Reviewing Introduction Histories, Pathways, Invasiveness, and Impact of Non-Indigenous Species in Danish Marine Waters
Abstract
1. Introduction
2. Materials and Methods
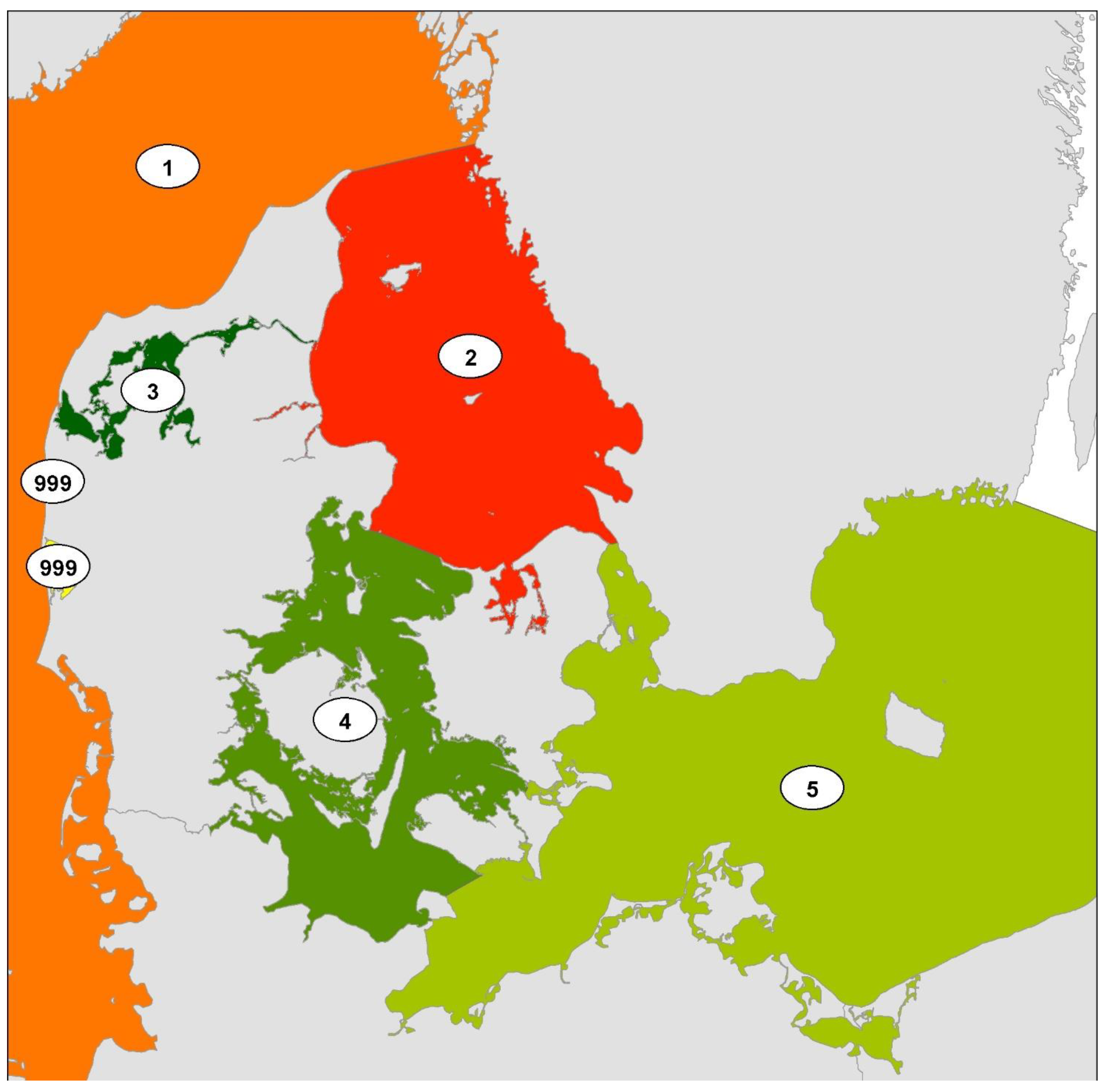
2.1. Study Area
2.2. Data Sources
2.3. Expert-Based Scoring
2.4. Geographic Distribution over Time of Selected Species
3. Results
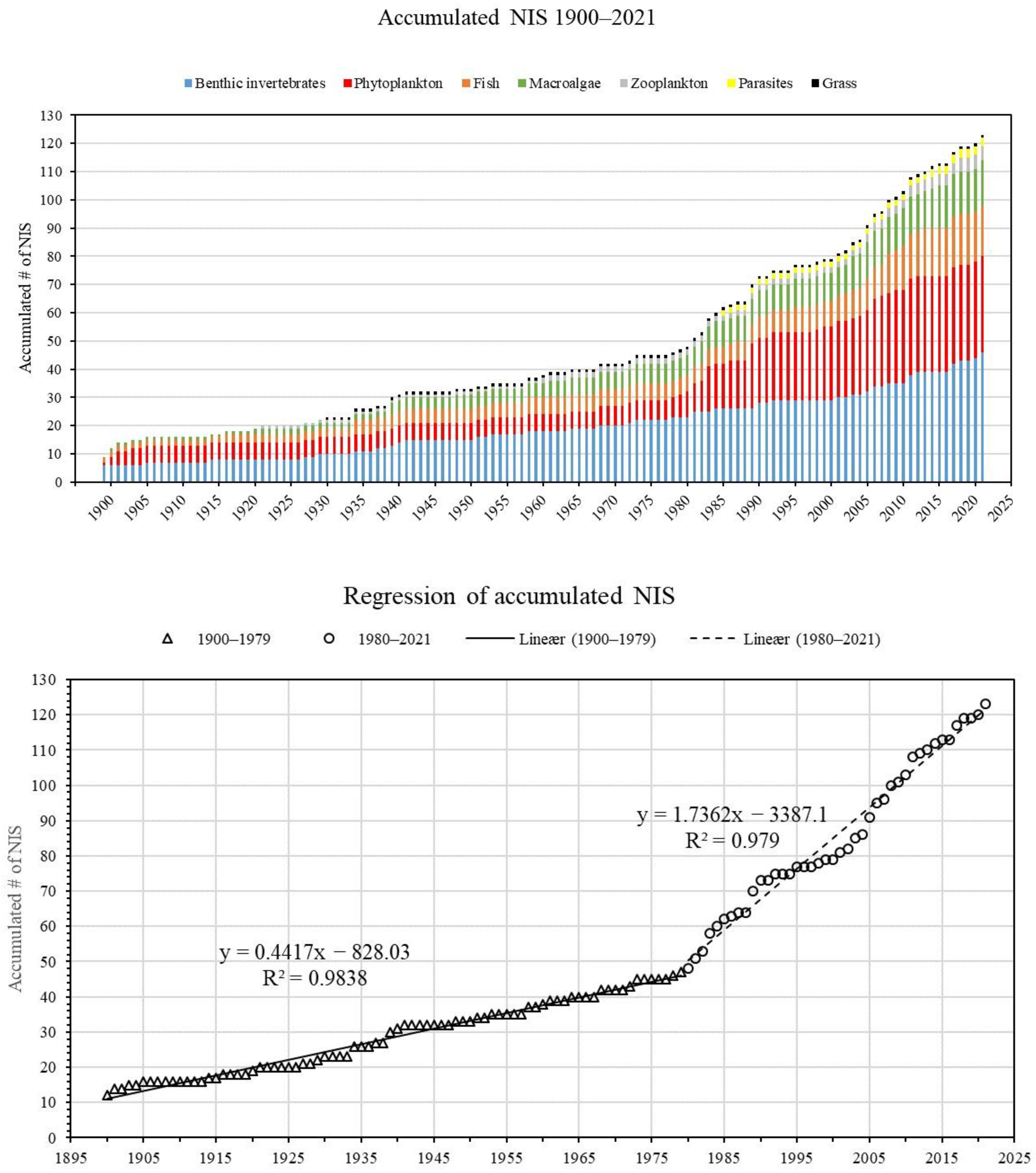
3.1. Maintaining and Updating the List of NIS in Danish Waters
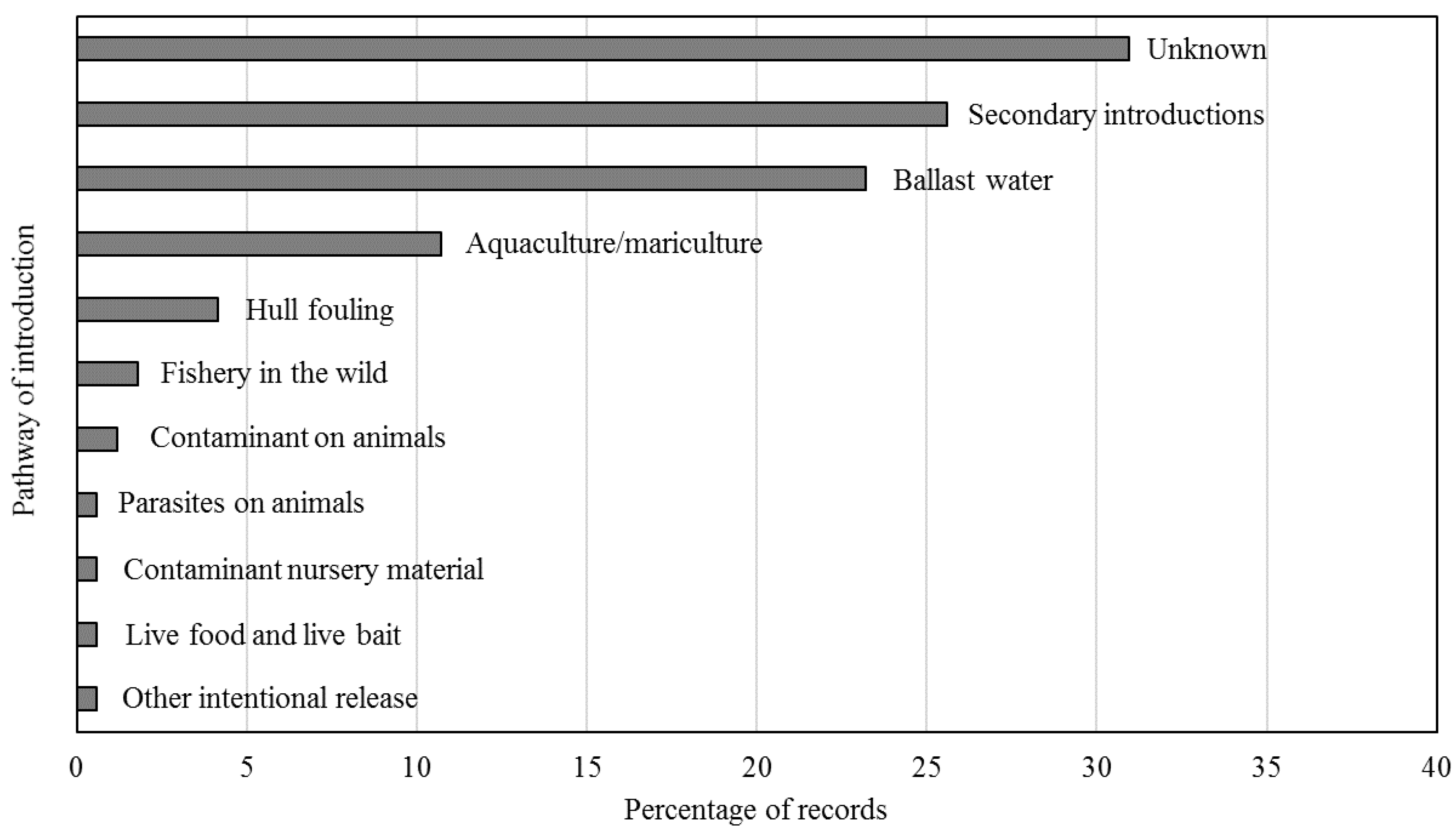
3.2. Problematic Taxa and Records
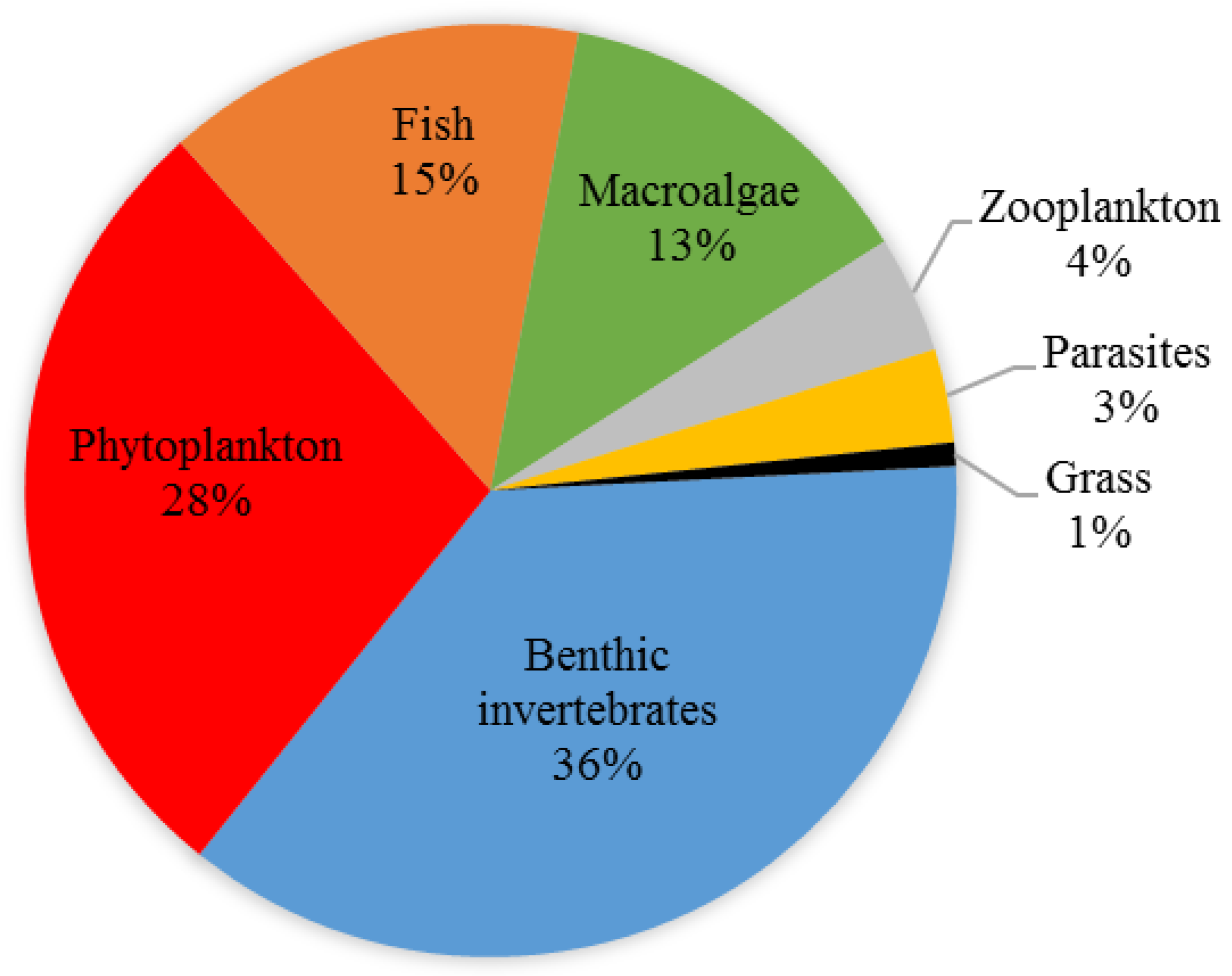
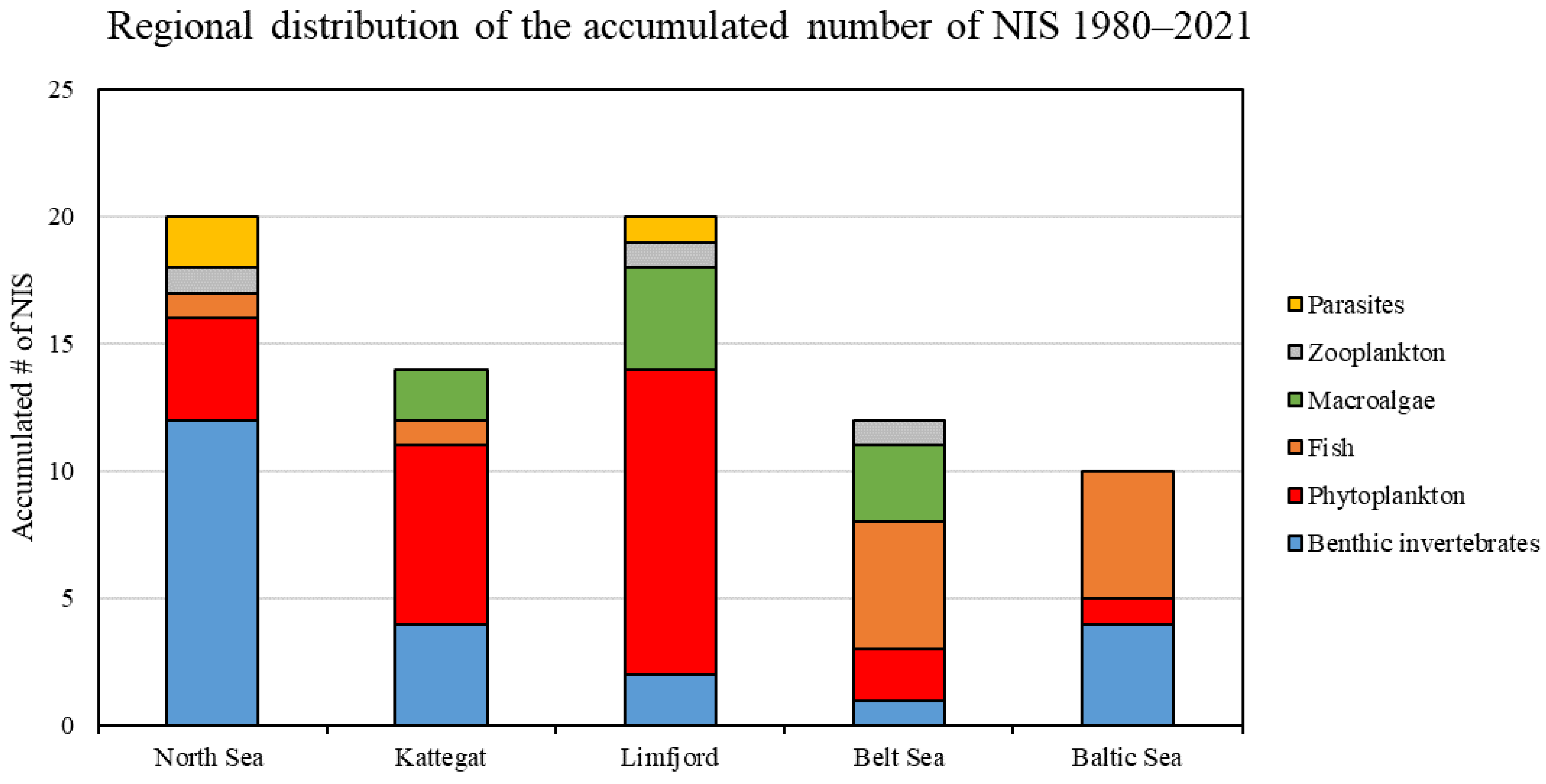
3.3. Analyses of the Updated List of NIS in Danish Waters
3.4. Expert-Based Scoring
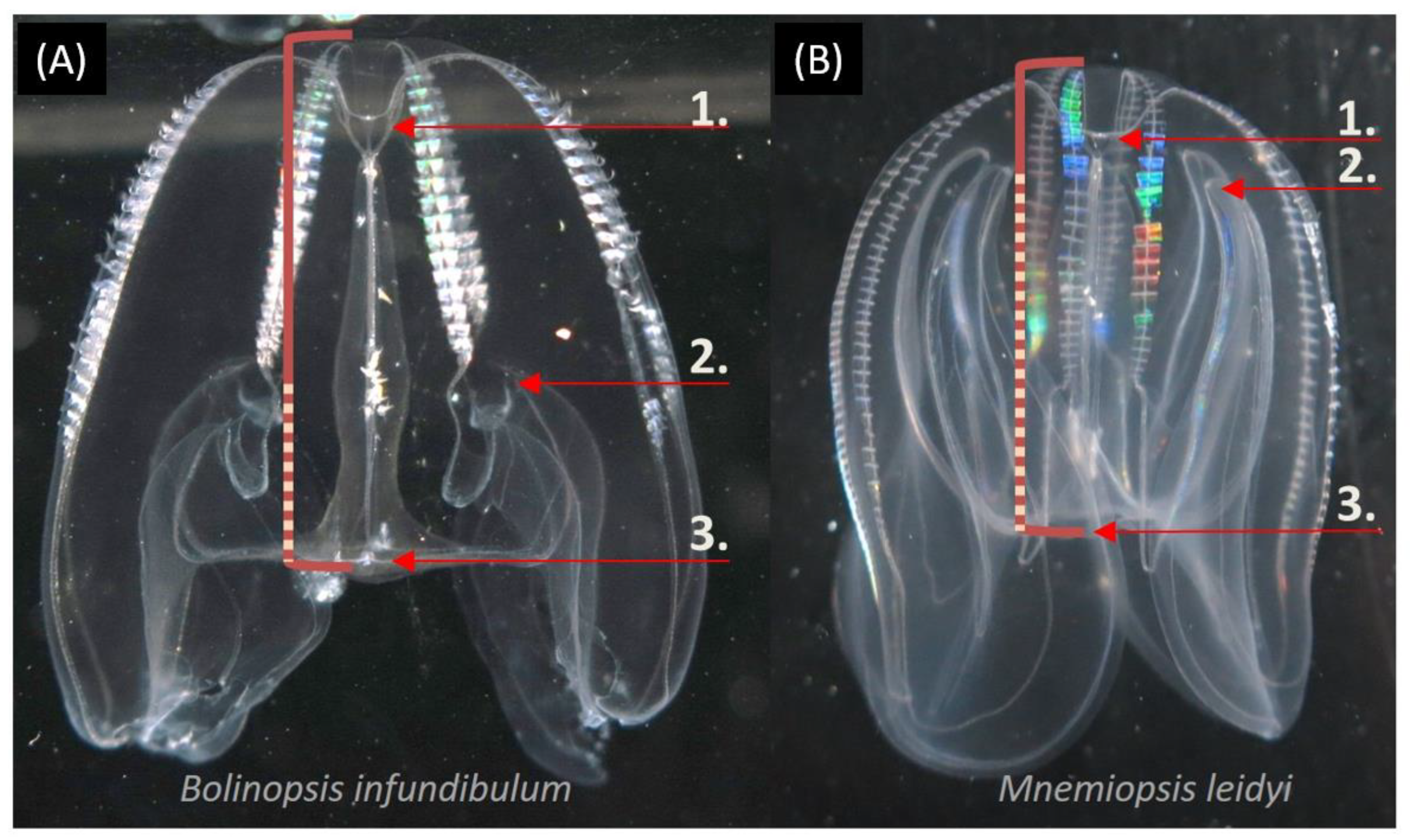
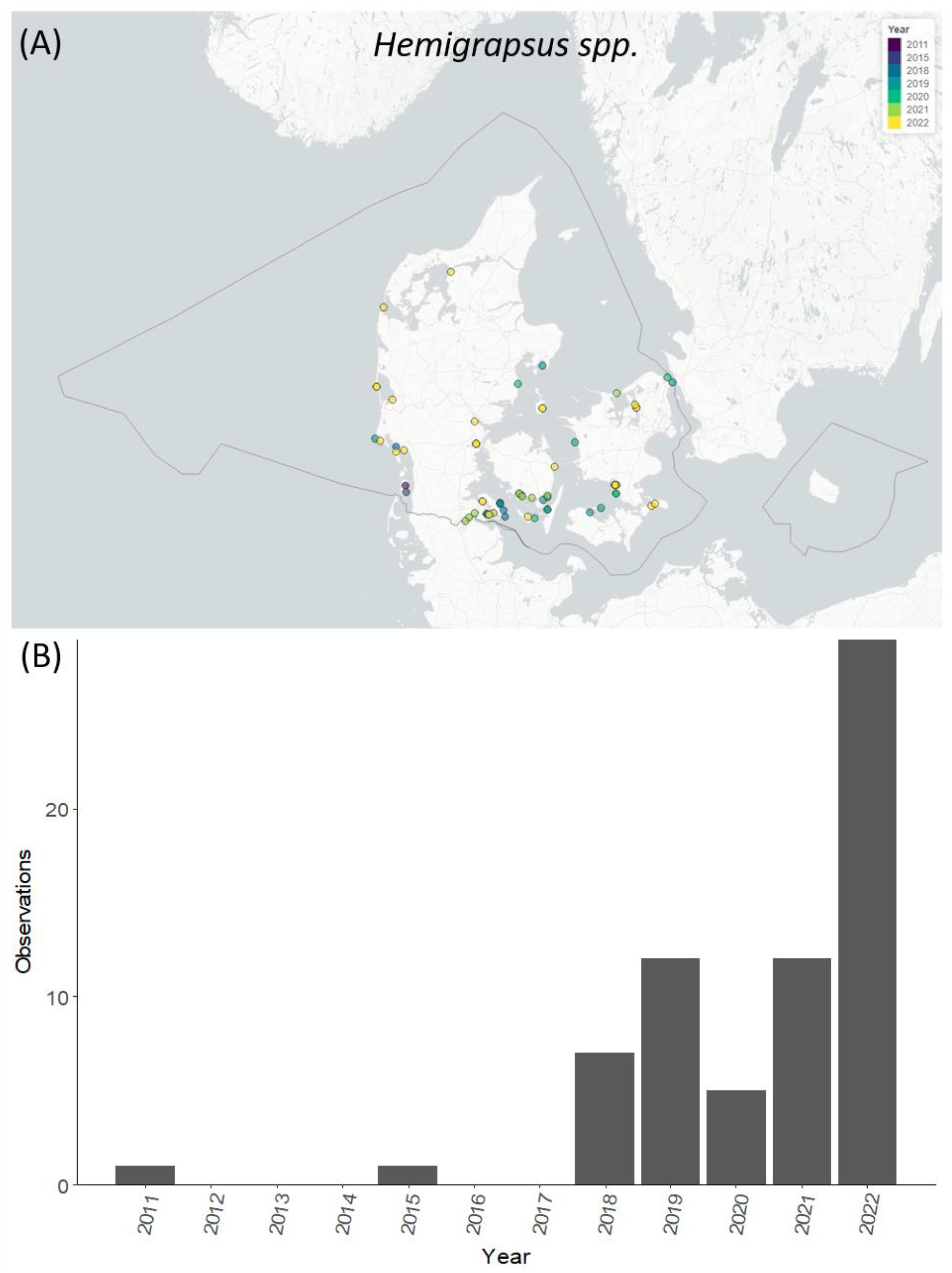
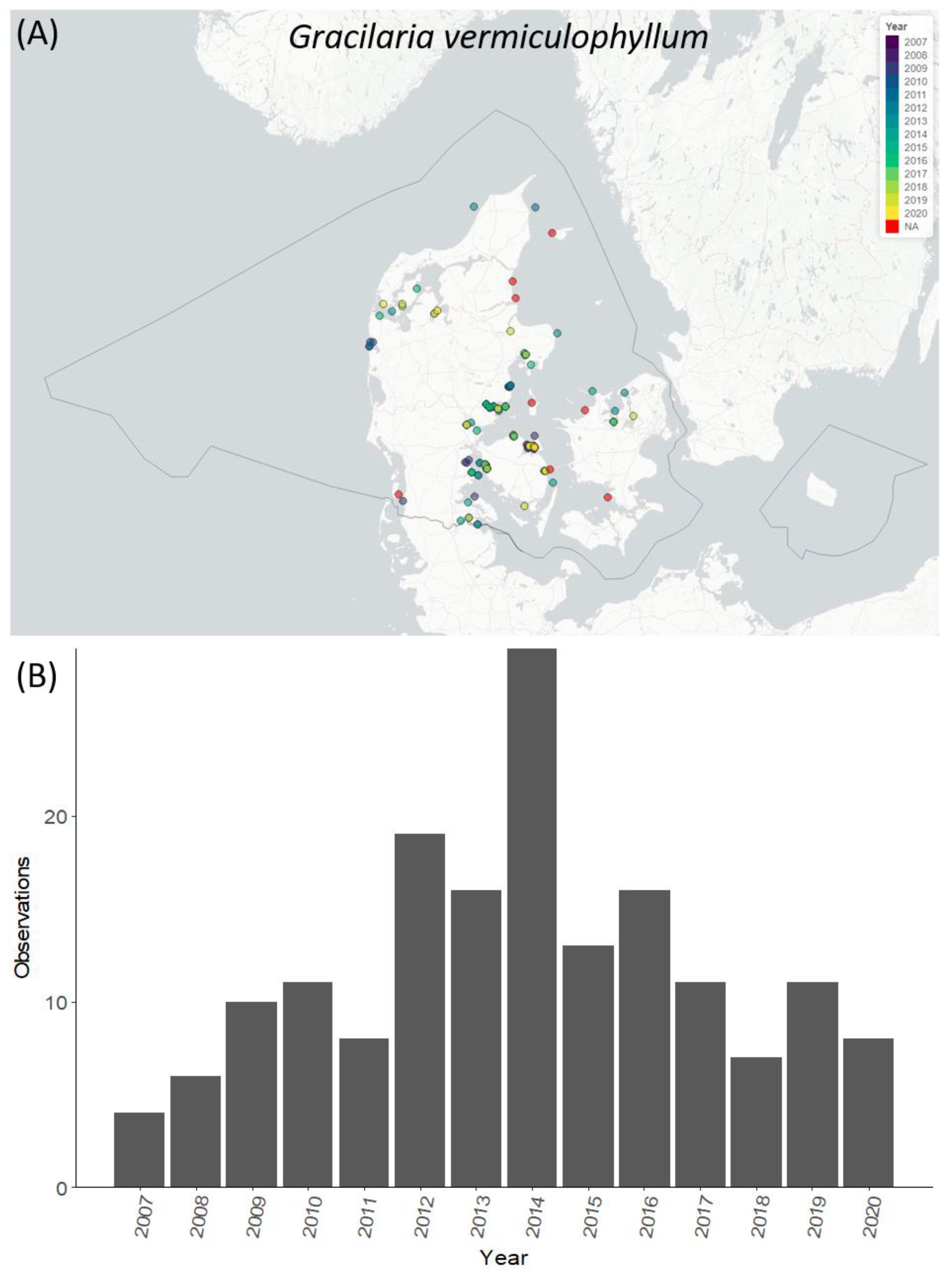
3.5. Temporal and Spatial Distribution of Selected Species
4. Discussion
4.1. Maintaining an Updated NIS List
4.2. Trends in New NIS Arrivals
4.3. Expert Evaluations
4.4. Temporal and Spatial Distribution of Selected NIS Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bax, N.; Williamson, A.; Aguero, M.; Gonzalez, E.; Geeves, W. Marine invasive alien species: A threat to global biodiversity. Mar. Policy 2003, 27, 313–323. [Google Scholar] [CrossRef]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Adams, T.P.; Miller, R.G.; Aleynik, D.; Burrows, M.T. Offshore marine renewable energy devices as stepping stones across biogeographical boundaries. J. Appl. Ecol. 2014, 51, 330–338. [Google Scholar] [CrossRef]
- Afonso, I.; Berecibar, E.; Castro, N.; Costa, J.L.; Frias, P.; Henriques, F.; Moreira, P.; Oliveira, P.M.; Silva, G.; Chainho, P. Assessment of the colonization and dispersal success of non-indigenous species introduced in recreational marinas along the estuarine gradient. Ecol. Indic. 2020, 113, 106147. [Google Scholar] [CrossRef]
- Occhipinti-Ambrogi, A. Global change and marine communities: Alien species and climate change. Mar. Pollut. Bull. 2007, 55, 342–352. [Google Scholar] [CrossRef]
- Floerl, O.; Rickard, G.; Inglis, G.; Roulston, H. Predicted effects of climate change on potential sources of non-indigenous marine species. Divers. Distrib. 2013, 19, 257–267. [Google Scholar] [CrossRef]
- Jaspers, C.; Marty, L.; Kiørboe, T. Selection for life-history traits to maximize population growth in an invasive marine species. Glob. Chang. Biol. 2018, 24, 1164–1174. [Google Scholar] [CrossRef]
- Geburzi, J.C.; McCarthy, M.L. How Do They Do It?—Understanding the Success of Marine Invasive Species. In Proceedings of the YOUMARES 8—Oceans Across Boundaries: Learning from Each Other, Cham, Switzerland, 30 August 2018; pp. 109–124. [Google Scholar]
