Oribatid Mites (Oribatida) Associated with Nests of Hollow-Nesting Birds, on the Example of a Model Species, the European Pied Flycatcher (Ficedula hypoleuca), in the Taiga Forests of the European North-East of Russia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region
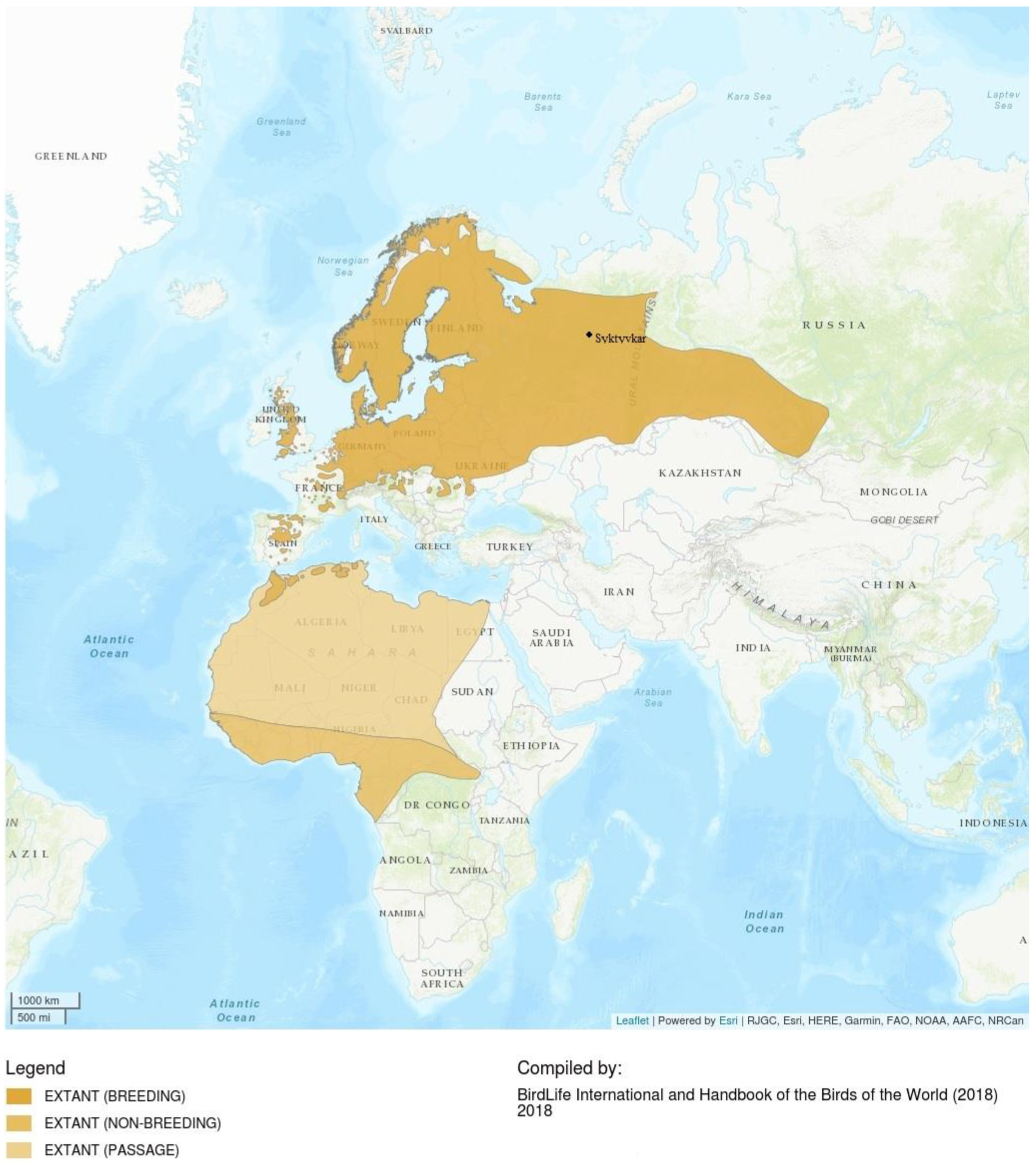
2.2. Pied Flycatcher, Distribution, Nesting Biology
2.3. Sampling Methods
2.4. Material Treatment
3. Results
3.1. Success of Pied Flycatcher Nesting
3.2. Abundance of Oribatid Mites
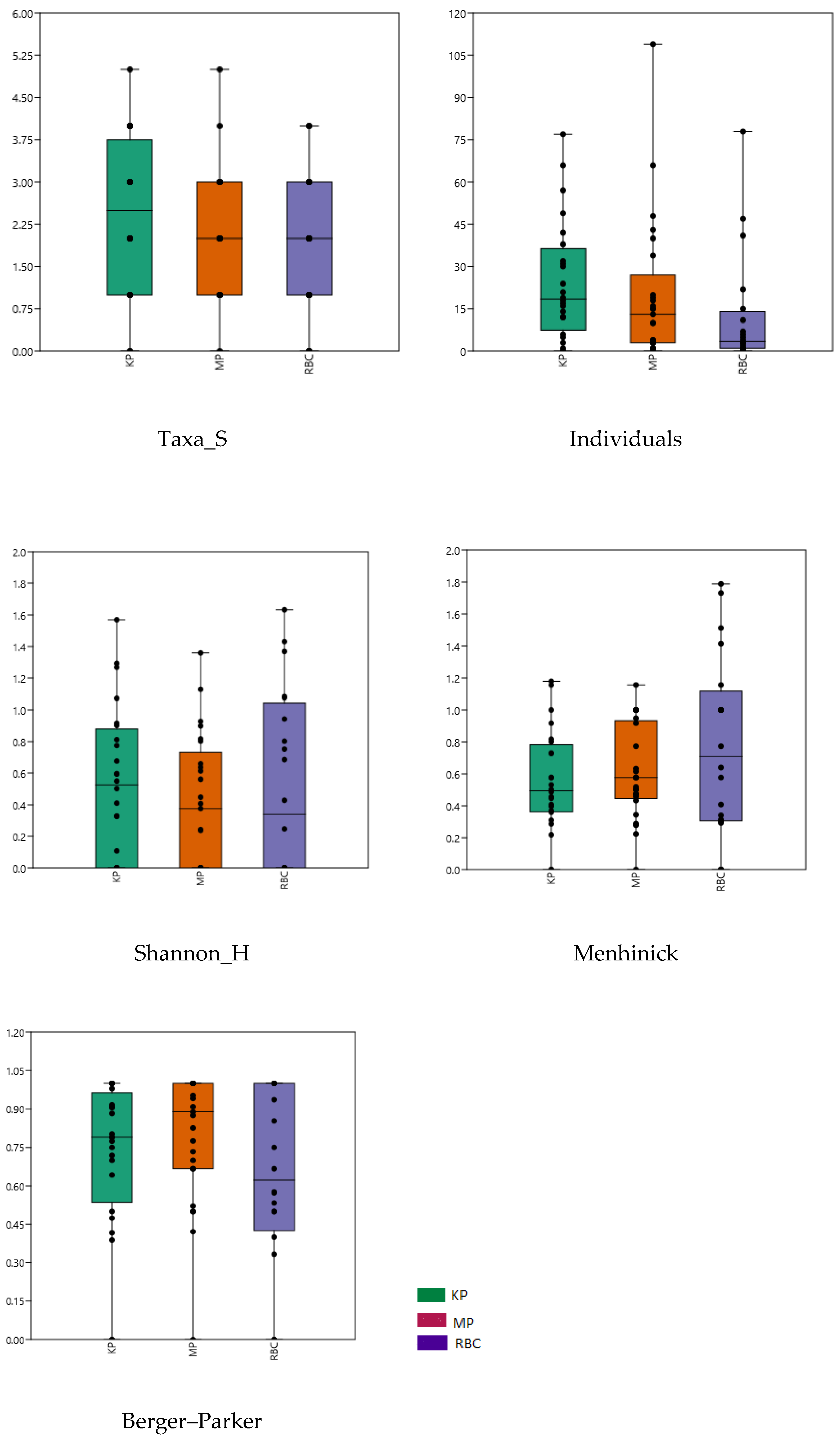
3.3. Taxonomic Composition and Diversity of Oribatid Mites
4. Discussion
4.1. Taxonomic Composition
4.2. Zoogeographic Structure of Fauna and Distribution of Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Nest-Box Number | Nest-Box Location Place | Nesting Start (Date) | Nesting End (Date) | Period the Birds Are in Nest, Days | Period the Birds Are Out of Nest, Days | Breeding Progress (%) | Sampling (Date) |
|---|---|---|---|---|---|---|---|
| 5 | MP | 13 June 2017 | 13 July 2017 | 30 | 307 | 100 | 16 May 2018 |
| 18 | MP | 27 June 2017 | 4 August 2017 | 38 | 285 | 100 | 16 May 2018 |
| 25 | MP | 11 June 2017 | 13 July 2017 | 32 | 307 | 100 | 16 May 2018 |
| 26 | MP | 10 June 2017 | 26 June 2017 | 16 | 324 | 0 | 16 May 2018 |
| 32 | MP | 10 June 2017 | 13 July 2017 | 33 | 307 | 50 | 16 May 2018 |
| 5 | KP | 30 June 2017 | 4 August 2017 | 35 | 285 | 43 | 16 May 2018 |
| 9 | KP | 11 June 2017 | 23 June 2017 | 12 | 285 | 0 | 16 May 2018 |
| 17 | KP | 12 June 2017 | 20 July 2017 | 38 | 300 | 100 | 16 May 2018 |
| 29 | KP | 11 June 2017 | 23 June 2017 | 12 | 327 | 0 | 16 May 2018 |
| 31 | KП | 10 June 2017 | 13 July 2017 | 33 | 307 | 57 | 16 May 2018 |
| б/н | KP | 27 June 2017 | 4 August 2017 | 38 | 285 | 100 | 16 May 2018 |
| 3 | MP | 13 June 2018 | 13 July 2018 | 30 | 7 | 100 | 13 July 2018 |
| 4 | MP | 21 May 2018 | 23 June 2018 | 33 | 13 | 86 | 6 July 2018 |
| 7 | MP | 3 June 2018 | 6 July 2018 | 33 | 7 | 100 | 13 July 2018 |
| 11 | MP | 26 May 2018 | 29 June 2018 | 34 | 7 | 86 | 6 July 2018 |
| 12 | MP | 25 May 2018 | 15 June 2018 | 21 | 21 | 0 | 6 July 2018 |
| 15 | MP | 13 June 2018 | 13 July 2018 | 30 | 7 | 83 | 13 July 2018 |
| 19 | MP | 25 May 2018 | 29 June 2018 | 35 | 13 | 71 | 6 July 2018 |
| 22 | MP | 25 May 2018 | 29 June 2018 | 35 | 13 | 43 | 6 July 2018 |
| 26 | MP | 25 May 2018 | 15 June 2018 | 21 | 21 | 0 | 6 July 2018 |
| 30 | MP | 12 June 2018 | 22 June 2018 | 10 | 14 | 0 | 6 July 2018 |
| 32 | MP | 31 May 2018 | 06 July 2018 | 36 | 7 | 100 | 13 July 2018 |
| 3 | KP | 22 June 2018 | 13 July 2018 | 21 | 19 | 0 | 1 August 2018 |
| 5 | KP | 20 May 2018 | 13 June 2018 | 24 | 7 | 0 | 20 July 2018 |
| 9 | KP | 11 June 2018 | 12 July 2018 | 31 | 7 | 83 | 20 July 2018 |
| 17 | KP | 25 May 2018 | 29 June 2018 | 35 | 7 | 43 | 6 July 2018 |
| 18 | KP | 29 June 2018 | 6 July 2018 | 7 | 14 | 0 | 20 July 2018 |
| 19 | KP | 21 June 2018 | 29 June 2018 | 8 | 21 | 0 | 20 July 2018 |
