Catching the Green—Diversity of Ruderal Spring Plants Traditionally Consumed in Bulgaria and Their Potential Benefit for Human Health
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Study and Research Approach
3. Results and Discussion
3.1. Plant Diversity and Consumption Practices
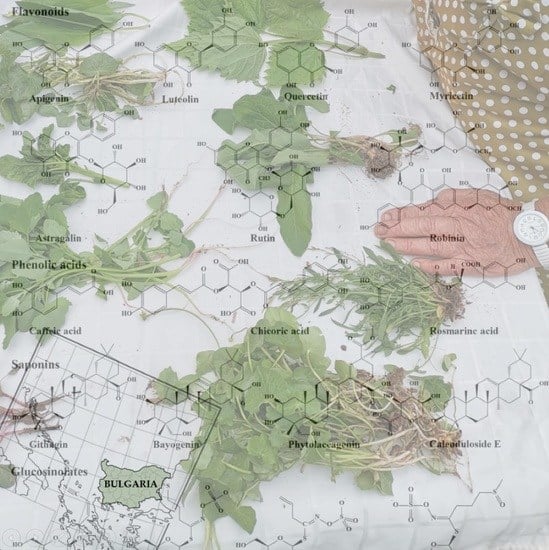
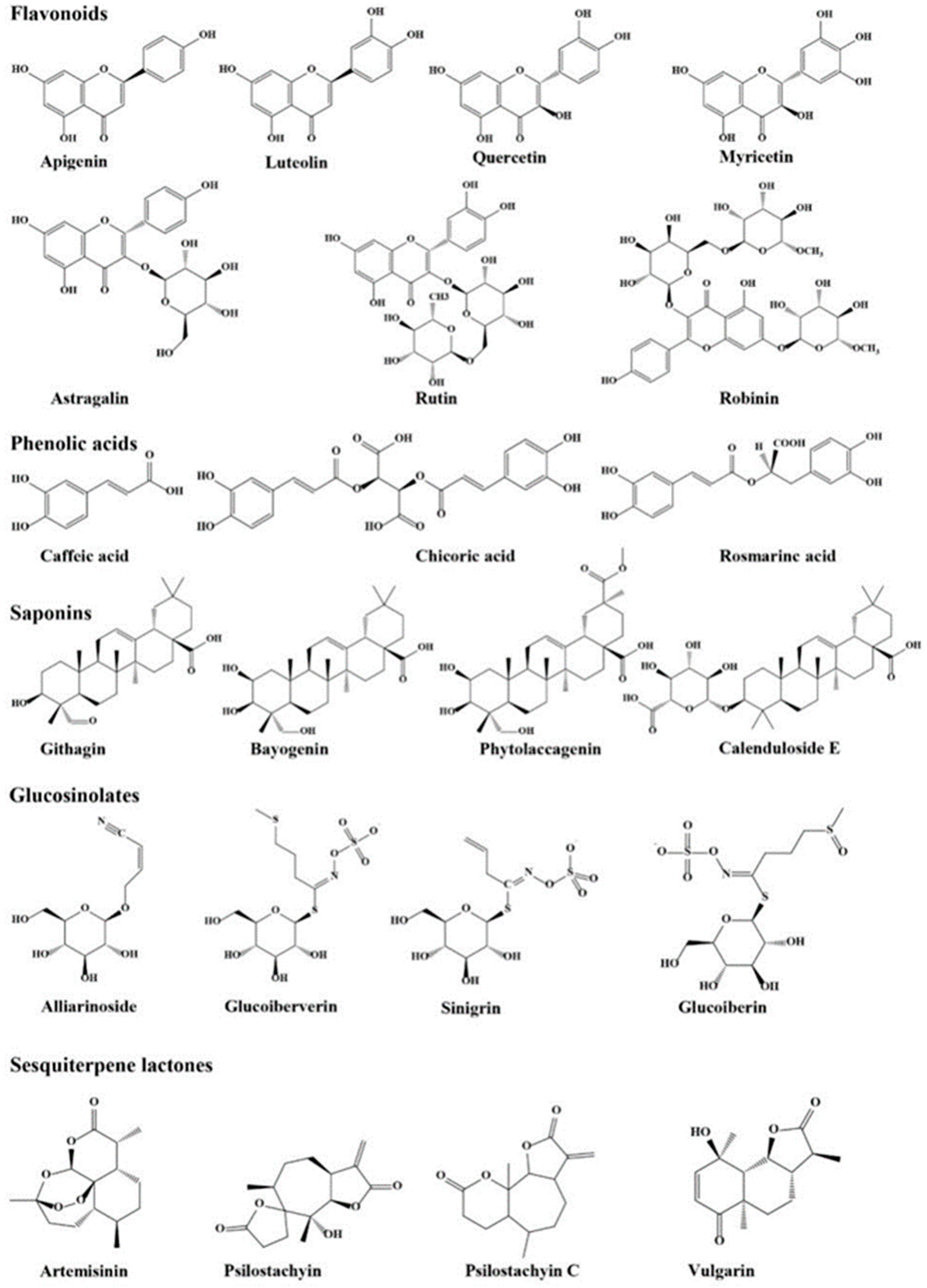
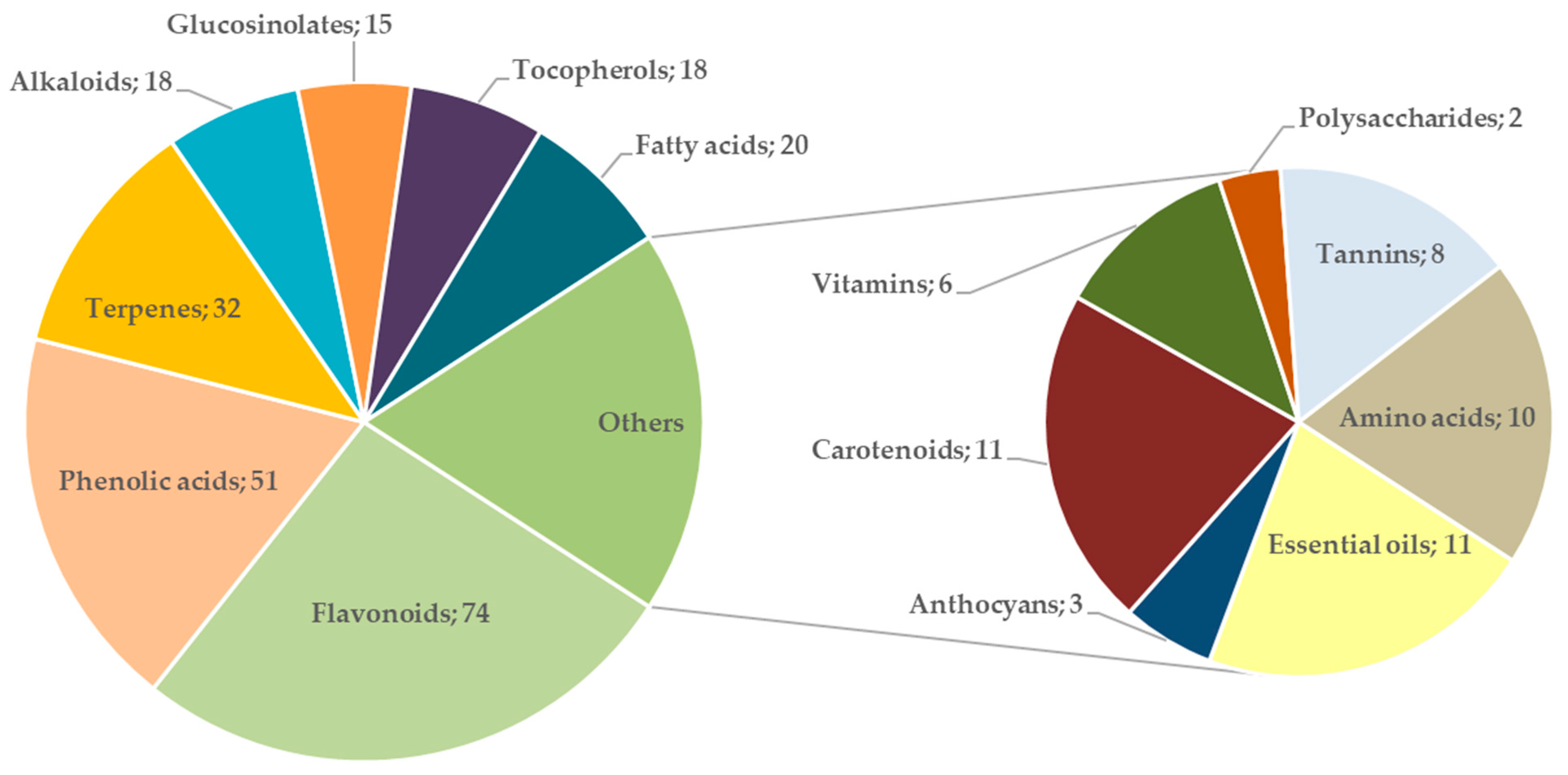
3.2. Bioactive Compounds and Potential Health Benefits
3.3. Potential Medicinal Benefits
3.4. Toxicity and Community Awareness
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berry, E.M.; Dernini, S.; Burlingame, B.; Meybeck, A.; Conforti, P. Food security and sustainability: Can one exist without the other? Public Health Nutr. 2015, 18, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Johns, T.; Eyzaguirre, P.B. Linking biodiversity, diet and health in policy and practice. Proc. Nutr. Soc. 2006, 65, 182–189. [Google Scholar] [CrossRef]
- Burlingame, B.; Dernini, S. Sustainable diets: The Mediterranean diet as an example. Public Health Nutr. 2011, 14, 2285–2287. [Google Scholar] [CrossRef]
- Ficiciyan, A.; Loos, J.; Sievers-Glotzbach, S.; Tscharntke, T. More than Yield: Ecosystem Services of Traditional versus Modern Crop Varieties Revisited. Sustainability 2018, 10, 2834. [Google Scholar] [CrossRef]
- Truzzi, M.L.; Puviani, M.B.; Tripodi, A.; Toni, S.; Farinetti, A.; Nasi, M.; Mattioli, A.V. Mediterranean Diet as a model of sustainable, resilient and healthy diet. Prog. Nutr. 2020, 22, 388–394. [Google Scholar] [CrossRef]
- Graça, J.; Godinho, C.A.; Truninger, M. Reducing meat consumption and following plant-based diets: Current evidence and future directions to inform integrated transitions. Trends Food Sci. Technol. 2019, 91, 380–390. [Google Scholar] [CrossRef]
- Cleveland, D.A.; Gee, Q. Plant-Based Diets for Mitigating Climate Change. In Vegetarian and Plant-Based Diets in Health and Disease Prevention; Mariotti, F., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 135–156. ISBN 9780128039694. [Google Scholar]
- Giampieri, F.; Mazzoni, L.; Cianciosi, D.; Alvarez-Suarez, J.M.; Regolo, L.; Sánchez-González, C.; Capocasa, F.; Xiao, J.; Mezzetti, B.; Battino, M. Organic vs conventional plant-based foods: A review. Food Chem. 2022, 383, 132352. [Google Scholar] [CrossRef] [PubMed]
- van Vliet, S.; Kronberg, S.L.; Provenza, F.D. Plant-Based Meats, Human Health, and Climate Change. Front. Sustain. Food Syst. 2020, 4, 128. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Shen, J.; Shan, J.; Zhong, L.; Liang, B.; Zhang, D.; Li, M.; Tang, H. Dietary Phytochemicals that Can Extend Longevity by Regulation of Metabolism. Plant Foods Hum. Nutr. 2022, 77, 12–19. [Google Scholar] [CrossRef]
- Lachat, C.; Raneri, J.E.; Smith, K.W.; Kolsteren, P.; Van Damme, P.; Verzelen, K.; Penafiel, D.; Vanhove, W.; Kennedy, G.; Hunter, D.; et al. Dietary species richness as a measure of food biodiversity and nutritional quality of diets. Proc. Natl. Acad. Sci. USA 2017, 115, 127–132. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Pieroni, A.; Tardío, J.; Pardo-De-Santayana, M.; Sõukand, R.; Svanberg, I.; Kalle, R. Wild food plant use in 21st century Europe: The disappearance of old traditions and the search for new cuisines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Tardío, J.; Pardo-De-Santayana, M.; Morales, R. Ethnobotanical review of wild edible plants in Spain. Bot. J. Linn. Soc. 2006, 152, 27–71. [Google Scholar] [CrossRef]
- Łuczaj, Ł. Changes in the utilization of wild green vegetables in Poland since the 19th century: A comparison of four ethnobotanical surveys. J. Ethnopharmacol. 2010, 128, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M.; Nebel, S.; Rivera, D.; Heinrich, M. Wild Gathered Food Plants in the European Mediterranean: A Comparative Analysis|SpringerLink. Econ. Bot. 2006, 60, 130–142. [Google Scholar] [CrossRef]
- Hadjichambis, A.C.; Paraskeva-Hadjichambi, D.; Della, A.; Elena Giusti, M.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Reyes Gonzales-Tejero, M.; Patricia Sanchez-Rojas, C.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2009, 59, 383–414. [Google Scholar] [CrossRef]
- Ebert, A.W. The Role of Vegetable Genetic Resources in Nutrition Security and Vegetable Breeding. Plants 2020, 9, 736. [Google Scholar] [CrossRef]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnobiol. Ethnomedicine 2018, 14, 14. [Google Scholar] [CrossRef]
- Biscotti, N.; Pieroni, A. The hidden Mediterranean diet: Wild vegetables traditionally gathered and consumed in the Gargano area, Apulia, SE Italy. Acta Soc. Bot. Pol. 2015, 84, 327–338. [Google Scholar] [CrossRef]
- Kalle, R.; Sõukand, R. Historical ethnobotanical review of wild edible plants of Estonia (1770s–1960s). Acta Soc. Bot. Pol. 2012, 81, 271–281. [Google Scholar] [CrossRef]
- Pieroni, A.; Sulaiman, N.; Sõukand, R. Chorta (Wild Greens) in Central Crete: The Bio-Cultural Heritage of a Hidden and Resilient Ingredient of the Mediterranean Diet. Biology 2022, 11, 673. [Google Scholar] [CrossRef]
- Pieroni, A.; Cattero, V. Wild vegetables do not lie: Comparative gastronomic ethnobotany and ethnolinguistics on the Greek traces of the Mediterranean Diet of southeastern Italy. Acta Bot. Bras. 2019, 33, 198–211. [Google Scholar] [CrossRef]
- Motti, R. Wild Plants Used as Herbs and Spices in Italy: An Ethnobotanical Review. Plants 2021, 10, 563. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M.; Savo, V. Wild food plants used in traditional vegetable mixtures in Italy. J. Ethnopharmacol. 2016, 185, 202–234. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, A.; Quave, C.; Giusti, M.E.; Papp, N. “We Are Italians!”: The Hybrid Ethnobotany of a Venetian Diaspora in Eastern Romania. Hum. Ecol. 2012, 40, 435–451. [Google Scholar] [CrossRef]
- Pasta, S.C.; La Rosa, A.; Garfì, G.; Marcenò, C.; Gristina, A.S.; Carimi, F.; Guarino, R. An Updated Checklist of the Sicilian Native Edible Plants: Preserving the Traditional Ecological Knowledge of Century-Old Agro-Pastoral Landscapes. Front. Plant Sci. 2020, 11, 388. [Google Scholar] [CrossRef]