- Thresher, R.E.; Kuris, A.M. Options for Managing Invasive Marine Species. Biol. Invasions 2004, 6, 295–300. [Google Scholar] [CrossRef]
- Pimentel, D.; McNair, S.; Janecka, J.; Wightman, J.; Simmonds, C.; O’Connell, C.; Wong, E.; Russel, L.; Zern, J.; Aquino, T.; et al. Economic and environmental threats of alien plant, animal, and microbe invasions. Agric. Ecosyst. Environ. 2001, 84, 1–20. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive Species, Environmental Change and Management, and Health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat. Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Parker, J.D.; Torchin, M.E.; Hufbauer, R.A.; Lemoine, N.P.; Alba, C.; Blumenthal, D.M.; Bossdorf, O.; Byers, J.E.; Dunn, A.M.; Heckman, R.W.; et al. Do invasive species perform better in their new ranges? Ecology 2013, 94, 985–994. [Google Scholar] [CrossRef] [PubMed]
- EU. Marine Strategy Framework Directive 2008/56/EC; Official Journal of the European Union: Maastricht, The Netherlands, 2008; pp. 19–40.
- EC. Commission Decision (EU) 2017/848 of 17 May 2017 Laying Down Criteria and Methodological Standards on Good Environmental Status of Marine Waters and Specifications and Standardized Methods for Monitoring and Assessment, and Repealing Decision 2010/477/EU; European Commission: Brussels, Belgium; Official Journal of the European Union: Maastricht, The Netherlands, 2017. Available online: https://eur-lex.europa.eu/eli/dec/2017/848/oj (accessed on 16 January 2023).
- Zenetos, A.; Tsiamis, K.; Galanidi, M.; Carvalho, N.; Bartilotti, C.; Canning-Clode, J.; Castriota, L.; Chainho, P.; Comas-González, R.; Costa, A.C.; et al. Status and Trends in the Rate of Introduction of Marine Non-Indigenous Species in European Seas. Diversity 2022, 14, 1077. [Google Scholar] [CrossRef]
- EU. Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32014R1143 (accessed on 17 January 2023).
- The Danish Environmental Protection Agency. Action Plan Against Invasive Species; Ministry of Environment and Food of Denmark, Danish Environmental Protection Agency: Copenhagen, Denmark, 2017; p. 75. Available online: https://eng.mst.dk/media/191170/04_uk_handlingsplan_invasive-arter_a4.pdf (accessed on 18 December 2022).
- Strandberg, B.; Andersen, P.; Bruhn, A.; Buur, H.; Carl, H.; Elmeros, M.; Fox, A.; Holmstrup, M.; Kjær, C.; Kristensen, H.V.; et al. Konsensus omkring vurdering af ikkehjemmehørende arter i Danmark. Vidensk. Rapp. Fra DCE 2023, in press. [Google Scholar]
