Klok, C. The ecological implications of physiological diversity in dung beetles. In Ecology and Evolution of Dung Beetles; Simmons, L.W., Rids-dill-Smith, T.J., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2011; pp. 200–219. [Google Scholar]
- Martin-Piera, F.; Lobo, J.M. A comparative discussion of trophic preferences in dung beetle communities. Misc. Zool. 1996, 19, 13–31. [Google Scholar]
- Noriega, J.A.; Floate, K.D.; Génier, F.; Reid, C.A.M.; Kohlmann, B.; Horgan, F.G.; Davis, A.L.V.; Forgie, S.A.; Aguilar, C.; Ibarra, M.G.; et al. Global distribution patterns provide evidence of niche shift by the introduced African dung beetle Digitonthophagus gazella. Entomol. Exp. Appl. 2020, 168, 766–782. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundances, 6th ed.; Pearson: Essex, UK, 2014; 653p. [Google Scholar]
- Martínez, N.; García, H.; Pulido, L.; Ospino, D.; Narváez, J. Escarabajos coprófagos (Coleoptera: Scarabaeinae) de la vertiente noroccidental, Sierra Nevada de Santa Marta, Colombia. Neotrop. Entomol. 2009, 38, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Raine, E.H.; Slade, E.M. Dung beetle mammal associations: Methods, research trends and future directions. Proc. R. Soc. B 2019, 286, 20182002. [Google Scholar] [CrossRef] [PubMed]
- Amézquita, S.; Favila, M.E. Removal rates of native and exotic dung by dung beetles (Scarabaeidae: Scarabaeinae) in a fragmented Tropical Rain Forest. Environ. Èntomol. 2010, 39, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Slade, E.M.; Kirwan, L.; Bell, T.; Philipson, C.D.; Lewis, O.T.; Roslin, T. The importance of species identity and interactions for multifunctionality depends on how ecosystem functions are valued. Ecology 2017, 98, 2626–2639. [Google Scholar] [CrossRef]
- Tonelli, M.; Verdú, J.R.; Zunino, M. Effects of the progressive abandonment of grazing on dung beetle biodiversity: Body size matters. Biodivers. Conserv. 2017, 27, 189–204. [Google Scholar] [CrossRef]
- Barraza, J.; Montes, J.; Martínez, N.; Deloya, C. Ensamblaje de escarabajos coprófagos (Scarabaeidae: Scarabaeinae) del Bosque Tropical Seco, Bahía Concha, Santa Marta (Colombia). Rev. Colomb. Entomol. 2010, 36, 285–291. [Google Scholar] [CrossRef]
- Escobar, F. Excremento, Coprófago y Deforestación en Bosque de Montaña al Suroriente de Colombia. Bachelor’s Thesis, Universidad del Valle, Cali, Colombia, 1994. [Google Scholar]
- Amat-Garcia, G.; Lopera, A.; Amézquita, S. Patrones de distribución de escarabajos coprófagos (Coleoptera: Scarabaeidae) en relictos del bosque altoandino, cordillera oriental de Colombia. Caldasia 1997, 19, 191–204. [Google Scholar]
- González-Tokman, D.; Martínez, M.I.; Villalobos-Ávalos, Y.; Munguía-Steyer, R.; Ortiz-Zayas, M.D.R.; Cruz-Rosales, M.; Lumaret, J.-P. Ivermectin alters reproductive success, body condition and sexual trait expression in dung beetles. Chemosphere 2017, 178, 129–135. [Google Scholar] [CrossRef]
- Verdú, J.R.; Cortez, V.; Ortiz, A.J.; Lumaret, J.-P.; Lobo, J.M.; Sánchez-Piñero, F. Biomagnification and body distribution of ivermectin in dung beetles. Sci. Rep. 2020, 10, 9073. [Google Scholar] [CrossRef] [PubMed]
- Errouissi, F.; Alvinerie, M.; Galtier, P.; Kerboeuf, D.; Lumaret, J.-P. The negative effects of the residues of ivermectin in cattle dung using a sustained-release bolus on Aphodius constans (Duft.) (Coleoptera: Aphodiidae). Vet. Res. 2001, 32, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Dadour, I.R.; Cook, D.F.; Hennessy, D. Reproduction and survival of the dung beetle Onthophagus binodis (Coleoptera: Scarabaeidae) exposed to Abamectin and Doramectin residues in cattle dung. Environ. Èntomol. 2000, 29, 1116–1122. [Google Scholar] [CrossRef]
- Martínez, I.M.; Lumaret, J.-P.; Cruz, M.R. Suspected side effects of a herbicide on dung beetle populations (Coleoptera: Scarabaeidae). C.R. L’académie Sci. Paris Life Sci. 2001, 324, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Verdú, J.R.; Morelli, F.; Zunino, M. Dung beetles: Functional identity, not functional diversity, accounts for ecological process disruption caused by the use of veterinary medical products. J. Insect Conserv. 2020, 24, 643–654. [Google Scholar] [CrossRef]
- Errouissi, F.; Lumaret, J.-P. Field effects of faecal residues from ivermectin slow-release boluses on the attractiveness of cattle dung to dung beetles. Med Vet.-Èntomol. 2010, 24, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Römbke, J.; Coors, A.; Alonso Fernández, A.; Förster, B.; Fernández, C.; Jensen, J.; Lumaret, J.P.; Porcel Cots, M.A.; Liebig, M. Effects of the parasiticide ivermectin on the structure and function of dung and soil invertebrate communities in the field (Madrid, Spain). Appl. Soil Ecol. 2010, 45, 284–292. [Google Scholar] [CrossRef]
- Bernal, J.L.; Del Nozal, M.J.; Salas, M.; Galante, E.; Lumaret, J.P. Determination of residual Ivermectin in cattle dung following subcutaneous injection. J. Liq. Chromatogr. 1994, 17, 2429–2444. [Google Scholar] [CrossRef]
- Nervo, B.; Tocco, C.; Caprio, E.; Palestrini, C.; Rolando, A. The effects of body mass on dung removal efficiency in dung beetles. PLoS ONE 2014, 9, e107699. [Google Scholar] [CrossRef]
- Laliberté, E.; Wells, J.A.; DeClerck, F.; Metcalfe, D.J.; Catterall, C.P.; Queiroz, C.; Aubin, I.; Bonser, S.P.; Ding, Y.; Fraterrigo, J.M.; et al. Land-use intensification reduces functional redundancy and response diversity in plant communities. Eco. Lett. 2010, 13, 76–86. [Google Scholar] [CrossRef]
- Anziani, O.; Suarez, V.; Guglielmone, A.; Wanker, O.; Grande, H.; Coles, G. Resistance to benzimidazole and avermectin anthelmintics in cattle nematodes in Argentina. Vet. Parasitol. 2004, 122, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Geurden, T.; Chartier, C.; Fanke, J.; di Regalbono, A.F.; Traversa, D.; von Samson-Himmelstjerna, G.; Demeler, J.; Vanimisetti, H.B.; Bartram, D.J.; Denwood, M.J. Anthelmintic resistance to ivermectin and moxidectin in gastrointestinal nematodes of cattle in Europe. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 163–171. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).