- Vitasović-Kosić, I.; Hodak, A.; Łuczaj, Ł.; Marić, M.; Juračak, J. Traditional Ethnobotanical Knowledge of the Central Lika Region (Continental Croatia)—First Record of Edible Use of Fungus Taphrina pruni. Plants 2022, 11, 3133. [Google Scholar] [CrossRef]
- Corrêa, R.C.G.; Di Gioia, F.; Ferreira, I.C.F.R.; Petropoulos, S.A. Wild Greens Used in the Mediterranean Diet. In The Mediter-ranean Diet; Preedy, V., Watson, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 209–228. [Google Scholar]
- Chatzopoulou, E.; Carocho, M.; Gioia, F.; Petropoulos, S. The Beneficial Health Effects of Vegetables and Wild Edible Greens: The Case of the Mediterranean Diet and Its Sustainability. Appl. Sci. 2020, 10, 9144. [Google Scholar] [CrossRef]
- Ozturk, H.I.; Nas, H.; Ekinci, M.; Turan, M.; Ercisli, S.; Narmanlioglu, H.K.; Yildirim, E.; Assouguem, A.; Almeer, R.; Sayed, A.A.; et al. Antioxidant Activity, Phenolic Composition, and Hormone Content of Wild Edible Vegetables. Horticulturae 2022, 8, 427. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Köhler, P.; Piroznikow, E.; Graniszewska, M.; Pieroni, A.; Gervasi, T. Wild Edible Plants of Belarus: From Ros-tafiński’s Questionnaire of 1883 to the Present. J. Ethnobiol. Ethnomed. 2013, 9, 21. [Google Scholar] [CrossRef]
- Gras, A.; Garnatje, T.; Marín, J.; Parada, M.; Sala, E.; Talavera, M.; Vallès, J. The Power of Wild Plants in Feeding Humanity: A Meta-Analytic Ethnobotanical Approach in the Catalan Linguistic Area. Foods 2020, 10, 61. [Google Scholar] [CrossRef]
- Sardeshpande, M.; Shackleton, C. Urban foraging: Land management policy, perspectives, and potential. PLoS ONE 2020, 15, e0230693. [Google Scholar] [CrossRef] [PubMed]
- Kolosova, V.; Belichenko, O.; Rodionova, A.; Melnikov, D.; Sõukand, R. Foraging in Boreal Forest: Wild Food Plants of the Republic of Karelia, NW Russia. Foods 2020, 9, 1015. [Google Scholar] [CrossRef]
- Pieroni, A.; Hovsepyan, R.; Manduzai, A.K.; Sõukand, R. Wild food plants traditionally gathered in central Armenia: Archaic ingredients or future sustainable foods? Environ. Dev. Sustain. 2020, 23, 2358–2381. [Google Scholar] [CrossRef]
- Stryamets, N.; Mattalia, G.; Pieroni, A.; Khomyn, I.; Sõukand, R. Dining Tables Divided by a Border: The Effect of Socio-Political Scenarios on Local Ecological Knowledge of Romanians Living in Ukrainian and Romanian Bukovina. Foods 2021, 10, 126. [Google Scholar] [CrossRef]
- Schunko, C.; Brandner, A. Urban nature at the fingertips: Investigating wild food foraging to enable nature interactions of urban dwellers. AMBIO 2022, 51, 1168–1178. [Google Scholar] [CrossRef]
- Markova, M. Food and Nutrition: Between Nature and Culture; Prof. Marin Drinov Academic Publishing House: Sofia, Bulgaria, 2011; ISBN 978-954-322-462-3. [Google Scholar]
- Shishkov, S. What Is the Food of the Pomaks. Ethnographic Notes. Rhodope Prog. 1908, 6, 1–13. [Google Scholar]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61 (Suppl. 6), 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- García-Conesa, M.-T.; Philippou, E.; Pafilas, C.; Massaro, M.; Quarta, S.; Andrade, V.; Jorge, R.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; et al. Exploring the Validity of the 14-Item Mediterranean Diet Adherence Screener (MEDAS): A Cross-National Study in Seven European Countries around the Mediterranean Region. Nutrients 2020, 12, 2960. [Google Scholar] [CrossRef]
- Quarta, S.; Massaro, M.; Chervenkov, M.; Ivanova, T.; Dimitrova, D.; Jorge, R.; Andrade, V.; Philippou, E.; Zisimou, C.; Maksimova, V.; et al. Persistent Moderate-to-Weak Mediterranean Diet Adherence and Low Scoring for Plant-Based Foods across Several Southern European Countries: Are We Overlooking the Mediterranean Diet Recommendations? Nutrients 2021, 13, 1432. [Google Scholar] [CrossRef] [PubMed]
- Kitanov, B. Materials on Utilization of Wild Plants in National Economy. Bull. Bot. Inst. Bulg. Acad. Sci. 1953, 3, 257–260. [Google Scholar]
- Ivanova, T.; Bosseva, Y.; Ganeva-Raycheva, V.; Dimitrova, D. Ethnobotanical Knowledge on Edible Plants Used in Zelnik Pastries from Haskovo Province (Southeast Bulgaria). Phytol. Balc. 2018, 24, 389–395. [Google Scholar]
- Nedelcheva, A. An ethnobotanical study of wild edible plants in Bulgaria. EurAsian J. Biosci. 2013, 7, 77–94. [Google Scholar] [CrossRef]
- Pieroni, A.; Nedelcheva, A.; Dogan, Y. Local knowledge of medicinal plants and wild food plants among Tatars and Romanians in Dobruja (South-East Romania). Genet. Resour. Crop. Evol. 2014, 62, 605–620. [Google Scholar] [CrossRef]
- Kreuz, A.; Marinova, E. Archaeobotanical evidence of crop growing and diet within the areas of the Karanovo and the Linear Pottery Cultures: A quantitative and qualitative approach. Veg. Hist. Archaeobotany 2017, 26, 639–657. [Google Scholar] [CrossRef]
- Hrisrova, I.; Atanassova, J.; Marinova, E. Plant economy and vegetation of the Iron Age in Bulgaria: Archaeobotanical evidence from pit deposits. Archaeol. Anthr. Sci. 2017, 9, 1481–1494. [Google Scholar] [CrossRef]
- Kathe, W.; Honnef, S.; Heym, A. Medicinal and Aromatic Plants in Albania, Bosnia-Herzegovina, Bulgaria, Croatia and Romania; Bundesamt für Naturschutz: Bonn, Germany, 2003. [Google Scholar]
- Evstatieva, L.; Hardalova, R.; Stoyanova, K. Medicinal Plants in Bulgaria: Diversity, Legislation, Conservation and Trade. Phytol. Balc. 2007, 13, 415–427. [Google Scholar]
- Kozuharova, E.; Getov, I.N. Herbal Medicinal Products Registrations in the EU and the Implications for the Bulgarian Medi-canal Plant Resources. Comptes Rendus L’academie Bulg. Des Sci. 2012, 65, 1527–1534. [Google Scholar]
- Nebel, S.; Pieroni, A.; Heinrich, M. Ta chòrta: Wild edible greens used in the Graecanic area in Calabria, Southern Italy. Appetite 2006, 47, 333–342. [Google Scholar] [CrossRef]
- Mikropoulou, E.V.; Vougogiannopoulou, K.; Kalpoutzakis, E.; Sklirou, A.D.; Skaperda, Z.; Houriet, J.; Wolfender, J.-L.; Trougakos, I.P.; Kouretas, D.; Halabalaki, M.; et al. Phytochemical Composition of the Decoctions of Greek Edible Greens (Chórta) and Evaluation of Antioxidant and Cytotoxic Properties. Molecules 2018, 23, 1541. [Google Scholar] [CrossRef]
- Redžić, S.; Barudanović, S.; Pilipović, S. Wild Mushrooms and Lichens Used as Human Food for Survival in War Conditions; Podrinje—Zepa Region (Bosnia and Herzegovina, W. Balkan) on JSTOR. Hum. Ecol. Rev. 2010, 17, 175–187. [Google Scholar]
