Abstract
Invasive non-indigenous crayfish species (NICS) are a major threat to the existence of native crayfish populations in European freshwater ecosystems. The discovery of signal crayfish Pacifastacus leniusculus, marbled crayfish Procambarus virginalis, and spiny-cheek crayfish Faxonius limosus in Estonia has increased the risk of extinction of Estonia’s only native crayfish species, the noble crayfish Astacus astacus. The aim of this study was to give an overview of the status, distribution, and impacts of P. leniusculus, F. limosus, and Procambarus virginalis on A. astacus populations and assess the effect of trapping on NICS abundance. Annual monitoring of crayfish has been carried out since 2008 as part of A. astacus conservation and the NICS eradication plan. In this study, we present data from nine sampling locations monitored from 2010 to 2022. The spread of NICS continues to increase beyond their distribution areas, and in two sampling locations P. leniusculus and A. astacus live in sympatry. Our results suggest that trapping has a limited effect on population abundance, as NICS have already caused the extinction of two A. astacus populations. However, intensive trapping should continue simultaneously with sensitive molecular techniques to monitor the spread of NICS.
1. Introduction
The noble crayfish Astacus astacus is the most valued and common freshwater crayfish species in northern Europe []. As the only native freshwater crayfish species in Estonia, A. astacus were reported to occur at varying densities in over 300 lakes, rivers, streams, and reservoirs across 15 Estonian counties []. More A. astacus localities and dense populations are present in Saaremaa Island and the south-eastern part of Estonia [], recording a catch per unit effort (CPUE) of above four [,]. Other regions have low population densities. For a long time, A. astacus populations have been facing the threat of over-exploitation, predation, habitat loss or alteration [,], crayfish plague [], and non-indigenous crayfish species (NICS) [], which have already been reported in most European countries. As of 2008, there was no known record of NICS in Estonia. Today, the once-abundant native crayfish species has suffered from long-term population decline due to introduced NICS of North American origin and crayfish plague and is, therefore, considered to be threatened. These NICS are often chronic carriers of the crayfish plague agent Aphanomyces astaci, and they have high resistance to the oomycete pathogen [,,]. Their introduction into the natural habitats of the indigenous crayfish has previously resulted in the transmission of crayfish plague, the most fatal disease to European indigenous crayfish species, leading to mass mortalities of the susceptible native species [,,,,].
From 2008 to 2022, three NICS, signal crayfish Pacifastacus leniusculus, marbled crayfish Procambarus virginalis, and spiny-cheek crayfish Faxonius limosus, were found in Estonia [,,]. The first report of the presence of P. leniusculus was in Mustjõgi River, Harju County, in 2008, the second one was in Riksu Stream on the island of Saaremaa, Saare County, in 2010, the third one was in Vääna River, Harju County, in 2012, and the fourth one was in Pärnu River, Pärnu County, in 2016 []. The spread of P. leniusculus, given its wide environmental tolerance [], has continued to threaten the existence of A. astacus, with crayfish plague causing the disappearance of the native crayfish from some Estonian waters. Procambarus virginalis were first found in 2017 in the Baltic Power Plant outflow channel in Narva Reservoir, Ida-Viru County, and their presence was confirmed in 2018 []. Procambarus virginalis is a parthenogenetic, all-female species [], which such as P. leniusculus, are highly adaptable to new habitats and harsh environmental conditions [,]. Therefore, their discovery in Estonia on top of the already existing invasive P. leniusculus populations increases the risk of invasion, thereby posing an additional threat not only to the native A. astacus population, but also to other indigenous communities in Estonian freshwater ecosystems. The threat of NICS to the native A. astacus populations in Estonia was further aggravated following the first sighting of F. limosus in Pärnu River in 2017 []. Some of the biological characteristics of F. limosus that support their population growth and invasive capabilities include rapid maturation, short lifespan, high fecundity, second mating period [], and ability to live and reproduce in brackish waters with a salinity of up to 7 ppt (parts per thousand) []. Even though it is considered to be of no commercial value, most people find it difficult to discriminate between F. limosus and A. astacus. This challenge, coupled with the biological advantages and migratory activity of F. limosus, appears to aid their continued spread.
NICS were possibly introduced into Estonia via the commercial aquarium trade [], illegal, human-assisted introductions, or accidental introduction by ballast water in ships, especially in Pärnu River [,]. Physical control by trapping is, so far, the only method that has been applied in Estonia to detect, monitor, and eradicate NICS, thereby safeguarding the native A. astacus population. This has been performed in line with the Estonian Nature Conservation Act and in keeping with Regulation (European Union) No 1143/2014 of the European Parliament and Council. Given the threat that these NICS pose to the native A. astacus population, continuous monitoring of their status and distribution remains essential. Advances in molecular research over the past decade have made it possible to improve the conservation of endangered aquatic species via continuous monitoring that allows the early detection of invasive species and pathogens [,,]. Trapping used simultaneously with molecular techniques, such as environmental DNA (eDNA) for monitoring, allow the detection of NICS, harmful pathogens, and native crayfish at low densities and at any season or life stage [,,].
In the last decade, most studies that assessed the distribution of NICS and their threat to the native crayfish in Europe using trapping focused largely on reporting the presence or absence of these alien invasive species, with some providing possible eradication plans. Grandjean and others [] indicated that in addition to A. astaci being the main threat, P. leniusculus was already widespread in France and is a serious danger to the native species. In Croatia, studies showed the spread of P. leniusculus in the rivers of the continental part of that country is the highest in Europe [,,,], and thus, they are probably the most established among the NICS in the region. The spread of P. leniusculus also seems to be the most monitored and reported [,], even though studies in nearby countries such as Latvia also confirmed the presence of F. limosus in their inland waters []. Kaldre and colleagues [,,] reported the first record of the establishment of P. leniusculus, F. limosus, and Procambarus virginalis and their involvement in a series of crayfish plague outbreaks in Estonia and provided suggestions for their control.
Within the context of assessing the spread and distribution of NICS using traps, a few additional studies have incorporated the effect of trappings on population abundance as part of the scope of their work. The few that did this or entirely aimed at evaluating traps as an effective control method for eradicating NICS arrived at different conclusions. On the one hand, intensive trapping when it is used either alone, with other fisheries management strategies [], or assessed indirectly along other objectives [,] was found to reduce the crayfish population density or abundance [,,]. On the other hand, the method had no negative effect on population growth, especially when it was performed continuously every month for a longer period []. Using a new method for estimating the size of red swamp crayfish (Procambarus clarkii) populations before and after year-long monthly trapping periods, Loureiro and others [] showed a high population growth rate. The disparity in findings from one study to another points to the need to periodically test the effectiveness of the control method in use.
Instances where indigenous and non-indigenous crayfish are living in sympatry and the native species remain less susceptible to crayfish plague have been reported as part of the findings of the status, spread, and distribution of NICS. In rivers where the coexistence of P. leniusculus and A. astacus occurs, it is difficult to say with certainty that the crayfish populations are free from crayfish plague because low levels of A. astaci are very hard to detect. Nonetheless, in habitats such as River Alling in Denmark, where sensitive molecular tests did not detect the presence of crayfish plague agent A. astaci in either of the species [], the threat of disease transmission is minor. However, trapping and eradicating NICS in these habitats still remains crucial to safeguard the future prospects of the native crayfish populations from possible competitive exclusion by alien invasive species [].