| 23 | KP | 18 June 2018 | 20 July 2018 | 32 | 7 | 100 | 20 July 2018 |
| 24 | KP | 1 June 2018 | 6 July 2018 | 35 | 13 | 100 | 6 July 2018 |
| 28 | KP | 24 May 2018 | 29 June 2018 | 36 | 7 | 100 | 6 July 2018 |
| 29 | KP | 5 June 2018 | 6 July 2018 | 31 | 7 | 100 | 20 July 2018 |
| 30 | KP | 26 May 2018 | 29 June 2018 | 34 | 13 | 57 | 6 July 2018 |
| 38 | KP | 31 May 2018 | 29 June 2018 | 29 | 7 | 86 | 6 July 2018 |
| 41 | KP | 13 June 2018 | 22 June 2018 | 9 | 6 | 0 | 1 August 2018 |
| 45 | KP | 25 May 2018 | 29 June 2018 | 35 | 7 | 86 | 6 July 2018 |
| 8 | RBC | 8 June 2018 | 12 July 2018 | 34 | 1 | 86 | 13 July 2018 |
| 10 | RBC | 20 May 2018 | 29 June 2018 | 40 | 0 | 71 | 2 July 2018 |
| 11 | RBC | 4 June 2018 | 12 July 2018 | 38 | 0 | 100 | 12 July 2018 |
| 13 | RBC | 29 May 2018 | 6 July 2018 | 38 | 0 | 83 | 6 July 2018 |
| 15 | RBC | 21 May 2018 | 2 July 2018 | 42 | 0 | 100 | 3 July 2018 |
| 2 | MP | 31 May 2019 | 4 July 2019 | 34 | 7 | 43 | 11 July 2019 |
| 4 | MP | 16 May 2019 | 21 June 2019 | 36 | 7 | 86 | 28 June 2019 |
| 9 | MP | 30 May 2019 | 4 July 2019 | 35 | 8 | 88 | 11 July 2019 |
| 14 | MP | 1 June 2019 | 4 July 2019 | 33 | 13 | 17 | 11 July 2019 |
| 19 | MP | 22 May 2019 | 6 June 2019 | 15 | 7 | 0 | 13 June 2019 |
| 29 | MP | 20 May 2019 | 22 June 2019 | 33 | 7 | 50 | 28 June 2019 |
| 32 | MP | 17 May 2019 | 7 June 2019 | 21 | 7 | 0 | 13 June 2019 |
| 1 | KP | 1 June 2019 | 4 July 2019 | 33 | 7 | 71 | 11 July 2019 |
| 3 | KP | 24 June 2019 | 25 July 2019 | 31 | 7 | 20 | 25 July 2019 |
| 6 | KP | 16 May 2019 | 22 June 2019 | 37 | 7 | 67 | 28 June 2019 |
| 9 | KP | 28 June 2019 | 25 July 2019 | 27 | 4 | 25 | 29 July 2019 |
| 18 | KP | 16 May 2019 | 22 June 2019 | 37 | 7 | 70 | 28 June 2019 |
| 19 | KP | 6 June 2019 | 13 July 2019 | 37 | 7 | 89 | 19 July 2019 |
| 20 | KP | 24 May 2019 | 1 June 2019 | 8 | 7 | 0 | 6 June 2019 |
| 23 | KP | 27 May 2019 | 28 June 2019 | 32 | 7 | 63 | 4 July 2019 |
| 24 | KP | 27 May 2019 | 28 June 2019 | 32 | 7 | 0 | 4 July 2019 |
| 28 | KP | 16 May 2019 | 31 May 2019 | 15 | 13 | 0 | 13 June 2019 |
| 29 | KP | 15 May 2019 | 22 June 2019 | 38 | 7 | 88 | 28 June 2019 |
| 37 | KP | 5 June 2019 | 4 July 2019 | 29 | 7 | 43 | 4 July 2019 |
| 38 | KP | 6 June 2019 | 11 July 2019 | 35 | 7 | 100 | 11 July 2019 |
| 45 | KP | 16 May 2019 | 20 June 2019 | 35 | 8 | 100 | 28 June 2019 |
| 2 | RBC | 30 May 2019 | 8 July 2019 | 39 | 0 | 86 | 8 July 2019 |
| 4 | RBC | 3 June 2019 | 8 July 2019 | 35 | 0 | 71 | 8 July 2019 |
| 8 | RBC | 14 June 2019 | 17 July 2019 | 33 | 12 | 100 | 29 July 2019 |
| 9 | RBC | 16 June 2019 | 4 July 2019 | 18 | 4 | 56 | 8 July 2019 |
| 11 | RBC | 15 May 2019 | 28 May 2019 | 13 | 5 | 0 | 5 June 2019 |
| 12 | RBC | 16 June 2019 | 20 June 2019 | 4 | 11 | 0 | 1 July 2019 |
| 13 | RBC | 30 May 2019 | 1 July 2019 | 32 | 8 | 83 | 9 July 2019 |
| 14 | RBC | 30 May 2019 | 4 July 2019 | 35 | 1 | 67 | 9 July 2019 |
| 15 | RBC | 9 June 2019 | 14 July 2019 | 35 | 15 | 83 | 29 July 2019 |