- Łuczaj, Ł.; Zovkokončić, M.; Miličević, T.; Dolina, K.; Pandža, M. Wild vegetable mixes sold in the markets of Dalmatia (southern Croatia). J. Ethnobiol. Ethnomed. 2013, 9, 2. [Google Scholar] [CrossRef]
- Pieroni, A.; Sulaiman, N.; Polesny, Z.; Sõukand, R. From Şxex to Chorta: The Adaptation of Maronite Foraging Customs to the Greek Ones in Kormakitis, Northern Cyprus. Plants 2022, 11, 2693. [Google Scholar] [CrossRef] [PubMed]
- Dogan, Y.; Nedelcheva, A. Wild Plants from Open Markets on Both Sides of the Bulgarian-Turkish Border. Indian J. Tradit. Knowl. 2015, 14, 351–358. [Google Scholar]
- Badalamenti, N.; Sottile, F.; Bruno, M. Ethnobotany, Phytochemistry, Biological, and Nutritional Properties of Genus Crepis—A Review. Plants 2022, 11, 519. [Google Scholar] [CrossRef]
- Torrens, F.; Castellano, G. Ethnobotanical Studies of Medicinal Plants: Underutilized Wild Edible Plants, Food, and Medicine. In Molecular Chemistry and Biomolecular Engineering; Pogliani, L., Torrens, F., Haghi, A.K., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2019; pp. 63–71. ISBN 9780429060649. [Google Scholar]
- Patel, B.; Sharma, S.; Nair, N.; Majeed, J.; Goyal, R.K.; Dhobi, M. Therapeutic opportunities of edible antiviral plants for COVID-19. Mol. Cell. Biochem. 2021, 476, 2345–2364. [Google Scholar] [CrossRef]
- Süntar, I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochem. Rev. 2019, 19, 1199–1209. [Google Scholar] [CrossRef]
- Holzner, W. Concepts, Categories and Characteristics of Weeds. In Biology and Ecology of Weeds; Holzner, W., Numata, M., Eds.; Springer: The Hague, The Netherlands, 1982; pp. 3–20. [Google Scholar]
- Fischer, L.K.; Brinkmeyer, D.; Karle, S.J.; Cremer, K.; Huttner, E.; Seebauer, M.; Nowikow, U.; Schütze, B.; Voigt, P.; Völker, S.; et al. Biodiverse edible schools: Linking healthy food, school gardens and local urban biodiversity. Urban For. Urban Green. 2019, 40, 35–43. [Google Scholar] [CrossRef]
- Rupprecht, C.D.; Byrne, J.A.; Garden, J.G.; Hero, J.-M. Informal urban green space: A trilingual systematic review of its role for biodiversity and trends in the literature. Urban For. Urban Green. 2015, 14, 883–908. [Google Scholar] [CrossRef]
- Toffolo, C.; Gentili, R.; Banfi, E.; Montagnani, C.; Caronni, S.; Citterio, S.; Galasso, G. Urban plant assemblages by land use type in Milan: Floristic, ecological and functional diversities and refugium role of railway areas. Urban For. Urban Green. 2021, 62, 127175. [Google Scholar] [CrossRef]
- Bocheva, L.; Trifonova, L.; Marinova, T.; Malcheva, K. Climate Profile of Bulgaria in the Period 1988–2016 and Brief Climatic Assessment of 2017. Bulg. J. Meteorol. Hydrol. 2017, 22, 2–15. [Google Scholar]
- Petrova, A.; Vladimirov, V. Recent Progress in Floristic and Taxonomic Studies in Bulgaria. Bot. Serb. 2018, 42, 35–69. [Google Scholar] [CrossRef]
- European Environment Agency. The European Environment—State and Outlook 2020; Publications Office of the European Union: Luxembourg City, Luxembourg, 2019; ISBN 978-92-9480-090-9.
- Dancheva, A.; Dimitrova, D.; Jordanova, E.; Cheshmedzhieva, G.; Nikolova, G.; Gergova, M.; Kolev, M.; Panagonova, S.; Petkova, R.; Filipovich, S.; et al. Statistical Reference Book 2022; National Statistical Institute: Sofia, Bulgaria, 2022. [Google Scholar]
- International Society of Ethnobiology ISE Code of Ethics. Available online: http://ethnobiology.net/code-of-ethics/ (accessed on 21 June 2021).
- Delipavlov, D.; Cheshmedzhiev, I.; Popova, M.; Terziyski, D.; Kovachev, I. Handbook of Bulgarian Vascular Flora; Delipavlov, D., Cheshmedzhiev, I., Eds.; Academic Publishing House of Agricultural University-Plovdiv: Plovdiv, Bulgaria, 2003. [Google Scholar]
- Royal Botanic Gardens Kew Garden; Missouri Botanical Garden The Plantlist Database. Available online: http://www.theplantlist.org (accessed on 26 May 2022).
- Sõukand, R.; Pieroni, A.; Biró, M.; Dénes, A.; Dogan, Y.; Hajdari, A.; Kalle, R.; Reade, B.; Mustafa, B.; Nedelcheva, A.; et al. An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. J. Ethnopharmacol. 2015, 170, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Boycheva, P.; Marinova, V. Ethnobotanical Study of Medicinal Plants for Culinary Purposes in the North Black Sea Coast, Varna Region (Bulgaria). Annu. L’université Sofia “St. Kliment Ohridski” Fac. Biol. 2018, 103, 172–182. [Google Scholar]
- Georgiev, M. Bulgarian Folk Medicine, 2nd ed.; Vasileva, M., Georgiev, M., Georgieva, I., Penchev, V., Popov, R., Simeonova, G., Troeva, E., Tsaneva, E., Eds.; Prof. Marin Drinov Academic Publishing House: Sofia, Bulgaria, 2013; ISBN 978-954-322-542-8. [Google Scholar]
- Stojanov, N.; Kitanov, B. Wild Useful Plants in Bulgaria; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1960. [Google Scholar]
- Ivanova, T.; Bosseva, Y.; Chervenkov, M.; Dimitrova, D. Enough to Feed Ourselves!—Food Plants in Bulgarian Rural Home Gardens. Plants 2021, 10, 2520. [Google Scholar] [CrossRef] [PubMed]
- Petrova, A.; Vladimirov, V.; Georgiev, V. Invasive Alien Species of Vascular Plants in Bulgaria; NeoPrint: Sofia, Bulgaria, 2013; ISBN 9789549746303. [Google Scholar]
- Dogan, Y.; Nedelcheva, A.; Łuczaj, Ł.; Drăgulescu, C.; Stefkov, G.; Maglajlić, A.; Ferrier, J.; Papp, N.; Hajdari, A.; Mustafa, B.; et al. Of the importance of a leaf: The ethnobotany of sarma in Turkey and the Balkans. J. Ethnobiol. Ethnomedicine 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Hakanova, A. Lenten and Vegetarian Dishes; Women Newspaper—Sofia: Sofia, Bulgaria, 1939. [Google Scholar]
- Hakanova, A. Bulgarian Folk Dishes; Women Newspaper—Sofia: Sofia, Bulgaria, 1939. [Google Scholar]
- Cholcheva, P. 1000 Well-Tried Coocking Recipes; Women Newspaper—Sofia: Sofia, Bulgaria, 1952. [Google Scholar]
- Women Library New Cookbook; Women Newspaper—Sofia: Sofia, Bulgaria, 1930.
- Women Library 500 Cooking Recipes; Women Newspaper—Sofia: Sofia, Bulgaria, 1927.
- Slaveykov, P. Cookbook or How to Make Any Kind of Dish, 5th ed.; Millenium: Sofia, Bulgaria, 1870; ISBN 978-954-515-299-3. [Google Scholar]
- Bulgarian Almanac Home Cookbook; Reprint (2020); Bulgarian History: Sofia, Bulgaria, 1895; ISBN 978-619-7496-48-2.