- Jaspers, C.; Bezio, N.; Hinrichsen, H.-H. Diversity and Physiological Tolerance of Native and Invasive Jellyfish/Ctenophores along the Extreme Salinity Gradient of the Baltic Sea. Diversity 2021, 13, 57. [Google Scholar] [CrossRef]
- Middelboe, A.L.; Sand-Jensen, K.; Brodersen, K. Patterns of macroalgal distribution in the Kattegat-Baltic region. Phycologia 1997, 36, 208–219. [Google Scholar] [CrossRef]
- Josefson, A.B.; Hansen, J.L.S. Species richness of benthic macrofauna in Danish estuaries and coastal areas. Glob. Ecol. Biogeogr. 2004, 13, 273–288. [Google Scholar] [CrossRef]
- Staehr, P.A.U.; Dahl, K.; Buur, H.; Göke, C.; Sapkota, R.; Winding, A.; Panova, M.; Obst, M.; Sundberg, P. Environmental DNA Monitoring of Biodiversity Hotspots in Danish Marine Waters. Front. Mar. Sci. 2022, 8, 800474. [Google Scholar] [CrossRef]
- Conley, D.J.; Kaas, H.; Møhlenberg, F.; Rasmussen, B.; Windolf, J. Characteristics of Danish Estuaries. Estuaries 2000, 23, 820–837. [Google Scholar] [CrossRef]
- Hansen, F.T.; Gabellini, A.P.; Christensen, A. Ranking of Danish Ports According to Shipping Activities and to the Potential of Natural Dispersal of Nonindigenous Species; National Institute of Aquatic Resources, Technical University of Denmark: Lyngby, Denmark, 2020; p. 93. [Google Scholar]
- Stæhr, P.A.; Jakobsen, H.H.; Hansen, J.L.S.; Andersen, P.; Storr-Paulsen, M.; Christensen, J.; Lundsteen, S.; Göke, C.; Carausu, M.C. Trends in Records and Contribution of Nonindigenous Species (NIS) to Biotic Communities in Danish Marine Waters; Scientific Report No. 179; DCE—Danish Centre for Environment and Energy, Aarhus University: Aarhus, Denmark, 2016; p. 44. [Google Scholar]
- Jensen, K.R.; Knudsen, J. A summary of alien marine benthic invertebrates in Danish waters. Oceanol. Hydrobiol. Stud. 2005, 34 (Suppl. S1), 137–162. [Google Scholar]
- Thomsen, M.S.; Wernberg, T.; Staehr, P.; Krause-Jensen, D.; Risgaard-Petersen, N.; Silliman, B.R. Alien macroalgae in Denmark—A broad-scale national perspective. Mar. Biol. Res. 2007, 3, 61–72. [Google Scholar] [CrossRef]
- Fossing, H.; Stæhr, P.A. Non-Native Marine Species; Version 1; NOVANA Technical Instruction for Marine Monitoring; M30; Danish Centre for Environment and Energy, Aarhus University (DCE): Aarhus, Denmark, 2017. [Google Scholar]
- Andersen, J.H.; Brink, M.; Kallenbach, E.; Hesselsøe, M.; Knudsen, S.W.; Støttrup, J.G.; Møller, P.R.; Eikrem, W.; Fagerli, C.W.; Oug, E. Sampling Protocol for Monitoring of Non-Indigenous Species in Selected Danish Harbours; NIVA-rapport, 7175; Norwegian Institute for Water Research: Oslo, Norway, 2017; Available online: http://hdl.handle.net/11250/2449904 (accessed on 11 June 2022).