| 2 | MP | 19 May 2020 | 23 June 2020 | 35 | 7 | 71 | 23 June 2020 |
| 4 | MP | 31 May 2020 | 30 June 2020 | 30 | 7 | 86 | 7 July 2020 |
| 12 | MP | 20 May 2020 | 23 June 2020 | 34 | 7 | 71 | 30 June 2020 |
| 17 | MP | 12 June 2020 | 14 July 2020 | 32 | 7 | 60 | 22 July 2020 |
| 18 | MP | 29 June 2020 | 30 June 2020 | 1 | 7 | 0 | 30 June 2020 |
| 19 | MP | 20 May 2020 | 23 June 2020 | 34 | 7 | 71 | 30 June 2020 |
| 25 | MP | 20 May 2020 | 23 June 2020 | 34 | 7 | 63 | 30 June 2020 |
| 28 | MP | 24 May 2020 | 23 June 2020 | 30 | 7 | 0 | 23 June 2020 |
| 31 | MP | 11 June 2020 | 14 July 2020 | 33 | 7 | 67 | 14 July 2020 |
| 33 | MP | 29 May 2020 | 17 June 2020 | 19 | 7 | 0 | 23 June 2020 |
| 1 | KP | 19 May 2020 | 23 June 2020 | 35 | 7 | 71 | 30 June 2020 |
| 3 | KP | 22 May 2020 | 23 June 2020 | 32 | 7 | 0 | 23 June 2020 |
| 8 | KP | 21 May 2020 | 23 June 2020 | 33 | 7 | 0 | 23 June 2020 |
| 9 | KP | 27 May 2020 | 23 June 2020 | 27 | 7 | 29 | 7 July 2020 |
| 14 | KP | 21 May 2020 | 30 June 2020 | 40 | 7 | 0 | 30 June 2020 |
| 19 | KP | 9 June 2020 | 7 July 2020 | 28 | 15 | 20 | 22 July 2020 |
| 23 | KP | 29 May 2020 | 14 July 2020 | 46 | 8 | 0 | 22 July 2020 |
| 24 | KP | 23 May 2020 | 23 June 2020 | 31 | 7 | 0 | 23 June 2020 |
| 28 | KP | 6 June 2020 | 8 July 2020 | 32 | 7 | 33 | 14 July 2020 |
| 29 | KP | 18 May 2020 | 23 June 2020 | 36 | 7 | 100 | 23 June 2020 |
| 41 | KP | 13 June 2020 | 22 July 2020 | 39 | 7 | 0 | 28 July 2020 |
| 43 | KP | 28 May 2020 | 1 July 2020 | 34 | 7 | 100 | 7 July 2020 |
| 2 | RBC | 23 May 2020 | 29 June 2020 | 37 | 7 | 86 | 30 June 2020 |
| 5 | RBC | 27 May 2020 | 30 June 2020 | 34 | 0 | 86 | 30 June 2020 |
| 6 | RBC | 28 May 2020 | 30 June 2020 | 33 | 3 | 71 | 3 July 2020 |
| 8 | RBC | 24 May 2020 | 29 June 2020 | 36 | 1 | 75 | 30 June 2020 |
| 10 | RBC | 28 May 2020 | 30 June 2020 | 33 | 3 | 83 | 3 July 2020 |
| 12 | RBC | 15 May 2020 | 22 June 2020 | 38 | 0 | 100 | 22 June 2020 |
| 13 | RBC | 23 May 2020 | 26 June 2020 | 34 | 0 | 100 | 26 June 2020 |
| 15 | RBC | 9 June 2020 | 14 July 2020 | 35 | 0 | 83 | 14 July 2020 |
| 4 | MP | 11 June 2021 | 12 July 2021 | 31 | 7 | 100 | 12 July 2021 |
| 5 | MP | 22 May 2021 | 1 June 2021 | 10 | 7 | 0 | 7 June 2021 |
| 10 | MP | 20 May 2021 | 14 June 2021 | 25 | 15 | 100 | 29 June 2021 |
| 14 | MP | 21 May 2021 | 23 June 2021 | 33 | 6 | 63 | 29 June 2021 |
| 18 | MP | 9 June 2021 | 12 July 2021 | 33 | 7 | 100 | 12 July 2021 |
| 21 | MP | 25 May 2021 | 29 June 2021 | 35 | 6 | 89 | 5 July 2021 |
| 25 | MP | 2 June 2021 | 14 June 2021 | 12 | 9 | 0 | 23 June 2021 |
| 28 | MP | 25 May 2021 | 29 June 2021 | 35 | 7 | 86 | 29 June 2021 |
| 30 | MP | 6 June 2021 | 29 June 2021 | 23 | 7 | 0 | 5 July 2021 |
| 32 | MP | 17 May 2021 | 17 May 2021 | 0 | 20 | 0 | 23 June 2021 |
| 3 | KP | 19 May 2021 | 23 June 2021 | 35 | 7 | 100 | 23 June 2021 |
| 8 | KP | 11 June 2021 | 12 July 2021 | 31 | 7 | 80 | 19 July 2021 |
| 9 | KP | 19 May 2021 | 23 June 2021 | 35 | 9 | 100 | 23 June 2021 |