- Tardío, J.; Pardo-De-Santayana, M. Ethnobotanical Analysis of Wild Fruits and Vegetables Traditionally Consumed in Spain. In Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Springer: New York, NY, USA, 2016; pp. 57–79. ISBN 9781493933297. [Google Scholar]
- Abbet, C.; Mayor, R.; Roguet, D.; Spichiger, R.; Hamburger, M.; Potterat, O. Ethnobotanical survey on wild alpine food plants in Lower and Central Valais (Switzerland). J. Ethnopharmacol. 2014, 151, 624–634. [Google Scholar] [CrossRef]
- Brunnbauer, U. Gebirgsgesellschaften Auf Dem Balkan: Wirtschaft Und Familienstrukturen Im Rhodopengebirge (19/20. Jahrhundert); Böhlau Verlag: Wien, Austria, 2004; ISBN 9783205771463. [Google Scholar]
- Yarkov, D.; Stankov, K.; Stankov, I. Historical Review of the Development of Bulgarian Livestock Production. Bulg. J. Agric. Sci. 2022, 28, 564–578. [Google Scholar]
- Stranski, I. Wild and Cultivated Plants in Bulgaria. Names, Distribution, Utilization. In Plants in the Folk Customs and Songs; Dinev, L., Ilchev, S., Levenson, E., Eds.; Bulgarian Academy of Sciences: Sofia, Bulgaria, 1963. [Google Scholar]
- Łuczaj, Ł.J.; Kujawska, M. Botanists and their childhood memories: An underutilized expert source in ethnobotanical research. Bot. J. Linn. Soc. 2012, 168, 334–343. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Pieroni, A. Nutritional Ethnobotany in Europe: From Emergency Foods to Healthy Folk Cuisines and Contemporary Foraging Trends. In Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; de Cortes Sánchez-Mata, M., Tardío, J., Eds.; Springer: New York, NY, USA, 2016; pp. 33–56. ISBN 9781493933297. [Google Scholar]
- Ugulu, I.; Unver, M.C.; Dogan, Y. Potentially toxic metal accumulation and human health risk from consuming wild Urtica urens sold on the open markets of Izmir. Euro-Mediterranean J. Environ. Integr. 2019, 4, 36. [Google Scholar] [CrossRef]
- Stark, P.B.; Miller, D.; Carlson, T.J.; de Vasquez, K.R. Open-source food: Nutrition, toxicology, and availability of wild edible greens in the East Bay. PLoS ONE 2019, 14, e0202450. [Google Scholar] [CrossRef]
- Petrov, L.; Dzhelepov, N.; Jordanov, E.; Uzunova, S. Bulgarian National Cuisine; Zemizdat: Sofia, Bulgaria, 1978. [Google Scholar]
- Baldi, A.; Bruschi, P.; Campeggi, S.; Egea, T.; Rivera, D.; Obón, C.; Lenzi, A. The Renaissance of Wild Food Plants: Insights from Tuscany (Italy). Foods 2022, 11, 300. [Google Scholar] [CrossRef]
- Smith, J.; Jehlička, P. Stories around food, politics and change in Poland and the Czech Republic. Trans. Inst. Br. Geogr. 2007, 32, 395–410. [Google Scholar] [CrossRef]
- Dogan, Y. Traditionally used wild edible greens in the Aegean Region of Turkey. Acta Soc. Bot. Pol. 2012, 81, 329–342. [Google Scholar] [CrossRef]
- Bianco, V.; Santamaria, P.; Elia, A. Nutritional value and nitrate content in edible wild species used in southern italy. Acta Hortic. 1998, 46, 71–87. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Dolina, K. A hundred years of change in wild vegetable use in southern Herzegovina. J. Ethnopharmacol. 2015, 166, 297–304. [Google Scholar] [CrossRef]
- Pieroni, A.; Nebel, S.; Santoro, R.F.; Heinrich, M. Food for two seasons: Culinary uses of non-cultivated local vegetables and mushrooms in a south Italian village. Int. J. Food Sci. Nutr. 2009, 56, 245–272. [Google Scholar] [CrossRef]
- Pardo-De-Santayana, M.; Tardío, J.; Morales, R. The gathering and consumption of wild edible plants in the Campoo (Cantabria, Spain). Int. J. Food Sci. Nutr. 2009, 56, 529–542. [Google Scholar] [CrossRef]
- Turner, N.J.; Łuczaj, Ł.J.; Migliorini, P.; Pieroni, A.; Dreon, A.L.; Sacchetti, L.E.; Paoletti, M.G. Edible and Tended Wild Plants, Traditional Ecological Knowledge and Agroecology. Crit. Rev. Plant Sci. 2011, 30, 198–225. [Google Scholar] [CrossRef]
- Tardío, J.; De Cortes Sánchez-Mata, M.; Morales, R.; Molina, M.; García-Herrera, P.; Morales, P.; Díez-Marqués, C.; Fernández-Ruiz, V.; Cámara, M.; Pardo-De-Santayana, M.; et al. Ethnobotanical and Food Composition Monographs of Selected Mediterranean Wild Edible Plants. In Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Springer: New York, NY, USA, 2016; pp. 273–470. ISBN 9781493933297. [Google Scholar]
- Motti, R.; Bonanomi, G.; Lanzotti, V.; Sacchi, R. The Contribution of Wild Edible Plants to the Mediterranean Diet: An Ethnobotanical Case Study along the Coast of Campania (Southern Italy). Econ. Bot. 2020, 74, 249–272. [Google Scholar] [CrossRef]
- Pinke, G.; Kapcsándi, V.; Czúcz, B. Iconic Arable Weeds: The Significance of Corn Poppy (Papaver rhoeas), Cornflower (Centaurea cyanus), and Field Larkspur (Delphinium consolida) in Hungarian Ethnobotanical and Cultural Heritage. Plants 2022, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Toscano, S.; Rizzo, V.; Muratore, G.; Romano, D. Edible Wild Flowers: An Innovative but Ancient Food. Proceedings 2020, 70, 32. [Google Scholar] [CrossRef]
- Feduraev, P.; Skrypnik, L.; Nebreeva, S.; Dzhobadze, G.; Vatagina, A.; Kalinina, E.; Pungin, A.; Maslennikov, P.; Riabova, A.; Krol, O.; et al. Variability of Phenolic Compound Accumulation and Antioxidant Activity in Wild Plants of Some Rumex Species (Polygonaceae). Antioxidants 2022, 11, 311. [Google Scholar] [CrossRef] [PubMed]
- Im, M.H.; Park, Y.-S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Ham, K.-S.; Kang, S.-G.; Najman, K.; Gorinstein, S. The thermostability, bioactive compounds and antioxidant activity of some vegetables subjected to different durations of boiling: Investigation in vitro. LWT-Food Sci. Technol. 2011, 44, 92–99. [Google Scholar] [CrossRef]
- Murador, D.; Braga, A.R.; Da Cunha, D.; De Rosso, V. Alterations in phenolic compound levels and antioxidant activity in response to cooking technique effects: A meta-analytic investigation. Crit. Rev. Food Sci. Nutr. 2017, 58, 169–177. [Google Scholar] [CrossRef]
- Vuković, A.J.; Terzić, A. Gastronomy and Regional Identity: Balkan versus National Cuisine. In Gastronomy for Tourism Development; Peštek, A., Kukanja, M., Renko, S., Eds.; Emerald Publishing Limited: Bingley, UK, 2020; pp. 1–25. ISBN 978-1-78973-756-1. [Google Scholar]
- Jasar, D.; Curcic, B.; Kubelka-Sabit, K.; Filipovski, V.; Kakurinov, V. Health Issues and Nutrition in the Balkans. J. Hyg. Eng. Des. 2021, 35, 96–105. [Google Scholar]
- Gostin, A.-I. Traditional Balkan Foods in a Global Context: An Introduction. In Nutritional and Health Aspects of Food in the Balkans; Academic Press: Cambridge, MA, USA, 2021; pp. 1–8. [Google Scholar]
- Watson, R.R.; Preedy, V.R.; Zibadi, S. (Eds.) Polyphenols: Mechanisms of Action in Human Health and Disease; Elsevier: Amsterdam, Netherlands, 2018; ISBN 9780128130063. [Google Scholar]
- Zheng, Y.; Choi, Y.-H.; Lee, J.-H.; Lee, S.-Y.; Kang, I.-J. Anti-Obesity Effect of Erigeron annuus (L.) Pers. Extract Containing Phenolic Acids. Foods 2021, 10, 1266. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Woźniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Guo, W.; Cao, P.; Wang, X.; Hu, M.; Feng, Y. Medicinal Plants for the Treatment of Gastrointestinal Cancers from the Metabolomics Perspective. Front. Pharmacol. 2022, 13, 909755. [Google Scholar] [CrossRef]
- Jakovljević, M.R.; Milutinović, M.; Djurdjević, P.; Todorović, Ž.; Stanković, M.; Milošević-Djordjević, O. Cytotoxic and apoptotic activity of acetone and aqueous Artemisia vulgaris L. and Artemisia alba Turra extracts on colorectal cancer cells. Eur. J. Integr. Med. 2023, 57, 102204. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Pereira, J.A.; Saraiva, J.A.; Casal, S. Borage, Camellia, Centaurea and Pansies: Nutritional, Fatty Acids, Free Sugars, Vitamin E, Carotenoids and Organic Acids Characterization. Food Res. Int. 2020, 132, 109070. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; El-Kashef, D.H.; Müller, W.E.; Proksch, P. Cytotoxic eudesmane sesquiterpenes from Crepis sancta. Phytochem. Lett. 2019, 33, 46–48. [Google Scholar] [CrossRef]
- Arora, S.K.; Itankar, P.R.; Verma, P.R.; Bharne, A.P.; Kokare, D.M. Involvement of NFκB in the antirheumatic potential of Chenopodium album L., aerial parts extracts. J. Ethnopharmacol. 2014, 155, 222–229. [Google Scholar] [CrossRef]
- Choudhary, N.; Prabhakar, P.K.; Khatik, G.L.; Chamakuri, S.R.; Tewari, D.; Suttee, A. Evaluation of Acute toxicity, In-vitro, In-vivo Antidiabetic Potential of the Flavonoid Fraction of the plant Chenopodium album L. Pharmacogn. J. 2021, 13, 765–779. [Google Scholar] [CrossRef]
- Parisa, N.; Hidayat, R.; Maritska, Z.; Prananjaya, B.A. Evaluation of the anti-gout effect of Sonchus Arvensis on monosodium urate crystal-induced gout arthritis via anti-inflammatory action—An in vivo study. Med. Pharm. Rep. 2021, 94, 358. [Google Scholar] [CrossRef]
- Neha, K.; Haider, R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Arumugam, R.; Sarikurkcu, C.; Ozer, M.S. Comparison of methanolic extracts of Doronicum orientale and Echium angustifolium in terms of chemical composition and antioxidant activities. Biocatal. Agric. Biotechnol. 2021, 33, 101984. [Google Scholar] [CrossRef]
- Di Ferdinando, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Bautista, I.; Boscaiu, M.; Lidón, A.; Llinares, J.V.; Lull, C.; Donat, M.P.; Mayoral, O.; Vicente, O. Environmentally induced changes in antioxidant phenolic compounds levels in wild plants. Acta Physiol. Plant. 2015, 38, 9. [Google Scholar] [CrossRef]