The aim of this study was to give an overview of the status, distribution, and impact of P. leniusculus, F. limosus, and Procambarus virginalis on A. astacus populations in Estonia, in addition to assessing the effect of trappings on NICS abundance.
2. Materials and Methods
2.1. Study Area
Sample collection was carried out in nine locations across five of the fifteen Estonian counties. These included Riksu Stream (with Riksu Lake and Koimla Ditch), Pärnu River with tributaries, Mustjõgi River, Vääna River, Reo Quarry, Ropka Water Reservoir, outflow channels of the Baltic and Estonian Power Plants (with Narva Water Reservoir and Narva River, respectively), which were grouped into two locations, and Narva Quarry, its outflow channel, and Metsküla Stream, which was recorded as one location (Figure 1, Table 1). Most NICS were found during the annual monitoring of A. astacus. After the initial discovery of NICS, annual monitoring was continued at these sites by the researchers from the chair of aquaculture of the Estonian University of Life Sciences between 2008 and 2022. However, since no NICS were caught in 2009, our study presents data from 2010 to 2022.
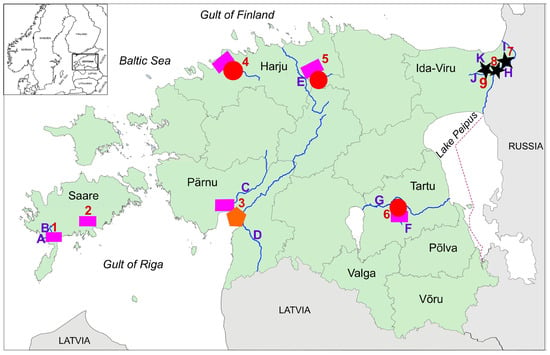
Figure 1.
Sampling locations and the distribution of NICS and Astacus astacus (noble crayfish) in Estonia. Pink squares indicate Pacifastacus leniusculus (signal crayfish), orange pentagons indicate Faxonius limosus (spiny-cheek crayfish), red circles indicate Astacus astacus (noble crayfish), and black stars indicate Procambarus virginalis (marbled crayfish).

Table 1.
Description of the sampled locations in Estonia. Location numbers and capital letters in brackets correspond to those in Figure 1.
2.2. Test Trapping of Crayfish
Test trappings were carried out according to the standard Swedish protocol []. In brief, cylindrical traps with two conical entrances and 9–15 mm mesh size (from knot to knot) were used. Test areas, which were predicted to be a suitable habitat for crayfish, was selected in each lake, river, or stream after screening longer stretches of the shoreline. Mostly frozen fish or sometimes fresh fish (caught from the same sampled water body), mainly roach (Rutilus rutilus) or bream (Abramis brama), was used as bait. The traps were applied as lines consisting mostly of 10–20 traps with an interval of 10 m and kept in the water overnight. Mostly, from three to ten lines were used depending on the size of the water body. Most of the trapped crayfish (NICS or A. astacus) were identified at the species level, counted, measured (total length (TL)), and weighed (precision of 0.5 g). The trapped NICS were frozen (killed) and sent for incineration, while A. astacus were released back to where they had been caught. There were separate traps in Reo Quarry and Ropka Water Reservoir, but in the other locations, the traps were used multiple times and were dried prior to use at another site as a means of disinfection to prevent the potential spread of crayfish plague. Test trappings were carried out from April to November (more frequently from July to September) each year, and a special license required for this activity was issued by the Ministry of Environment. The number of trap nights (number of traps per night) differed from one sampling location to another one across the trapping period or years (Table 2). In 2022, the number of trap nights were increased at all sampling locations to increase the NICS eradication efforts. In locations such as Reo Quarry and Ropka Water Reservoir the traps were constantly in the water and emptied around two times per week in 2022. For every trapping session, the catch per unit effort (CPUE), which is the number of crayfish caught per trap per trapping night at each site, was calculated and recorded. At the same time, the CPUE max was also determined and recorded. Since test trappings were carried out in different sections at different times in the distribution area, CPUE max denotes the most abundant catch in the most abundant crayfish area. CPUE is a commonly used measure of relative fishing efficiency [] and was applied in this study to estimate the relative abundance of NICS or A. astacus within each sampling location. The classification of Tulonen and colleagues [,] was applied to describe the population density of crayfish based on the CPUE results. Based on this classification, a CPUE above 4 was estimated as a high density, a value between 1 and 4 was moderate, and a value below 1 was low, indicating only the presence of the crayfish population []. The effect of traps on NICS abundance was estimated and assessed on the basis of CPUE max recorded as part of the above-described methodology.
2.3. Data Analysis
The map was made using ArcMap in the ArcGIS 10.1 (Environmental Systems Research Institute, Inc., Esri, CA, USA, 2012) program package. Statistical analyses were performed using R statistical software version 4.2.1 (The R Foundation for Statistical Computing: Vienna, Austria, 2022) []. As the sample size was relatively small and, according to the QQ (quantile–quantile) plot and Shapiro–Wilk normality test, the data did not follow normal distributions. A one-sided Wilcoxon signed-rank test was used to compare the mean difference between the NICS and A. astacus population within the same sampling location.
3. Results and Discussion
The continuous monitoring of NICS in Estonia is crucial for determining their status, distribution, and threat to A. astacus, and the adoption of effective control and eradication measures such as intensive trapping, biocidal and biological control is also essential [,]. In this study, we present the results of crayfish monitoring over a 13-year period (2010–2022). In each of the nine sampling locations (Figure 1, Table 1), the case of crayfish distribution is presented separately.
3.1. Riksu Stream
Since their first discovery in Riksu Stream, the population abundance of P. leniusculus has fluctuated between low (CPUE < 1), moderate (1 ≤ CPUE ≤ 4), and high (CPUE > 4) across different years (Table 3). The population density of P. leniusculus increased from low to moderate between 2013 and 2014, and it rose to high in 2018. Since then, the abundance has fluctuated between high and moderate (Table 3). The fluctuations reported in P. leniusculus abundance are attributed to the stream characteristics, such as the water regime that affects the numbers of crayfish caught. Considering the seasonal variations of water levels in Riksu Stream, with some sections remaining dry in drought seasons, catches from test trappings varied were based on water volumes and time of the catch. This may explain the moderate-to-high abundance in Riksu Stream and in Koimla Ditch and the low abundance in Lake Riksu, which is a sediment-rich lake [], and thus, it not such a suitable habitat for crayfish [].

Table 3.
The estimated crayfish population abundance per year within each sampling location based on capture per unit effort (CPUE) values. All comparisons were made against Astacus astacus (noble crayfish).