| 14 | KP | 17 May 2021 | 23 June 2021 | 37 | 9 | 86 | 23 June 2021 |
| 19 | KP | 25 May 2021 | 29 June 2021 | 35 | 6 | 83 | 29 June 2021 |
| 20 | KP | 10 June 2021 | 12 July 2021 | 32 | 7 | 100 | 12 July 2021 |
| 23 | KP | 20 May 2021 | 23 June 2021 | 34 | 9 | 100 | 29 June 2021 |
| 28 | KP | 28 May 2021 | 29 June 2021 | 32 | 6 | 100 | 5 July 2021 |
| 29 | KP | 19 May 2021 | 23 June 2021 | 35 | 9 | 100 | 23 June 2021 |
| 31 | KP | 30 May 2021 | 1 July 2021 | 32 | 6 | 0 | 7 June 2021 |
| 43 | KP | 17 May 2021 | 17 May 2021 | 0 | 29 | 0 | 14 June 2021 |
| 45 | KP | 21 May 2021 | 1 July 2021 | 41 | 6 | 0 | 7 June 2021 |
| 1 | RBC | 18 May 2021 | 23 June 2021 | 36 | 1 | 86 | 24 June 2021 |
| 4 | RBC | 17 May 2021 | 24 June 2021 | 38 | 0 | 100 | 24 June 2021 |
| 8 | RBC | 1 June 2021 | 30 June 2021 | 29 | 4 | 83 | 2 July 2021 |
| 9 | RBC | 18 May 2021 | 24 June 2021 | 37 | 0 | 100 | 24 June 2021 |
| 11 | RBC | 10 June 2021 | 12 July 2021 | 32 | 3 | 75 | 14 July 2021 |
| 12 | RBC | 17 May 2021 | 21 June 2021 | 35 | 0 | 86 | 21 June 2021 |
| 15 | RBC | 18 May 2021 | 21 June 2021 | 34 | 3 | 67 | 21 June 2021 |
| 2 | RBC | 4 June 2022 | 7 July 2022 | 33 | 0 | 100 | 8 July 2022 |
| 8 | RBC | 29 May 2022 | 30 June 2021 | 32 | 3 | 83 | 4 July 2022 |
| 11 | RBC | 2 June 2022 | 5 July 2022 | 33 | 2 | 86 | 7 July 2022 |
| 12 | RBC | 5 June 2022 | 7 July 2022 | 32 | 1 | 100 | 11 July 2022 |
| 14 | RBC | 30 May 2022 | 4 July 2022 | 35 | 4 | 100 | 4 July 2022 |
References
- Shakhab, S.V. Oribatid mites (Oribatei, Acariformes) in nests of passerine birds. Entomol. Rev. 2006, 86, 173–176. [Google Scholar] [CrossRef]
- Coulson, S.J.; Moe, B.; Monson, F.; Gabrielsen, G.W. The invertebrate fauna of High Arctic seabird nests: The microarthropod community inhabiting nests on Spitsbergen, Svalbard. Polar Biol. 2009, 32, 1041–1046. [Google Scholar] [CrossRef]
- Makarova, O.L.; Osadtchy, A.V.; Melnikov, M.V. Gamasid Mites (Parasitiformes, Mesostigmata) in Nests of Passerine Birds on the Arctic Seven Islands Archipelago, the Barents Sea. Entomol. Rev. 2010, 90, 643–649. [Google Scholar] [CrossRef]
- Lebedeva, N.V.; Melekhina, E.N.; Gwiazdowicz, D.J. New data on soil mites in the nests of the Glaucous Gull Larus hyperboreus L. in the Svalbard archipelago. Bull. Southern Sci. Center RAS 2012, 8, 70–75. (In Russian) [Google Scholar]
- Makarova, O.L.; Rozenfeld, S.B. Gamasid Mites (Parasitiformes, Mesostigmata) Inhabiting Goose Nests on Kolguev Island, the Pechora Sea. In Complex Studies of the Nature of Spitsbergen and the Adjacent Shelf; Geos: Moscow, Russia, 2014; Issue 12, pp. 182–190. (In Russian) [Google Scholar]
- Melekhina, E.N.; Matyukhin, A.V.; Glazov, P.M. Oribatid mites in nests of the Lapland Bunting (Calcarius lapponicus) on the arctic island of Vaygach (with analysis of the islands fauna). Proc. Karelian Sci. Cent. Russ. Acad. Sci. 2019, 8, 108–122. (In Russian) [Google Scholar] [CrossRef]
