1. Introduction
Recent research on litter arthropods in some areas of Central and South America revealed the important role of weevils within the litter community of tropical forests, both in terms of their abundance, species richness and rate of endemism, and also as a possible indicator taxon of the biodiversity of tropical forests [
1]. The weevils, along with the rove beetles, are the two most species-rich beetle taxa and are numerically dominant within the litter community of tropical forests [
1], thus constituting a key component in the biodiversity of these ecosystems and providing ecosystem services as decomposers of organic matter [
2]. The communities of litter weevils are therefore useful to determine forest health and to evaluate the effect of forest management or damage to forest ecosystems [
3] (and references therein).
Litter weevils can be considered good indicators of soil diversity in tropical montane cloud forests (TMCFs herein) as they reflect variations in species richness and community composition more accurately than other taxa that are not as well represented or are insufficiently diverse within these habitats [
1]. They meet criteria that are used in the selection of litter bioindicator organisms [
4], mainly that they are normally quite frequent in the targeted area, allowing collection of enough data for their analysis, and are usually characterised by low mobility and inability to fly, thus being closely tied to very circumscribed habitats. The litter weevils of TMCFs include numerous species that have a range of different ecological needs, making it possible to assess the causes of the presence or absence of species in the various survey areas and to recognise taxa indicators of specific coenoses. Importantly, the sampling techniques are simple, reproducible and can be easily standardised. Litter weevils are therefore suited for comparative studies between different sites.
TMCFs are one of the most threatened ecosystems in the world [
5,
6]. They globally occupy less than 380,000 square kilometres, which corresponds to 0.26% of the world’s landmass. Of these cloud forests, 25.3% are found in tropical America, where, however, they account for less than 1.2% of the forested surface [
7]. These forests are found in the tropical belt in mountainous areas, and are characterised by an almost continuous presence of clouds and fog, as moisture-laden air from the nearby oceans ascends towards the mountain ranges and, while cooling, it reaches the condensation point. TMCFs are extremely important sites of plant and animal diversity, with a high rate of endemism [
8] (and references therein). In Ecuador, in the Cordillera Occidental (in which this research was conducted) the TMCFs are found in an elevation range between 1300 and 3000 m, most of them being located below 2600 m a.s.l. [
9].
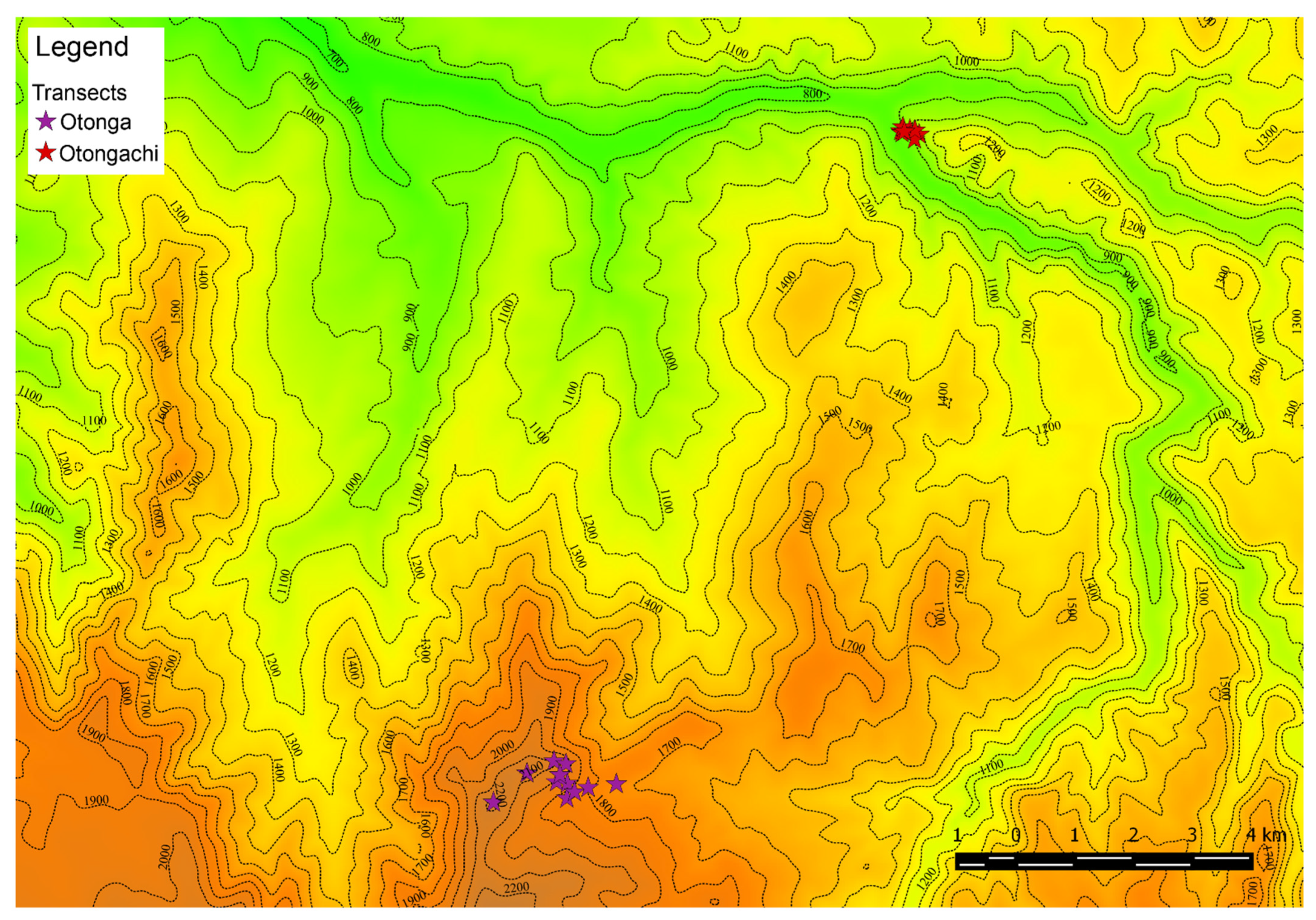
The purpose of our study was to gather semi-quantitative information on the litter weevil fauna of two Integral Reserves in Ecuador, Otonga and Otongachi. Particular effort was made to evaluate the composition of the litter weevil communities of the Otonga TMCF, selecting plots at various elevation levels and comparing patches in primary and secondary forest. We aimed at recognizing the degree of fidelity of each species to the type of forest and the elevation level, while also considering the response of this fauna to several other habitat variables. Samplings were also carried out in the degraded Otongachi secondary forest. In this case, other than comparing the weevil communities of the two reserves, we attempted to evaluate the utility of litter weevils as bioindicators of habitats with a high degree of naturalness as well as habitats severely impacted by anthropic activities. Changes in vegetation (determined by felling of forest areas, fires and other human activities) result in variation in the structure of the litter [
10], which in turn influences, usually negatively, the composition of litter communities [
11]. The different levels of impact given by disturbance on vegetation have been assessed through an approach that included soil arthropods as indicators of environmental quality [
11,
12].
Current knowledge on the litter weevils of the Otonga region.
Only few, scattered data are available, but no complete study of the Otonga weevil fauna has ever been attempted.
Voisin [
13,
14,
15,
16,
17,
18] described seven new species of Molytinae: Anchonini (
sensu Voisin, 2013). Four of them belong to the genus
Baillytes Voisin, 1992:
B. propinquus Voisin, 1993,
B. loricatus Voisin, 1996,
B. bartolozzii Voisin, 1996 and
B. rotundicollis Voisin, 1996. The other three species belong to three different genera, also new and simultaneously described by the author:
Boudinotes Voisin, 2000, with
B. franciscanus Voisin, 2000,
Capsonotus Voisin, 2007, with
C. smilodon Voisin, 2007 and
Mallanchonus Voisin, 2009, with
M. lalisae Voisin, 2009. All these species were recollected by one of the authors (C.B.) in more or less deep litter in TMCF.
Following the work of a few individual researchers from 1997 to 2002, but especially the research organised by the World Biodiversity Association (WBA) in 2004 and 2006, Bellò and Osella [
19] described two new species of Cossoninae belonging to the genus
Howdeniola Osella, 1980. One of these,
H. margheritae Bellò & Osella, 2008 was found to be widespread in forest litter from 1500 to 2200 m in the Otonga TMCF.
Finally, Baviera, Bellò and Osella [
20], from the material collected predominantly in the expedition organised by the WBA named “Ecuador 2008”, reported for the first time the presence in Ecuador of the genus
Bordoniola Osella, 1987, attributed to the subfamily Raymondionyminae, and described five new species, among which was
B. otongana Baviera, Bellò & Osella, 2012, whose typical locality is the Otonga Integral Reserve, at 2000 m.
These data evidence that the knowledge of the Curculionoidea of Otonga is limited to only very few species, and the vast majority of the weevils native to the region are still undescribed.
3. Results
3.1. Habitat Variables
The habitat variables collected at each of the five points along each transect were merged and led to the following results (
Supplementary Material Table S1).
The rotten_log index shows more transects with index = 1 in Otonga (8 plots out of 11) than in Otongachi (2 plots out of 8). There is no substantial difference between the two types of forest in Otonga: in the primary forest the plots with rotten_log index = 1 are 4 out of 6, while in the secondary forest they are 4 out of 5.
The moss_index shows a slightly higher number of plots with index = 1 in Otonga (5 plots out of 11, all in primary forest) compared to Otongachi (3 plots out of 8). There are no plots with moss_index = 1 in the secondary forest of Otonga.
Litter is generally less humid in Otonga (dryness_index = 3 for 8 plots out of 11) than in Otongachi (dryness_index = 3 for 1 plot out of 8), where more plots with a low dryness_index were found (dryness_index = 1 for 5 plots out of 8). No substantial differences were seen between primary and secondary forests in Otonga.
The comparison of canopy coverage from the plots in Otonga and Otongachi primary and secondary forests shows no particular differences, with mean coverage ranging from 56.3% in Otonga primary forest (56.6% in secondary forest) to 59% in Otongachi.