Despite the fluctuations in abundance, P. leniusculus seem to be spreading continuously every year and at an increasing pace, given their detection in Riksu Lake and Koimla Ditch (Figure 1). As P. leniusculus spread further upstream, A. astacus disappeared from those areas. The population density of A. astacus in Riksu Stream remained low on the occasions reported, and in 2014–2022, none were caught from this location (Table 3). Statistical analysis further demonstrated that the density of the P. leniusculus population was significantly higher than that of A. astacus (Table 3). The increasing spread of P. leniusculus indicates that the habitat conditions of Riksu Stream are advantageous for their population growth. In addition, there is no direct competitive pressure and no obstacles for P. leniusculus to hinder their spread in both directions (either downstream, via Riksu Lake towards the sea, or upstream) []. It is very likely that the growing population of P. leniusculus in Riksu Stream was responsible for the disappearance of the A. astacus population through competition, with crayfish plague giving P. leniusculus a competitive edge. Through stable isotope analysis, Olsson and colleagues [] showed that P. leniusculus and native A. astacus in a set of Swedish streams had similar niche widths at the population level, and thus, seem to use resources (i.e., food items and habitats) in a similar manner. Westman and Savolainen [] demonstrated earlier that at sites with small populations of A. astacus and P. leniusculus, competitive exclusion along with other interaction mechanisms led to the collapse of A. astacus population. While we may not have recently carried out tests to detect A. astaci (the last test was conducted in 2016) in this P. leniusculus population, it is possible that crayfish plague may have contributed to the extinction of A. astacus in Riksu stream. Kaldre and colleagues [] indicated that crayfish plague was detected for the first time on the island of Saaremaa in 2006. They later confirmed the occurrence of A. astaci pathogen in Riksu Stream in 2011 after conducting crayfish plague studies using experimental cages with A. astacus [].
The fluctuating pattern of P. leniusculus population density in Riksu Stream was also observed when we were assessing the effect of trappings on NICS abundance. Our data show that the relative abundance (CPUE max) of P. leniusculus fluctuated throughout the sampling period (Figure 2a). It is possible that the reductions of the CPUE max of P. leniusculus during certain years between 2012 and 2022 may have been a result of trapping pressure. At the same time, the increases in relative abundance of the population can be attributed to the growth of the juvenile individuals. Ercoli and others [] showed that in Italian streams, the diets of adult and juvenile P. leniusculus between the summer and autumn seasons were the same. Therefore, the selective removal of the adult P. leniusculus population from the habitat may have, in turn, spurred the growth of juveniles. Overall, based on our results, it may be difficult to determine if trapping is causing a reduction of the population abundance of P. leniusculus in Riksu Stream. It has been shown before that changes in water temperature [] and seasonal variations in stream water levels [,] can cause fluctuations in the P. leniusculus population density. Perhaps undertaking test trapping by simultaneously using cylindrical traps and fine-mesh nets may, in the future, provide sufficient data to determine the overall effect of trapping on P. leniusculus population density in this seasonal stream. García-de-Lomas [] showed, in their study, that using both cylindrical traps and horizontal hauls of fine-mesh netting had higher selectivity and efficiency for catching the smaller red swamp crayfish than traps alone did. Equally, the CPUE max of A. astacus in Riksu Stream decreased from 7.5 to zero between 2010 and 2014 (Figure 2a). In 2010 and 2011, 19 and 7 A. astacus were caught, respectively, from the upper part of Riksu stream. In 2013, 14 A. astacus were caught from under Riksu bridge; presumably, someone stocked them there given the location. Nonetheless, the CPUE max of A. astacus remaining zero in 2014 suggests their disappearance following the increased spread of P. leniusculus (Figure 2a).
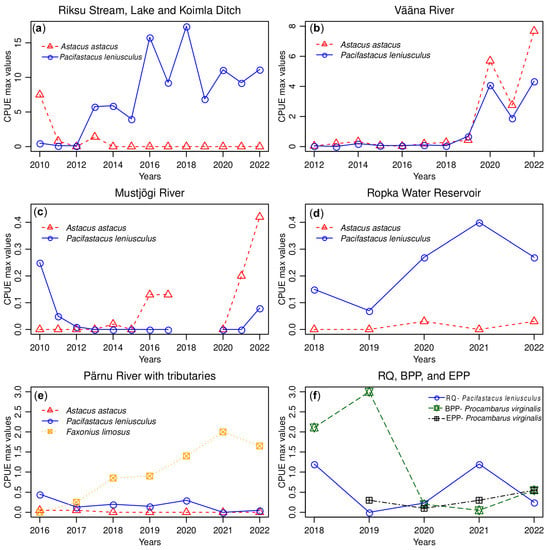
Figure 2.
Changes in relative crayfish abundance (CPUE max) of NICS and Astacus astacus (noble crayfish) or NICS during the years at different sampling locations. (a) CPUE max in Riksu Stream; (b) CPUE max in Vääna River; (c) CPUE max in Mustjõgi River; (d) CPUE max in Ropka Water Reservoir; (e) CPUE max in Pärnu River; (f) CPUE max in Reo Quarry (RQ), Baltic Power Plant outlet (BPP), and Estonian Power Plant outlet (EPP).
3.2. Vääna River
According to the Vääna River sampling data, the population density of A. astacus and P. leniusculus remained low (between <0.1 and 0.7) for eight years, and then increased to moderate in 2020 and 2022 for A. astacus and P. leniusculus, respectively, (Table 3). Statistical analysis showed that the difference in abundance between the two species was not significant (Table 3). Irrespective of the moderate population abundances of A. astacus and P. leniusculus in 2022, our results indicate only the presence of few crayfish individuals (CPUE < 1) for almost a decade. The moderate crayfish abundances in Vääna River might be explained by the rivers’ characteristics (e.g., there are a few hiding places and a high sediment content), which makes it a less suitable habitat for crayfish. Nonetheless, with the moderate population density, the distribution of both species has expanded up- and downstream from the initial site of the river. The situation in this river is unique because P. leniusculus live in sympatry with A. astacus in a small area. At the population level, P. leniusculus and A. astacus have been shown to have the same niche widths []. Since most individuals use the same resources, such as food and shelter, in the same way in a given habitat, the niche width at the population level basically reflects the available resources []. Additionally, the population density is usually determined by the availability of shelters []. Therefore, it is possible that the coexistence of P. leniusculus and A. astacus in Vääna River has been supported by reduced competition for food and shelter. However, based on our previous analysis of a few individuals of A. astacus that were caught alive in 2016, which showed weak positive results for A. astaci detection (K. Kaldre’s unpublished data), we equally suspect that the sympatry in Vääna River was made possible by the presence of the low prevalence of the A. astaci pathogen. Schrimpf and others [] demonstrated, in their study, that a consistent absence or a very low prevalence of A. astaci appears to enable permanent coexistence between A. astacus and F. limosus or P. leniusculus. Additionally, to claim that the threat of crayfish plague is insignificant, the use of more sensitive molecular methods are essential []. Furthermore, this will also provide additional evidence that all NICS of North American origin may not be carriers of A. astaci []. Since the pathogen prevalence varies widely among P. leniusculus populations [], crayfish plague studies employing modern molecular methods will help in identifying the virulent and less virulent strains of A. astaci, which are vital for undertaking a risk assessment for native A. astacus populations.