- Laoué, J.; Fernandez, C.; Ormeño, E. Plant Flavonoids in Mediterranean Species: A Focus on Flavonols as Protective Metabolites under Climate Stress. Plants 2022, 11, 172. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Brahmi-Chendouh, N.; Piccolella, S.; Nigro, E.; Hamri-Zeghichi, S.; Madani, K.; Daniele, A.; Pacifico, S. Urtica dioica L. leaf chemical composition: A never-ending disclosure by means of HR-MS/MS techniques. J. Pharm. Biomed. Anal. 2021, 195, 113892. [Google Scholar] [CrossRef]
- Carvalho, A.R.; Costa, G.; Figueirinha, A.; Liberal, J.; Prior, J.A.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Urtica spp.: Phenolic composition, safety, antioxidant and anti-inflammatory activities. Food Res. Int. 2017, 99, 485–494. [Google Scholar] [CrossRef]
- Muccilli, V.; Kubik, J.; Waszak, Ł.; Adamczuk, G.; Humeniuk, E.; Iwan, M.; Adamczuk, K.; Michalczuk, M.; Korga-Plewko, A.; Józefczyk, A. Phytochemical Analysis and Anti-Cancer Properties of Extracts of Centaurea castriferrei Borbás & Waisb Genus of Centaurea L. Molecules 2022, 27, 7537. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Vasilopoulou, E.; Hollman, P.; Chamalides, C.; Foufa, E.; Kaloudis, T.; Kromhout, D.; Miskaki, P.; Petrochilou, I.; Poulima, E.; et al. Nutritional Composition and Flavonoid Content of Edible Wild Greens and Green Pies: A Potential Rich Source of Antioxidant Nutrients in the Mediterranean Diet. Food Chem. 2000, 70, 319–323. [Google Scholar] [CrossRef]
- Ceylan, S.; Cetin, S.; Camadan, Y.; Saral, O.; Ozsen, O.; Tutus, A. Antibacterial and antioxidant activities of traditional medicinal plants from the Erzurum region of Turkey. Ir. J. Med. Sci. 2019, 188, 1303–1309. [Google Scholar] [CrossRef]
- Bilić, V.L.; Gašić, U.; Milojković-Opsenica, D.; Nemet, I.; Rončević, S.; Kosalec, I.; Rodriguez, J.V. First Extensive Polyphenolic Profile of Erodium Cicutarium with Novel Insights to Elemental Composition and Antioxidant Activity. Chem. Biodivers. 2020, 17, e2000280. [Google Scholar] [CrossRef]
- Tomczyk, M.; Gudej, J.; Sochacki, M. Flavonoids from Ficaria verna Huds. Z. Fur Nat.-Sect. C. 2002, 57, 440–444. [Google Scholar] [CrossRef]
- Karpiuk, V.; Konechna, R. Total phenolic and flavonoid content, antioxidant activity of Ficaria verna. Sci. J. Pol. Univ. 2021, 46, 229–234. [Google Scholar] [CrossRef]
- Bhatti, M.Z.; Ali, A.; Ahmad, A.; Saeed, A.; Malik, S.A. Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC Res. Notes 2017, 8, 279. [Google Scholar] [CrossRef]
- Chen, W.-C.; Wang, S.-W.; Li, C.-W.; Lin, H.-R.; Yang, C.-S.; Chu, Y.-C.; Lee, T.-H.; Chen, J.-J. Comparison of Various Solvent Extracts and Major Bioactive Components from Portulaca oleracea for Antioxidant, Anti-Tyrosinase, and Anti-α-Glucosidase Activities. Antioxidants 2022, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Boskovic, I.; Đukić, D.A.; Mašković, P.; Mandić, L.; Perovic, S. Phytochemical composition and antimicrobial, antioxidant and cytotoxic activities of Anchusa officinalis L. extracts. Biologia 2018, 73, 1035–1041. [Google Scholar] [CrossRef]
- Brown, A.W.; Stegelmeier, B.L.; Colegate, S.M.; Gardner, D.R.; Panter, K.E.; Knoppel, E.L.; Hall, J.O. The comparative toxicity of a reduced, crude comfrey (Symphytum officinale) alkaloid extract and the pure, comfrey-derived pyrrolizidine alkaloids, lycopsamine and intermedine in chicks (Gallus gallus domesticus). J. Appl. Toxicol. 2016, 36, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Miere, F.; Teușdea, A.C.; Laslo, V.; Cavalu, S.; Fritea, L.; Dobjanschi, L.; Zdrinca, M.; Zdrinca, M.; Ganea, M.; Pașc, P.; et al. Evaluation of In Vitro Wound-Healing Potential, Antioxidant Capacity, and Antimicrobial Activity of Stellaria Media (L.) Vill. Appl. Sci. 2021, 11, 11526. [Google Scholar] [CrossRef]
- Rahman, M.; Khatun, A.; Liu, L.; Barkla, B.J. Brassicaceae Mustards: Traditional and Agronomic Uses in Australia and New Zealand. Molecules 2018, 23, 231. [Google Scholar] [CrossRef] [PubMed]
- Narzary, H.; Basumatary, S. Amino Acid Profiles and Anti-Nutritional Contents of Traditionally Consumed Six Wild Vege-tables. Curr. Chem. Lett. 2019, 8, 137–144. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Naidu, K.A.; Shang, X.; Keum, Y.-S. Omega−3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Zekić, M.; Radonić, A.; Marijanović, Z. Glucosinolate Profiling of Calepina irregularis. Nat. Prod. Commun. 2016, 11, 1329–1332. [Google Scholar] [CrossRef]
- Stojković, D.; Drakulić, D.; Schwirtlich, M.; Rajčević, N.; Stevanović, M.; Soković, M.; Gašić, U. Extract of Herba Anthrisci cerefolii: Chemical Profiling and Insights into Its Anti-Glioblastoma and Antimicrobial Mechanism of Actions. Pharmaceuticals 2021, 14, 55. [Google Scholar] [CrossRef]
- Sergazy, S.; Vetrova, A.; Orhan, I.E.; Deniz, F.S.S.; Kahraman, A.; Zhang, J.-Y.; Aljofan, M. Antiproliferative and cytotoxic activity of Geraniaceae plant extracts against five tumor cell lines. Futur. Sci. OA 2022, 8, 0109. [Google Scholar] [CrossRef]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Ślesak, H.; Szopa, A. Significance of Artemisia vulgaris L. (Common Mugwort) in the History of Medicine and Its Possible Contemporary Applications Substantiated by Phytochemical and Pharmacological Studies. Molecules 2020, 25, 4415. [Google Scholar] [CrossRef]
- Kokanova-Nedialkova, Z.; Nedialkov, P.T.; Nedialkov, P.T.; Nikolov, S.D. The Genus Chenopodium: Phytochemistry, Eth-nopharmacology and Pharmacology. Pharmacogn. Rev. 2009, 3, 280–306. [Google Scholar]
- Korpelainen, H.; Pietiläinen, M. Hop (Humulus lupulus L.): Traditional and Present Use, and Future Potential. Econ. Bot. 2021, 75, 302–322. [Google Scholar] [CrossRef]
- Escher, G.B.; Santos, J.S.; Rosso, N.D.; Marques, M.B.; Azevedo, L.; do Carmo, M.A.V.; Daguer, H.; Molognoni, L.; do Prado-Silva, L.; Sant’Ana, A.S.; et al. Chemical study, antioxidant, anti-hypertensive, and cytotoxic/cytoprotective activities of Centaurea cyanus L. petals aqueous extract. Food Chem. Toxicol. 2018, 118, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, O.S.; Oyebamiji, A.K. Stellaria media (L.) Vill.-A plant with immense therapeutic potentials: Phytochemistry and pharmacology. Heliyon 2020, 6, e04150. [Google Scholar] [CrossRef] [PubMed]
- Kokanova-Nedialkova, Z.; Nedialkov, P.T.; Kokanova-Nedialkova, Z.; Nedialkov, P. Antioxidant Properties of 6-Methoxyflavonol Glycosides from the Aerial Parts of Chenopodium Bonus-Henricus L. Artic. Bulg. Chem. Com-Munications 2017, 49, 253–258. [Google Scholar]
- Luczaj, L. Ethnobotanical Review of Wild Edible Plants of Slovakia. Acta Soc. Bot. Pol. 2012, 81, 198–225. [Google Scholar] [CrossRef]
- Gupta, S.; Lakshmi, A.J.; Manjunath, M.; Prakash, J. Analysis of nutrient and antinutrient content of underutilized green leafy vegetables. LWT-Food Sci. Technol. 2005, 38, 339–345. [Google Scholar] [CrossRef]
- Acık, D.Y.; Yilmaz, M.; Sahin, H.H.; Sayıner, Z.; Koruk, I.; Tiryaki, O.; Okan, V.; Pehlivan, M. Management of Chenopodium polyspermum toxicity with plasma exchange and hemodialysis. J. Clin. Apher. 2012, 27, 278–281. [Google Scholar] [CrossRef]
- Günthardt, B.F.; Hollender, J.; Hungerbühler, K.; Scheringer, M.; Bucheli, T.D. Comprehensive Toxic Plants-Phytotoxins Database and Its Application in Assessing Aquatic Micropollution Potential. J. Agric. Food Chem. 2018, 66, 7577–7588. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Davis, T.Z.; Clayton, M.J. Plants Containing Urinary Tract, Gastrointestinal, or Miscellaneous Toxins that Affect Livestock. Veter-Clin. N. Am. Food Anim. Pract. 2020, 36, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Kopp, T.; Abdel-Tawab, M.; Mizaikoff, B. Extracting and Analyzing Pyrrolizidine Alkaloids in Medicinal Plants: A Review. Toxins 2020, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- El-Shazly, A.; Wink, M. Diversity of Pyrrolizidine Alkaloids in the Boraginaceae Structures, Distribution, and Biological Properties. Diversity 2014, 6, 188–282. [Google Scholar] [CrossRef]
- Akbulut, S.; Semur, H.; Kose, O.; Ozhasenekler, A.; Celiktas, M.; Basbug, M.; Yagmur, Y. Phytocontact dermatitis due to Ranunculus arvensis mimicking burn injury: Report of three cases and literature review. Int. J. Emerg. Med. 2011, 4, 7. [Google Scholar] [CrossRef]
- Kocak, A.O.; Saritemur, M.; Atac, K.; Guclu, S.; Ozlu, I. A rare chemical burn due to Ranunculus arvensis: Three case reports. Ann. Saudi Med. 2016, 36, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Bender, N.; Chiu, Y. Photosensitivity. In Nelson Textbook of Pediatrics; Kliegman, R., Geme, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 1, pp. 3496–3501. ISBN 9780323529501. [Google Scholar]
- Wink, M. Modes of Action of Herbal Medicines and Plant Secondary Metabolites. Medicines 2015, 2, 251–286. [Google Scholar] [CrossRef]
- Munjal, K.; Amin, S.A.; Mir, S.R.; Gauttam, V.K.; Gupta, S. Furanocoumarins and Lectins as Food Toxins. In Plant-Derived Bioactives: Chemistry and Mode of Action; Swamy, M.K., Ed.; Springer: Singapore, 2020; pp. 67–80. ISBN 9789811523618. [Google Scholar]
- Levorato, S.; Dominici, L.; Fatigoni, C.; Zadra, C.; Pagiotti, R.; Moretti, M.; Villarini, M. In vitro toxicity evaluation of estragole-containing preparations derived from Foeniculum vulgare Mill. (fennel) on HepG2 cells. Food Chem. Toxicol. 2018, 111, 616–622. [Google Scholar] [CrossRef]
- Koulman, A.; Kubbinga, M.E.; Batterman, S.; Woerdenbag, H.J.; Pras, N.; Woolley, J.G.; Quax, W.J. A Phytochemical Study of Lignans in Whole Plants and Cell Suspension Cultures of Anthriscus Sylvestris. Planta Med. 2003, 69, 733–738. [Google Scholar] [CrossRef]
- Orčić, D.; Berežni, S.; Škorić, D.; Mimica-Dukić, N. Comprehensive study of Anthriscus sylvestris lignans. Phytochemistry 2021, 192, 112958. [Google Scholar] [CrossRef]