3.2. Species Richness
A total of 510 specimens of Curculionoidea, identified as belonging to 100 different morphospecies, included in 24 different morphogenera, were collected (
Supplementary Material Table S2). The species considered as typical of the soil litter share several functional traits. All of them are apterous, usually small, microphthalmic or even anophthalmic, and most of them have integument with broad setae, tubercles and granules. Even though functional traits of weevils associated with forest litter have never been evaluated, this combination of characters is often found in these taxa, in tropical as well as temperate forests (M.M., unpublished data). Very few winged, macrophthalmic species were sampled, and these were excluded from the analyses, since they were considered to be “tourists”, casual findings of species not normally associated with the habitat investigated. If added to the analyses, they could interfere with the results. In the 11 plots of Otonga, 393 specimens belonging to 85 morphospecies were collected, while 117 specimens belonging to 15 morphospecies were collected in the eight plots of Otongachi. The majority of species were Molytinae: Anchonini; other taxa belonged to Raymondionyminae, and in particular to the genus
Bordoniola Osella, 1987. However, we did not attempt at any formal identification, which may be carried out later.
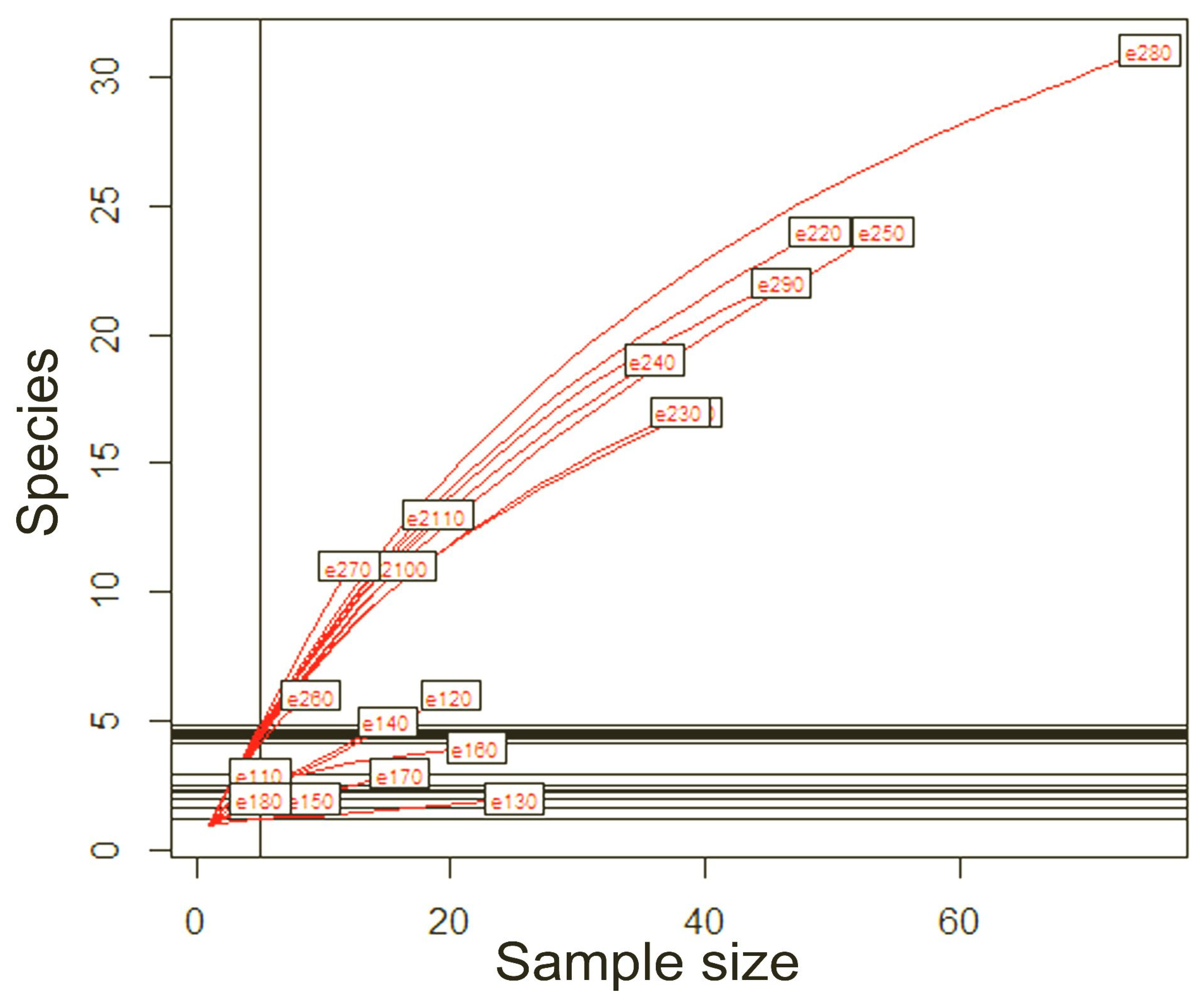
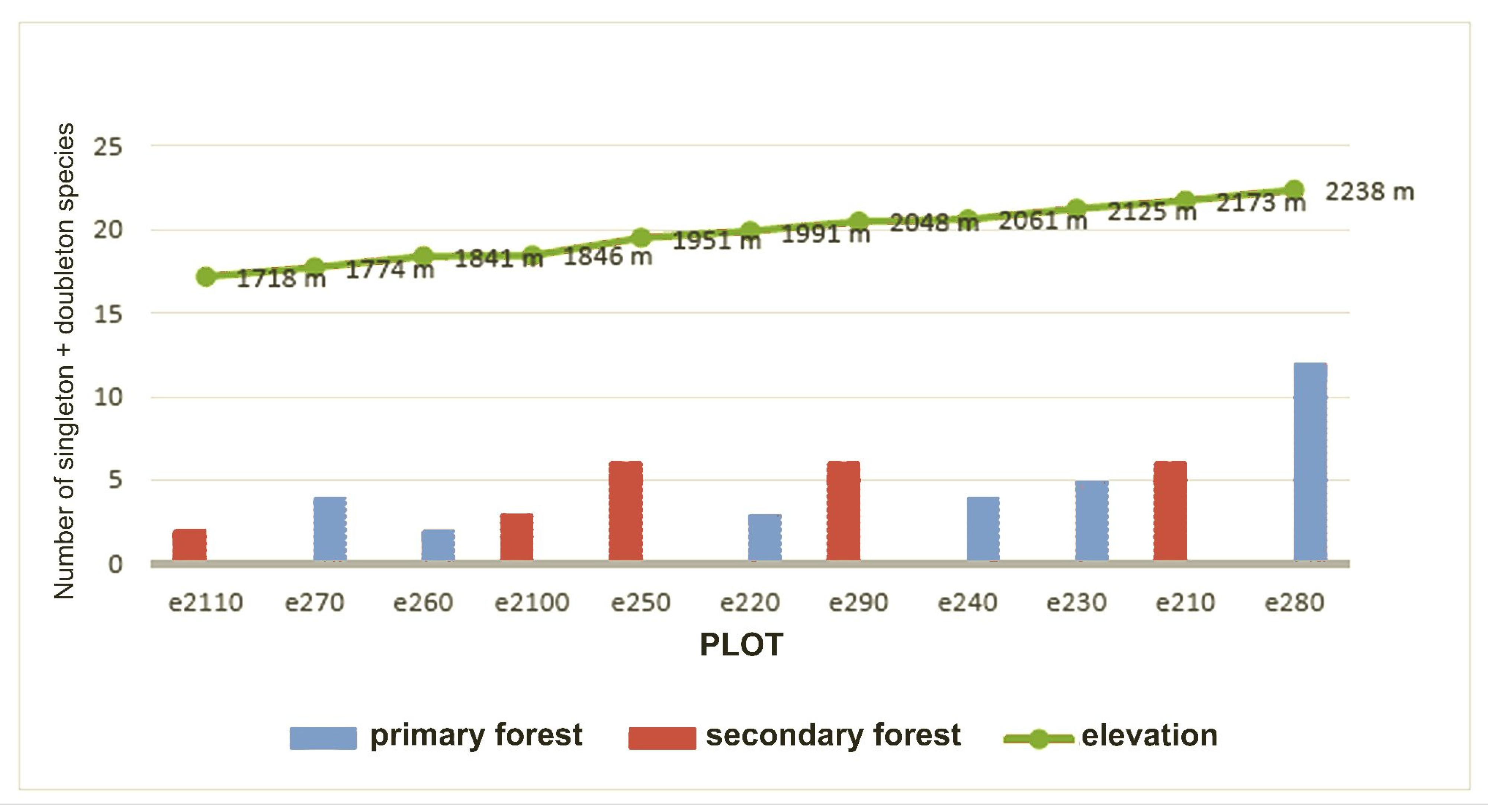
Rarefaction curves were calculated for all transects monitored and then represented on a graph correlating sample size and morphospecies richness per sampling station. Looking at the graph, it can be seen that the entire estimated community was never sampled, as no curve reaches the asymptote (growth close to zero as the number of specimens sampled increases). A markedly different pattern in the development of the rarefaction curve can be recognised for the plots in Otonga. These have faster growing curves and show a higher mean richness of species per plot, even in the case of relatively small sample sizes, than the plots in Otongachi. In particular, the Otonga plot “e270” has the fastest growing rarefaction curve. The only exception for Otonga is plot “e260”, which shows a rarefaction curve that is intermediate between those of Otonga and those of Otongachi (
Figure 3).
The estimated species richness was calculated for each plot as the mean of the corresponding values obtained from the Chao1, ACE, Jacknife 1 and Jacknife 2 estimators (
Table 1).