- EPA. Danmarks Havstrategi II. In Anden del Overvågningsprogram; Danish Environmental Protection Agency (Miljøstyrelsen): Odense, Denmark, 2020; 67p. Available online: https://www2.mst.dk/Udgiv/publikationer/2020/07/978-87-7038-209-0.pdf (accessed on 20 February 2021).
- CBD. Pathways of introduction of invasive species, their prioritization and management. In Proceedings of the Eighteenth Meeting, Montreal, QC, Canada, 23–28 June 2014. Available online: https://www.cbd.int/doc/meetings/sbstta/sbstta-18/official/sbstta-18-09-add1-en.pdf (accessed on 20 December 2022).
- Branquart, E.E. ISEIA Guidelines, Harmonia Information System: Guidelines for Environmental Impact Assessment and List Classification of Nonnative Organisms in Belgium, Version 2.6 (07/12/2009); ISEIA_protocol (biodiversity.be); Belgian Forum on Invasive Species. 2009. Available online: https://ias.biodiversity.be/documents/ISEIA_protocol.pdf (accessed on 21 September 2021).
- Tiselius, P.; Møller, L.F. Community cascades in a marine pelagic food web controlled by the non-visual apex predator Mnemiopsis leidyi. J. Plankton. Res. 2017, 39, 271–279. [Google Scholar] [CrossRef]
- Jaspers, C.; Huwer, B.; Antajan, E.; Hosia, A.; Hinrichsen, H.-H.; Biastoch, A.; Angel, D.; Asmus, R.; Augustin, C.; Bagheri, S.; et al. Ocean current connectivity propelling the secondary spread of a marine invasive comb jelly across western Eurasia. Glob. Ecol. Biogeogr. 2018, 27, 814–827. [Google Scholar] [CrossRef]
- Tsiamis, K.; Palialexis, A.; Connor, D.; Antoniadis, S.; Bartilotti, C.; Bartolo, A.G.; Berggreen, U.C.; Boschetti, S.; Buschbaum, C.; Canning-Clode, J.; et al. Marine Strategy Framework Directive—Descriptor 2, Non-Indigenous Species; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-32257-3. [CrossRef]
- Kirkegaard, J.B. Havbørsteorme II. In Sedentaria. Danmarks Fauna 86; Dansk Naturhistorisk Forening: København, Denmark, 1996; pp. 1–451. [Google Scholar]
- Rasmussen, E. Systematics and ecology of the Isefjord marine fauna (Denmark). Ophelia 1973, 11, 1–507. [Google Scholar] [CrossRef]
- Smidt, E.L.B. Animal production in the Danish Wadden Sea. In Meddelelser fra Kommissionen for Danmarks Fiskeri- og Havundersøgelser; Fiskeri 11: 1–151; C.A. Reitzels Forlag: Copenhagen, Denmark, 1951; pp. 63–65. [Google Scholar]
- Andersen, J.H.; Kallenbach, E.; Kjeldgaard, M.B.; Knudsen, S.W.; Eikrem, W.; Fagerli, C.; Oug, E.; Dahle, T.; Thaulow, J.; Gitmark, J.; et al. A Baseline Study of the Occurrence of Non-Indigenous Species in Danish Harbours; NIVA Denmark Rapport L.nr. 7769-2022; NIVA Denmark, DTULitehauz: Kongens Lyngby, Denmark, 2022; 170p. [Google Scholar]
- Blake, J.A.; Göransson, P. Redescription of Tharyx killariensis (Southern) from Ireland and description of two new species of Tharyx from the Kattegat, Sweden (Polychaeta, Cirratulidae). Zootaxa 2015, 4039, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.T. Macrozoobenthos on an intertidal mudflat in the Danish Wadden Sea: Comparisons of surveys made in the 1930s, 1940s and 1980s. Helgoländer Meeresunters 1992, 46, 363–376. [Google Scholar] [CrossRef]
- Reise, K.; Buschbaum, C.; Lackschewitz, D.; Thieltges, D.W.; Waser, A.M.; Wegner, K.M. Introduced species in a tidal ecosystem of mud and sand: Curse or blessing? Mar. Biodivers. 2023, 53, 5. [Google Scholar] [CrossRef]
- Bastrop, R.; Blank, M. Multiple Invasions—A Polychaete Genus Enters the Baltic Sea. Biol. Invasions 2006, 8, 1195–1200. [Google Scholar] [CrossRef]
- Blank, M.; Laine, A.O.; Jürss, K.; Bastrop, R. Molecular identification key based on PCR/RFLP for three polychaete sibling species of the genus Marenzelleria, and the species’ current distribution in the Baltic Sea. Helgol. Mar. Res. 2008, 62, 129–141. [Google Scholar] [CrossRef]
- Blank, M.; Bastrop, R. Phylogeny of the mud worm genus Marenzelleria (Polychaeta, Spionidae) inferred from mitochondrial DNA sequences. Zool. Scr. 2009, 38, 313–321. [Google Scholar] [CrossRef]
- Kirkegaard, J.B. Ny amerikansk havbørsteorm i Ringkøbing Fjord. Flora Og Fauna 1990, 96, 63–65. [Google Scholar]
- Kristensen, E.; Hansen, T.; Delefosse, M.; Banta, G.T.; Quintana, C.O. Contrasting effects of the polychaetes Marenzelleria viridis and Nereis diversicolor on benthic metabolism and solute transport in sandy coastal sediment. Mar. Ecol. Prog. Ser. 2011, 425, 125–139. [Google Scholar] [CrossRef]
- Delefosse, M.; Banta, G.T.; Canal-Vergés, P.; Penha-Lopes, G.; Quintana, C.O.; Valdemarsen, T.; Kristensen, E. Macrobenthic community response to the Marenzelleria viridis (Polychaeta) invasion of a Danish estuary. Mar. Ecol. Prog. Ser. 2012, 461, 83–94. [Google Scholar] [CrossRef]
- Quintana, C.O.; Kristensen, E.; Valdemarsen, T. Impact of the invasive polychaete Marenzelleria viridis on the biogeochemistry of sandy marine sediments. Biogeochemistry 2013, 115, 95–109. [Google Scholar] [CrossRef]
- Radashevsky, V.I.; Pankova, V.V.; Malyar, V.V.; Cerca, J.; Struck, T.H. A review of the worldwide distribution of Marenzelleria viridis, with new records for M. viridis, M. neglecta and Marenzelleria sp. (Annelida: Spionidae). Zootaxa 2021, 5081, 353–372. [Google Scholar] [CrossRef]
- Stæhr, P.A.; Jakobsen, H.H.; Hansen, J.L.S.; Andersen, P.; Christensen, J.; Göke, C.; Thomsen, M.S.; Stebbing, P.D. Trends in records and contribution of non-indigenous and cryptogenic species to marine communities in Danish waters: Potential indicators for assessing impact. Aquat. Invasions 2020, 15, 217–244. [Google Scholar] [CrossRef]
- Andersen, J.H.; Knudsen, S.W.; Murray, C.; Carl, H.; Møller, P.R.; Hesselsøe, M. Ikke-Hjemme-Hørende Arter I Marine Områder. In NIVA Danmark Rapport; Norwegian Institute for Water Research: Oslo, Norway, 2021; 59p, Available online: https://niva.brage.unit.no/niva–xmlui/bitstream/handle/11250/2789601/7658-2021%2Bhigh.pdf?sequence=1 (accessed on 29 October 2022).
- Schuchert, P. Revision of the European athecate hydroids and their medusae (Hydrozoa, Cnidaria): Families Oceanidae and Pachycordylidae. Rev. Suisse Zool. 2004, 111, 315–369. [Google Scholar] [CrossRef]
- ICES. Report of the Working Group on Introduction and Transfers of Marine Organisms (WGITMO) 16–18 March 2011, Nantes, France. ICES CM 2011/ACOM 2011, 29, 180. [Google Scholar]
- Stephensen, K.; Storkrebs, I. Skjoldkrebs. In Danmarks Fauna 9. Naturhistorisk Forening; Gads Forlag: København, Danmark, 1910; 193p. [Google Scholar]
- Reuschel, S.; Cuesta, J.A.; Schubart, C.D. Marine biogeographic boundaries and human introduction along the European coast revealed by phylogeography of the prawn Palaemon elegans. Mol. Phylogenet Evol. 2010, 55, 765–775. [Google Scholar] [CrossRef]
- González-Castellano, I.; González-López, J.; González-Tizón, A.M.; Martínez-Lage, A. Genetic diversity and population structure of the rockpool shrimp Palaemon elegans based on microsatellites: Evidence for a cryptic species and differentiation across the Atlantic–Mediterranean transition. Sci. Rep. 2020, 10, 10784. [Google Scholar] [CrossRef] [PubMed]
- González-Castellano, I.; Pons, J.; González-Ortegón, E.; Martínez-Lage, A. Mitogenome phylogenetics in the genus Palaemon (Crustacea: Decapoda) sheds light on species crypticism in the rockpool shrimp P. elegans. PLoS ONE 2020, 15, e0237037. [Google Scholar] [CrossRef]
- Nielsen, R.; Lundsteen, S.; Brodie, J. Seaweeds of Denmark. In Red Algae (Rhodophyta); The Royal Danish Academy of Sciences and Letters: Copenhagen, Denmark, 2022; Volume 1. [Google Scholar]
- Nielsen, R.; Lundsteen, S.; Brodie, J. Seaweeds of Denmark. In Brown Algae (Phaeophyceae). Green Algae (Chlorophyta); The Royal Danish Academy of Sciences and Letters: Copenhagen, Denmark, 2022; Volume 2. [Google Scholar]
- Dolmer, P.; Holm, M.W.; Strand, Å.; Lindegarth, S.; Bodvin, T.; Norling, P.; Mortensen, S. The invasive Pacific oyster, Crassostrea gigas, in Scandinavian coastal waters: A risk assessment on the impact in different habitats and climate conditions. In Fisken og Havet; Institute of Marine Research: Bergen, Norway, 2014; Volume 12, 67p, Available online: https://imr.brage.unit.no/imr-xmlui/bitstream/handle/11250/193021/FoH_2-2014.pdf (accessed on 7 January 2023).