- Napierała, A.; Maziarz, M.; Hebda, G.; Broughton, R.K.; Rutkowski, T.; Zacharyasiewicz, M.; Błoszyk, J. Lack of specialist nidicoles as a characteristic of mite assemblages inhabiting nests of the ground-nesting wood warbler, Phylloscopus sibilatrix (Aves: Passeriformes). Exp. Appl. Acarol. 2021, 84, 149–170. [Google Scholar] [CrossRef]
- Gwiazdowicz, D.J.; Niedbała, W.; Skarżyński, D.; Zawieja, B. Occurrence of mites (Acari) and springtails (Collembola) in bird nests on King George Island (South Shetland Islands, Antarctica). Polar Biol. 2022, 45, 1035–1044. [Google Scholar] [CrossRef]
- Lebedeva, N.V.; Krivolutsky, D.A. Birds Spread Soil Microarthropods to Arctic Islands. Doklady Biol. Sci. 2003, 391, 329–332. [Google Scholar] [CrossRef]
- Krivolutsky, D.A.; Lebedeva, N.V. Oribatid mites (Oribatei) in bird feathers. Part 2. Passeriformes. Acta Zool. Litu. 2004, 14, 19–38. [Google Scholar] [CrossRef]
- Krivolutsky, D.A.; Lebedeva, N.V.; Gavrilo, M.V. Soil microartrhropods in the feathers of Antarctic birds. Doklady Biol. Sci. 2004, 397, 342–345. [Google Scholar] [CrossRef]
- Pilskog, H.E.; Solhoy, T.; Gwiazdowicz, D.J.; Grytnes, J.-A.; Coulson, S.J. Invertebrate communities inhabiting nests of migrating passerine, wild fowl and sea birds breeding in the High Arctic, Svalbard. Polar Biol. 2014, 37, 981–998. [Google Scholar] [CrossRef]
- Lebedeva, N.V.; Melekhina, E.N.; Lebedev, V.D. Oribatid Mites in the Snow Bunting Habitats and Nests in the High Arctic. In Complex Studies of the Nature of Spitsbergen and the Adjacent Shelf; Geos: Moscow, Russia, 2014; Issue 12, pp. 162–168. (In Russian) [Google Scholar]
- Forests of the Komi Republic; Kozubov, G.M., Taskaev, A.I., Eds.; Publishing Centre Design. Information. Cartography: Moscow, Russia, 1999; p. 332. (In Russian) [Google Scholar]
- Atlas of the Komi Republic on Climate and Hydrology; Drofa: Moscow, Russia, 1997; p. 116. (In Russian)
- Vlasova, V.V.; Dronova, T.I.; Degteva, S.V.; Elsakov, V.V.; Zherebtsov, I.L.; Zainullin, V.G.; Zakharov, A.B.; Matsuk, M.A.; Sharapov, V.E.; Sazhina, S.A.; et al. Atlas of the Republic of Komi; Theoria: Moscow, Russia, 2011; p. 448. (In Russian) [Google Scholar]
- Stepanyan, L.S. Synopsis of the Ornithological Fauna of Russia and Adjacent Territories (within the Boundaries of the USSR as a Historical Region); Pavlov, D.S., Ed.; Akademkniga: Moscow, Russia, 2003; p. 808. (In Russian) [Google Scholar]
- Ryabitsev, V.K. Birds of the Urals, the Pre-Urals and Western Siberia: A Guide-Determinant; Ryabitsev, V.C., Ed.; Ural University Press: Yekaterinburg, Russia, 2008; p. 634. (In Russian) [Google Scholar]
- Teplova, E.N. Birds of the Pechoro-Ilychsky Reserve. In Proceedings of the Pechoro-Ilychsky Reserve; Komi Book Publishing House: Syktyvkar, Russia, 1957; Issue 6, pp. 5–115. (In Russian) [Google Scholar]
- Demetriades, K.K. The composition of the avifauna of the taiga of the Middle Timan. In Animal World of the Forest Zone of the European Part of the USSR; Kalinin State University: Kalinin, Russia, 1988; pp. 15–23. (In Russian) [Google Scholar]