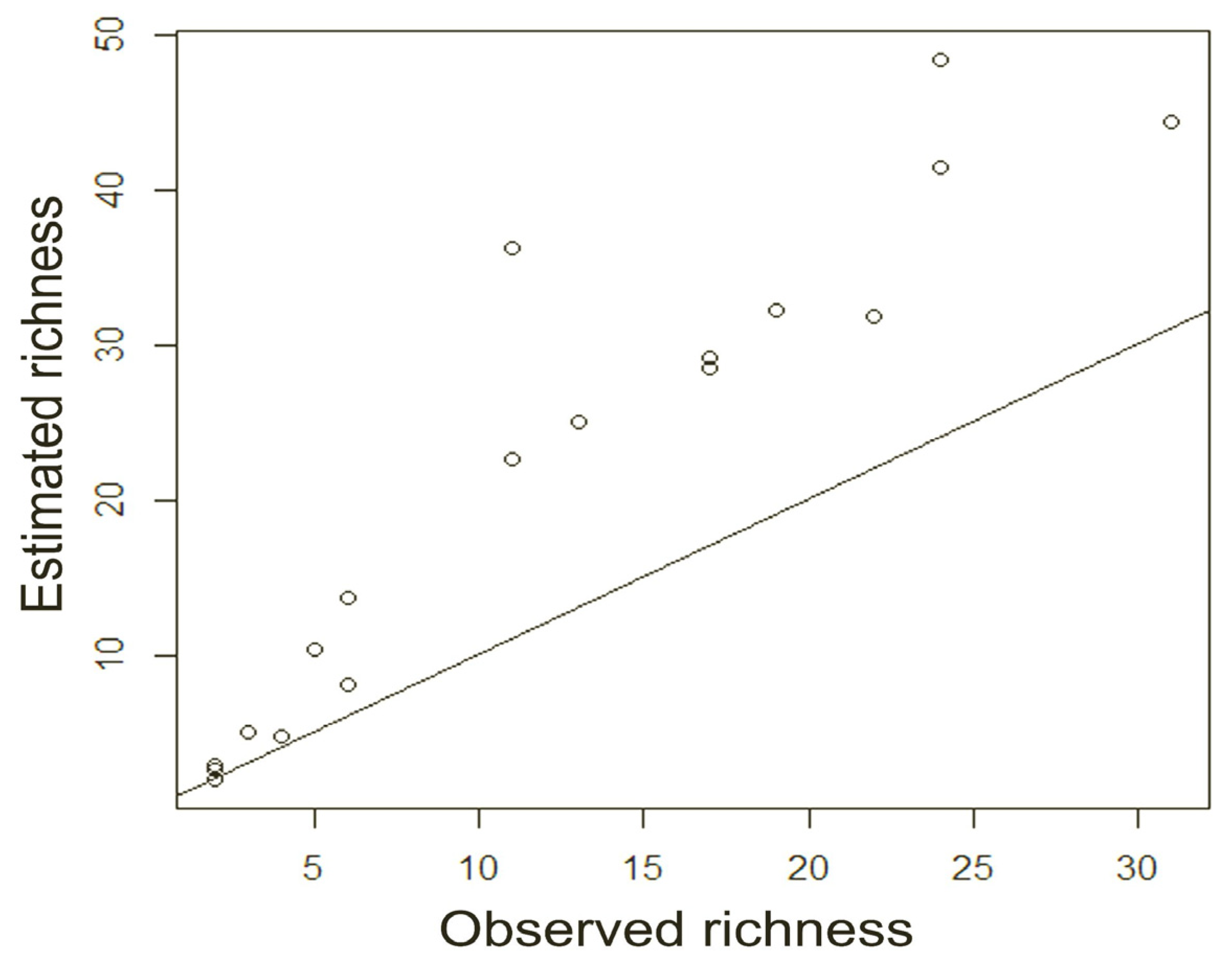
The graph shows that the observed species richness is the result of an underestimation in all areas sampled in both reserves, and the gap with the estimated species richness value is most pronounced (it reaches a value greater than or equal to twice the observed value) in two plots in Otongachi (“e140”, “e120”) and in three plots in Otonga (“e250”, “e270”, “e2100”).
The scatter plot and Spearman’s correlation coefficient (0.953,
p < 0.001) show, however, that there is a positive and significant correlation between the observed and estimated values of species richness (
Figure 4).
The abundance and diversity indices (Shannon and Simpson) were found to be positively and significantly correlated with species richness (Spearman, S-N, ρ = 0.798,
p < 0.001; S-Shannon, ρ = 0.974,
p < 0.001, S-Simpson, ρ = 0.931,
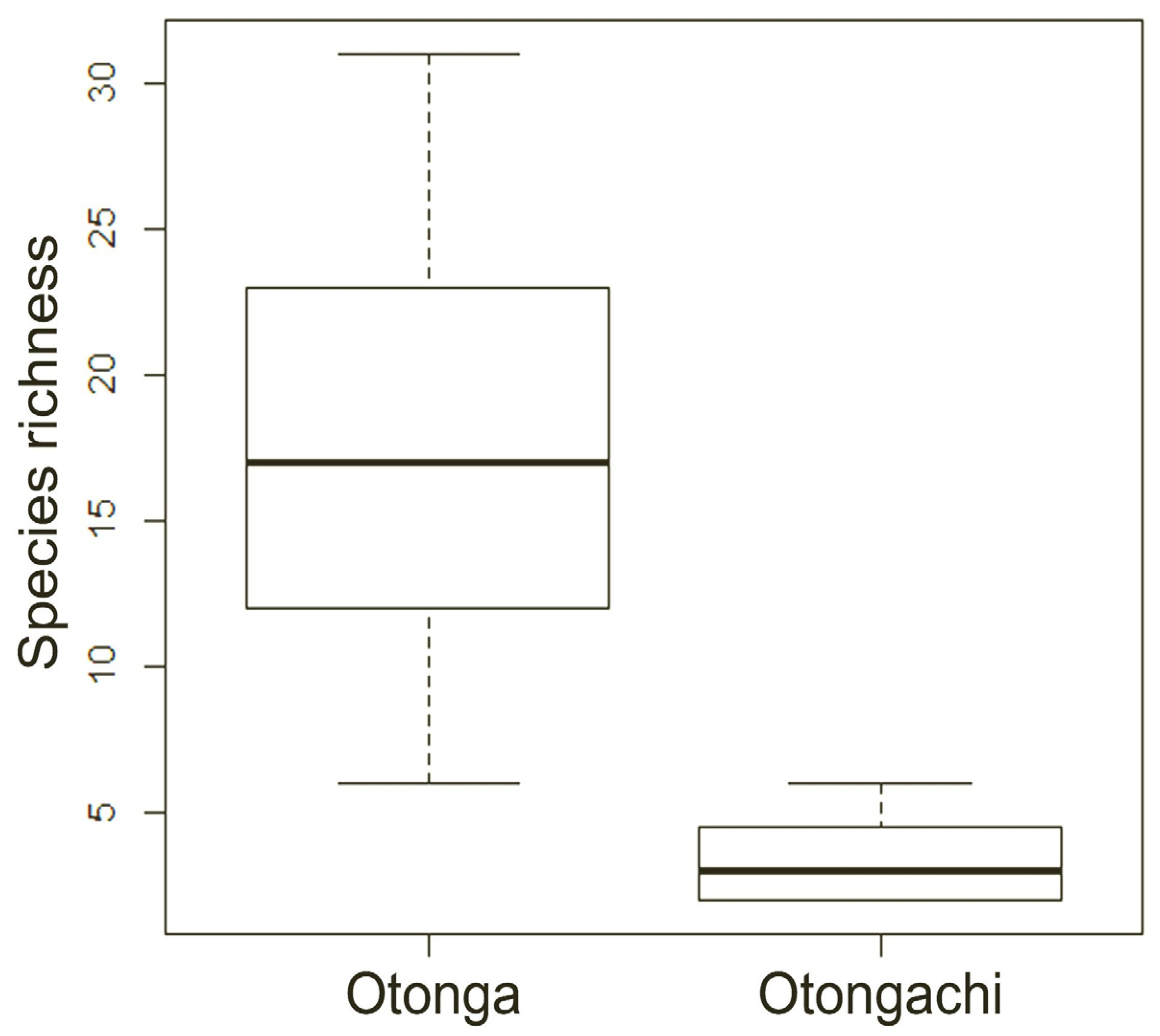
p < 0.001), so that only species richness was considered in the subsequent analyses. Significant differences in mean species richness per plot were observed between the Otonga and Otongachi Reserves. The mean abundance of species and the mean number of specimens per plot are higher in Otonga (17.7 ± 2.2 species per plot, with 35.7 ± 6.1 specimens) than in Otongachi (3.4 ± 0.5 species per plot and 14.6 ± 2.7 specimens) (MW test: V = 87.5,
p-value < 0.01) (
Figure 5).