- Mortensen, S.; Bodvin, T.; Strand, Å.; Holm, M.W.; Dolmer, P. Effects of a bio-invasion of the Pacific oyster, Crassostrea gigas (Thunberg, 1793) in five shallow water habitats in Scandinavia. Manag. Biol. Invasions 2017, 8, 543–552. [Google Scholar] [CrossRef]
- Nielsen, M.; Hansen, B.W.; Vismann, B. Feeding traits of the European flat oyster, Ostrea edulis, and the invasive Pacific oyster, Crassostrea gigas. Mar. Biol. 2016, 164, 6. [Google Scholar] [CrossRef]
- Hansen, B.W.; Dolmer, P.; Vismann, B. Too late for regulatory management on Pacific oysters in European coastal waters? J. Sea Res. 2023, 191, 102331. [Google Scholar] [CrossRef]
- Petersen, J.K. Menneskeskabte Påvirkninger af Havet—Andre Presfaktorer end Næringsstoffer og Klimaforandringer. DTU Aqua-Rapport nr. 336–2018; Institut for Akvatiske Ressourcer, Danmarks Tekniske Universitet. 2018. 118p. + bilag. Available online: https://mst.dk/natur-vand/vandmiljoe/vandomraadeplaner/vandplanprojekter/kystvandsprojekter/andre-presfaktorer-end-naeringsstoffer-og-klima/ (accessed on 19 February 2021).
- Petersen, J.K.; Behrens, J.; van Deurs, M.; Dinesen, G.; Jaspers, C.; Møller, L.F.; Plet-Hansen, S.K. Andre presfaktorer end næringsstoffer og klimaforandringer—Vurdering af de invasive arter amerikansk ribbegople og sortmundet kutling. DTU Aqua-RapportNr. 365–2020, 33 p. 2020. Available online: https://mst.dk/media/205229/vurdering-af-de-invasive-arter-amerikansk-ribbegople-og-sortmundet-kutling-rapport-fra-dtu-nr-365-2020.pdf (accessed on 19 February 2021).
- EU. Regulation (EU) 2016/1141 of 13 July 2016 Adopting a List of Invasive Alien Species of Union Concern Pursuant to Regulation (EU) No 1143/2014 of the European Parliament and of the Council. EU Regulation 2016/1141. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L:2016:189:FULL&from=EN (accessed on 17 January 2023).
- Herborg, L.M.; Rushton, S.P.; Clare, A.S.; Bentley, M.G. Spread of the Chinese mitten crab (Eriocheir sinensis H. Milne Edwards) in Continental Europe: Analysis of a historical data set. Hydrobiologia 2003, 503, 21–28. [Google Scholar] [CrossRef]
- Möbius, K. On experiments, begun in 1880, to plant American oysters in the western Baltic, and the usefulness of continuing these experiments with the aid of the German Fishery Association. Bull. United States Fish Comm. 1883, 3, 213–217. Available online: https://www.biodiversitylibrary.org/item/22564#page/225/mode/1up (accessed on 27 January 2023).
- Möbius, K. Report on planting Canadian oysters near the Island of Aaröe, in the Little Belt, November 6, 1884. Bull. United States Fish Comm. 1885, 5, 257–260. Available online: https://www.biodiversitylibrary.org/item/22628#page/275/mode/1up (accessed on 27 January 2023).
- Gittenberger, A. Recent population expansions of non-native ascidians in The Netherlands. J. Exp. Mar. Biol. Ecol. 2007, 342, 122–126. [Google Scholar] [CrossRef]
- Graham, J.; Collins, C.; Lacaze, J.-P.; Brown, L.; McCollin, T. Molecular identification of Didemnum vexillum Kott, 1982 from sites around the UK coastline. BioInvasions Rec. 2015, 4, 171–177. [Google Scholar] [CrossRef]
- Carlton, J.T.; Thompson, J.K.; Schemel, L.E.; Nichols, F.H. Remarkable invasion of San Francisco Bay (California, USA), by the Asian clam Potamocorbula amurensis. I. Introduction and dispersal. Mar. Ecol. Prog. Ser. 1990, 66, 81–94. [Google Scholar] [CrossRef]
- Charles, L. Premier signalement de Musculista senhousia (Benson in Cantor, 1842) (Bivalvia, Mytilidae) sur la côte atlantique française; nouvelle espèce invasive dans le basin d’Arcachon. Bull. Soc. Linn. Bordeaux 2007, 35, 45–52. [Google Scholar]
- Faasse, M.; Bayha, K.M. The ctenophore Mnemiopsis leidyi A. Agassiz 1865 in coastal waters of the Netherlands: An unrecognized invasion? Aquat. Invasions 2006, 1, 270–277. [Google Scholar] [CrossRef]
- Watson, G.J.; Dyos, J.; Barfield, P.; Stebbing, P.; Dey, K.G. Evidence for self-sustaining populations of Arcuatula senhousia in the UK and a review of this species’ potential impacts within Europe. Sci. Rep. 2021, 11, 9678. [Google Scholar] [CrossRef]
- Tendal, O.S.; Flintegaard, H. Et fund af en sjælden krabbe i danske farvande: Den blå svømmekrabbe, Callinectes sapidus (Crustacea; Decapoda; Portunidae). Flora Og Fauna 2007, 113, 53–56. [Google Scholar]
- Shiganova, T.A.; Riisgard, H.U.; Ghabooli, S.; Tendal, O.S. First report on Beroe ovata in an unusual mixture of ctenophores in the Great Belt (Denma. Aquat. Invasions 2014, 9, 111–116. [Google Scholar] [CrossRef]
- Riisgård, H.U.; Goldstein, J. Jellyfish and Ctenophores in Limfjorden (Denmark)—Mini-Review, with Recent New Observations. J. Mar. Sci. Eng. 2014, 2, 593–615. [Google Scholar] [CrossRef]
- Shiganova, T.A.; Bulgakova, Y.V.; Volovik, S.P.; Mirzoyan, Z.A.; Dudkin, S.I. The new invader Beroe ovata Mayer 1912 and its effect on the ecosystem in the northeastern Black Sea. Hydrobiol. 2001, 451, 187–197. [Google Scholar] [CrossRef]
- Wootton, M.; Fischer, A.C.; Ostle, C.; Skinner, J.; Stevens, D.P.; Johns, D.G. Using the continuous plankton recorder to study the distribution and ecology of marine pelagic copepods. In Chapter 2 in: Trends in Copepod Studies—Distribution, Biology and Ecology; Uttieri, M., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 13–42. ISBN 978-1-53612-593-1. [Google Scholar]
- Rewicz, T.; Grabowski, M.; Tończyk, G.; Konopacka, A.; Bącela-Spychalska, K. Gammarus tigrinus Sexton, 1939 continues its invasion in the Baltic Sea: First record from Bornholm (Denmark). BioInvasions Rec. 2019, 8, 862–870. [Google Scholar] [CrossRef]
- Jänes, H.; Kotta, J.; Herkül, K. High fecundity and predation pressure of the invasive Gammarus tigrinus cause decline of indigenous gammarids. Estuar. Coast. Shelf Sci. 2015, 165, 185–189. [Google Scholar] [CrossRef]
- Kotta, J.; Pärnoja, M.; Katajisto, T.; Lehtiniemi, M.; Malavin, S.A.; Reisalu, G.; Panov, V.E. Is a rapid expansion of the invasive amphipod Gammarus tigrinus Sexton, 1939 associated with its niche selection: A case study in the Gulf of Finland, the Baltic Sea. Aquat. Invasions 2013, 8, 319–332. [Google Scholar] [CrossRef]
- Jazdzewski, K.; Konopacka, A.; Grabowski, M. Four Ponto-Caspian and one American gammarid species (Crustacea, Amphipoda) recently invading Polish waters. Contrib. Zool. 2002, 71, 115–122. [Google Scholar] [CrossRef]
- Meßner, U.; Zettler, M.L. The conquest (and avoidance?) of the brackish environment by Ponto-Caspian amphipods: A case study of the German Baltic Sea. BioInvasions Rec. 2018, 7, 269–278. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Graca, B. Effect of salinity on the distribution of Ponto-Caspian gammarids in a non-native area—Environmental and experimental study. Mar. Biol. Res. 2018, 14, 183–190. [Google Scholar] [CrossRef]
- Schiller, J.; Lackschewitz, D.; Buschbaum, C.; Reise, K.; Pang, S.; Bischof, K. Heading northward to Scandinavia: Undaria pinnatifida in the northern Wadden Sea. Bot. Mar. 2018, 61, 365–371. [Google Scholar] [CrossRef]
- Sundström, B. The Marine Diatom Genus Rhizosolenia: A New Approach to the Taxonomy; Lund University: Lund, Sweden, 1986. [Google Scholar]
- Karpinsky, M.G. Pseudosolenia calcar-avis (Bacillariophyta, Centrophyceae) in the Caspian Sea. Russ. J. Biol. Invasions 2010, 1, 81–86. [Google Scholar] [CrossRef]
- Boonprakob, A.; Lundholm, N.; Medlin, L.K.; Moestrup, Ø. The morphology and phylogeny of the diatom genera Rhizosolenia, Proboscia, Pseudosolenia and Neocalyptrella from Gulf of Thailand and the Andaman Sea, with description of Rhizosolenia loanicola sp. nov., Proboscia siamensis sp. nov. and Probosciales ord. nov. Diatom Res. 2021, 36, 143–184. [Google Scholar] [CrossRef]
- Yun, S.M.; Lee, J.H. Morphology and distribution of some marine diatoms, family Rhizosoleniaceae, genus Proboscia, Neocalyptrella, Pseudosolenia, Guinardia, and Dactyliosolen in Korean coastal waters. Algae 2011, 26, 299–315. [Google Scholar] [CrossRef]
- Hansen-Ostenfeld, C. De Danske farvandes plankton I aarene 1898—1901. Phytoplankton og protozoer. 1. Phytoplanktonets livskaar og biologi, samt de i vore farvande iagttagne phytoplanktonters optræden og forekomst. In Mémoires de l’Académie Royale des Sciences et des Lettres de Danemark, Copenhague; 7me série; Section des Sciences: Copenhague, Danemark, 1913; 364p. [Google Scholar]
- Henriksen, P. Long-term changes in phytoplankton in the Kattegat, the Belt Sea, the Sound and the western Baltic Sea. J. Sea Res. 2009, 61, 114–123. [Google Scholar] [CrossRef]
- Daugbjerg, N.; Hansen, G.; Larsen, J.; Moestrup, Ø. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia 2000, 39, 302–317. [Google Scholar] [CrossRef]
- Gómez, F. Phytoplankton invasions: Comments on the validity of categorizing the non-indigenous dinoflagellates and diatoms in European Seas. Mar. Pollut. Bull. 2008, 56, 620–628. [Google Scholar] [CrossRef]
- Bjergskov, T.; Larsen, J.; Moestrup, O.; Sørensen, H.M.; Krogh, P. Toksiske og Potentielt Toksiske Alger i Danske Farvande, 1st ed.; Fiskeriministeriets Industritilsyn: Copenhagen, Denmark, 1990.