- Kochanov, S.K. Anthropogenic and Geographical Factors in the Formation of the Avifauna of Large Cities of the European North-East of Russia; Abstract for the Degree of Candidate of Biological Sciences; Institute of Biology Komi Scientific Center of the Ural Branch of the RAS: Syktyvkar, Russia, 2000; p. 20. (In Russian) [Google Scholar]
- Neyfeld, N.D.; Teplov, V.V. Birds of the southeastern part of the Komi Republic. In Materials for the Distribution of Birds in the Urals, the Pre-Urals and Western Siberia; Ryabitsev, V.C., Ed.; Publishing house “Yekaterinburg”: Yekaterinburg, Russia, 2000; pp. 132–154. (In Russian) [Google Scholar]
- Sotnikov, V.N. Birds of the Kirov Region and Adjacent Territories. Passeriformes; Solovyov, A.N., Ed.; Triada Plus: Kirov, Russia, 2008; p. 423. (In Russian) [Google Scholar]
- Semenov, S.M. Materials on the nutrition of the Pied Flycatcher (Ficedula hypoleuca) during the nesting period. In Ways and Methods of Using Birds in the Fight against Harmful Insects; Publishing House of the Ministry of Agriculture of the RSFSR: Moscow, Russia, 1956; pp. 38–39. (In Russian) [Google Scholar]
- Bakkal, S.N. On the role of dipterous insects in the nutrition of Pied Flycatcher chicks Ficedula hypoleuca. Russ. Ornithol. J. 1997, 11, 3–9. (In Russian) [Google Scholar]
- Krivolutsky, D.A.; Lebren, F.; Kunst, M.; Akimov, I.A.; Bayartogtokh, B.; Vasiliu, N.; Golosova, L.D.; Grishina, L.G.; Karppinen, E.; Kramnoy, V.J.; et al. Oribatid Mites: Morphology, Development, Phylogeny, Ecology, Research Methods, and Characteristics of the Model Species Nothrus Palustris C.L. Koch, 1839; Nauka: Moscow, Russia, 1995; p. 223. (In Russian) [Google Scholar]
- Gilyarov, M.S. The Key to Identify Soil Mites. Sarcoptiformes; Nauka: Moscow, Russia, 1975; p. 488. (In Russian) [Google Scholar]
- Subías, L.S. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (excepto fósiles). Monogr. Electrónicas 2022, 12, 1–538. [Google Scholar]
- Karppinen, E.; Krivolutsky, D.A. List of oribatid mites (Acarina, Oribatei) of northern palaearctic region. 1. Europe. Acta Entomol. Fenn. 1982, 41, 1–18. [Google Scholar]
- Karppinen, E.; Krivolutsky, D.A.; Poltavskaja, M.P. List of oribatid mites (Acarina, Oribatei) of northern palaearctic region. III. Arid lands. Ann. Entomol. Fenn. 1986, 52, 81–94. [Google Scholar]
- Golosova, L.; Karppinen, E.; Krivolutsky, D.A. List of oribatid mites (Acarina, Oribatei) of northern palaearctic region. II. Siberia and the Far East. Acta Entomol. Fenn. 1983, 43, 1–14. [Google Scholar]
- Melekhina, E.N. Analysis of oribatid fauna of the eastern European tundra with first reported data from Subpolar Urals. Diversity 2020, 12, 235. [Google Scholar] [CrossRef]
- Melekhina, E.N.; Kanev, V.A.; Deneva, S.V. Karst Ecosystems of Middle Timan, Russia: Soils, Plant Communities, and Soil Oribatid Mites. Diversity 2022, 14, 718. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Melekhina, E.N. Oribatid Mites as Inhabitants of Lichens in the Taiga Zone of Northeastern Europe: Biotopic Association and Ecological Groups of Species. Biol. Bull. 2020, 47, 522–534. [Google Scholar] [CrossRef]