Our results on the effect of trapping on NICS abundance in this river show that the CPUE max of both species remained generally low (below one) from 2012 to 2018 (Figure 2b). In 2020, the CPUE max of A. astacus increased to 5.7, and then fell to 2.8 in 2021, while that of P. leniusculus increased to 4.1 and fell to 1.9 during the same period (Figure 2b). The decrease was subsequently followed by an increase in the CPUE max of both species in 2021 (Figure 2b). In 2021, test trapping was conducted only in October, which probably influenced the reduction of the CPUE max and gave rise to the perception of a population decline and an immediate increase afterwards, which most likely is the result of fewer traps being used at night and might not reflect the real situation in the water body. Based on the CPUE max results, it is possible that prior to 2019, the continuous removal of adult P. leniusculus was helping to reduce the predatory pressure and minimise interspecific competition, which initially may have slowed down P. leniusculus population establishment [,], and later (after 2019), resulted in population growth. The increase in the CPUE max of both species after 2019 may also be a consequence of reduced intraspecific competition, which has been shown to result in population growth []. Most studies have shown that, in the long run, the likely outcome of the co-existence between indigenous crayfish species and NICS is always the competitive exclusion of native crayfish [,,,]. However, when competition between two species for territory, food, and hiding places is not strong, the continuous removal of P. leniusculus is necessary for the survival of A. astacus.
3.3. Mustjõgi River
The abundance of P. leniusculus and A. astacus in Mustjõgi River has remained low (CPUE < 1), with no significant difference between the two species (Table 3). Before 2022, only a few crayfish individuals of both species were caught in separate traps; however, since 2022, they started to appear together. The two species coexist in the middle section (about 2 km) of Mustjõgi River. It is possible that this coexistence is supported by reduced competition for food and habitat, since P. leniusculus has been shown to have greater feeding plasticity than A. astacus does, despite both species having similar niche widths at the population level []. Furthermore, the distribution area of both species has expanded, and the 2022 catches of P. leniusculus show their spread 1–2 km upstream from the previous location. Previous reports on the crayfish trappings between 1994 and 2009 revealed that until the mid-1990s, A. astacus were still present in Mustjõgi River []. However, from 2008, following P. leniusculus detection, to 2014, the presence of A. astacus had still not been detected in this river (Table 3). We suspect that the disappearance of A. astacus from Mustjõgi River was caused by the competitive P. leniusculus, which are carriers of A. astaci. Jussila and Edsman [] showed that P. leniusculus could be more competitive than A. astacus can, and usually, they are a carrier of crayfish plague agent A. astaci, which gives it a competitive advantage because it might not die from the crayfish plague infection. The presence of A. astaci in Mustjõgi River was confirmed in 2009 by carrying out the cage experiments (full trial period in 2009–2013) []. However, we believe that the one A. astacus caught in 2014 (CPUE < 0.1) was probably from the 2013 cage experiment.
Between 2015 and 2016, a total of 1000 A. astacus were stocked in the Mustjõgi River in an effort to restore their populations. Our results on the effect of trapping on NICS abundance show that the CPUE max of P. leniusculus and A. astacus in Mustjõgi River remained low, between <0.1 and 0.4, within the survey period (Figure 2c). Considering that in 2016, 2017, and 2021, five, four, and four A. astacus were caught, respectively, and that in 2022, both species were caught further upstream, it is possible that A. astacus individuals caught may be from the restocking event. Overall, the numbers of A. astacus and P. leniusculus individuals caught from Mustjõgi River over the survey period were very low, and low CPUE values indicate the presence of a poorly established population. Therefore, an informative interpretation of the relative abundance of both species in this river can only be made after additional trappings in the future. Nonetheless, these results show the coexistence of A. astacus and P. leniusculus in Mustjõgi River. Initially, we believed that from 2013 to 2020, the recreational fishing ban and control of NICS via test trapping had resulted in the successful elimination of the few P. leniusculus individuals from this river. However, our results showed that P. leniusculus are still present in Mustjõgi River. Studies on P. leniusculus’ range of expansion in Croatia have shown their ability to completely displace narrow-clawed crayfish (Astacus leptodactylus) from sites of overlapping populations []. Considering the positive results of restocking in Mustjõgi River, we suggest continuing with this practice to increase the number of A. astacus individuals and continue with P. leniusculus monitoring and eradication.
3.4. Ropka Water Reservoir
In Ropka Water Reservoir, the population densities of A. astacus and P. leniusculus, which co-inhabit the same site, remained low, between <0.1 and 0.4, from 2018 to 2022 (Table 3), indicating the presence of a few crayfish individuals. Statistical analysis also showed that the difference in abundance between P. leniusculus and A. astacus was significant (Table 3). The entire lake can be regarded as a suitable habitat for crayfish, even though P. leniusculus were mostly caught from the northern shore. The abundance of A. astacus in Ropka Water Reservoir before the discovery of P. leniusculus is unknown because no crayfish monitoring has been conducted in this water body. However, A. astacus have been reported to occur in the river immediately downstream of Ropka Water Reservoir and in areas further downstream. The impact of P. leniusculus on the A. astacus population is not clear due to a low number (three) of A. astacus being trapped during the survey period, indicating a poorly established population. Furthermore, differences in the frequency of checking the traps that were constantly in the water possibly resulted in the low reported CPUE values (between <0.1 and 0.4). The size and the artificial nature of the Ropka Water Reservoir and the low number of P. leniusculus (CPUE < 1) make it a good location for biocidal control. However, when one is using this eradication method, it is important to be aware of the negative impacts on non-target biota, in addition to the potential for crayfish surviving in burrows or roaming to other sites []. As an alternative, biological control measures (e.g., predatory native fish species) can be used in this water body.
3.5. Pärnu River
The abundance of A. astacus, P. leniusculus, and F. limosus in Pärnu River has remained low (between <0.1 and 0.5) during seven years of sampling (Table 3). Despite the low abundance, A. astacus and P. leniusculus were found together in 2016, but from 2018 to 2022, no A. astacus were found within the sampling location (Table 3). The three species co-inhabited Pärnu River only in 2017 (Table 3). The numbers of P. leniusculus and F. limosus individuals have remained significantly higher than those of A. astacus (Table 3). In Pärnu River, F. limosus are much more widespread than P. leniusculus are. They have been found upstream and in areas closer to the mouths of the Sauga River and Reiu River (locations C and D on the map, respectively). A. astacus have also been reported to occur further upstream of Pärnu River and its tributaries (outside of our sampling area), where NICS have not yet spread.
Pärnu River is unique because during the different sampling years, A. astacus, P. leniusculus, and F. limosus lived in sympatry in different sections of the river (Table 3, Figure 2). The occurrence of A. astacus in this river before the discovery of P. leniusculus is unknown; thus, it is possible that A. astacus were either absent or rarely present in very low numbers. However, their presence was confirmed after the detection of P. leniusculus in 2016 []. The co-existence of these three species, i.e., A. astacus, P. leniusculus, and F. limosus, in the Pärnu River in the same year probably led to the subsequent decline and disappearance of A. astacus through competitive exclusion. A. astacus has been shown as being incapable of withstanding competition for food, territory, and hiding places from the two invasive species, P. leniusculus and F. limosus, even in waters that may be free of A. astaci []. Kaldre and others [] screened tissue samples of six F. limosus specimens from Pärnu River in 2017, but they did not detect the presence of A. astaci. Given the small sample size that was analysed for crayfish plague and the size of the Pärnu River system, we cannot completely rule out the crayfish plague as one of the possible causes of A. astacus extinction from this river. We suggest the continued monitoring and trapping of NICS as a control measure, as well as screening for the pathogenic agent A. astaci to effectively understand and assess the threats to A. astacus.