- Bjørnsen, P.K.; Nielsen, T.G. Decimeter scale heterogeneity in the plankton during a pycnocline bloom of Gyrodinium aureolum. Mar. Ecol. Prog. Ser. 1991, 73, 263–267. [Google Scholar] [CrossRef]
- Chang, F.H. Toxic effects of three closely-related dinoflagellates, Karenia concordia, K. brevisulcata and K. mikimotoi (Gymnodiniales, Dinophyceae) on other microalgal species. Harmful Algae 2011, 10, 181–187. [Google Scholar] [CrossRef]
- Niu, X.; Xu, S.; Yang, Q.; Xu, X.; Zheng, M.; Li, X.; Guan, W. Toxic effects of the dinoflagellate Karenia mikimotoi on zebrafish (Danio rerio) larval behavior. Harmful Algae 2021, 103, 101996. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Bullock, A.; Turners, M.; Jones, K.; Tett, P. Mortalities of Salmo gairdneri exposed to cultures of Gyrodinium aureolum. J. Mar. Biol. Assoc. UK 1983, 63, 741–743. [Google Scholar] [CrossRef]
- Mitchell, S.; Rodger, H. Pathology of wild and cultured fish affected by a Karenia mikimotoi bloom in Ireland, 2005. Bull. Eur. Assoc. Fish Pathol. 2007, 27, 39. [Google Scholar]
- Colin, S.P.; Costello, J.H.; Hansson, L.J.; Titelman, J.; Dabiri, J.O. Stealth predation and the predatory success of the invasive ctenophore Mnemiopsis leidyi. Proc. Natl. Acad. Sci. USA 2010, 107, 17223–17227. [Google Scholar] [CrossRef]
- Sullivan, L.J.; Gifford, D.J. Diet of the larval ctenophore Mnemiopsis leidyi A. Agassiz (Ctenophora, Lobata). J. Plankton. Res. 2004, 26, 417–431. [Google Scholar] [CrossRef]
- Tendal, O.S.; Jensen, K.; Riisgard, H.U. Invasive ctenophore Mneniopsis leidyi widely distributed in Danish waters. Aquat. Invasions 2007, 2, 455–460. [Google Scholar] [CrossRef]
- Antajan, E.; Bastian, T.; Raud, T.; Brylinski, J.-M.; Hoffman, S.; Breton, G.; Cornille, V.; Delegrange, A.; Vincent, D. The invasive ctenophore Mnemiopsis leidyi A. Agassiz, 1865 along the English Channel and the North Sea French coasts: Another introduction pathway in northern European waters? Aquat. Invasions 2014, 9, 167–173. [Google Scholar] [CrossRef]
- Oliveira, O.M.P. The presence of the ctenophore Mnemiopsis leidyi in the Oslofjorden and considerations on the initial invasion pathways to the North and Baltic Seas Aquat. Invasions 2007, 2, 185–189. [Google Scholar] [CrossRef]
- Jaspers, C.; Møller, L.F.; Kiørboe, T. Salinity gradient of the Baltic Sea limits the reproduction and population expansion of the newly invaded comb jelly Mnemiopsis leidyi. PLoS ONE 2011, 6, e24065. [Google Scholar] [CrossRef] [PubMed]
- Haraldsson, M.; Jaspers, C.; Tiselius, P.; Aksnes, D.L.; Andersen, T.; Titelman, J. Environmental constraints of the invasive Mnemiopsis leidyi in Scandinavian waters. Limnol. Oceanogr. 2013, 58, 37–48. [Google Scholar] [CrossRef]
- Jaspers, C.; Costello, J.H.; Colin, S.P. Carbon content of Mnemiopsis leidyi eggs and specific egg production rates in northern Europe. J. Plankton. Res. 2014, 37, 11–15. [Google Scholar] [CrossRef]
- Jaspers, C.; Møller, E.F.; Kiørboe, T. Reproduction rates under variable food conditions and starvation in Mnemiopsis leidyi: Significance for the invasion success of a ctenophore. J. Plankton Res. 2015, 37, 1011–1018. Available online: https://academic.oup.com/plankt/article/37/5/1011/1552108 (accessed on 2 February 2023). [CrossRef]
- Riisgård, H.U.; Bøttiger, L.; Madsen, C.V.; Purcell, J.E. Invasive ctenophore Mnemiopsis leidyi in Limfjorden (Denmark) in late summer 2007—Assessment of abundance and predation effects. Aquat. Invasions 2007, 2, 395–401. [Google Scholar] [CrossRef]
- Jaspers, C.; Haraldsson, M.; Lombard, F.; Bolte, S.; Kiørboe, T. Seasonal dynamics of early life stages of invasive and native ctenophores give clues to invasion and bloom potential in the Baltic Sea. J. Plankton Res. 2013, 35, 582–594. [Google Scholar] [CrossRef]
- Gorokhova, E.; Lehtiniemi, M.; Viitasalo-Frösen, S.; Haddock, S.H.D. Molecular evidence for the occurrence of ctenophore Mertensia ovum in the northern Baltic Sea and implications for the status of the Mnemiopsis leidyi invasion. Limnol. Oceanogr. 2009, 54, 2025–2033. [Google Scholar] [CrossRef]
- Jensen, K.R. NOBANIS—Invasive Alien Species Fact Sheet—Crepidula fornicata. Identification Key to Marine Invasive Species in Nordic Waters—NOBANIS. 2010. Available online: www.nobanis.org (accessed on 30 January 2023).
- Spärck, R. On the occurrence of Crepidula fornicata (L.) in the Limfjord. Rep. Dan. Biol. Stn. 1935, 40, 43–44. [Google Scholar]
- Thieltges, D.; Strasser, M.; Reise, K. The American slipper limpet Crepidula fornicata (L.) in the northern Wadden Sea 70 years after its introduction. Helgol. Mar. Res. 2003, 57, 27–33. [Google Scholar] [CrossRef]
- Spärck, R. On the distribution of the slipper-limpet (Crepidula fornicata) in Danish waters. Rep. Dan. Biol. Stn. 1950, 52, 48–50. [Google Scholar]
- Thieltges, D.W.; Strasser, M.; van Beusekom, J.E.E.; Reise, K. Too cold to prosper—Winter mortality prevents population increase of the introduced American slipper limpet Crepidula fornicata in northern Europe. J. Exp. Mar. Biol. Ecol. 2004, 311, 375–391. [Google Scholar] [CrossRef]
- Noisette, F.; Richard, J.; Le Fur, I.; Peck, L.S.; Davoult, D.; Martin, S. Metabolic responses to temperature stress under elevated pCO2 in Crepidula fornicata. J. Molluscan Stud. 2014, 81, 238–246. [Google Scholar] [CrossRef]
- Newell, R.C.; Kofoed, L.H. Adjustment of the components of energy balance in the gastropod Crepidula fornicata in response to thermal acclimation. Mar. Biol. 1977, 44, 275–286. [Google Scholar] [CrossRef]
- Pechenik, J.A. The relationship between temperature, growth rate, and duration of planktonic life for larvae of the gastropod Crepidula fornicata (L.). J. Exp. Mar. Biol. Ecol. 1984, 74, 241–257. [Google Scholar] [CrossRef]
- Rayment, W.J. Crepidula Fornicata. Slipper Limpet; Marine Biological Association of the United Kingdom: Plymouth, UK, 2008. [Google Scholar]
- Richard, J.; Huet, M.; Thouzeau, G.; Paulet, Y.-M. Reproduction of the invasive slipper limpet, Crepidula fornicata, in the Bay of Brest, France. Mar. Biol. 2006, 149, 789–801. [Google Scholar] [CrossRef]
- Pechenik, J.A.; Diederich, C.M.; Browman, H.I.; Jelmert, A. Fecundity of the invasive marine gastropod Crepidula fornicata near the current northern extreme of its range. Invertebr. Biol. 2017, 136, 394–402. [Google Scholar] [CrossRef]
- Vogensen, T.K.; Nielsen, M.R.; Jensen, K.T. Klippekrabber i Vadehavet: En trussel mod den lokale flora og fauna? Flora Fauna 2020, 125, 15–23. [Google Scholar]
- Nour, O.M.; Pansch, C.; Lenz, M.; Wahl, M.; Clemmesen, C.; Stumpp, M. Impaired larval development at low salinities could limit the spread of the non-native crab Hemigrapsus takanoi in the Baltic Sea. Aquat. Biol. 2021, 30, 85–99. [Google Scholar] [CrossRef]
- van den Brink, A.; Godschalk, M.; Smaal, A.; Lindeboom, H.; McLay, C. Some like it hot: The effect of temperature on brood development in the invasive crab Hemigrapsus takanoi (Decapoda: Brachyura: Varunidae). J. Mar. Biol. Assoc. UK 2013, 93, 189–196. [Google Scholar] [CrossRef]
- Epifanio, C.E. Invasion biology of the Asian shore crab Hemigrapsus sanguineus: A review. J. Exp. Mar. Biol. Ecol. 2013, 441, 33–49. [Google Scholar] [CrossRef]
- Geburzi, J.C.; Brandis, D.; Buschbaum, C. Recruitment patterns, low cannibalism and reduced interspecific predation contribute to high invasion success of two Pacific crabs in northwestern Europe. Estuar. Coast. Shelf Sci. 2018, 200, 460–472. [Google Scholar] [CrossRef]
- Bleile, N.; Thieltges, D.W. Prey preferences of invasive (Hemigrapsus sanguineus, H. takanoi) and native (Carcinus maenas) intertidal crabs in the European Wadden Sea. J. Mar. Biol. Assoc. UK 2021, 101, 811–817. [Google Scholar] [CrossRef]
- Cornelius, A.; Wagner, K.; Buschbaum, C. Prey preferences, consumption rates and predation effects of Asian shore crabs (Hemigrapsus takanoi) in comparison to native shore crabs (Carcinus maenas) in northwestern Europe. Mar. Biodivers. 2021, 51, 75. [Google Scholar] [CrossRef]
- Landschoff, J.; Lackschewitz, D.; Kesy, K.; Reise, K. Globalisation pressure and habitat change: Pacific rocky shore crabs invade armored shorelines in the Atlantic Wadden Sea. Aquat. Invasions 2013, 8, 77–87. [Google Scholar] [CrossRef]
- Christensen, T. Sargassotang, en ny algeslægt i Danmark. Urt 1984, 4, 99–104. [Google Scholar]
- Stæhr, P.A.; Nielsen, M.M.; Göke, C.; Petersen, J.K. Andre presfaktorer end næringsstoffer og klimaforanderinger—Effeketer af sargassotang på den øvrige marine vegetation. In DTU Aqua-Rapport nr. 353–2019; Institut for Akvatiske Ressourcer, Tekniske Universitet: Copenhagen, Danmark, 2019; 28p. [Google Scholar]
- Stæhr, P.A.; Pedersen, M.F.; Thomsen, M.; Wernberg, T.; Krause-Jensen, D. Invasion of Sargassum muticum in Limfjorden (Denmark) and its possible impact on the indigenous macroalgal community. Mar. Ecol. Prog. Ser. 2000, 207, 79–88. [Google Scholar] [CrossRef]
- Canal-Vergés, P.; Potthoff, M.; Hansen, F.T.; Holmboe, N.; Rasmussen, E.K.; Flindt, M.R. Eelgrass re-establishment in shallow estuaries is affected by drifting macroalgae—Evaluated by agent-based modeling. Ecol. Model. 2014, 272, 116–128. [Google Scholar] [CrossRef]
- Pedersen, M.F.; Staehr, P.A.; Wernberg, T.; Thomsen, M.S. Biomass dynamics of exotic Sargassum muticum and native Halidrys siliquosa in Limfjorden, Denmark—Implications of species replacements on turnover rates. Aquat. Bot. 2005, 83, 31–47. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Nejrup, L.B.; Pedersen, M.F. The effect of temporal variability in salinity on the invasive red alga Gracilaria vermiculophylla. Eur. J. Phycol. 2012, 47, 254–263. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Holmer, M. Potential effects of the invasive species Gracilaria vermiculophylla on Zostera marina metabolism and survival. Mar. Environ. Res. 2010, 69, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Staehr, P.A.; Nejrup, L.B.; Schiel, D.R. Effects of the invasive macroalgae Gracilaria vermiculophylla on two co-occurring foundation species and associated invertebrates. Aquat. Invasions 2013, 8, 133–145. [Google Scholar] [CrossRef]
- Hammann, M.; Buchholz, B.; Karez, R.; Weinberger, F. Direct and indirect effects of Gracilaria vermiculophylla on native Fucus vesiculosus. Aquat. Invations 2013, 8, 121–132. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current Status of the Algae Production Industry in Europe: An Emerging Sector of the Blue Bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Jude, D.J.; Reider, R.H.; Smith, G.R. Establishment of Gobiidae in the Great Lakes Basin. Can. J. Fish. Aquat. Sci. 1992, 49, 416–421. [Google Scholar] [CrossRef]
- van Beek, G.C.W. The round goby Neogobius melanostomus first recorded in the Netherlands Aquat. Invasions 2006, 1, 42–43. [Google Scholar] [CrossRef]
- Czugala, A.; Wozniczka, A. The River Odra estuary—Another Baltic Sea area colonized by the round goby Neogobius melanostomus Pallas, 1811. Aquat. Invasions 2010, 5, S61–S65. [Google Scholar] [CrossRef]
- Azour, F.; van Deurs, M.; Behrens, J.; Carl, H.; Hussy, K.; Greisen, K.; Ebert, R.; Moller, P.R. Invasion rate and population characteristics of the round goby Neogobius melanostomus: Effects of density and invasion history. Aquat. Biol. 2015, 24, 41–52. [Google Scholar] [CrossRef]
- Greisen, K.; Ebert, R.B. Tæthed Og Antal Af Den Sortmundede Kutling Neogobius melanostomus I Guldborgsund; Bachelorprojekt, Statens Naturhistoriske Museum, Københavns Universitet: Copenhagen, Denmark, 2012. [Google Scholar]
- Sapota, M.R. NOBANIS—Invasive Alien Species Fact Sheet—Neogobius melanostomus. Online Database of the European Network on Invasive Alien Species—NOBANIS. Available online: www.nobanis.org (accessed on 7 December 2022).
- Kornis, M.S.; Mercado-Silva, N.; Vander Zanden, M.J. Twenty years of invasion: A review of round goby Neogobius melanostomus biology, spread and ecological implications. J. Fish Biol. 2012, 80, 235–285. [Google Scholar] [CrossRef]
- Charlebois, P.M.; Marsden, J.E.; Goettel, R.G.; Wolfe, R.K.; Jude, D.J.; Rudnicka, S. The round goby, Neogobius melanostomus (Pallas), a review of European and North American literature. Illinois-Indiana Sea Grant Program and Illinois Natural History Survey. Aquatic Ecology Technical Report 96/10. Available online: https://hdl.handle.net/2142/111690 (accessed on 2 February 2023).