- Melekhina, E.N. Lichen-Associated Oribatid Mites in the Taiga Zone of Northeast European Russia: Taxonomical Composition and Geographical Distribution of Species. Diversity 2023, 15, 599. [Google Scholar] [CrossRef]
- Shakhab, S.V. The life forms of oribatids dwelling in bird nests. In Problems of Soil Zoology, Proceedings of the XVII National Conference on Soil Zoology, Syktyvkar, Russia, 22–26 September 2014; Striganova, B.R., Ed.; KMK: Moscow, Russia, 2014; pp. 251–252. (In Russian) [Google Scholar]
- Vysotskaya, S.O.; Shakhab, S.V.; Gushtan, G.G.; Kaprus, I.Y.; Roshko, V.G. Oribatid mites (Acari: Oribatida) of small mammal nests of Zakarpat’ye. ScienceRise 2015, 6, 22–30. [Google Scholar] [CrossRef]
- Shakhab, S.V. Oribatid mites (Acariformes, Oribatida) from the nests of some species of waterfowl and near-water birds of the Sea of Azov basin. In Terrestrial and Marine Ecosystems of the Black Sea Region and their Protection; Proceedings of the Collection of abstracts of the scientific-practical school-conference, Novorossiysk, Russia, April 23–27, 2018; Federal State Budgetary Scientific Institution “Institute of Natural and Technical Systems”: Sevastopol, Russia, 2018; pp. 166–167. (In Russian) [Google Scholar]
- Coulson, S.J.; Fjellberg, A.; Melekhina, E.N.; Taskaeva, A.A.; Lebedeva, N.V.; Belkina, O.A.; Seniczak, S.; Seniczak, A.; Gwiazdowicz, D.J. Microarthropod communities of industrially disturbed or imported soils in the High Arctic; the abandoned coal mining town of Pyramiden, Svalbard. Biodivers. Conserv. 2015, 24, 1671–1690. [Google Scholar] [CrossRef]


| Title 1 | Life Forms | Distribution | N | D% | F% |
|---|---|---|---|---|---|
| Trhypochthoniidae Willmann, 1931 Trhypochthonius tectorum s. str. (Berlese, 1896) | |||||
| hemiedaphic | Semi-cosmopolitan | 4 | 0.23 | 1.54 | |
| Crotoniidae Thorell, 1876 Heminothrus (Platynothrus) peltifer s. str. (Koch, 1839) | |||||
| hemiedaphic | Semi-cosmopolitan | 2 | 0.11 | 1.54 | |
| Damaeidae (Berlese, 1896) | |||||
| Damaeus (Epidamaeus) bituberculatus (Kulczynski, 1902) | epigeic | Palearctic | 30 | 1.7 | 9.23 |
| Liacaridae Sellnick, 1928 | |||||
| Adoristes (A.) ovatus (Koch, 1839) | epigeic | Holarctic | 3 | 0.17 | 1.54 |
| Oppiidae (Sellnick, 1937) | |||||
| Dissorhina ornata s. str. (Oudemans, 1900) | euedaphic | Holarctic | 31 | 1.77 | 6.15 |
| Graptoppia (Apograptoppia) foveolata (Paoli, 1908) | euedaphic | Holarctic | 17 | 0.96 | 4.61 |
| Suctobelbidae Jacot, 1938 | |||||
| Suctobelbella (S.) acutidens sarekensis (Forsslund, 1941) | euedaphic | Holarctic | 2 | 0.11 | 1.54 |
| Carabodidae Koch, 1843 | |||||
| Carabodes (C.) subarcticus Trägårdh, 1902 | epigeic | Palearctic | 8 | 0.45 | 3.08 |
| Tectocepheidae (Grandjean, 1954) | |||||