- Piechowska, K.; Mizerska-Kowalska, M.; Zdzisińska, B.; Cytarska, J.; Baranowska-Łączkowska, A.; Jaroch, K.; Łuczykowski, K.; Płaziński, W.; Bojko, B.; Kruszewski, S.; et al. Tropinone-Derived Alkaloids as Potent Anticancer Agents: Synthesis, Tyrosinase Inhibition, Mechanism of Action, DFT Calculation, and Molecular Docking Studies. Int. J. Mol. Sci. 2020, 21, 9050. [Google Scholar] [CrossRef]
- Todd, F.G.; Stermitz, F.R.; Schultheis, P.; Knight, A.P.; Traub-Dargatz, J. Tropane Alkaloids and Toxicity of Convolvulus Arvensis. Phytochemistry 1995, 39, 301–303. [Google Scholar] [CrossRef]
- Shaghaghi, A.; Alirezalu, A.; Nazarianpour, E.; Sonboli, A.; Nejad-Ebrahimi, S. Opioid alkaloids profiling and antioxidant capacity of Papaver species from Iran. Ind. Crop. Prod. 2019, 142, 111870. [Google Scholar] [CrossRef]
- Grauso, L.; de Falco, B.; Motti, R.; Lanzotti, V. Corn poppy, Papaver rhoeas L.: A critical review of its botany, phytochemistry and pharmacology. Phytochem. Rev. 2020, 20, 227–248. [Google Scholar] [CrossRef]
- Wencai, Y.; Ji, M.; Shouxun, Z. Bioactive Amino Acids from Agrostemma Githago L. with the Effects of Increasing Production of Wheat. J. China Pharm. Univ. 1997, 28, 65–68. [Google Scholar]
- Alaca, K.; Okumuş, E.; Bakkalbaşi, E.; Javidipour, I. Phytochemicals and antioxidant activities of twelve edible wild plants from Eastern Anatolia, Turkey. Food Sci. Technol. 2021, 42, 18021. [Google Scholar] [CrossRef]
- Woolum, J.A.; Akpunonu, P.; Johnson, M.; Webb, A.N. Human exposures to Phytolacca americana in Kentucky. Toxicon 2022, 220, 106962. [Google Scholar] [CrossRef]
- Qneibi, M.; Jaradat, N.; Zaid, A.N.Z.A.N.; Abu-Khalaf, N.; Natsheh, A.-R.; Hussen, F. Evaluation of Taste, Total Phenols and Antioxidant for Fresh, Roasted, Shade dried and Boiled leaves of Edible Arum palaestinum Bioss. Marmara Pharm. J. 2018, 22, 52–58. [Google Scholar] [CrossRef]
- Hali´nski, A.; Bhatti, K.H.; Boeri, L.; Cloutier, J.; Davidoff, K.; Elqady, A.; Fryad, G.; Gadelmoula, M.; Hui, H.; Petkova, K.; et al. Stone composition of renal stone formers from different global regions. Arch. Ital. Urol. E Androl. 2021, 93, 307–312. [Google Scholar] [CrossRef]
- Marais, J.P. Nitrate and Oxalates. In Handbook of Plant and Fungal Toxicants; D’Mello, J.P.F., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 205–218. ISBN 9780429281952. [Google Scholar]
- Mahlangeni, N.T.; Moodley, R.; Jonnalagadda, S.B. The distribution of macronutrients, anti-nutrients and essential elements in nettles, Laportea pedunculariss usp. peduncularis (River nettle) and Urtica dioica (Stinging nettle). J. Environ. Sci. Health Part B 2015, 51, 160–169. [Google Scholar] [CrossRef]
- Bello, O.M.; Fasinu, P.S.; Bello, O.E.; Ogbesejana, A.B.; Adetunji, C.O.; Dada, A.O.; Ibitoye, O.S.; Aloko, S.; Oguntoye, O.S. Wild vegetable Rumex acetosa Linn.: Its ethnobotany, pharmacology and phytochemistry—A review. S. Afr. J. Bot. 2019, 125, 149–160. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The Antioxidant Properties of Alcoholic Extracts from Sambucus Nigra L. (Antioxidant Properties of Extracts). LWT-Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive Properties of Sambucus Nigra L. as a Functional Ingredient for Food and Pharmaceutical Industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Mocanu, M.L.; Amariei, S. Elderberries-A Source of Bioactive Compounds with Antiviral Action. Plants 2022, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, S.; D’Abrosca, B.; Golino, A.; Mastellone, C.; Piccolella, S.; Fiorentino, A.; Monaco, P. Antioxidant Evaluation of Polyhydroxylated Nerolidols from Redroot Pigweed (Amaranthus Retroflexus) Leaves. LWT-Food Sci. Technol. 2008, 41, 1665–1671. [Google Scholar] [CrossRef]
- Conforti, F.; Marrelli, M.; Carmela, C.; Menichini, F.; Valentina, P.; Uzunov, D.; Statti, G.A.; Duez, P.; Menichini, F. Bioactive Phytonutrients (Omega Fatty Acids, Tocopherols, Polyphenols), in Vitro Inhibition of Nitric Oxide Production and Free Radical Scavenging Activity of Non-Cultivated Mediterranean Vegetables. Food Chem. 2011, 129, 1413–1419. [Google Scholar] [CrossRef]
- Kongdang, P.; Dukaew, N.; Pruksakorn, D.; Koonrungsesomboon, N. Biochemistry of Amaranthus Polyphenols and Their Potential Benefits on Gut Ecosystem: A Comprehensive Review of the Literature. J. Ethnopharmacol. 2021, 281, 114547. [Google Scholar] [CrossRef]
- Bylka, W.; Stobiecki, M.; Frański, R. Sulphated Flavonoid Glycosides from Leaves of Atriplex Hortensis. Acta Physiol. Plant 2001, 23, 285–290. [Google Scholar] [CrossRef]
- Bueno, M.; Lendínez, M.L.; Aparicio, C.; Cordovilla, M.P. Germination and Growth of Atriplex Prostrata and Plantago Coronopus: Two Strategies to Survive in Saline Habitats. Flora 2017, 227, 56–63. [Google Scholar] [CrossRef]
- Rizk, A.M. The Phytochemistry of the Flora of Qatar; Scientific and Applied Research Centre, University of Qatar: Doha, Qatar, 1986; ISBN 9780855462246. [Google Scholar]
- Mynarski, A.; Dawiec, E.; Grabowska, K.; Pietrzak, W.; Nowak, R.; Podolak, I. Qualitative and Quantitative Determination of Flavonoids in Different Organs of Atriplex Nitens Schkuhr and Evaluation of Anti-Hyaluronidase Activity. Planta Med. 2019, 85, P215. [Google Scholar] [CrossRef]
- Van Niekerk, W.A.; Sparks, C.F.; Rethman, N.F.G.; Coertze, R.J. Mineral Composition of Certain Atriplex Species and Cassia Sturtii | South African Journal of Animal Science. S. Afr. J. Anim. Sci. 2004, 34, 94387. [Google Scholar] [CrossRef]
- Ali, B.; Musaddiq, S.; Iqbal, S.; Rehman, T.; Shafiq, N.; Hussain, A. The Therapeutic Properties, Ethno Pharmacology and Phytochemistry of Atriplex Species: A Review. Pak. J. Biochem. Biotechnol. 2021, 2, 49–64. [Google Scholar] [CrossRef]
- Usman, L.A.; Hamid, A.A.; Muhammad, N.O.; Olawore, N.O.; Edewor, T.I.; Saliu, B.K. Chemical Constituents and Anti-Inflammatory Activity of Leaf Essential Oil of Nigerian Grown Chenopodium Album L. EXCLI J. 2010, 9, 186. [Google Scholar]
- Laghari, A.H.; Memon, S.; Nelofar, A.; Khan, K.M.; Yasmin, A. Determination of Free Phenolic Acids and Antioxidant Activity of Methanolic Extracts Obtained from Fruits and Leaves of Chenopodium Album. Food Chem. 2011, 126, 1850–1855. [Google Scholar] [CrossRef] [PubMed]
- Chamkhi, I.; Charfi, S.; El Hachlafi, N.; Mechchate, H.; Guaouguaou, F.E.; El Omari, N.; Bakrim, S.; Balahbib, A.; Zengin, G.; Bouyahya, A. Genetic Diversity, Antimicrobial, Nutritional, and Phytochemical Properties of Chenopodium Album: A Comprehensive Review. Food Res. Int. 2022, 154, 110979. [Google Scholar] [CrossRef]
- Hanganu, D.; Olah, N.; Vlase, L.; Mărculescu, A.; Pintea, A. Chemical Research of Carotenoids from Chenopodium Bonus Henricus L. (Chenopodiaceae). Farmacia 2012, 60, 840–849. [Google Scholar]
- Kokanova-Nedialkova, Z.; Nedialkov, P.; Kondeva-Burdina, M. Ultra-High-Performance Liquid Chromatography—High-Resolution Mass Spectrometry Profiling and Hepatoprotective Activity of Purified Saponin and Flavonoid Fractions from the Aerial Parts of Wild Spinach (Chenopodium Bonus-Henricus L.). Z. Fur Nat.-Sect. C 2021, 76, 261–271. [Google Scholar] [CrossRef]
- Iwashina, T. Flavonoid Properties in Plant Families Synthesizing Betalain Pigments (Review). Nat. Prod. Commun. 2015, 10, 1103–1114. [Google Scholar] [CrossRef]
- Nowak, R.; Szewczyk, K.; Gawlik-Dziki, U.; Rzymowska, J.; Komsta, Ł. Antioxidative and Cytotoxic Potential of Some Chenopodium L. Species Growing in Poland. Saudi J. Biol. Sci. 2016, 23, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Fejes, S.; Lemberkovics, É.; Balázs, A.; Apáti, P.; Kristó, T.S.; Szoke, É.; Kéry, Á.; Blázovics, A. Antioxidant Activity of Different Compounds from Anthriscus Cerefolium L. (Hoffm.). Acta Hortic. 2004, 597, 191–198. [Google Scholar] [CrossRef]
- Chizzola, R. Composition of the Essential Oils from Anthriscus Cerefolium Var. Trichocarpa and A. Caucalis Growing Wild in the Urban Area of Vienna (Austria). Nat. Prod. Commun. 2011, 6, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Slimestad, R.; Rathe, B.A.; Aesoy, R.; Diaz, A.E.C.; Herfindal, L.; Fossen, T. A Novel Bicyclic Lactone and Other Polyphenols from the Commercially Important Vegetable Anthriscus Cerefolium. Sci. Rep. 2022, 12, 7805. [Google Scholar] [CrossRef] [PubMed]
- Kokkalou, E.; Stefanou, E. The Volatiles of Chaerophyllum bulbosum L. ssp. Bulbosum Growing Wild in Greece. Pharm. Acta Helv. 1989, 64, 133–134. [Google Scholar]
- Stamenković, J.G.; Đorđević, A.S.; Stojanović, G.S.; Mitić, V.D.; Petrović, G.M. Phytochemical Analysis of Volatiles and Biological Activities of Chaerophyllum Bulbosum L. Essential Oils. J. Serb. Chem. Soc. 2021, 86, 257–267. [Google Scholar] [CrossRef]
- Molo, Z.; Tel-Çayan, G.; Deveci, E.; Öztürk, M.; Duru, M.E. Insight into Isolation and Characterization of Compounds of Chaerophyllum Bulbosum Aerial Part with Antioxidant, Anticholinesterase, Anti-Urease, Anti-Tyrosinase, and Anti-Diabetic Activities. Food Biosci. 2021, 42, 101201. [Google Scholar] [CrossRef]
- Senatore, F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; De Falco, E. Chemical Composition, Antimicrobial and Antioxidant Activities of Anethole-Rich Oil from Leaves of Selected Varieties of Fennel [Foeniculum Vulgare Mill. Ssp. Vulgare Var. Azoricum (Mill.) Thell]. Fitoterapia 2013, 90, 214–219. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. The Nutritional Composition of Fennel (Foeniculum Vulgare): Shoots, Leaves, Stems and Inflorescences. LWT-Food Sci. Technol. 2010, 43, 814–818. [Google Scholar] [CrossRef]