The difference between the species richness per transect between the primary and secondary forests, considering both reserves, was also significant (MW, V = 63,
p = 0.04), with a
p-value higher than that obtained from the reserve comparison. However, these results were essentially determined by the differences in species richness between Otonga and Otongachi. No significant difference emerged, in fact, in species richness between primary and secondary forest in Otonga (mean number of species per plot 18.0 ± 3.7, with 36.5 ± 10.0 specimens in the primary forest, 17.4 ± 7.5 species with 34.8 ± 7.5 specimens per plot in the secondary forest, MW: V = 15.5,
p = 1.0). There was no significant difference in the mean species richness per plot observed in any of the following cases: between plots with high or low moss_index (MW: V = 31,
p-value = 0.3); between plots with high or low rotten_log index (MW: V = 26,
p-value = 0.13); between plots with different litter moisture (dryness_index, KW: χ2 = 5.34, df = 2,
p-value = 0.07); between plots with different canopy coverage (KW: χ2 = 3.15, df = 3,
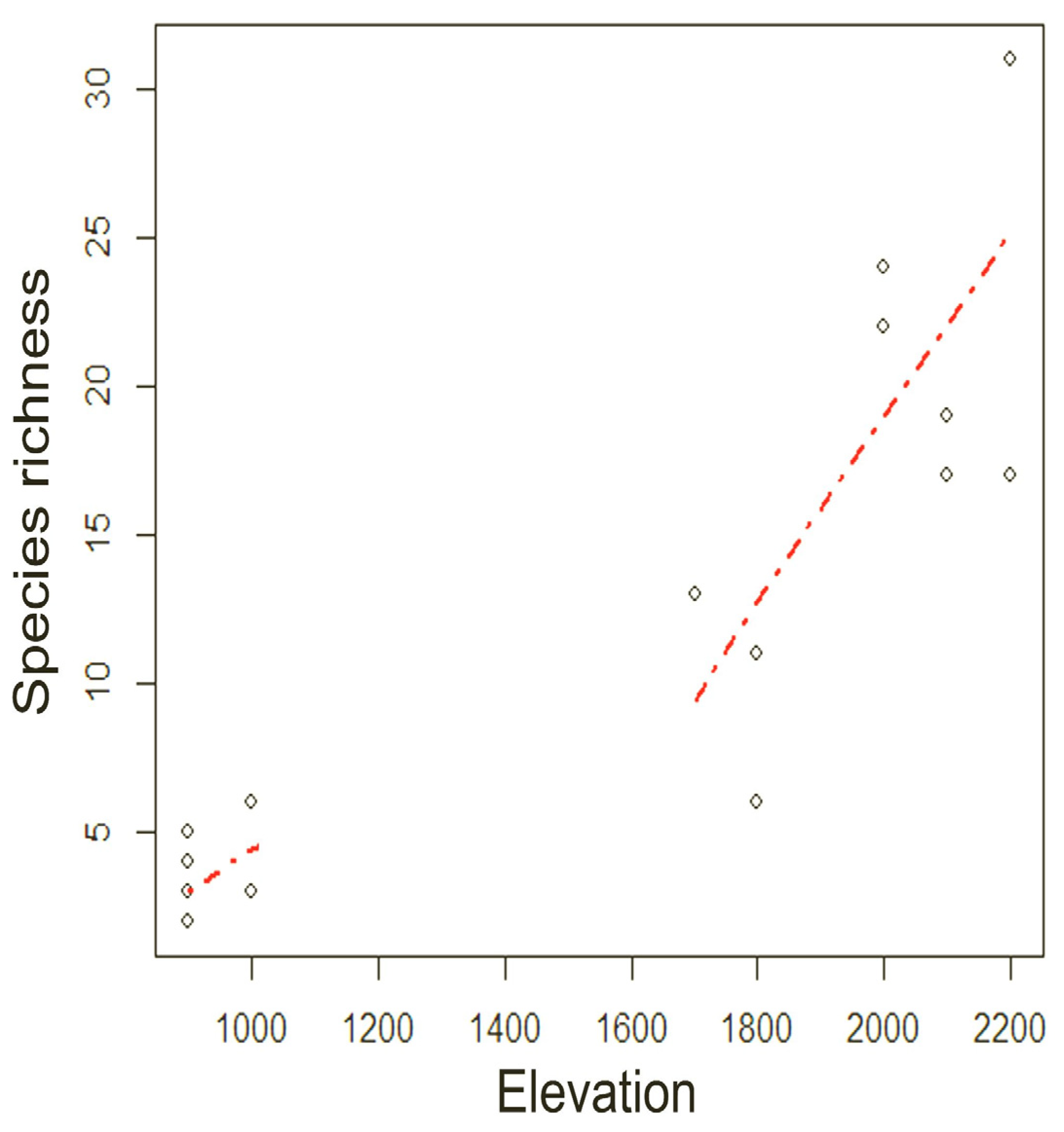
p-value = 0.37). A significant correlation was found between elevation and species richness (Spearman’s correlation, ρ = 0.895,
p < 0.001). Species richness increased as a function of elevation, apparently more markedly above 1700 m, although information on species richness at intermediate elevations between Otongachi and Otonga would be needed to confirm this finding (
Figure 6).
Within each plot, the percentage trend of singletons and doubletons was analysed as a function of environmental variables. The number of rare species was related to the total species richness (ρ = 0.990, p < 0.001), but not their percentage trend (ρ = 0.300, p = 0.212). The relationship between the proportion of rare species and the variables characterising the sampling stations yielded the following results: differences at the limits of significance between the Otonga and Otongachi Reserves (MW, V = 67, p-value = 0.062), with mean values higher in Otonga; no difference between primary and secondary forests, considering both Otonga and Otongachi (MW, V = 49.5, p-value = 0.378); no difference between primary and secondary forests, considering only Otonga (MW, V = 14.5, p-value = 1); no difference between plots with high or low moss_index (MW, V = 40, p-value = 0.77); no difference between plots with high or low rotten_log index (MW, V = 36.5, p-value = 0.51); no difference between plots with different litter moisture index (KW, χ2 = 0.12, df = 2, p-value = 0.94); no difference between plots with different canopy coverage (KW, χ2 = 2.94, df = 3, p-value = 0.40). There is no clear linear relationship in the proportion of singletons and doubletons per transect as a function of elevation (Spearman’s correlation, ρ = 0.209, p = 0.391). There is a growth in the percentage of rare species per plot directly proportional to elevation in Otongachi, and a growth inversely proportional to elevation in Otonga, but since Otongachi extends only for two elevation levels, the difference in percentage may also be determined by random factors due to non-exhaustive sampling.