Regarding the effect of trapping, the CPUE max of F. limosus in Pärnu River rose steadily throughout the sampling period (Figure 2e). It is possible that the increase in F. limosus population abundance was a result of a feedback mechanism [], where the crayfish perceived a reduction of their numbers, and in response, invested in reproductive strategies []. The steady increase in the CPUE max of F. limosus during the survey period may also indicate that despite the effect of the trapping pressure, the time frame between the annual continuous test trappings was sufficient for the untrapped population to adapt and grow. Furthermore, their increased spread in Pärnu River also indicates their ability to live and reproduce in brackish waters [] and adapt to Estonian climatic conditions. It may also mean that F. limosus are slowly dominating P. leniusculus [], as the CPUE max of P. leniusculus remained low (between 0.1 and 0.5) during the sampling period (Figure 2e).
3.6. Reo Quarry
The population density of P. leniusculus in Reo Quarry remained between low and moderate (CPUE 0.2 and 1.2) between 2018 and 2022 (Table 3). Despite continuous monitoring, no A. astacus were caught in this location. The entire lake can be regarded as a suitable habitat for crayfish, even though most P. leniusculus were caught on the northern side. The CPUE max of P. leniusculus in this location remained low, between 0.2 and 1.2, throughout the sampling period (Figure 2f), indicating the presence of only a few P. leniusculus individuals. It is possible that these CPUE values were affected by the frequency of checking the traps, which were constantly kept in the water and checked twice per week. Nonetheless, the pressure from test trapping has not been effective in eradicating P. leniusculus from this water body. Considering these findings, we suggest that, besides trapping, additional eradication measures such as biocidal and biological control could be considered to limit their spread to other locations.
3.7. Estonian and Baltic Power Plant Outflow Channels
In the Baltic Power Plant (BPP) outflow channel (and Narva Water Reservoir) and the Estonian Power Plant (EPP) outflow channel (and the Narva River), the population density of Procambarus virginalis remained low, between <0.1 and 0.9, during the sampling period (Table 3). The Narva Quarry (with outlet and Metsküla Stream) had an equally low abundance (CPUE ≤ 0.1) of Procambarus virginalis and A. astacus during the two years that sampling was conducted (Table 3). Even though these low CPUE values of Procambarus virginalis indicate a poorly established population (or presence of few crayfish individuals), it is vital to keep monitoring these water bodies, as Procambarus virginalis reproduces through parthenogenesis [,], and a single individual is sufficient to establish a new population [,]. Therefore, the detection of even one Procambarus virginalis individual may imply a serious threat to the native crayfish. The occurrence of A. astacus before the discovery of Procambarus virginalis is unknown in the power plants’ outflow channels.
The cooling water that is used by the Estonian and Baltic Power Plants (EPP and BPP) has been reported to raise the temperature of the surface water by an average of 7 °C []. The optimal thermal range of Procambarus virginalis has been shown to be between 18 °C and 25 °C [], and they can survive and reproduce in low winter temperatures between 5.1 °C and 9.5 °C []. Therefore, it is possible that the warmer temperatures in the BPP and EPP outflow channels provide a suitable habitat for the growth and reproduction of Procambarus virginalis. However, occasionally during the summer period, the temperatures can reach to extreme levels, i.e., above 30 °C (we measured and recorded temperatures as high as 34.5 °C and 32.8 °C in the BPP and EPP outflow channels, respectively, in the summer of 2020), which may be fatal to Procambarus virginalis, lowering their population densities.
In 2020, members of Wildlife Estonia (a non-governmental organisation) caught twelve Procambarus virginalis and four A. astacus individuals in the Narva Quarry and outlet using ten crayfish traps. Our data later confirmed the co-existence of these two species during the 2021 test trappings conducted in the sampling location. While it may be possible that, in 2022, a few A. astacus individuals may have already been displaced by Procambarus virginalis in this location, continuous trapping remains crucial in eradicating NICS. The CPUE max of Procambarus virginalis in BPP increased from two to three between 2018 and 2019, followed by a decrease to less than one during the subsequent sampling period (Figure 2f) possibly due to trapping pressure. In EPP, the CPUE max of Procambarus virginalis remained low, between 0.1 and 0.6, throughout the sampling period (Figure 2f).
3.8. General Observations
When the results are examined all together as a single case, this study demonstrates that regardless of the NICS population dynamics in each sampling location, physical control by trapping has a limited effect on population abundance and has limitations as a monitoring tool. Our results indicate that trapping does not eradicate them, but it slows down the spread and increase of the population densities (or individual numbers) of P. leniusculus, F. limosus, and Procambarus virginalis. The CPUE max of NICS (apart from that of F. limosus in Pärnu River) slightly varied during the sampling period, possibly as a result of trapping pressure or season of test trapping. Considering the mesh size of the traps used, and the missing juvenile crayfish in traps, it seems that trapping acted more as a size selector [,]. Furthermore, it is possible that the increase in abundance was a result of the speedy growth of the younger, untrapped individuals. These observations imply that trapping only detected species (NICS and A. astacus) that were already well established in the water body. However, early detection during the monitoring process is critical for the effective control and eradication of invasive NICS and the conservation of the endangered A. astacus species [,]. We suggest the adoption of molecular techniques such as eDNA to supplement trapping in the monitoring of NICS in Estonia.
4. Conclusions
From 2008 to 2022, Pacifastacus leniusculus, Procambarus virginalis, and Faxonius limosus detected in Estonia spread beyond their earlier detection or distribution areas. The population dynamics of these species within each sampling location remain different, with slight changes in abundance, and in three locations, P. leniusculus and A. astacus coexist. The population densities of P. leniusculus and Procambarus virginalis have remained low, with slight variations over the years, while that of F. limosus slowly rose. The impact of NICS on A. astacus has been devastating since they have already caused the extinction of two A. astacus populations, despite the complexity in the case of Mustjõgi River. Our findings suggest that, as a monitoring tool, trapping has limitations, and as a control and eradication method, it has only a little effect on the population abundance of NICS. For the successful conservation of A. astacus and the effective control and eradication of NICS, intensive trapping should continue simultaneously with sensitive molecular techniques to monitor the spread of the invasive species. Future research on the distribution of NICS and A. astacus populations using both tools will improve the conservation efforts of the only native crayfish species in Estonia.
Author Contributions
Conceptualization, K.K.; methodology, K.K., M.H. and M.O.A.; investigation, M.H. and K.K.; writing—original draft preparation, M.O.A.; writing—review and editing, M.O.A., K.K., L.P. and M.H.; visualization, M.O.A., K.K., L.P. and M.H.; supervision, K.K. and L.P.; funding acquisition, K.K. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Climate Change Mitigation and Adaptation Programme financed by the European Economic Area Financial Mechanism and the Environmental Investment Centre project “Eradication of aquatic invasive species in Estonian freshwaters” number 4-17/16674.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are publicly unavailable due to privacy reasons, but can be provided upon request.
Acknowledgments
The authors express their gratitude to Mati Kivistik, Albert Hurt, Jaanus Tuusti, Härmo Hiiemäe, Taigor Veevo, and Fabio Ercoli, who participated in test fishing.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript or in the decision to publish the results.