- van Deurs, M.; Moran, N.P.; Plet-Hansen, K.S.; Dinesen, G.E.; Azour, F.; Carl, H.; Moller, P.R.; Behrens, J.W. Impacts of the invasive round goby (Neogobius melanostomus) on benthic invertebrate fauna: A case study from the Baltic Sea. Neobiota 2021, 68, 19–30. [Google Scholar] [CrossRef]
- Bzoma, S.; Stempniewicz, L. Great cormorants (Phalacrocorax carbo) diet in the Gulf of Gdansk in 1998 and 1999. In Proceedings of the Third International Symposium on Functioning of Coastal Ecosystems in Various Geographical Regions, Gdansk, Poland, 19–22 June 2001; Institute of Oceanography, University of Gdansk: Gdansk, Poland, 2001. [Google Scholar]
- Wolff, W.J. Non-indigenous marine and estuarine species in the Netherlands. Zool. Med. Leiden 2005, 79, 1–116. [Google Scholar]
- Leppäkoski, E.; Olenin, S. Non-native Species and Rates of Spread: Lessons from the Brackish Baltic Sea. Biol. Invasions 2000, 2, 151–163. [Google Scholar] [CrossRef]
- Kerckhof, F.; Haelters, J.; Gollasch, S. Alien species in the marine and brackish ecosystem: The situation in Belgian waters. Aquat. Invasions 2007, 2, 243–257. [Google Scholar] [CrossRef]
- Gollasch, S.; Haydar, D.; Minchin, D.; Wolff, W.J.; Reise, K. Introduced Aquatic Species of the North Sea Coasts and Adjacent Brackish Waters. In Biological Invasions in Marine Ecosystems: Ecological, Management, and Geographic Perspectives; Rilov, G., Crooks, J.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 507–528. [Google Scholar]
- Gittenberger, A.; Rensing, M.; Stegenga, H.; Hoeksema, B.W. Native and non-native species of hard substrata in the Dutch Wadden Sea. Ned. Faun. Meded. 2010, 33, 21–75. [Google Scholar]
- Miljøstyrelsen. NOVANA. Det Nationale Overvågningsprogram for Vandmiljø Og Natur 2017–2021; Mlijøstyrelsen Miljøog Fødevareministeriet: Copenhagen, Denmark, 2017.
- Miljøstyrelsen. NOVANA. Det Nationale Overvågningsprogram for Vandmiljø Og Natur 2022; Miljøstyrelsen Miljøministeriet: Odense, Denmark, 2022.
- Outinen, O.; Puntila-Dodd, R.; Barda, I.; Brzana, R.; Hegele-Drywa, J.; Kalnina, M.; Kostanda, M.; Lindqvist, A.; Normant-Saremba, M.; Ścibik, M.; et al. The role of marinas in the establishment and spread of non-indigenous species in Baltic Sea fouling communities. Biofouling 2021, 37, 984–997. [Google Scholar] [CrossRef]
- Andersen, J.H.; Kallenbach, E.; Thaulow, J.; Hesselsøe, M.; Bekkevold, D.; Hansen, B.K.; Jacobsen, L.M.W.; Olesen, C.A.; Møller, P.R.; Knudsen, S.W. Development of Species-Specific eDNA-Based Test Systems for Monitoring of Non-Indigenous Species in Danish Marine Waters; Norsk Institutt for Vannforskning: Oslo, Norway, 2018; Rapport L. Nr. 7204-2017; 77p. [Google Scholar]
- Andersen, J.H.; Møller, P.R.; Kallenbach, E.; Hesselsøe, M.; Knudsen, S.W.; Bekkevold, D.; Hansen, B.K.; Thaulow, J. Steps toward Nation-Wide Monitoring of Non-Indigenous Species in Danish Marine Waters under the Marine Strategy Framework Directive; Norsk Institutt for Vannforskning: Oslo, Norway, 2016; Rapport L. Nr. 7022-2016; 122p. [Google Scholar]
- Knudsen, S.W.; Andersen, J.H.; Møller, P.R. Development of Species-Specific eDNA-Based Test Systems for Monitoring of Non-indigenous Decapoda in Danish Marine Waters; Report SNO 7544–2020; NIVA Denmark and Natural History Museum of Denmark: Copenhagen, Denmark, 2020; 56p. [Google Scholar]
- DONG-Energy. The Danish Offshore Wind Farm Demonstration Project: Horns Rev and Nysted Offshore Wind Farm Environmental Impact Assessment and Monitoring; DONG Energy and Vattenfall A/S; Review Report 2005; Ørsted: Fredericia, Denmark, 2006; 150p. [Google Scholar]
- Rambøll. Ny Miljøundersøgelse af “Disken”, Øresund. Report to Miljøstyrelsen (Denmark) and Länsstyrelsen Skåne (Sweden). 2018. 103p. Available online: https://mst.dk/media/170945/180226_1100029996-001-003-ny-disken-rapport-2017-final-version.pdf (accessed on 6 February 2019).
- Lehtiniemi, M.; Outinen, O.; Puntila-Dodd, R. Citizen science provides added value in the monitoring for coastal non-indigenous species. J. Environ. Manag. 2020, 267, 110608. [Google Scholar] [CrossRef] [PubMed]
- Jaspers, C.; Ehrlich, M.; Pujolar, J.M.; Künzel, S.; Bayer, T.; Limborg, M.T.; Lombard, F.; Browne, W.E.; Stefanova, K.; Reusch, T.B.H. Invasion genomics uncover contrasting scenarios of genetic diversity in a widespread marine invader. Proc. Natl. Acad. Sci. USA 2021, 118, e2116211118. [Google Scholar] [CrossRef] [PubMed]
- Madsen, L. Fund af Bonamia ostreae i danske østers fra Limfjord. Dan Vet. 2015, 98, 41. [Google Scholar]
- Robbins, J.R.; Bouchet, P.J.; Miller, D.L.; Evans, P.G.H.; Waggitt, J.; Ford, A.T.; Marley, S.A. Shipping in the north-east Atlantic: Identifying spatial and temporal patterns of change. Mar. Pollut. Bull. 2022, 179, 113681. [Google Scholar] [CrossRef]
- Stæhr, P.; Guerin, L.; Viard, F.; Tidbury, H.; Carbonell, A.; Kabuta, S.H. Trends in New Records of Non-Indigenous Species (NIS) Intro-duced by Human Activities. In OSPAR, 2023: The 2023 Quality Status Re-port for the Northeast Atlantic; OSPAR Commission: London, UK, 2023. [Google Scholar]
- Pinsky, M.L.; Worm, B.; Fogarty, M.J.; Sarmiento, J.L.; Levin, S.A. Marine Taxa Track Local Climate Velocities. Science 2013, 341, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Poloczanska, E.S.; Burrows, M.T.; Brown, C.J.; García Molinos, J.; Halpern, B.S.; Hoegh-Guldberg, O.; Kappel, C.V.; Moore, P.J.; Richardson, A.J.; Schoeman, D.S.; et al. Responses of Marine Organisms to Climate Change across Oceans. Front. Mar. Sci. 2016, 3, 62. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Belchior, C.; Cardoso, A.C. Invading European Seas: Assessing pathways of introduction of marine aliens. Ocean Coast. Manag. 2013, 76, 64–74. [Google Scholar] [CrossRef]
- Copp, G.H.; Vilizzi, L.; Tidbury, H.; Stebbing, P.D.; Tarkan, A.S.; Miossec, L.; Goulletquer, P. Development of a genericdecision-support tool for identifying potentially invasiveaquatic taxa: AS-ISK. Manag. Biol. Invasions 2016, 7, 343–350. [Google Scholar] [CrossRef]
- COWI/IGN. Revision af lister over invasive arter. Proces og resultater Naturstyrelsen januar. 2016. Available online: https://view.officeapps.live.com/op/View.aspx?src=https%3A%2F%2Fmst.dk&2Fmedia%2F120416%2Finvasive-arter (accessed on 9 December 2020).
- Miossec, L.; Le Deuff, R.-M.; Goulletquer, P. Alien species alert: Crassostrea gigas (Pacific oyster). In ICES Cooperative Research Report No. 299; ICES: Copenhagen, Denmark, 2009; 42p. [Google Scholar]
- Gollasch, S.; Kerckhof, F.; Craeymeersch, J.; Goulletquer, P.; Jensen, K.; Jelmert, A.; Minchin, D. Alien Species Alert: Ensis directus. Current status of invasions by the marine bivalve Ensis directus. In ICES Cooperative Research Reports (CRR); ICES: Copenhagen, Denmark, 2015. [Google Scholar]
- Tsiamis, K.; Azzurro, E.; Bariche, M.; Çinar, M.E.; Crocetta, F.; De Clerck, O.; Galil, B.; Gómez, F.; Hoffman, R.; Jensen, K.R.; et al. Prioritizing marine invasive alien species in the European Union through horizon scanning. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 794–845. [Google Scholar] [CrossRef]
- Olenin, S.; Minchin, D.; Daunys, D. Assessment of biopollution in aquatic ecosystems. Mar. Pollut. Bull. 2007, 55, 379–394. [Google Scholar] [CrossRef]
- Ojaveer, H.; Kotta, J.; Outinen, O.; Einberg, H.; Zaiko, A.; Lehtiniemi, M. Meta-analysis on the ecological impacts of widely spread non-indigenous species in the Baltic Sea. Sci. Total Environ. 2021, 786, 147375. [Google Scholar] [CrossRef]
- Knudsen, J. Den amerikanske knivmusling, Ensis americanus Gould, 1870, en nyindvandret art i de danske farvande. Flora og Fauna 1989, 95, 17–18. [Google Scholar]
- Rasmussen, E. Nyt om den amerikanske knivmusling, Ensis americanus Gould, 1870 (=E. directus Conrad) i danske farvande. Flora Og Fauna 1996, 101, 53–60. [Google Scholar]
- Tendal, O.S.; Olesen, J.; Lundholm, B.S. Den østamerikanske brakvandskrabbe Rhithropanopeus harrisii i Danmark: Gammel gæst og ny invasiv art. Flora og Fauna 2011, 117, 23–27. [Google Scholar]
- Wrange, A.-L.; Valero, J.; Harkestad, L.S.; Strand, Ø.; Lindegarth, S.; Christensen, H.T.; Dolmer, P.; Kristensen, P.S.; Mortensen, S. Massive settlements of the Pacific oyster, Crassostrea gigas, in Scandinavia. Biological. Invasions 2010, 12, 1453–1458. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Hummel, H.; Wijnhoven, S. Long-term patterns in the establishment, expansion and decline of invading macrozoobenthic species in the brackish and marine waters of Southwest Netherlands. Mar. Ecol. 2014, 35, 50–55. [Google Scholar] [CrossRef]
- Baird, D.; Asmus, H.; Asmus, R. Effect of invasive species on the structure and function of the Sylt-Rømø Bight ecosystem, northern Wadden Sea, over three time periods. Mar. Ecol. Prog. Ser. 2012, 462, 143–161. [Google Scholar] [CrossRef]