Rare species over the totality of the samplings were classified according to two rarity criteria: low abundance (rare_num) and low frequency (rare_freq). These values, expressed as a percentage of plot species richness, were found to be positively correlated with each other (Spearman correlation, ρ = 0.981, p < 0. 001), but neither with plot species richness (rare_num, ρ = 0.105, p = 0.668; rare_freq, ρ = 0.187, p = 0.444), nor with the percentage of singletons and doubletons (rare_num, ρ = 0.358, p = 0.132; rare_freq, ρ = 0.278, p = 0.250). No significant difference for these two categories of rare species was observed either in the comparison between study areas, or between the two forest types, or as a function of environmental variables. The only case in which significant differences were observed is as a function of the dryness_index (KW: rare_num, χ2 = 7.576, df = 2, p = 0.023; rare_freq, χ2 = 8.984, df = 2, p = 0.011), indicating that intermediate values of litter moisture have a lower percentage of rare species.
In none of the following cases was a significant influence of environmental variables on the percentage of rare morphospecies observed: between the Otonga and Otongachi Reserves (MW, rare_num, V = 39.5, p-value = 0.740; rare_freq, V = 40.5, p-value = 0.804); between primary and secondary forests (MW, rare_num, V = 41, p-value = 0.895; rare_freq, V = 45.5, p-value = 0.598); between these two forest types in Otonga (MW, rare_num, V = 18.5, p-value = 0.582; rare_freq, V = 22, p-value = 0.247); between plots with high or low moss_index (MW, rare_num, V = 39, p-value = 0.709; rare_freq, V = 33.5, p-value = 0.408); between plots with high or low rotten_log index (MW, rare_num, V = 51, p-value = 0.652; rare_freq, V = 51, p-value = 0.653); between plots with different canopy coverage (canopy, KW, rare_num, χ2 = 4.8, df = 3, p-value = 0.187; rare_freq, χ2 = 5.112, df = 3, p-value = 0.164).
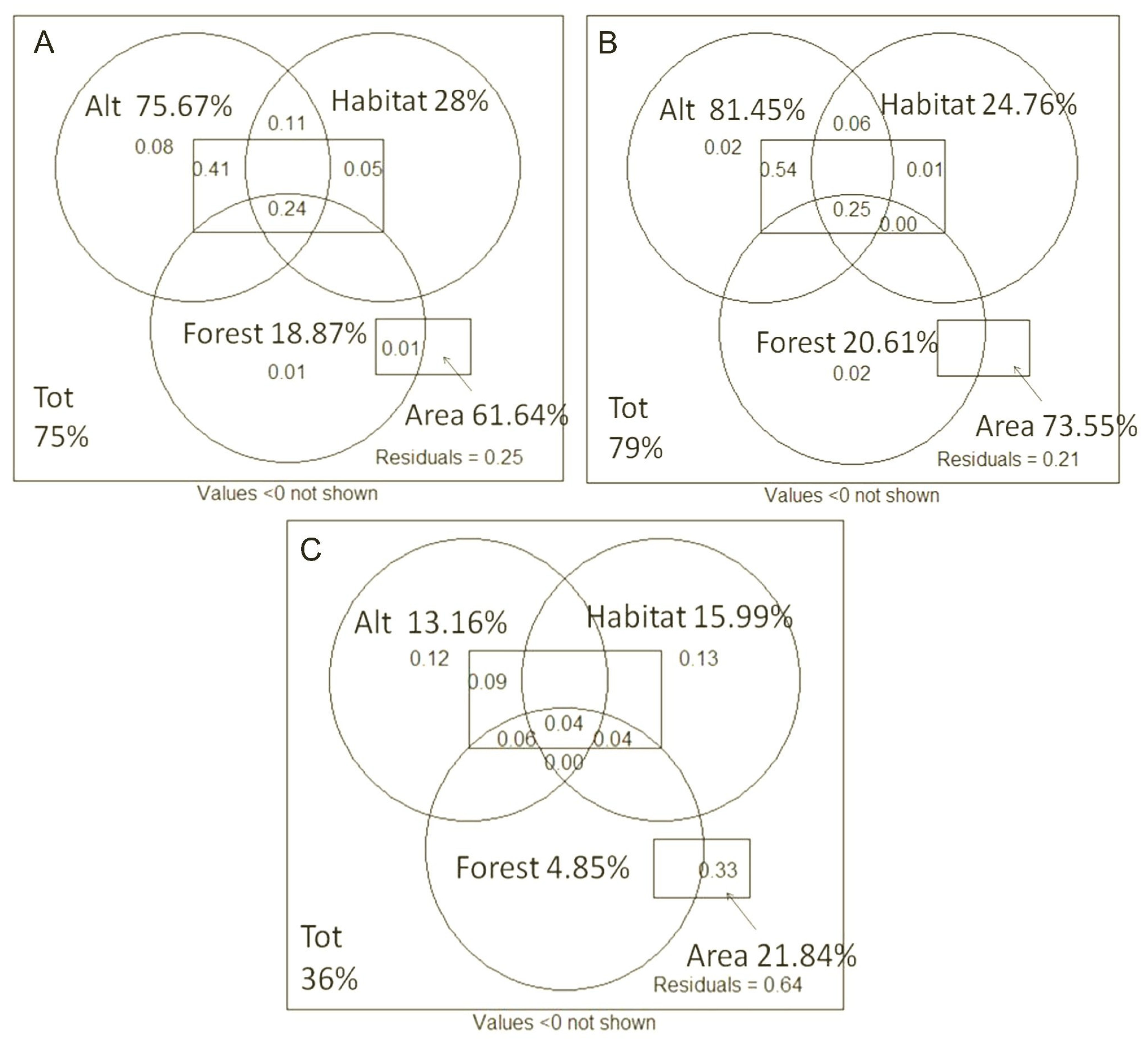
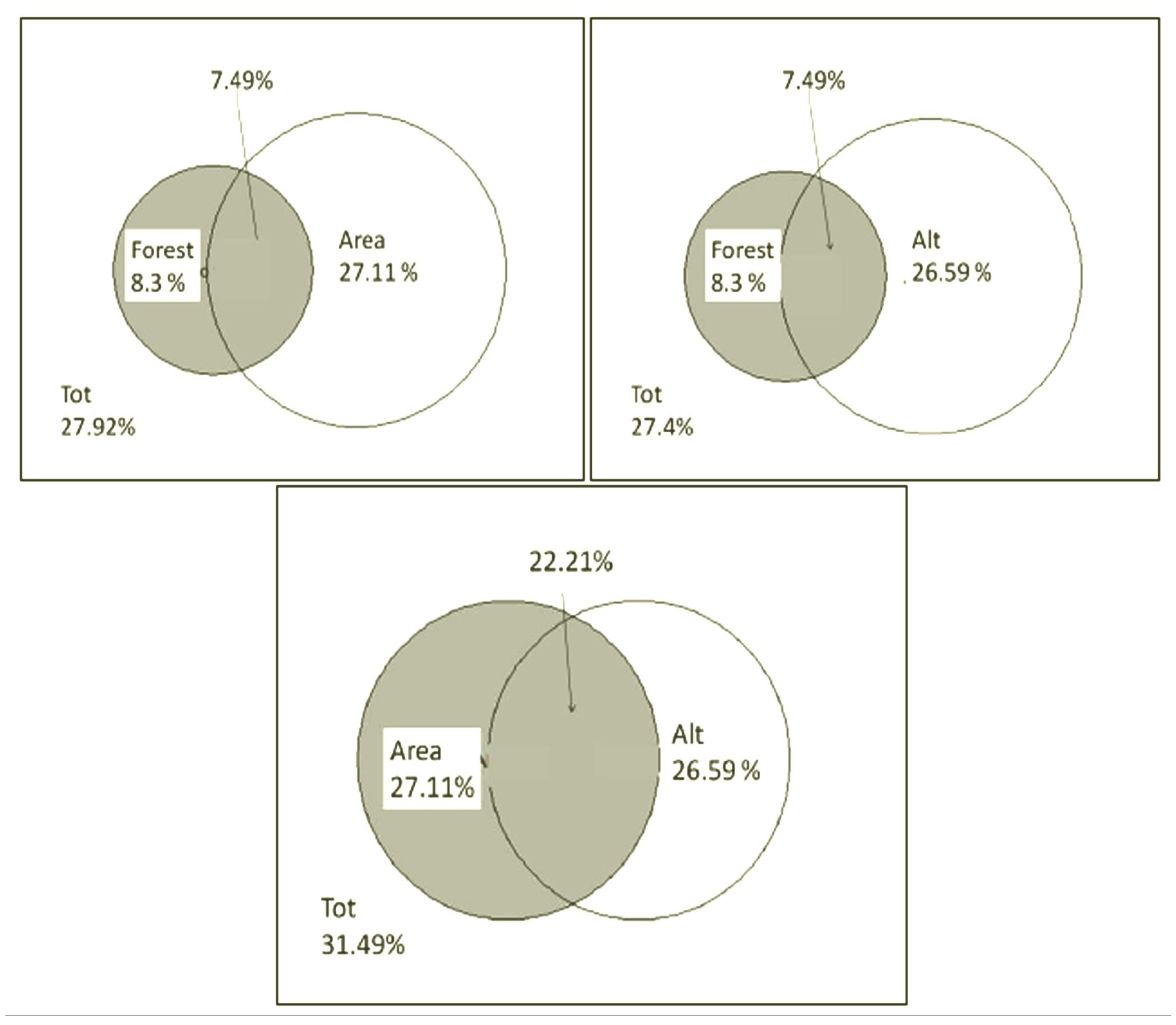
A variation partitioning analysis was applied to assess the relative roles of the different groups of variables (elevation, study area, forest type and microhabitat characteristics) in determining the observed patterns of species richness. The following variables were analysed: the partitioning of the variability of species richness by transect; the partition of the variability of the number of rare species per transect; the partition of the variability of the percentage of rare species per transect. The percentage of total variability explained by the model consisting of the variables considered was: 75% on the data of species richness per transect; 79% on the data of number of rare species per transect; 36% on the data of percentage of rare species per transect. In two out of three models (species richness and number of rare species), most of the variability was determined by the elevation factor. In the third model (percentage of rare species), the variability was determined by the area factor, when considered in relation to the other variables. The second element in terms of explanatory importance is the area factor in two out of three models (species richness and number of rare species) and the elevation factor in the third model, when considered as a function of the other variables. The categories “forest” and “habitat” almost entirely lose their explanatory power when conditioned to the other variables. All these components present overlaps, as can be seen in
Figure 7. In most cases, the elevation component is explanatory of a greater percentage of variability than the area component.
3.3. Distribution of Species
No ubiquitous species, present in both the reserves, were found and no species widespread in Otonga was sampled (
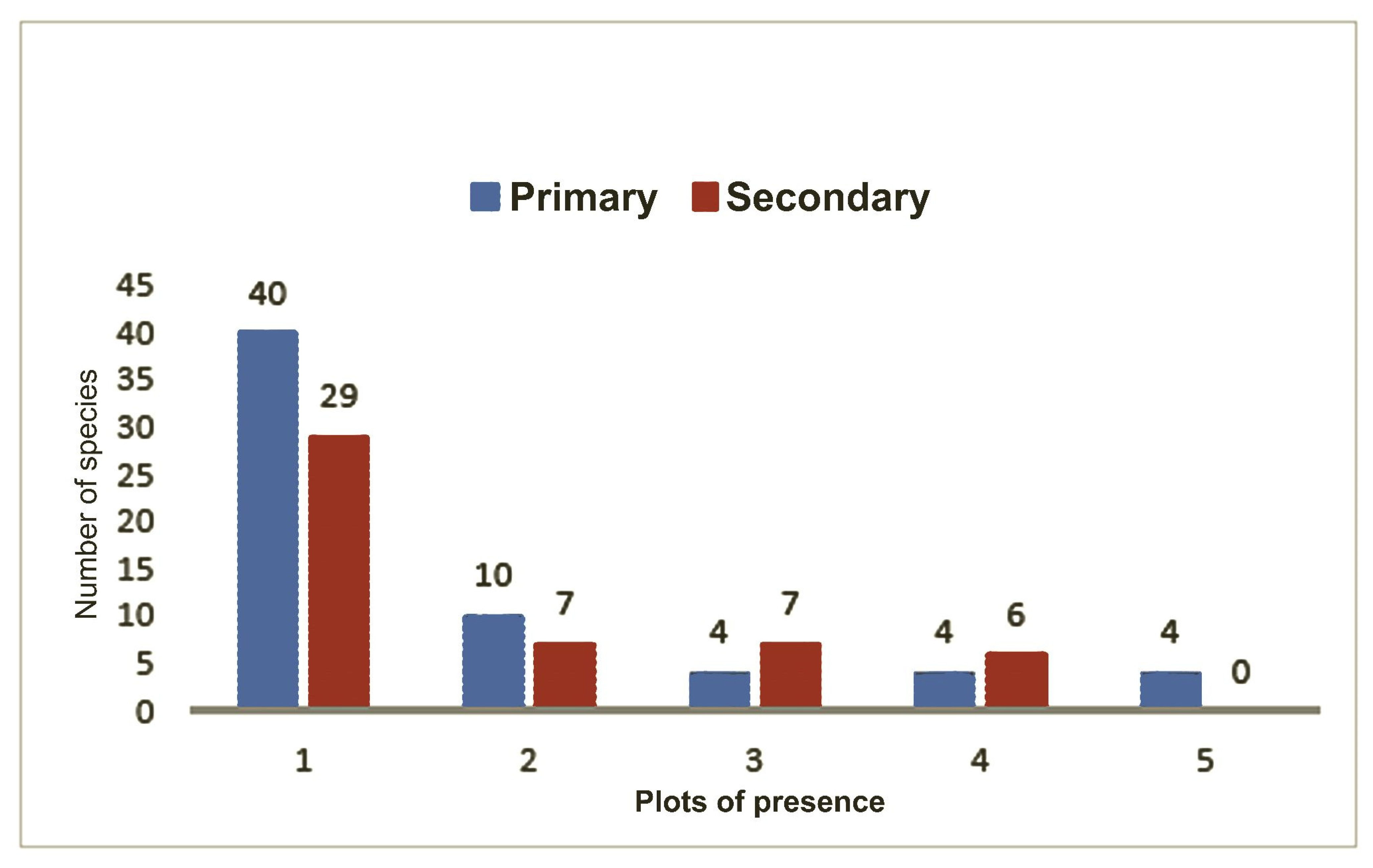
Supplementary Material Table S2). On the contrary, the species “n5” was found in all the plots in Otongachi. Regarding the forest types in Otonga (primary vs. secondary), 36 species were found in the primary forest only, 23 in the secondary forest only and 26 were shared between the two forest types (
Figure 8).