- Comlekcioglu, N.; Çolak, S.; Aygan, A. A Study on the Bıoactıvıty of Plant Extracts Obtaıned from Arum Maculatum Leaves by Dıfferent Extractıon Technıques. Croat. J. Food Technol. 2021, 16, 41–46. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Esmaeilzadeh Kenari, R.; Asnaashari, M.; Shahrampour, D.; Bakhshandeh, T. Bioactive Compounds, Antioxidant and Antimicrobial Activities of Arum Maculatum Leaves Extracts as Affected by Various Solvents and Extraction Methods. Food Sci. Nutr. 2019, 7, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Kozuharova, E.; Naychov, Z.; Kochmarov, V.; Benbassat, N.; Gibernau, M.; Momekov, G. The Potential of Arum Spp. as a Cure for Hemorrhoids: Chemistry, Bioactivities, and Application. Adv. Tradit. Med. 2020, 20, 133–141. [Google Scholar] [CrossRef]
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Piné, R.; Švarc-Gajić, J.; Segura-Carretero, A. The Potential of Artemisia Vulgaris Leaves as a Source of Antioxidant Phenolic Compounds. J. Funct. Foods 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Tigno, X.T.; de Guzman, F.; Flora, A.M.T.V. Phytochemical Analysis and Hemodynamic Actions of Artemisia vulgaris L. Clin. Hemorheol. Microcirc. 2000, 23, 167–175. [Google Scholar] [PubMed]
- Thangjam, N.M.; Taijong, J.; Kumar, A. Phytochemical and Pharmacological Activities of Methanol Extract of Artemisia vulgaris L. Leaves. Clin. Phytoscience 2020, 6, 72. [Google Scholar] [CrossRef]
- Sharonova, N.; Nikitin, E.; Terenzhev, D.; Lyubina, A.; Amerhanova, S.; Bushmeleva, K.; Rakhmaeva, A.; Fitsev, I.; Sinyashin, K. Comparative Assessment of the Phytochemical Composition and Biological Activity of Extracts of Flowering Plants of Centaurea Cyanus L., Centaurea Jacea L. and Centaurea Scabiosa L. Plants 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Litvinenko, V.I.; Bubenchikova, V.N. Phytochemical Study of Centaurea Cyanus. Chem. Nat. Compd. 1988, 24, 672–674. [Google Scholar] [CrossRef]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical Characterization of Borago Officinalis L. and Centaurea Cyanus L. during Flower Development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef]
- Ebada, S.S.; Al-Jawabri, N.A.; Youssef, F.S.; Albohy, A.; Aldalaien, S.M.; Disi, A.M.; Proksch, P. In Vivo Antiulcer Activity, Phytochemical Exploration, and Molecular Modelling of the Polyphenolic-Rich Fraction of Crepis Sancta Extract. Inflammopharmacology 2019, 28, 321–331. [Google Scholar] [CrossRef]
- Boskabadi, J.; Askari, Z.; Zakariaei, Z.; Fakhar, M.; Tabaripour, R. Mild-to-Severe Poisoning Due to Conium Maculatum as Toxic Herb: A Case Series. Clin. Case Rep. 2021, 9, e04509. [Google Scholar] [CrossRef]
- Badalamenti, N.; Modica, A.; Ilardi, V.; Bruno, M. Chemical Constituents and Biological Properties of Genus Doronicum (Asteraceae). Chem. Biodivers. 2021, 18, e2100631. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.-H.; Nam, E.-K.; Shim, K.-H. Chemical Components in Different Parts of Erigeron Annuus. J. Korean Soc. Food Sci. Nutr. 2005, 34, 857–861. [Google Scholar] [CrossRef]
- Poljuha, D.; Sladonja, B.; Šola, I.; Šenica, M.; Uzelac, M.; Veberič, R.; Hudina, M.; Famuyide, I.M.; Eloff, J.N.; Mikulic-Petkovsek, M. LC–DAD–MS Phenolic Characterisation of Six Invasive Plant Species in Croatia and Determination of Their Antimicrobial and Cytotoxic Activity. Plants 2022, 11, 596. [Google Scholar] [CrossRef]
- Fontanel, D.; Galtier, C.; Viel, C.; Gueiffier, A. Caffeoyl Quinic and Tartaric Acids and Flavonoids from Lapsana Communis L. Subsp. Communis (Asteraceae). Z. Fur Nat.-Sect. C 1998, 53, 1090–1092. [Google Scholar] [CrossRef]
- Seal, T. Quantitative HPLC Analysis of Phenolic Acids, Flavonoids and Ascorbic Acid in Four Different Solvent Extracts of Two Wild Edible Leaves, Sonchus Arvensis and Oenanthe Linearis of North-Eastern Region in India. J. Appl. Pharm. Sci. 2016, 6, 157–166. [Google Scholar] [CrossRef]
- Itam, A.; Ismail, A.M.S.A.M.; Ismail, Z. Antioxidant and Antiangiogenic Properties, and Gas Chromatographic-Time of Flight Analysis of Sonchus Arvensis Leaves Extracts. J. Chem. Soc. Pak. 2015, 37, 1239. [Google Scholar]
- De Paula Filho, G.X.; Barreira, T.F.; Pinheiro-Sant’Ana, H.M. Chemical Composition and Nutritional Value of Three Sonchus Species. Int. J. Food Sci. 2022, 2022, 4181656. [Google Scholar] [CrossRef]
- Jimoh, F.; Adedapo, A.A.; Afolayan, A.J. Comparison of the Nutritive Value, Antioxidant and Antibacterial Activities of Sonchus Asper and Sonchus Oleraceus. Rec. Nat. Prod. 2011, 5, 29–42. [Google Scholar]
- Wang, L.; Xu, M.L.; Liu, J.; Wang, Y.; Hu, J.H.; Wang, M.H. Sonchus Asper Extract Inhibits LPS-Induced Oxidative Stress and pro-Inflammatory Cytokine Production in RAW264.7 Macrophages. Nutr. Res. Pract. 2015, 9, 579–585. [Google Scholar] [CrossRef]
- Biel, W.; Jaroszewska, A.; Łysoń, E.; Telesiński, A. The Chemical Composition and Antioxidant Properties of Common Dandelion Leaves Compared with Sea Buckthorn. Can. J. Plant Sci. 2017, 97, 1165–1174. [Google Scholar] [CrossRef]
- Hussain, F.H.; Hama Saeed Hussain, F.; Ahamad, J.; Shafiq Osw, P. A Comprehensive Review on Pharmacognostical and Pharmacological Characters of Anchusa Azurea. Adv. Med. Dent. Health Sci. 2019, 2, 33–37. [Google Scholar] [CrossRef]
- Kuruüzüm-Uz, A.; Güvenalp, Z.; Kazaz, C.; Salih, B.; Demirezer, L.Ö. Four New Triterpenes from Anchusa Azurea Var. Azurea. Helv. Chim. Acta 2010, 93, 457–465. [Google Scholar] [CrossRef]
- Baghiani, A.; Naouel, B.; Trabsa, H.; Aouachria, S.; Boussoualim, N.; Arrar, L.; Boumerfeg, S. In Vivo Free Radical Scavenging, Antihemolytic Activity and Antibacterial Effects of Anchusa Azurea Extracts. Int. J. Med. Med. Sci. 2013, 46, 2051–5731. [Google Scholar]
- Paun, G.; Neagu, E.; Albu, C.; Savin, S.; Radu, G.L. In Vitro Evaluation of Antidiabetic and Anti-Inflammatory Activities of Polyphenolic-Rich Extracts from Anchusa Officinalis and Melilotus Officinalis. ACS Omega 2020, 5, 13014–13022. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Tumer, T.B.; Ozleyen, A.; Rodríguez-Pérez, C.; Ezzat, S.M.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I.; et al. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef] [PubMed]
- Haribal, M.; Renwick, J.A.A. Isovitexin 6″-O-β-d-Glucopyranoside: A Feeding Deterrent to Pieris Napi Oleracea from Alliaria Petiolata. Phytochemistry 1998, 47, 1237–1240. [Google Scholar] [CrossRef]
- Cipollini, D.; Gruner, B. Cyanide in the Chemical Arsenal of Garlic Mustard, Alliaria Petiolata. J. Chem. Ecol. 2007, 33, 85–94. [Google Scholar] [CrossRef]
- Frisch, T.; Agerbirk, N.; Davis, S.; Cipollini, D.; Olsen, C.E.; Motawia, M.S.; Bjarnholt, N.; Møller, B.L. Glucosinolate-Related Glucosides in Alliaria Petiolata: Sources of Variation in the Plant and Different Metabolism in an Adapted Specialist Herbivore, Pieris Rapae. J. Chem. Ecol. 2014, 40, 1063–1079. [Google Scholar] [CrossRef]
- Kumarasamy, Y.; Byres, M.; Cox, P.J.; Delazar, A.; Jaspars, M.; Nahar, L.; Shoeb, M.; Sarker, S.D. Isolation, Structure Elucidation, and Biological Activity of Flavone 6-C-Glycosides from Alliaria Petiolata. Chem. Nat. Compd. 2004, 40, 122–128. [Google Scholar] [CrossRef]
- Al-Snafi, A.E.; Al-Snafi, A.E. The Chemical Constituents and Pharmacological Effects of Capsella Bursa-Pastoris-A Review. Int. J. Pharmacol. Toxicol. 2015, 5, 76–81. [Google Scholar]
- Duke, J.; Ayensu, E. Medicinal Plants of China; Reference Publications: Algonac, MO, USA, 1985; ISBN 9780917256202. [Google Scholar]
- Pehlivan, M.; Akgul, H.; Yayla, F. The Some Nutrient and Trace Elements Content of Wild Plants Using as Ethno Botanical and Grown in the Gaziantep Region. J. Appl. Pharm. Sci. 2013, 3, 143–145. [Google Scholar] [CrossRef]
- Song, N.; Xu, W.; Guan, H.; Liu, X.; Wang, Y.; Nie, X. Several Flavonoids from Capsella Bursa-Pastoris (L.) Medic. Asian J. Tradit. Med. 2007, 2, 218–222. [Google Scholar]
- Kubínová, R.; Špačková, V.; Švajdlenka, E.; Lučivjanská, K. Antioxidant Activity of Extracts and HPLC Analysis of Flavonoids from Capsella Bursa-Pastoris (L.) Medik. Ceska. Slov. Farm. 2013, 62, 174–176. [Google Scholar] [PubMed]
- Riaz, I.; Bibi, Y.; Ahmad, N.; Nisa, S.; Qayyum, A. Evaluation of Nutritional, Phytochemical, Antioxidant and Cytotoxic Potential of Capsella Bursa-Pastoris, a Wild Vegetable from Potohar Region of Pakistan. Kuwait J. Sci. 2021, 48. [Google Scholar] [CrossRef]
- Montaut, S.; Bleeker, R.S. Cardamine sp.—A Review on Its Chemical and Biological Profiles. Chem. Biodivers. 2011, 8, 955–975. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Glauser, G.; Defossez, E.; Rasmann, S. Ecological Convergence of Secondary Phytochemicals along Elevational Gradients. New Phytol. 2021, 229, 1755–1767. [Google Scholar] [CrossRef]
- Kejariwal, M.; Rodrigues, D.; Gowda, H. Study of Growth, Secondary Metabolities and Glucosinolate Content in Cardamine Hirsuta v/s Brassica Juncea (Indian Mustard). J. Harmon. Res. Appl. Sci. 2018, 6, 20–27. [Google Scholar] [CrossRef]
- Agerbirk, N.; Hansen, C.C.; Olsen, C.E.; Kiefer, C.; Hauser, T.P.; Christensen, S.; Jensen, K.R.; Ørgaard, M.; Pattison, D.I.; Lange, C.B.A.; et al. Glucosinolate Profiles and Phylogeny in Barbarea Compared to Other Tribe Cardamineae (Brassicaceae) and Reseda (Resedaceae), Based on a Library of Ion Trap HPLC-MS/MS Data of Reference Desulfoglucosinolates. Phytochemistry 2021, 185, 112658. [Google Scholar] [CrossRef]
- Bandara, M.; Savidov, N.; Driedger, D. Evaluation of Field Pepperweed (Lepidium Campestre L.) as a Source for Glucoraphanin Production. Acta Hortic. 2008, 765, 165–172. [Google Scholar] [CrossRef]