| Tectocepheus velatus (Michael, 1880) | eurybiontic | Cosmopolitan | 13 | 0.74 | 3.08 |
| Ameronothridae Vitzthum, 1943 | |||||
| Ameronothrus oblongus Sitnikova, 1975 | hydrobiontic | Holarctic | 1 | 0.06 | 1.54 |
| Ceratozetidae Jacot, 1925 | |||||
| Melanozetes mollicomus (Koch, 1839) | epigeic | Holarctic | 9 | 0.51 | 3.08 |
| Sphaerozetes piriformis (Nicolet, 1855) | epigeic | Palearctic | 3 | 0.17 | 1.54 |
| Trichoribates (T.) berlesei (Jacot, 1929) | epigeic | Holarctic | 4 | 0.23 | 4.61 |
| Chamobatidae Thor, 1937 | |||||
| Chamobates (C.) pusillus (Berlese, 1895) | epigeic | Holarctic | 7 | 0.40 | 4.61 |
| Humerobatidae Grandjean, 1971 | |||||
| Diapterobates oblongus (L. Koch, 1879) | epigeic | Palearctic | 9 | 0.51 | 6.15 |
| Diapterobates humeralis (Hermann, 1804) | epigeic | Holarctic | 7 | 0.40 | 3.08 |
| Oribatulidae Thor, 1929 | |||||
| Oribatula (Oribatula) tibialis (Nicolet, 1855) | eurybiontic | Holarctic | 21 | 1.19 | 7.69 |
| Oribatula (Zygoribatula) exilis (Nicolet, 1855) | eurybiontic | Holarctic | 76 | 4.31 | 9.23 |
| Oribatula (Z.) propinqua (Oudemans, 1902) | eurybiontic | Palearctic | 1006 | 57.09 | 41.53 |
| Scheloribatidae Grandjean, 1933 | |||||
| Scheloribates (S.) laevigatus (Koch, 1835) | eurybiontic | Semi-cosmopolitan | 487 | 27.64 | 30.77 |
| Parakalummidae Grandjean, 1936 | |||||
| Neoribates (N.) aurantiacus (Oudemans, 1914) | epigeic | Holarctic | 15 | 0.85 | 3.08 |
| Galumnidae Jacot, 1925 | |||||
| Pergalumna (P.) willmanni (Zachvatkin, 1953) | epigeic | Palearctic | 7 | 0.40 | 4.61 |
| Total | 1762 | 100 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melekhina, E.N.; Korolev, A.N.; Selivanova, N.P. Oribatid Mites (Oribatida) Associated with Nests of Hollow-Nesting Birds, on the Example of a Model Species, the European Pied Flycatcher (Ficedula hypoleuca), in the Taiga Forests of the European North-East of Russia. Diversity 2023, 15, 765. https://doi.org/10.3390/d15060765
Melekhina EN, Korolev AN, Selivanova NP. Oribatid Mites (Oribatida) Associated with Nests of Hollow-Nesting Birds, on the Example of a Model Species, the European Pied Flycatcher (Ficedula hypoleuca), in the Taiga Forests of the European North-East of Russia. Diversity. 2023; 15(6):765. https://doi.org/10.3390/d15060765
Chicago/Turabian StyleMelekhina, Elena N., Andrey N. Korolev, and Natalia P. Selivanova. 2023. "Oribatid Mites (Oribatida) Associated with Nests of Hollow-Nesting Birds, on the Example of a Model Species, the European Pied Flycatcher (Ficedula hypoleuca), in the Taiga Forests of the European North-East of Russia" Diversity 15, no. 6: 765. https://doi.org/10.3390/d15060765
APA StyleMelekhina, E. N., Korolev, A. N., & Selivanova, N. P. (2023). Oribatid Mites (Oribatida) Associated with Nests of Hollow-Nesting Birds, on the Example of a Model Species, the European Pied Flycatcher (Ficedula hypoleuca), in the Taiga Forests of the European North-East of Russia. Diversity, 15(6), 765. https://doi.org/10.3390/d15060765