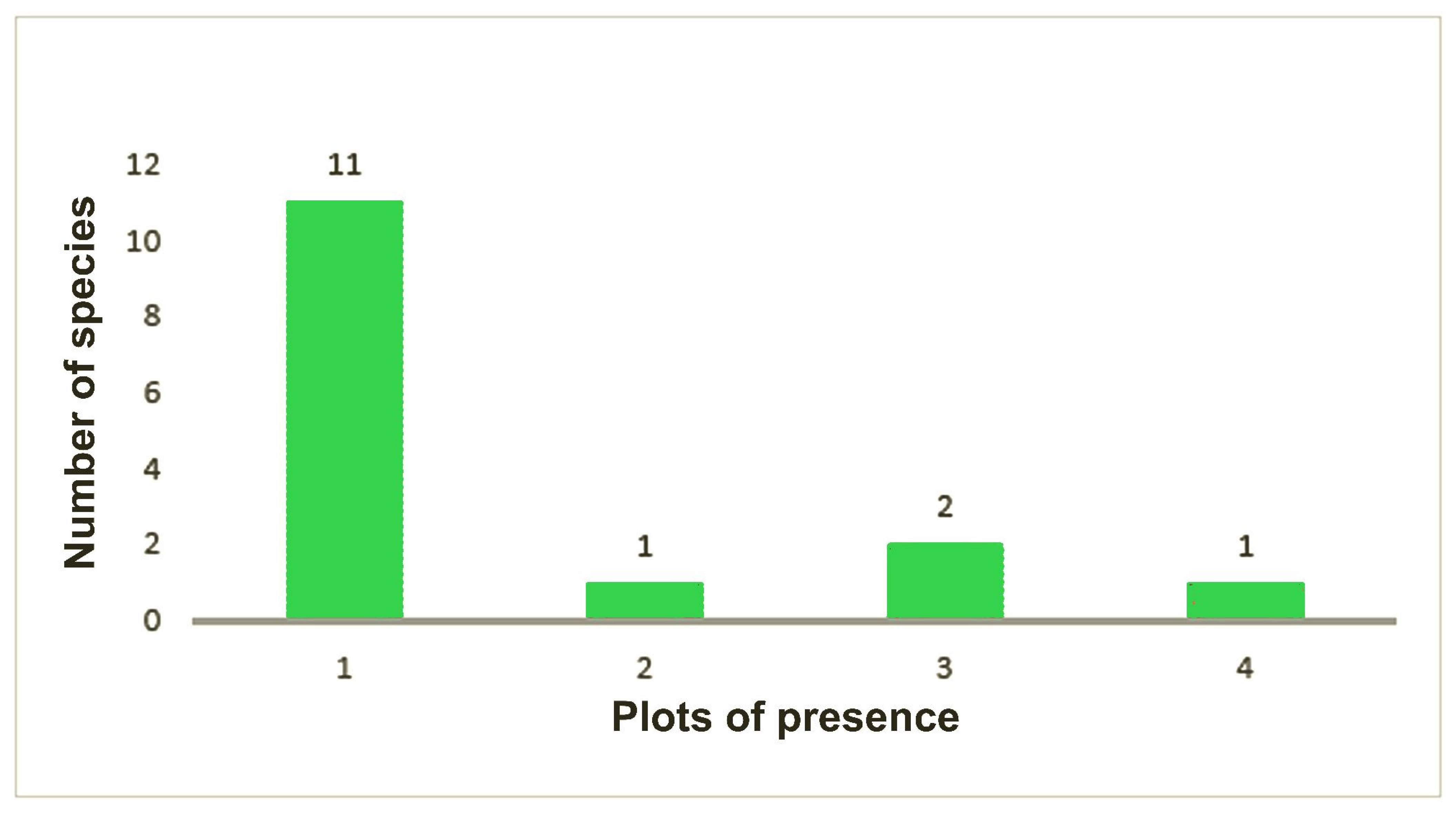
This comparison was not possible in Otongachi, where there is only a secondary forest. The data gathered in both reserves reveal a large number of species for which only a few specimens were collected (rare_num). The number of singletons or doubletons is high: 50 singletons and 18 doubletons were found out of the 100 species collected in total (68% altogether). These data are different when referring to Otonga or Otongachi singularly: in Otongachi, 10 singletons and 2 doubletons out of 15 species were found (80% altogether), whereas in Otonga 40 singletons and 16 doubletons out of the total 85 species were sampled (66% altogether). In the primary forest, 22 singletons and 8 doubletons out of 36 species (83% altogether) were exclusive to this habitat. These species are distributed throughout the entire elevation range, with a slight increase towards the higher elevation; 17 of them were found in the two plots located above 2100 m; in the plot located at the highest elevation, “e280”, 12 species in singletons or doubletons were found. Only 3 out of the 26 species that were present in both primary and secondary forest at Otonga were doubletons (“f1”, “i4”, “n2”). The 23 species found only in the secondary forest of Otonga were either singletons (18) or doubletons (5) (
Figure 9).
The most abundant species found in the two reserves was “n5”, which is ubiquitous in Otongachi and of which 87 specimens were found. There are no other taxa whose abundance or distribution is remarkable in this reserve: 11 out of the 15 species collected here were found only in one plot (
Figure 10).
Comparison of the abundance and distribution of the species collected in the different forest types was only possible in Otonga, given the absence of primary forest in Otongachi. There are a few species relatively rich in specimens in Otonga, but all of them have fewer specimens than “n5”: the commonest one is “o5” (34 specimens), which is present in 9 out of the 11 plots (five in primary and four in secondary forest). The second most abundant species in Otonga is the related “o4”, with 25 specimens from the same nine plots as “o5”. Other relatively abundant species in Otonga are “a2”, with 27 specimens from seven plots, followed by “p2”, with 26 specimens from eight plots. These two species are sympatric in the seven plots where “a2” is present. Another species relatively frequent in Otonga is “h5”, found in 20 specimens from eight different plots. None of the 36 species found only in the primary forest was particularly abundant or widespread: “p1-1”, “e6” and “i3” consisted of four specimens each, whereas in this forest type the most widespread species were present in no more than three plots each (“h3” and “i3”). The same trend was recorded for the 23 species exclusive to the secondary forest, all singletons or doubletons, and with only one species present in two plots (“k6”).
3.4. IndVal Species
Through the application of the IndVal method, 14 species were significantly associated with a specific grouping of sites (
Table 2).
Two species were exclusively associated with the Otonga primary forest, “h3” and “i3” (both with IndVal = 0.71, p-value = 0.03), while two were found to be associated with the Otonga secondary forest, “d1” (IndVal = 0.83, p-value = < 0.01) and “m1” (IndVal = 0.75, p-value = 0.03). One species, “n5” (IndVal = 1, p-value < 0.01), was associated with Otongachi. Eight IndVal species were associated with Otonga as a whole, some of them with high IndVal values, such as “o4” and the related “o5” (IndVal = 0.91, p-value < 0.01).
The mean IndVal for groups of species gave a significantly higher value for Otonga as the entire weevil community (IndVal = 0.66,
p-value = 0.04), compared to the Otonga primary forest only (IndVal = 0.45,
p-value = 0.02) and secondary forest only communities (IndVal = 0.49,
p-value = 0.02) (
Table 3).
3.5. Community Composition
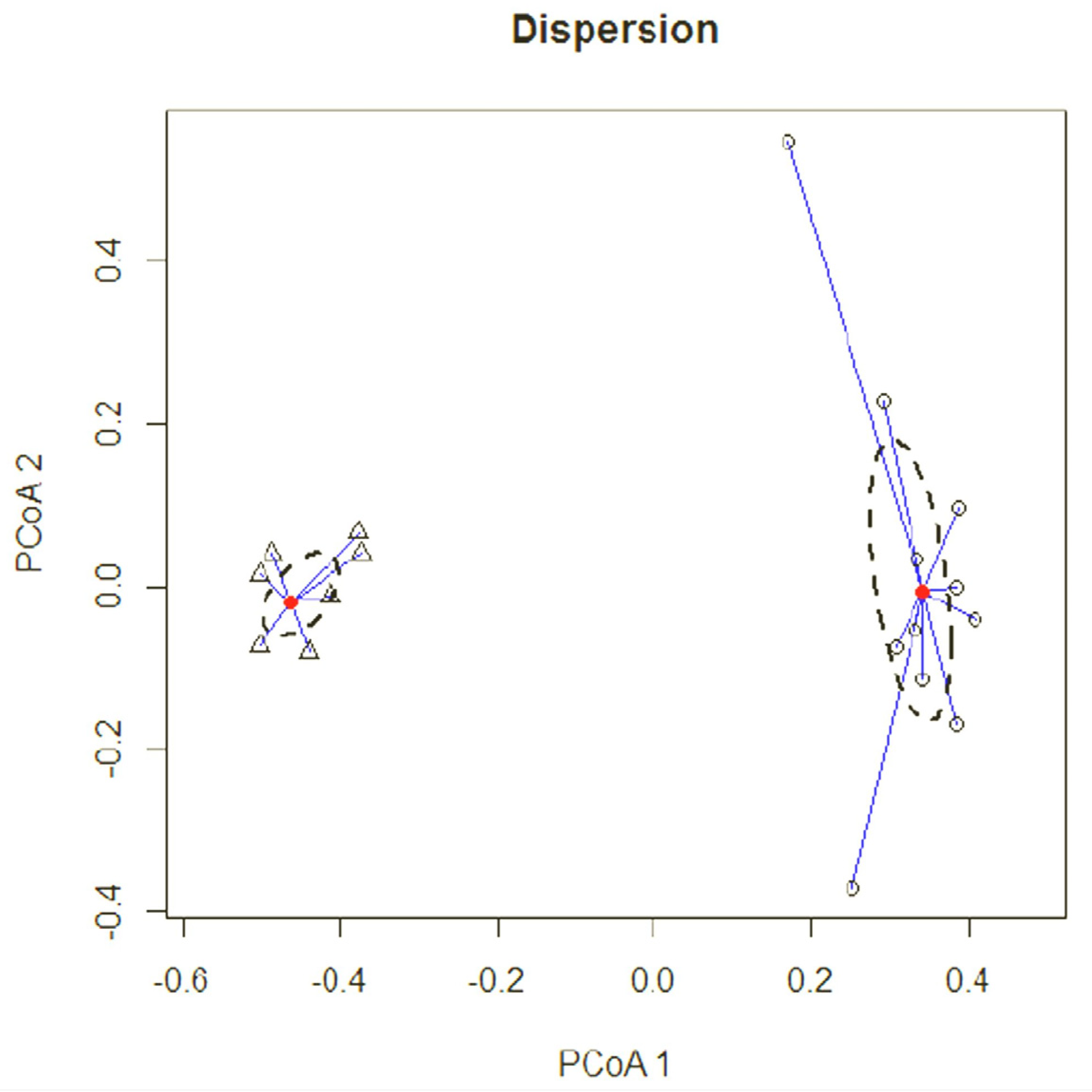
The sampling stations were represented in a scatter plot, in which the two axes of the graph represent the first two principal components extracted from the source data matrix (explained variability, PCoA1 = 12.34%, PCoA2 = 10.21%), where the distance between communities in the plots was calculated using the Jaccard index. Considering the abundance data, a clear distinction appeared in the scatter plot between Otonga and Otongachi in the distribution of samples along the first axis. Furthermore, the confidence interval (delimited in the graph by the dotted line) was larger for Otonga, revealing a greater variability in terms of community composition in Otonga than in Otongachi. Most of the plots in Otonga diverged markedly from the centroid, outside the 95% confidence interval, the most distant being “e260” and “e2100”. The plots in Otongachi were much less divergent, with only a few plots outside the 95% confidence interval (
Figure 11).