| Species | Functional Group | I | II | I + II | III | IV | III + IV | V | VI | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Magallana gigas | Benthic invertebrate | 3 | 3 | 6 | 3 | 3 | 6 | 2 | 2 | 16 |
| Mnemiopsis leidyi | Zooplankton | 3 | 3 | 6 | 3 | 3 | 6 | 2 | 0 | 14 |
| Neogobius melanostomus | Fish | 3 | 3 | 6 | 3 | 2 | 5 | 3 | 0 | 14 |
| Sargassum muticum | Macroalga | 3 | 3 | 6 | 3 | 3 | 6 | 2 | 0 | 14 |
| Spartina anglica | Grass | 3 | 3 | 6 | 3 | 3 | 6 | 2 | 0 | 14 |
| Gracilaria vermiculophyllum | Macroalga | 3 | 3 | 6 | 3 | 3 | 6 | 1 | 0 | 13 |
| Ensis leei | Benthic invertebrate | 3 | 3 | 6 | 2 | 2 | 4 | 2 | 0 | 12 |
| Hemigrapsus sanguineus | Benthic invertebrate | 3 | 3 | 6 | 2 | 2 | 4 | 1 | 0 | 11 |
| Hemigrapsus takanoi | Benthic invertebrate | 3 | 3 | 6 | 2 | 2 | 4 | 1 | 0 | 11 |
| Styela clava | Benthic invertebrate | 2 | 3 | 5 | 3 | 2 | 5 | 1 | 0 | 11 |
| Marenzelleria neglecta | Benthic invertebrate | 3 | 3 | 6 | 2 | 2 | 4 | 0 | 0 | 10 |
| Marenzelleria viridis | Benthic invertebrate | 3 | 3 | 6 | 2 | 2 | 4 | 0 | 0 | 10 |
| Ficopomatus enigmaticus | Benthic invertebrate | 2 | 2 | 4 | 2 | 2 | 4 | 1 | 0 | 9 |
| Fucus distichus | Macroalga | 3 | 3 | 6 | 2 | 1 | 3 | 0 | 0 | 9 |
| Rhithropanopeus harrisii | Benthic invertebrate | 2 | 2 | 4 | 2 | 2 | 4 | 1 | 0 | 9 |
| Crepidula fornicata | Benthic invertebrate | 3 | 2 | 5 | 1 | 2 | 3 | 0 | 0 | 8 |
| Ocinebrellus inornatus | Benthic invertebrate | 1 | 2 | 3 | 2 | 1 | 3 | 1 | 1 | 8 |
| Cordylophora caspia | Benthic invertebrate | 2 | 2 | 4 | 1 | 2 | 3 | 1 | 0 | 8 |
| Caprella mutica | Benthic invertebrate | 2 | 2 | 4 | 2 | 1 | 3 | 0 | 0 | 7 |
| Anguillicola crassus Kuwahara | Parasite | 1 | 1 | 2 | 3 | 1 | 4 | 3 | 1 | 10 |
| Pseudodactylogyrus anguillae | Parasite | 1 | 1 | 2 | 3 | 1 | 4 | 3 | 1 | 10 |
| Amphibalanus improvisus | Benthic invertebrate | 3 | 3 | 6 | 1 | 1 | 2 | 1 | 0 | 9 |
| Austrominius modestus | Benthic invertebrate | 3 | 2 | 5 | 1 | 1 | 2 | 2 | 0 | 9 |
| Colpomenia peregrina | Macroalga | 3 | 3 | 6 | 1 | 1 | 2 | 1 | 0 | 9 |
| Codium fragile ssp. fragile | Macroalga | 3 | 3 | 6 | 1 | 1 | 2 | 1 | 0 | 9 |
| Petricolaria pholadiformis | Benthic invertebrate | 2 | 3 | 5 | 1 | 1 | 2 | 1 | 1 | 9 |
| Teredo navalis | Benthic invertebrate | 2 | 1 | 3 | 1 | 1 | 2 | 3 | 1 | 9 |
| Bonnemaisonia hamifera | Macroalga | 3 | 3 | 6 | 1 | 1 | 2 | 0 | 0 | 8 |
| Dasya baillouviana | Macroalga | 3 | 3 | 6 | 1 | 1 | 2 | 0 | 0 | 8 |
| Dasysiphonia japonica | Macroalga | 3 | 3 | 6 | 1 | 1 | 2 | 0 | 0 | 8 |
| Dictyota dichotoma | Macroalga | 2 | 3 | 5 | 1 | 1 | 2 | 1 | 0 | 8 |
| Eriocheir sinensis | Benthic invertebrate | 3 | 3 | 6 | 1 | 1 | 2 | 0 | 0 | 8 |
| Melanothamnus harveyi | Macroalga | 3 | 3 | 6 | 1 | 1 | 2 | 0 | 0 | 8 |
| Mya arenaria | Benthic invertebrate | 3 | 2 | 5 | 1 | 1 | 2 | 0 | 1 | 8 |
| Mytilicola intestinalis | Parasite | 1 | 1 | 2 | 2 | 1 | 3 | 2 | 1 | 8 |
| Crassostrea virginica | Benthic invertebrate | 1 | 1 | 2 | 2 | 1 | 3 | 1 | 1 | 7 |
| Spartina alterniflora × maritima | Grass | 0 | 3 | 3 | 2 | 2 | 4 | 0 | 0 | 7 |
| Diadumene lineata | Benthic invertebrate | 2 | 2 | 4 | 1 | 1 | 2 | 0 | 0 | 6 |
| Gonionemus vertens | Zooplankton | 2 | 2 | 4 | 1 | 1 | 2 | 0 | 0 | 6 |
| Potamopyrgus antipodarum | Benthic invertebrate | 2 | 2 | 4 | 1 | 1 | 2 | 0 | 0 | 6 |
| Oncorhynchus gorbuscha | Fish | 1 | 2 | 3 | 1 | 0 | 1 | 0 | 0 | 4 |
| Oncorhynchus kisutch | Fish | 1 | 2 | 3 | 1 | 0 | 1 | 0 | 0 | 4 |
| Oncorhynchus mykiss | Fish | 0 | 2 | 2 | 1 | 1 | 2 | 0 | 0 | 4 |
| Salvelinus alpinus | Fish | 0 | 3 | 3 | 1 | 0 | 1 | 0 | 0 | 4 |
| Salvelinus fontinalis | Fish | 0 | 2 | 2 | 1 | 1 | 2 | 0 | 0 | 4 |
| Acipenser baerii | Fish | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 3 |
| Acipenser gueldenstaedtii | Fish | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 0 | 3 |
| Acipenser stellatus | Fish | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 2 |
| Species Name | Functional Group | I | II | I + II | III | IV | III + IV | V | VI | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Didemnum vexillum | Benthic invertebrate | 3 | 3 | 6 | 2 | 3 | 5 | 2 | 2 | 15 |
| Callinectes sapidus * | Benthic invertebrate | 2 | 3 | 5 | 3 | 3 | 6 | 1 | 1 | 13 |
| Potamocorbula amurensis | Benthic invertebrate | 3 | 2 | 5 | 2 | 2 | 4 | 3 | 1 | 13 |
| Undaria pinnatifida | Macroalga | 3 | 3 | 6 | 3 | 3 | 6 | 1 | 0 | 13 |
| Arcuatula senhousia | Benthic invertebrate | 3 | 3 | 6 | 2 | 2 | 4 | 1 | 1 | 12 |
| Charybdis (Charybdis) japonica | Benthic invertebrate | 3 | 2 | 5 | 2 | 1 | 3 | 2 | 1 | 11 |
| Paralithodes camtschaticus | Benthic invertebrate | 2 | 2 | 4 | 2 | 2 | 4 | 2 | 1 | 11 |
| Perna viridis | Benthic invertebrate | 2 | 2 | 4 | 1 | 2 | 3 | 3 | 1 | 11 |
| Homarus americanus * | Benthic invertebrate | 1 | 2 | 3 | 2 | 2 | 4 | 2 | 1 | 10 |
| Mulinia lateralis | Benthic invertebrate | 3 | 2 | 5 | 2 | 1 | 3 | 1 | 1 | 10 |
| Mytilopsis leucophaeata | Benthic invertebrate | 2 | 2 | 4 | 2 | 2 | 4 | 1 | 1 | 10 |
| Urosalpinx cinerea | Benthic invertebrate | 1 | 2 | 3 | 2 | 1 | 3 | 3 | 1 | 10 |
| Cancer irroratus | Benthic invertebrate | 2 | 2 | 4 | 2 | 2 | 4 | 2 | 0 | 10 |
| Gammarus tigrinus * | Benthic invertebrate | 2 | 1 | 3 | 3 | 2 | 5 | 1 | 0 | 9 |
| Dikerogammarus villosus | Benthic invertebrate | 1 | 3 | 4 | 3 | 2 | 5 | 0 | 0 | 9 |
| Obesogammarus crassus | Benthic invertebrate | 1 | 3 | 4 | 3 | 2 | 5 | 0 | 0 | 9 |
| Pontogammarus robustoides | Benthic invertebrate | 1 | 3 | 4 | 3 | 2 | 5 | 0 | 0 | 9 |
| Gmelinoides fasciatus | Benthic invertebrate | 1 | 3 | 4 | 3 | 2 | 5 | 0 | 0 | 9 |
| Celtodoryx ciocalyptoides | Benthic invertebrate | 2 | 3 | 5 | 2 | 2 | 4 | 0 | 0 | 9 |
| Chama pacifica | Benthic invertebrate | 3 | 2 | 5 | 2 | 2 | 4 | 0 | 0 | 9 |
| Palaemon macrodactylus | Benthic invertebrate | 3 | 1 | 4 | 2 | 2 | 4 | 1 | 0 | 9 |
| Rapana venosa | Benthic invertebrate | 1 | 1 | 2 | 2 | 2 | 4 | 2 | 1 | 9 |
| Schizoporella japonica * | Benthic invertebrate | 2 | 2 | 4 | 2 | 2 | 4 | 1 | 0 | 9 |
| Corbicula fluminalis | Benthic invertebrate | 2 | 3 | 5 | 2 | 2 | 4 | 0 | 0 | 9 |
| Procambarus acutus | Benthic invertebrate | 1 | 3 | 4 | 2 | 2 | 4 | 1 | 0 | 9 |
| Boccardia proboscidea | Benthic invertebrate | 2 | 1 | 3 | 2 | 2 | 4 | 1 | 0 | 8 |
| Cercopagis (Cercopagis) pengoi | Zooplankton | 2 | 2 | 4 | 2 | 1 | 3 | 1 | 0 | 8 |
| Bugula neritina | Benthic invertebrate | 3 | 2 | 5 | 1 | 1 | 2 | 1 | 0 | 8 |
| Pseudodiaptomus marinus * | Zooplankton | 2 | 2 | 4 | 2 | 1 | 3 | 1 | 0 | 8 |
| Cornigerius maeoticus | Zooplankton | 2 | 2 | 4 | 2 | 1 | 3 | 1 | 0 | 8 |
| Corella eumyota | Benthic invertebrate | 2 | 1 | 3 | 2 | 1 | 3 | 1 | 0 | 7 |
| Ruditapes philippinarum | Benthic invertebrate | 3 | 2 | 5 | 1 | 1 | 2 | 1 | 0 | 8 |
| Rangia cuneata | Benthic invertebrate | 2 | 2 | 4 | 1 | 1 | 2 | 1 | 0 | 7 |
| Amphibalanus amphitrite | Benthic invertebrate | 3 | 1 | 4 | 1 | 1 | 2 | 1 | 0 | 7 |
| Echinogammarus ischnus | Benthic invertebrate | 1 | 3 | 4 | 2 | 1 | 3 | 0 | 0 | 7 |
| Echinogammarus trichiatus | Benthic invertebrate | 1 | 3 | 4 | 2 | 1 | 3 | 0 | 0 | 7 |
| Hesperibalanus fallax | Benthic invertebrate | 1 | 2 | 3 | 1 | 1 | 2 | 1 | 0 | 6 |
| Beroe ovata * | Zooplankton | 3 | 1 | 4 | 1 | 1 | 2 | 0 | 0 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jensen, K.R.; Andersen, P.; Andersen, N.R.; Bruhn, A.; Buur, H.; Carl, H.; Jakobsen, H.; Jaspers, C.; Lundgreen, K.; Nielsen, R.; et al. Reviewing Introduction Histories, Pathways, Invasiveness, and Impact of Non-Indigenous Species in Danish Marine Waters. Diversity 2023, 15, 434. https://doi.org/10.3390/d15030434
Jensen KR, Andersen P, Andersen NR, Bruhn A, Buur H, Carl H, Jakobsen H, Jaspers C, Lundgreen K, Nielsen R, et al. Reviewing Introduction Histories, Pathways, Invasiveness, and Impact of Non-Indigenous Species in Danish Marine Waters. Diversity. 2023; 15(3):434. https://doi.org/10.3390/d15030434
Chicago/Turabian StyleJensen, Kathe R., Per Andersen, Nikolaj R. Andersen, Annette Bruhn, Helle Buur, Henrik Carl, Hans Jakobsen, Cornelia Jaspers, Kim Lundgreen, Ruth Nielsen, and et al. 2023. "Reviewing Introduction Histories, Pathways, Invasiveness, and Impact of Non-Indigenous Species in Danish Marine Waters" Diversity 15, no. 3: 434. https://doi.org/10.3390/d15030434
APA StyleJensen, K. R., Andersen, P., Andersen, N. R., Bruhn, A., Buur, H., Carl, H., Jakobsen, H., Jaspers, C., Lundgreen, K., Nielsen, R., Strandberg, B., & Stæhr, P. A. U. (2023). Reviewing Introduction Histories, Pathways, Invasiveness, and Impact of Non-Indigenous Species in Danish Marine Waters. Diversity, 15(3), 434. https://doi.org/10.3390/d15030434