References
- Gross, R.; Palm, S.; Kõiv, K.; Prestegaard, T.; Jussila, J.; Paaver, T.; Geist, J.; Kokko, H.; Karjalainen, A.; Edsman, L. Microsatellite Markers Reveal Clear Geographic Structuring among Threatened Noble Crayfish (Astacus astacus) Populations in Northern and Central Europe. Conserv. Genet. 2013, 14, 809–821. [Google Scholar] [CrossRef]
- Hurt, M.; Kivistik, M. Tegevuskava Rakendamine Jõevähi Varude Kasutamiseks Ja Kaitseks 2021.a.—Keskkonnaameti Tellitud (Riigihanke Viitenumber 236237) Ja Eesti Maaülikooli Teostatud Projekti Aruanne.107 Lk. 2022. Available online: https://envir.ee/media/4550/download (accessed on 7 February 2023).
- Tulonen, J.; Erkamo, E.; Järvenpää, T.; Westman, K.; Savolainen, R.; Mannonen, A. Rapuvedet Tuottaviksi; Riistan-ja kalantutkimuslaitos: Helsinki, Finland, 1998; 152p. [Google Scholar]
- Tulonen, J.; Erkamo, E.; Jussila, J.; Mannonen, A. The Effects of Minimum Size Regulations and Exploitation on Population Dynamics of Noble Crayfish (Astacus astacus (Linnaeus)) in a Small Lake in Central Finland: A Seven Year Study. Freshw. Crayfish 2008, 16, 7–14. [Google Scholar]
- Tuusti, J.; Taugbøl, T.; Skurdal, J.; Kukk, L. Freshwater Crayfish in Estonia. I: Action Plan for Crayfish Management. II: Crayfish Status Report; Østlandsforskning: Oppland, Finland, 1998. [Google Scholar]
- Reynolds, J.D. A Review of Ecological Interactions between Crayfish and Fish, Indigenous and Introduced. Knowl. Manag. Aquat. Ecosyst. 2011, 401, 10p1–10p21. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Holdich, D.M.; Noel, P.Y.; Reynolds, J.D.; Haffner, P. Atlas of Crayfish in Europe; Muséum National d´Histoire naturelle: Paris, France, 2006; pp. 60–123. [Google Scholar]
- Holdich, D.M.; Reynolds, J.D.; Souty-Grosset, C.; Sibley, P.J. A Review of the Ever Increasing Threat to European Crayfish from Non-Indigenous Crayfish Species. Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 11p1–11p46. [Google Scholar] [CrossRef]
- Diéguez-Uribeondo, J.; Söderhäll, K. Procambarus clarkii Girard as a Vector for the Crayfish Plague Fungus, Aphanomyces astaci Schikora. Aquac. Res. 2008, 24, 761–765. [Google Scholar] [CrossRef]
- Kozubíková, E.; Viljamaa-Dirks, S.; Heinikainen, S.; Petrusek, A. Spiny-Cheek Crayfish Orconectes limosus Carry a Novel Genotype of the Crayfish Plague Pathogen Aphanomyces astaci. J. Invertebr. Pathol. 2011, 108, 214–216. [Google Scholar] [CrossRef]
- Keller, N.S.; Pfeiffer, M.; Roessink, I.; Schulz, R.; Schrimpf, A. First Evidence of Crayfish Plague Agent in Populations of the Marbled Crayfish (Procambarus fallax Forma virginalis). Knowl. Manag. Aquat. Ecosyst. 2014, 414, 15p1–15p8. [Google Scholar]
- Alderman, D.J. Crayfish Plague in Britain, the First Twelve Years. Freshw. Crayfish 1993, 9, 266–272. [Google Scholar]
- Alderman, D.J. History of the Spread of Crayfish Plague in Europe, in Crustaceans: Bacterial and Fungal Diseases. QIE Sci. Technol. Rev. 1997, 15, 15–23. [Google Scholar]
- Taugbøl, T.; Skurdal, J.; Håstein, T. Crayfish Plague and Management Strategies in Norway. Biol. Conserv. 1993, 63, 75–82. [Google Scholar] [CrossRef]
- Diéguez-Uribeondo, J.; Temiño, C.; Mùzquiz, J.L. The Crayfish Plague Fungus (Aphanomyces astaci) in Spain. Bull. Fr. Pêche Piscic. 1997, 347, 753–763. [Google Scholar] [CrossRef]
- Mirimin, L.; Brady, D.; Gammell, M.; Lally, H.; Minto, C.; Graham, C.T.; Slattery, O.; Cheslett, D.; Morrissey, T.; Reynolds, J.; et al. Investigation of the First Recent Crayfish Plague Outbreak in Ireland and Its Subsequent Spread in the Bruskey River and Surrounding Areas. Knowl. Manag. Aquat. Ecosyst. 2022, 423, 13. [Google Scholar] [CrossRef]
- Kaldre, K.; Paaver, T.; Hurt, M.; Grandjean, F. First Records of the Non-Indigenous Signal Crayfish (Pacifastacus leniusculus) and Its Threat to Noble Crayfish (Astacus astacus) Populations in Estonia. Biol. Invasions 2017, 19, 2771–2776. [Google Scholar] [CrossRef]
- Ercoli, F.; Kaldre, K.; Paaver, T.; Gross, R. First Record of an Established Marbled Crayfish Procambarus virginalis (Lyko, 2017) Population in Estonia. Bioinvasions Rec. 2019, 8, 675–683. [Google Scholar] [CrossRef]
- Kaldre, K.; Paaver, T.; Hurt, M.; Gross, R. Continuing Expansion of Non-Indigenous Crayfish Species in Northern Europe: First Established Spiny-Cheek Crayfish Faxonius limosus (Refinesque, 1817) Population in Estonia. BioInvasions Rec. 2020, 9, 127–132. [Google Scholar] [CrossRef]
- Martin, P.; Kohlmann, K.; Scholtz, G. The Parthenogenetic Marmorkrebs (Marbled Crayfish) Produces Genetically Uniform Offspring. Naturwissenschaften 2007, 94, 843–846. [Google Scholar] [CrossRef] [PubMed]
- Kaldre, K.; Meženin, A.; Paaver, T.; Kawai, T. A Preliminary Study on the Tolerance of Marble Crayfish Procambarus fallax f. virginalis to Low Temperature in Nordic Climate. In Freshwater Crayfish: A Global Overview; Taylor & Francis: Boca Raton, FL, USA, 2015; pp. 54–62. [Google Scholar]
- Lipták, B.; Veselý, L.; Ercoli, F.; Bláha, M.; Buřič, M.; Ruokonen, T.; Kouba, A. Trophic Role of Marbled Crayfish in a Lentic Freshwater Ecosystem. Aquat. Invasions 2019, 14, 299–309. [Google Scholar] [CrossRef]
- Kozák, P.; Buřič, M.; Policar, T. The Fecundity, Time of Egg Development and Juvenile Production in Spiny-Cheek Crayfish (Orconectes limosus) under Controlled Conditions. Bull. Fr. Pêche Piscic. 2006, 380–381, 1171–1182. [Google Scholar] [CrossRef]
- Jaszczołt, J.; Szaniawska, A. The Spiny-Cheek Crayfish Orconectes limosus (Rafinesque, 1817) as an Inhabitant of the Baltic Sea—Experimental Evidences for Its Invasion of Brackish Waters. Oceanol. Hydrobiol. Stud. 2011, 40, 52–60. [Google Scholar] [CrossRef]