A similar pattern was also evident in the scatter plot regarding presence/absence data: again, there was a clear distinction between Otonga and Otongachi in the distribution of the samples along the first axis (
Figure 12).
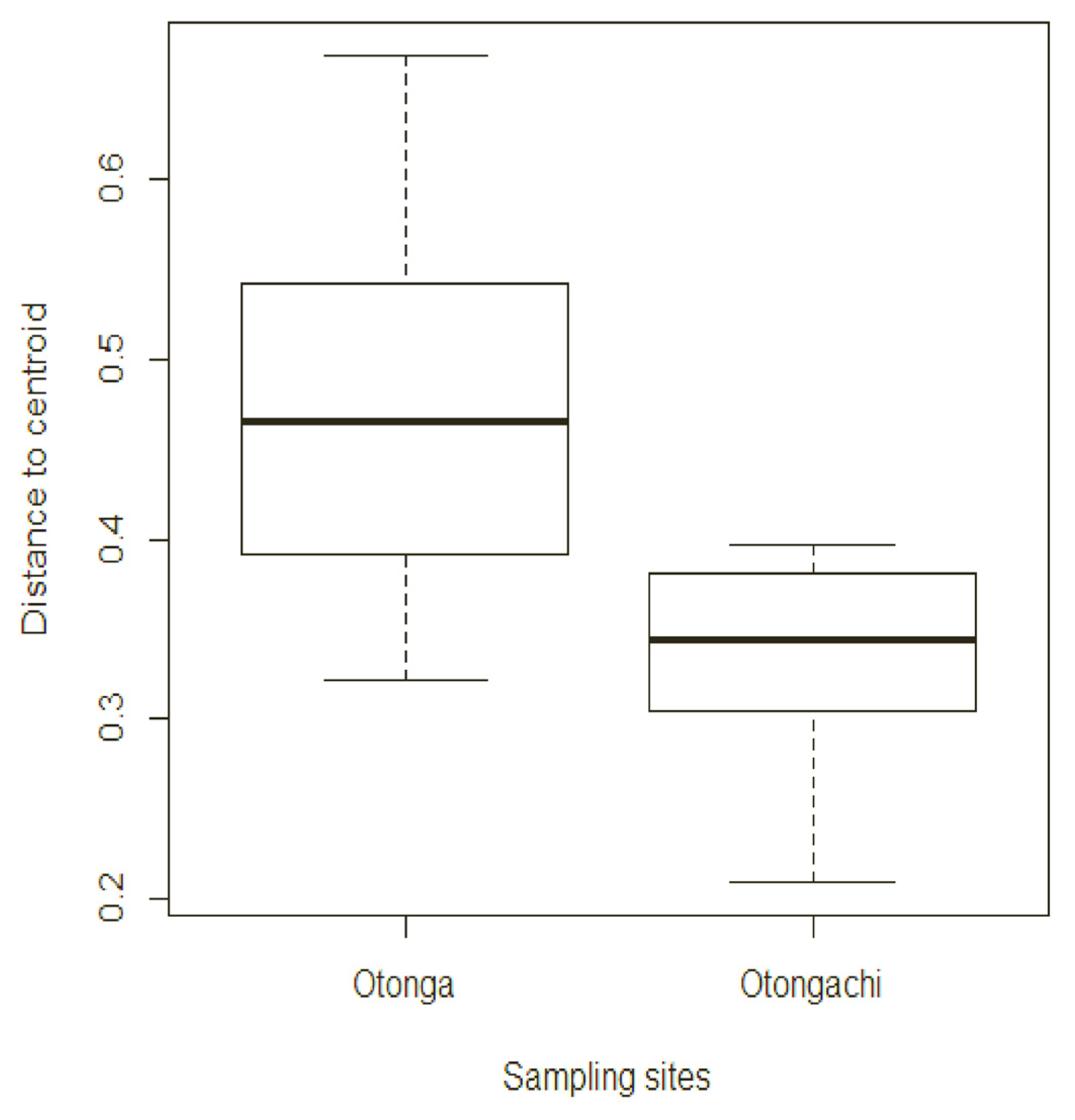
The mean value of the distance of each plot from the centroid in the dispersion analysis was significant when considering abundance data (F-value = 12.16;
p-value < 0.01). The mean value was 0.48 for Otonga and 0.33 for Otongachi (
Figure 13).
The trend was similar for the presence/absence data, but in this case the differences in the dispersion around the centroid were not significant (F-value 1.18, p-value = 0.29).
The PERMANOVA analysis was carried out by first taking into account the abundance data of plots belonging to either the Otonga Reserve or the Otongachi Reserve. Significant differences were observed between the two reserves (R2 = 0.46, p-value < 0.01). The PERMANOVA analysis of the presence/absence data produced similar results (R2 = 0.46, p-value < 0.01). When this analysis was applied to plot groupings to test for differences between primary and secondary forest, no significant values were found for both abundance data (p-value = 0.19) and presence/absence data (p-value = 0.16).
The main factors influencing the composition of the weevil communities sampled in the various transects were determined by partitioning the variability in community composition according to the various categories of environmental variables measured. The percentage of variability explained by the model consisting of the variables considered in the presence/absence data is over 22%, a relatively high value, particularly in view of the inherent variability of animal communities. Most of the variability was determined by the area factor (Otonga or Otongachi), when considered as a function of the other variables. The second element in terms of explanatory importance was the elevation component (which has a value very close to that of the area factor), when considered as a function of the other variables; it occupies the first position when considered as a whole. The forest category (primary or secondary) loses almost all explanatory power when conditioned to the other variables (
Table 4).
The variability partition data for the previous categories were corrected and rescaled to assess their influence on the abundance data; no significant differences from the previously obtained ranking were observed. The other environmental variables identified by the habitat category (derived from rotten_log, moss_index, dryness_index and canopy) were not considered in this comparison because the variability explained by these was found to be low and not significant. A more detailed comparison between the habitat components given by the type of forest (primary or secondary), the category identified by the area and the elevation made it possible to identify the relative value of these individual components for the model consisting of the variables considered on the presence/absence data. All these components showed overlap. The area component explained almost as much variability as the elevation component, and both of these components are more explanatory than the forest component (
Figure 14).
A very similar result was obtained by comparing the relative values of the same components for the model built on the abundance data.
4. Discussion
This is the first study that took into the litter weevil fauna of tropical forests in South America, comparing different types of forests (specifically, tropical montane cloud forest and foothill evergreen forest), primary and secondary forests and different elevation levels. The richness and density of weevils found in the Otonga (TMCF) and Otongachi (FEF) reserves during the study, 510 specimens belonging to 100 morphospecies, included in roughly 24 morphogenera, were notable, particularly considering the limited number of collecting sites and the short time during which the study was carried out. Possible variations in the composition of the communities at different times of year or during the relatively drier season could not be taken into account. However, even though we could not carry out a larger number of samplings, we are confident that the results of our research offer previously unavailable information about diversity of litter weevils of the Ecuadorian TMCFs and provide evidence of their role as bioindicators usable to characterise the biocoenoses of these tropical forests, helping to assess their environmental quality. According to Basset et al. [
45], in fact, “to determine the species diversity of a tropical rainforest, the total area sampled need not be overly large—provided that the sampling design adequately covers both microhabitats and plant species”.
The mean density of weevils was about five specimens per 3 L per each one-square-metre sampling point, which converts to about 50,000 per ha. Considering that the total surface of Otonga and Otongachi is about 1650 ha, this means approximately 80 million litter weevils in the two reserves, but only in 3 L of soil per square metre. In the plots that were sampled very often, the quantity of litter far exceeded 3 L per square metre, therefore it can be very roughly, but reasonably, estimated that Otonga and Otongachi contain hundreds of millions of specimens, an amazingly rich fauna. A rough count of the numerosity of the litter weevils was also attempted in a Mexican forest [
8], where 170,000 individuals per hectare were estimated. In this study, however, only 57 species, assigned to 10 genera, from over 2000 specimens sampled were identified (including a few species that appeared not to be true leaf litter obligate inhabitants, since they were winged). So, the Ecuadorian forests investigated host a much richer diversity, even though apparently the density of specimens is lower. As for the Mexican habitat, in Ecuador the vast majority of species were also undescribed.
The plots were selected so that a comprehensive example of the various coenoses could be investigated, with particular regard to the different elevation levels and forest types but, quite obviously, our sampling plots were undersampled, and only in a few cases did the collected species approach the expected richness. The communities in these ecosystems are apparently composed of a large number of species, the majority present in a small number of specimens, as suggested by the high percentage of singletons and doubletons, and rare species in general. A percentage of infrequent species, exceeding the expected, is a common finding when studying the arthropod fauna of tropical forests, not only for weevils, but for other arthropods associated with litter as well [
45,
46,
47,
48]. A more exhaustive sampling could not be applied to our study, mainly for practical reasons (research time and sorting equipment were limited). In any case, thanks to the relevant species richness of our samplings and its good correlation with the estimated one, it was possible to characterise the communities present in the two reserves, revealing the differences between them and assessing their association with the observed environmental variables. Otonga and Otongachi are colonised by distinct weevil communities, and thus can be considered as different coenoses. Otongachi differs from Otonga in the lower elevation (800–1000 m vs. 1700–2300 m), the type of forest, FEF instead of TMCF and EMF, the absence of primary habitats, the lower thickness of the litter and a much heavier anthropic impact.
Otonga hosts 85% of the species found in our study. A high fidelity to a single plot was detected, with a low number of species present in more plots. Almost no data on biotic and abiotic factors are available for each of the elevation levels, but it is possible that the distribution of some parameters (mean annual rainfall, cloud coverage, air and soil humidity, mean annual temperature, tree species, understory vegetation and more) varies to some extent in the different elevation levels. Elevation was found to be one of the most significant variables in determining the faunistic composition of the litter weevils. In Otonga, the species richness was found to increase from the lower to the higher elevation levels, with the highest number of species found in the plot at the top (32 species, plot “e280”), an area of primary forest with large trees and thick litter, with intermediate soil wetness, features that may have favoured the considerable number of weevils present. In this plot, the sampled species closely approached the number of estimated species. An increment of species towards higher elevations, within the range of presence of the TMCFs, was also observed in studies on different groups of arthropods [
21]. Even though only morphospecies were used, these were assigned to provisional genera. It appears that, in some cases, closely related vicariant species were present at different elevation levels. Among the Molytinae: Anchonini, the two morphological, and probably systematical, vicariant taxa “n3” and “n4” were present, respectively, in the level ranging from 1700 to 1800 m and in the level ranging from 1800 to 1900 m, without any overlap. Additionally, for the two morphologically vicariant taxa “m7_1” and “m7_2”, there is a separation according to the elevation. In this case, “m7_1” was found from 1700 to 1900 m, whereas “m7_2” was present in the 2000 to 2100 m level (
Figure 15).
These observations, if confirmed after a taxonomic study, could indicate that these species underwent allopatric speciation at different elevations. This would confirm observations that were made for other weevil species from tropical forests, such as the extremely speciose genus
Trigonopterus Fauvel, 1862, native to the tropical forests of South-East Asia, recently investigated [
49,
50,
51,
52]. This finding is also in agreement with the high β-biodiversity values demonstrated by other studies conducted in the TMCFs [
53] and with biogeographic models, according to which these forests are characterised by a high rate of allopatric speciation [
8]. An allopatric speciation that occurred in spatially or altitudinally close areas indicates that these are very highly specialised elements, strictly stenoecious, that seem to have adapted to a very narrow range of environmental variation and have a very low capability of dispersion or adaptation to modifications of their habitat. Their presence implies that the habitat did not suffer from extremely severe anthropic impacts, which these taxa could not tolerate. There is another observation suggesting that the TMCF of Otonga, probably thanks to the efforts of the Otonga Foundation, remained well preserved in the areas where the primary forest was impacted to some extent and is now replaced by secondary forest. We evidenced only limited differences between the primary and secondary forests in the composition of the weevil communities. The overall numbers of species and specimens were quite similar (mean number of species per plot 18.0 ± 3.7 with 36.5 ± 10.0 specimens in the primary and 17.4 ± 7.5 species with 34.8 ± 7.5 specimens in the secondary forest), and only four, out of 85 species found in Otonga, were statistically associated with Otonga primary (“h3” and “i3”) or secondary forest (“d1” and “m1”) (
Figure 16).
The species richness was similar between the two types of forest, and only the singletons and doubletons were more frequent in the primary patches. The blind species of morphogenus “p” give some more precise hints in the evaluation of the Otonga forests. These weevils are closely associated with the deeper part of the litter, require very specific and stable environmental conditions and have low mobility. As expected, they were mainly sampled in primary forests, but one species, namely, “p2”, was also present in two plots in secondary forests (“e210” and “e250”). At least in these plots, the habitat must have suffered a limited impact when the primary forest was replaced by a secondary forest, indicating that this succession occurred gradually, and that the primary forest was not completely removed and replaced by a uniform grassland, but at least some patches with forest remained untouched. A colonisation of the secondary forest that occurred after the time of its planting (less than 30 years ago) is in fact extremely unlikely. Quite interestingly, in one of these two plots of secondary forest (“e250”), 23 species were found, making it the second richest sampling site of Otonga. It is not so unusual that in secondary forests a high diversity can be detected. The litter fauna in the primary, undisturbed patches of TMCF appears to be well structured, quite stable for a long time and mostly composed of numerous strongly specialised species, most of which are present in a low number of specimens. A large part of this primary fauna could survive in plots of secondary forest that did not suffer from a particularly intense level of anthropic impact, but these communities may also include some species more tolerant and resilient to human disturbance and with a higher dispersion capability, which have been able to colonise the patches from more deteriorated habitats. The presence of abundant soil arthropods in secondary, or restored, forests was also detected in other cases [
54,
55], but in these studies, even though the soil arthropods were abundant, quite often the species composition was quite different from that of the primary forests, and in general it was dependent on the distance from the patches of untouched vegetation [
54]. Moreover, these studies did not specifically evaluate the litter weevils, which, including many specialised taxa, offer a more accurate response.
Despite the fact that no quantitative data on litter thickness were collected during the current study, random observations of the depth of litter present in the vicinity of the plots showed that the thickness was consistently greater in Otonga than in Otongachi. This is likely to be one of the most important factors responsible for the much more abundant weevil fauna present in Otonga, even though more habitat constraints may have contributed to determine the different aspects of the communities. When forest litter is well structured and quite deep, its conditions are more stable, an essential requirement for most species. The low number of species in the plot “e260”, compared with the very speciose nearby plots “e270” (primary forest) and “e250” (secondary forest), appears to be determined by the unsuitable conditions of the litter, whose structure is deficient due to its low thickness and the quite superficial rock layer, and is therefore subject to unstable conditions; food availability in shallow litter may be a limiting factor and water content can rapidly vary from dryer to wetter conditions. Intermediate humidity conditions were found in the plots with the highest number of species, and these conditions are granted by a thicker layer of litter, that acts as a buffer to stabilise humidity, and contains a much higher availability of decaying material. The presence of larger dead branches and logs did not significantly determine a variation in the species richness; most species, at the larval stage, are xylosaprophagous (such as the Molytinae: Anchonini)—but Raymondionyminae feed on roots. However, the adults can move around, feeding on smaller branches and small fragments of decaying organic matter at some distance from the logs.
As said, no species were present in both Otonga and Otongachi forests, despite the limited distance between the areas. This was not completely unexpected, since it is known that the spatial and ecological variation of the soil arthropods is particularly consistent, not only in the tropics but also in other regions [
45,
56]; moreover, the habitat in Otongachi has been severely impacted by anthropic disturbance, much more than in the secondary forests of Otonga. Comparisons between the two reserves give further details for evaluating the differences in the communities between well-preserved and severely disturbed secondary habitats. In particular, the presence of a dominant species in Otongachi, “n5”, widespread in all the plots and accounting for 71% of the specimens collected and the other taxa being found only in singletons or doubletons are remarkable. The presence of one, or at most very few, dominant species seems to be a typical feature of anthropogenically influenced and/or unsuitable ecosystems ([
57], and references therein).
The most sensitive and highly adapted species are not able to re-colonise recovered habitats after disturbance, so the ideal management would be to leave these habitats untouched, but anyway constantly checked to guarantee that the ecological conditions do not vary. However, the few differences that were detected between the Otonga primary and secondary TMCFs indicate that a limited disturbance can be tolerated, if proper management is implemented and special attention is paid to keeping the litter layer as unaltered as possible. Even if not including a tropical forest, a survey on litter weevils in Mexican oak forests with different disturbance regimes showed a correlation with the disturbance level regarding species richness and abundance, and concluded that, similarly to what we have observed in Otonga, “… leaf litter weevil communities in oak forests of central Mexico are similar in taxonomic composition and richness to cloud forests from southern Mexico and that even small, moderately disturbed fragments may be sufficient to maintain their populations” [
58]. The poor and highly altered communities found in Otongachi show that severe anthropic impact can render the forest unsuitable for the most specialised taxa. Only one tolerant species found proper conditions in this area, and the others, probably more specifically adapted to this low-elevation forest, are extremely rare and scattered, and some may have disappeared. In order to achieve a more complete understanding of the causes that determined the imbalance of the community of Otongachi, more research is needed in areas intermediate between Otonga and Otongachi, and in plots characterised by apparent good ecological conditions.
Litter weevils are demonstrated to be extremely useful bioindicators, and, in general, the study of these specialised litter arthropods is confirmed to be an indispensable tool in bringing detailed information on the biocoenoses in natural and altered tropical forests and for evaluating the results of forest management, not differently from what can be achieved from other taxa, such as ants [
59]. Information that can be recovered from their analysis can be used to refine data obtained from the entire weevil fauna, whose
ꞵ-diversity can be quite high even in “artificial” environments, such as oil palm plantations [
60]. In these cases, a comparatively rich diversity is determined by spatial turnover of many non-specialised elements. Knowledge of the range of the more specialised litter weevils of the montane tropical forest is almost absent, but a few revisory studies on single genera [
49,
50,
51,
52,
61] suggest that in many instances most taxa have a very narrow range, even limited to a single location. In the case of Otonga, we cannot exclude that some of the species, i.e., those that were found only at a single elevation level and are replaced by vicariant species at different elevation levels, are exclusive to this region and perhaps even limited to a very narrow area. These species are therefore intrinsically at risk, either as a consequence of anthropic impact or possibly also in the case that climate change modifies some characteristics of the litter, with particular regard to the humidity balance.
A critical use of morphospecies can overcome the taxonomic impediment so frequently encountered when studying tropical fauna. However, it cannot be forgotten that only with complete knowledge of the taxa and recognition of their phylogenetic relationships can a more precise evaluation of the habitats investigated be obtained, and the interrelations between them be disclosed. Moreover, cataloguing the diversity is essential to increase our knowledge of these habitats and assess conservation needs, particularly in view of the environmental changes that are rapidly occurring.
The data on the litter weevil coenoses of Otonga and Otongachi can have a broader application to other sites of TMCF, and tropical montane forests in general. In conservation strategies, quite often large vertebrates are well-known symbols and are used as indicators of habitat quality; they are also suitable to spread information outside the scientific community about the necessity of providing good protection to what remains of natural habitats in the tropics. Most vertebrates, however, can move and look for proper conditions, within the range of each species. These highly specialised arthropods are incapable of any significant movement, and the destruction of their habitats, or a strong impact on them, will inevitably result in their extinction. This is probably what has occurred in Otongachi, whereas full diversity seems to remain in Otonga and must be accurately conserved.