- Bohmann, K.; Evans, A.; Gilbert, M.T.P.; Carvalho, G.R.; Creer, S.; Knapp, M.; Yu, D.W.; de Bruyn, M. Environmental DNA for Wildlife Biology and Biodiversity Monitoring. Trends Ecol. Evol. 2014, 29, 358–367. [Google Scholar] [CrossRef]
- Strand, D.A.; Jussila, J.; Johnsen, S.I.; Viljamaa-Dirks, S.; Edsman, L.; Wiik-Nielsen, J.; Viljugrein, H.; Engdahl, F.; Vrålstad, T. Detection of Crayfish Plague Spores in Large Freshwater Systems. J. Appl. Ecol. 2014, 51, 544–553. [Google Scholar] [CrossRef]
- Dougherty, M.M.; Larson, E.R.; Renshaw, M.A.; Gantz, C.A.; Egan, S.P.; Erickson, D.M.; Lodge, D.M. Environmental DNA (EDNA) Detects the Invasive Rusty Crayfish Orconectes rusticus at Low Abundances. J. Appl. Ecol. 2016, 53, 722–732. [Google Scholar] [CrossRef]
- Wittwer, C.; Stoll, S.; Strand, D.; Vrålstad, T.; Nowak, C.; Thines, M. EDNA-Based Crayfish Plague Monitoring Is Superior to Conventional Trap-Based Assessments in Year-Round Detection Probability. Hydrobiologia 2018, 807, 87–97. [Google Scholar]
- Strand, D.A.; Johnsen, S.I.; Rusch, J.C.; Agersnap, S.; Larsen, W.B.; Knudsen, S.W.; Møller, P.R.; Vrålstad, T. Monitoring a Norwegian freshwater crayfish tragedy: eDNA snapshots of invasion, infection and extinction. J. Appl. Ecol. 2019, 56, 1661–1673. [Google Scholar] [CrossRef]
- Rusch, J.C.; Mojžišová, M.; Strand, D.A.; Svobodová, J.; Vrålstad, T.; Petrusek, A. Simultaneous Detection of Native and Invasive Crayfish and Aphanomyces astaci from Environmental DNA Samples in a Wide Range of Habitats in Central Europe. NeoBiota 2020, 58, 1–32. [Google Scholar] [CrossRef]
- Grandjean, F.; Roques, J.; Delaunay, C.; Petrusek, E.; Becking, T.; Collas, M. Status of Pacifastacus leniusculus and Its Role in Recent Crayfish Plague Outbreaks in France: Improving Distribution and Crayfish Plague Infection Patterns. Aquat. Invasions 2017, 12, 541–549. [Google Scholar] [CrossRef]
- Dragičević, P.; Faller, M.; Kutlesa, P.; Hudina, S. Update on the Signal Crayfish, Pacifastacus leniusculus (Dana, 1852) Range Expansion in Croatia: A 10-Year Report. BioInvasions Rec. 2020, 9, 793–807. [Google Scholar] [CrossRef]
- Hudina, S.; Žganec, K.; Lucić, A.; Trgovčić, K.; Maguire, I. Recent Invasion of the Karstic River Systems in Croatia through Illegal Introductions of the Signal Crayfish. Freshw. Crayfish 2013, 19, 21–27. [Google Scholar] [CrossRef]
- Hudina, S.; Kutleša, P.; Trgovčić, K. Duplić Dynamics of Range Expansion of the Signal Crayfish (Pacifastacus leniusculus) in a Recently Invaded Region in Croatia. Aquat. Invasions 2017, 12, 67–75. [Google Scholar] [CrossRef]
- Maguire, I.; Jelić, M.; Klobučar, G. Update on the Distribution of Freshwater Crayfish in Croatia. Knowl. Manag. Aquat. Ecosyst. 2011, 401, 31p1–31p10. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Skóra, M.E.; Raczyński, M.; Szaniawska, A. The Signal Crayfish Pacifastacus leniusculus—Distribution and Invasion in the Southern Baltic Coastal River. Pol. J. Ecol. 2017, 65, 445–452. [Google Scholar]
- Weinländer, M.; Füreder, L. The Continuing Spread of Pacifastacus leniusculus in Carinthia (Austria). Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 17p1–17p10. [Google Scholar]
- Birzaks, J.; Skute, A. Alien Crayfish Species in Latvian Inland Waters. Environ. Exp. Biol. 2019, 17, 21–25. [Google Scholar]
- Hein, C.L.; Vander Zanden, M.J.; Magnuson, J.J. Intensive Trapping and Increased Fish Predation Cause Massive Population Decline of an Invasive Crayfish. Freshw. Biol. 2007, 52, 1134–1146. [Google Scholar] [CrossRef]
- Moorhouse, T.P.; Poole, A.E.; Evans, L.C.; Bradley, D.C.; Macdonald, D.W. Intensive Removal of Signal Crayfish (Pacifastacus leniusculus) from Rivers Increases Numbers and Taxon Richness of Macroinvertebrate Species. Ecol. Evol. 2014, 4, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Moorhouse, T.P.; Macdonald, D.W. The Effect of Manual Removal on Movement Distances in Populations of Signal Crayfish (Pacifastacus leniusculus). Freshw. Biol. 2011, 56, 2370–2377. [Google Scholar] [CrossRef]
- Stancliffe-Vaughan, A.E. Sampling UK Pacifastacus leniusculus (Dana, 1852): The Effect of Trapping on Population Structure. Master’s Thesis, Anglia Ruskin University, Cambridge, UK, 2015. [Google Scholar]
- Loureiro, T.G.; Anastácio, P.M.; Bueno, S.L.d.S.; Araujo, P.B. Management of Invasive Populations of the Freshwater Crayfish Procambarus clarkii (Decapoda, Cambaridae): Test of a Population-Control Method and Proposal of a Standard Monitoring Approach. Environ. Monit. Assess. 2018, 190, 559. [Google Scholar] [CrossRef]
- Skov, C.; Aarestrup, K.; Sivebæk, F.; Pedersen, S.; Vrålstad, T.; Berg, S. Non-Indigenous Signal Crayfish Pacifastacus leniusculus Are Now Common in Danish Streams: Preliminary Status for National Distribution and Protective Actions. Biol. Invasions 2011, 13, 1269–1274. [Google Scholar] [CrossRef]
- Hudina, S.; Hock, K.; Radović, A.; Klobučar, G.; Petković, J.; Jelić, M.; Maguire, I. Species-Specific Differences in Dynamics of Agonistic Interactions May Contribute to the Competitive Advantage of the Invasive Signal Crayfish (Pacifastacus leniusculus) over the Native Narrow-Clawed Crayfish (Astacus leptodactylus). Mar. Freshw. Behav. Physiol. 2016, 49, 147–157. [Google Scholar] [CrossRef]
- Estonian Nature Information System Display of Water Body Data. Available online: https://eelis.ee/default.aspx?state=15;572247461;est;eelisand;;&comp=objresult=veekogu&obj_id=268239962 (accessed on 31 January 2023).
- Edsman, L.; Söderbäck, B. Standardised Sampling Methodology for Crayfish—The Swedish Protocol. Freshw. Crayfish 1999, 12, 705–713. [Google Scholar]
- Cheng, Z.; Folkins, M.H.; Huang, L.; Li, Y. Comparing Catch Efficiency of Crayfish (Procambarus clarkii) Traps with Different Entrance Numbers. J. Mar. Sci. Eng. 2022, 10, 1708. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 7 February 2023).
- Manfrin, C.; Souty-Grosset, C.; Anastácio, P.M.; Reynolds, J.; Giulianini, P.G. Detection and Control of Invasive Freshwater Crayfish: From Traditional to Innovative Methods. Diversity 2019, 11, 5. [Google Scholar] [CrossRef]
- Hudina, S.; Maguire, I.; Dragičević, P.; Galic, N. Evaluating the Efficacy of Approaches to Control Invasive Populations: A Conceptual Model Development for the Signal Crayfish. Ecologies 2022, 3, 78–95. [Google Scholar] [CrossRef]
- Eesti Energia Environmental Report. Available online: https://www.energia.ee/c/document_library/get_file?uuid=e704c22c-c190-469b-9e3e-f2a9bf95b777&groupId=10187 (accessed on 3 February 2023).
- Olsson, K.; Stenroth, P.; Nyström, P.; Granéli, W. Invasions and Niche Width: Does Niche Width of an Introduced Crayfish Differ from a Native Crayfish? Freshw. Biol. 2009, 54, 1731–1740. [Google Scholar] [CrossRef]
- Westman, K.; Savolainen, R. Long Term Study of Competition between Two Co-Occurring Crayfish Species, the Native Astacus astacus L. and the Introduced Pacifastacus leniusculus Dana, in a Finnish Lake. Bull. Fr. Pêche Piscic. 2001, 361, 613–627. [Google Scholar] [CrossRef]
- Ercoli, F.; Ghia, D.; Gruppuso, L.; Fea, G.; Bo, T.; Ruokonen, T.J. Diet and Trophic Niche of the Invasive Signal Crayfish in the First Invaded Italian Stream Ecosystem. Sci. Rep. 2021, 11, 8704. [Google Scholar] [CrossRef]
- Johnson, M.F.; Rice, S.P.; Reid, I. The Activity of Signal Crayfish (Pacifastacus leniusculus) in Relation to Thermal and Hydraulic Dynamics of an Alluvial Stream, UK. Hydrobiologia 2014, 724, 41–54. [Google Scholar] [CrossRef]
- Mathers, K.L.; White, J.C.; Fornaroli, R.; Chadd, R. Flow Regimes Control the Establishment of Invasive Crayfish and Alter Their Effects on Lotic Macroinvertebrate Communities. J. Appl. Ecol. 2020, 57, 886–902. [Google Scholar] [CrossRef]
- Larson, C.E.; Bo, T.; Candiotto, A.; Fenoglio, S.; Doretto, A. Predicting Invasive Signal Crayfish (Pacifastacus leniusculus) Spread Using a Traditional Survey and River Network Simulation. River Res. Appl. 2022, 38, 1424–1435. [Google Scholar] [CrossRef]
- García-de-Lomas, J.; Dana, E.D.; González, R. Traps and Netting, Better Together than Alone: An Innovative Approach to Improve Procambarus clarkii Management. Knowl. Manag. Aquat. Ecosyst. 2020, 421, 39. [Google Scholar] [CrossRef]
- Nyström, P.; Stenroth, P.; Holmqvist, N.; Berglund, O.; Larsson, P.; Granéli, W. Crayfish in Lakes and Streams: Individual and Population Responses to Predation, Productivity and Substratum Availability. Freshw. Biol. 2006, 51, 2096–2113. [Google Scholar] [CrossRef]
- Schrimpf, A.; Maiwald, T.; Vrålstad, T.; Schulz, H.K.; Śmietana, P.; Schulz, R. Absence of the Crayfish Plague Pathogen (Aphanomyces astaci) Facilitates Coexistence of European and American Crayfish in Central Europe. Freshw. Biol. 2013, 58, 1116–1125. [Google Scholar] [CrossRef]
- James, J.; Nutbeam-Tuffs, S.; Cable, J.; Mrugała, A.; Viñuela-Rodriguez, N.; Petrusek, A.; Oidtmann, B. The Prevalence of Aphanomyces astaci in Invasive Signal Crayfish from the UK and Implications for Native Crayfish Conservation. Parasitology 2017, 144, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Vanni, M.J.; Duncan, J.M.; González, M.J.; Horgan, M.J. Competition Among Aquatic Organisms. In Encyclopedia of Inland Waters; Likens, G.E., Ed.; Academic Press: Oxford, UK, 2009; pp. 395–404. [Google Scholar]
- Söderbäck, B. Interactions among Juveniles of Two Freshwater Crayfish Species and a Predatory Fish. Oecologia 1994, 100, 229–235. [Google Scholar] [CrossRef]
- Schulz, H.; Śmietana, P.; Maiwald, T.; Oidtmann, B.; Schulz, R. Case Studies on the Co-Occurrence of Astacus astacus (L.) and Orconectes limosus (Raf.): Snapshots of a Slow Displacement. Freshw. Crayfish 2006, 15, 212–219. [Google Scholar]
- Paaver, T.; Hurt, M. Status and Management of Noble Crayfish Astacus astacus in Estonia. Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 18. [Google Scholar] [CrossRef]
- Jussila, J.; Edsman, L. Relaxed Attitude towards Spreading of Alien Crayfish Species Affects Protection of Native Crayfish Species: Case Studies and Lessons Learnt from a Fennoscandian Viewpoint. Freshw. Crayfish 2020, 25, 39–46. [Google Scholar] [CrossRef]
- Krzywosz, T. Evaluation of Changes in the Abundance of Three Catchable Crayfish Species in Lake Pobłędzie (Northern Poland). Fish. Aquat. Life 2006, 14, 131–140. [Google Scholar]
- Gherardi, F.; Aquiloni, L.; Diéguez-Uribeondo, J.; Tricarico, E. Managing Invasive Crayfish: Is There a Hope? Aquat. Sci. 2011, 73, 185–200. [Google Scholar] [CrossRef]
- Jimenez, S.A.; Faulkes, Z. Establishment and Care of a Colony of Parthenogenetic Marbled Crayfish, Marmorkrebs. Invertebr. Rearing 2010, 1, 10–18. [Google Scholar]
- Linzmaier, S.M.; Goebel, L.S.; Ruland, F.; Jeschke, J.M. Behavioral Differences in an Over-Invasion Scenario: Marbled vs. Spiny-Cheek Crayfish. Ecosphere 2018, 9, e02385. [Google Scholar] [CrossRef]
- Vogt, G. The Marbled Crayfish: A New Model Organism for Research on Development, Epigenetics and Evolutionary Biology. J. Zool. 2008, 276, 1–13. [Google Scholar] [CrossRef]
- Sibley, P.; Noel, P. Control and Management of Alien Crayfish. Bull. Fr. Pêche Piscic. 2002, 367, 881–886. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).