- Yusifova, D.Y.; Movsumov, I.S.; Garaev, E.A.; Mahiou-Leddet, V.; Mabrouki, F.; Herbette, G.; Baghdikian, B.; Ollivier, E. Biologically Active Compounds from Lepidium Campestre and Pulp from Lemon-Juice Production. Chem. Nat. Compd. 2015, 51, 964–965. [Google Scholar] [CrossRef]
- Kaur, T.; Hussain, K.; Koul, S.; Vishwakarma, R.; Vyas, D. Evaluation of Nutritional and Antioxidant Status of Lepidium Latifolium Linn.: A Novel Phytofood from Ladakh. PLoS ONE 2013, 8, e69112. [Google Scholar] [CrossRef]
- Azimkhanova, B.B.; Ustenova, G.O.; Sharipov, K.O.; Rakhimov, K.D.; Sayakova, G.M.; Jumagaziyeva, A.B.; Flisyuk, E.V.; Gemejiyeva, N.G. Chemical Composition and Antimicrobial Activity of Subcritical CO2Extract of Lepidium Latifolium L. (Brassicaceae). Int. J. Biomater. 2021, 2021, 4389967. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, F.; Mohammadi, H.; Azarpeik, S.; Amiri, F.T.; Shahani, S. Attenuation of Liver Mitochondrial Oxidative Damage by the Extract and Desulfo Glucosinolate Fraction of Lepidium perfoliatum L. Seeds. S. Afr. J. Bot. 2021, 138, 377–385. [Google Scholar] [CrossRef]
- Khare, C.P. Indian Medicinal Plants: An Illustrated Dictionary; Springer: New York, NY, USA, 2007; ISBN 978-0-387-70637-5. [Google Scholar]
- García-Herrera, P.; Sánchez-Mata, M.C.; Cámara, M.; Tardío, J.; Olmedilla-Alonso, B. Carotenoid Content of Wild Edible Young Shoots Traditionally Consumed in Spain (Asparagus Acutifolius L., Humulus Lupulus L., Bryonia Dioica Jacq. and Tamus Communis L.). J. Sci. Food Agric. 2014, 94, 1914–1916. [Google Scholar] [CrossRef]
- Maietti, A.; Brighenti, V.; Bonetti, G.; Tedeschi, P.; Prencipe, F.P.; Benvenuti, S.; Brandolini, V.; Pellati, F. Metabolite Profiling of Flavonols and in Vitro Antioxidant Activity of Young Shoots of Wild Humulus lupulus L. (Hop). J. Pharm. Biomed. Anal. 2017, 142, 28–34. [Google Scholar] [CrossRef]
- Abiko, Y.; Paudel, D.; Uehara, O. Hops Components and Oral Health. J. Funct. Foods 2022, 92, 105035. [Google Scholar] [CrossRef]
- Weise, C.; Schrot, A.; Wuerger, L.T.D.; Adolf, J.; Gilabert-Oriol, R.; Sama, S.; Melzig, M.F.; Weng, A. An Unusual Type I Ribosome-Inactivating Protein from Agrostemma githago L. Sci. Rep. 2020, 10, 15377. [Google Scholar] [CrossRef] [PubMed]
- Bohlooli, S.; Bohlooli, S.; Aslanian, R.; Nouri, F.; Teimourzadeh, A. Aqueous Extract of Agrostemma Githago Seed Inhibits Caspase-3 and Induces Cell-Cycle Arrest at G1 Phase in AGS Cell Line. J. Ethnopharmacol. 2015, 175, 295–300. [Google Scholar] [CrossRef]
- Boukhira, S.; Bousta, D.; El Mansouri, L.; Nordine, A.; Hamsas El Youbi, A.; Daoudi, A. Phytochemical Studies, Antioxidant Activity and Protective Effect on DNA Damage and Deoxyribose of Silene Vulgaris Extract from Morocco Medicinal Plants View Project Activités Apoptotiques et Antiprolifératives Es Extraits Des Plantes View Project Smahane Boukhira. Artic. Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 1172–1178. [Google Scholar]
- Zengin, G.; Mahomoodally, M.F.; Aktumsek, A.; Ceylan, R.; Uysal, S.; Mocan, A.; Yilmaz, M.A.; Picot-Allain, C.M.N.; Ćirić, A.; Glamočlija, J.; et al. Functional Constituents of Six Wild Edible Silene Species: A Focus on Their Phytochemical Profiles and Bioactive Properties. Food Biosci. 2018, 23, 75–82. [Google Scholar] [CrossRef]
- Hu, Y.M.; Wang, H.; Ye, W.C.; Qian, L. New Triterpenoid from Stellaria media (L.) Cyr. Nat. Prod. Res. 2010, 23, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sharma, V.; Kumawat, T.K.; Seth, R. A Review on Antidermatophytic Efficiency of Plant Essential Oils. Int. J. Pure Appl. Biosci. 2014, 2, 265–278. [Google Scholar]
- Mojab, F.; Kamalinejad, M.; Ghaderi, N.; Vahidipour, H.R. Phytochemical Screening of Some Species of Iranian Plants. Iran. J. Pharm. Res. 2003, 2, 77–82. [Google Scholar] [CrossRef]
- Saleem, U.; Zaib, S.; Khalid, S.; Anwar, F.; Akhtar, M.; Ahmad, B. Chemical Characterization, Docking Studies, Anti-Arthritic Activity and Acute Oral Toxicity of Convolvulus arvensis L. Leaves. Asian Pac. J. Trop Biomed. 2020, 10, 442. [Google Scholar] [CrossRef]
- Menemen, Y.; Williams, C.; Jury, S. Flavonoid Patterns in Convolvulus L., (Convolvulaceae) Species from Morocco. Pak. J. Bot. 2002, 34, 291–295. [Google Scholar]
- Khaliq, A.; Taviano, F.; Acquaviva, R.; Malfa, G.A.; Khaliq, H.A.; Ortiz, S.; Alhouayek, M.; Muccioli, G.G.; Quetin-Leclercq, J. Dereplication and Quantification of Major Compounds of Convolvulus Arvensis L. Extracts and Assessment of Their Effect on LPS-Activated J774 Macrophages. Molecules 2022, 27, 963. [Google Scholar] [CrossRef]
- Syrchina, A.I.; Gorokhova, V.G.; Tyukavkina, N.A.; Babkin, V.A.; Voronkov, M.G. Flavonoid Glycosides of Spore-Bearing Stems of Equisetum Arvense. Chem. Nat. Compd. 1980, 16, 245–248. [Google Scholar] [CrossRef]
- Boeing, T.; Tafarelo Moreno, K.G.; Gasparotto Junior, A.; Mota Da Silva, L.; De Souza, P. Phytochemistry and Pharmacology of the Genus Equisetum (Equisetaceae): A Narrative Review of the Species with Therapeutic Potential for Kidney Diseases. Evid.-Based Complement. Altern. Med. 2021, 2021, 6658434. [Google Scholar] [CrossRef]
- Stankov, S.; Fidan, H.; Ivanova, T.; Stoyanova, A.; Damyanova, S.; Desyk, M. Chemical Composition and Application of Flowers of False Acacia (Robinia pseudoacacia L.). Ukr. Food J. 2018, 7, 577–588. [Google Scholar] [CrossRef]
- Fecka, I.; Cisowski, W. Tannins and Flavonoids from the Erodium Cicutarium Herb. Z. Fur Nat.-Sect. B 2005, 60, 555–560. [Google Scholar] [CrossRef]
- Radulović, N.; Dekić, M.; Stojanović-Radić, Z.; Palić, R. Volatile Constituents of Erodium cicutarium (L.) L’ Hérit. (Geraniaceae). Cent. Eur. J. Biol. 2009, 4, 404–410. [Google Scholar] [CrossRef]
- Stojanović-Radić, Z.; Čomić, L.; Radulović, N.; Dekić, M.; Randelović, V.; Stefanović, O. Chemical Composition and Antimicrobial Activity of Erodium Species: E. ciconium L., E. cicutarium L., and E. absinthoides Willd. (Geraniaceae). Chem. Pap. 2010, 64, 368–377. [Google Scholar] [CrossRef]
- Eilers, E.J. Intra-Individual and Intraspecific Terpenoid Diversity in Erodium Cicutarium. Plants 2021, 10, 1574. [Google Scholar] [CrossRef]
- Qahtan Mostafa Al-Smail, M.; Adnan Hussein, F. Isolation and Identification of Alkaloids and Glycosides Active Compound from Geranium Lucidum and Geranium Purpureum. J. Phys. Conf. Ser. 2019, 1294, 062040. [Google Scholar] [CrossRef]
- Ilić, M.D.; Marčetić, M.D.; Zlatković, B.K.; Lakušić, B.S.; Kovačević, N.N.; Drobac, M.M. Chemical Composition of Volatiles of Eight Geranium L. Species from Vlasina Plateau (South Eastern Serbia). Chem. Biodivers. 2020, 17, e1900544. [Google Scholar] [CrossRef]
- Fahamiya, N.; Shiffa, M.; Aslam, M. A Comprehensive Review on Althaea Rosea Linn. Indo Am. J. Pharm. Res. 2016, 6, 6888–6894. [Google Scholar]
- Akhi, M.A. An In-Vitro Study on Antioxidant Properties of Alcea Rosea Leaves; Brac University: Dhaka, Bangladesh, 2020. [Google Scholar]
- Abdel-salam, N.A.; Ghazy, N.M.; Sallam, S.M.; Radwan, M.M.; Wanas, A.S.; ElSohly, M.A.; El-Demellawy, M.A.; Abdel-Rahman, N.M.; Piacente, S.; Shenouda, M.L. Flavonoids of Alcea Rosea L. and Their Immune Stimulant, Antioxidant and Cytotoxic Activities on Hepatocellular Carcinoma HepG-2 Cell Line. Nat. Prod. Res. 2017, 32, 702–706. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Behbudi, G.; Mazraedoost, S.; Omidifar, N.; Gholami, A.; Chiang, W.H.; Babapoor, A.; Pynadathu Rumjit, N. A Review on Health Benefits of Malva Sylvestris L. Nutritional Compounds for Metabolites, Antioxidants, and Anti-Inflammatory, Anticancer, and Antimicrobial Applications. Evid.-Based Complement. Altern. Med. 2021, 2021, 5548404. [Google Scholar] [CrossRef]
- Unsal, C.; Sariyan, G.; Mat, A.; Oktayoglu, E.; Ozhatay, N. Distribution of Alkaloids in the Samples of Papaver Dubium Subsp. Lecoqii Var. Lecoqii from Turkey: A Potential Source for Thebaine Türkiye Geofitlerinin Kültüre Alınması Yeni Tür ve Çeşitlerin İlgili Sektörlere Kazandırılması View Project The Flora of Yıldız Mountains (Kırklareli) Biosphere Project View Project. Biochem. Syst. Ecol. 2006, 34, 170–173. [Google Scholar] [CrossRef]
- Turan, M.; Kordali, S.; Zengin, H.; Dursun, A.; Sezen, Y. Macro and Micro Mineral Content of Some Wild Edible Leaves Consumed in Eastern Anatolia. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2003, 53, 129–137. [Google Scholar] [CrossRef]
- Ünsal, Ç.; Özbek, B.; Saryar, G.; Mat, A. Antimicrobial Activity of Four Annual Papaver Species Growing in Turkey. Pharm. Biol. 2009, 47, 4–6. [Google Scholar] [CrossRef]
- Akrout, A.; El Jani, H.; Zammouri, T.; Mighri, H.; Neffati, M. Phytochemical Screening and Mineral Contents of Annual Plants Growing Wild in the Southern of Tunisia. J. Phytol. 2010, 2, 034–040. [Google Scholar]
- Ali Hijazi, M.; Aboul-Ela, M.; Bouhadir, K.; Fatfat, M.; Khalife, H.; Ellakany, A.; Gali-Muhtasib, H. Cytotoxic Activity of Alkaloids from Papaver Rhoeas Growing in Lebanon. Rec. Nat. Prod. 2017, 11, 211–216. [Google Scholar]
- Maurizi, A.; De Michele, A.; Ranfa, A.; Ricci, A.; Roscini, V.; Coli, R.; Bodesmo, M.; Buruni, G. Bioactive Compounds and Antioxidant Characterization of Three Edible Wild Plants Traditionally Consumed in the Umbria Region (Central Italy): Bunias Erucago L. (Corn Rocket), Lactuca Perennis L. (Mountain Lettuce) and Papaver Rhoeas L. (Poppy). J. Appl. Bot. Food Qual. 2015, 88, 109–114. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Majchrzak, D.; Wagner, K.H.; Elmadfa, I.; Kafatos, A. Lipid Concentrations of Wild Edible Greens in Crete. Food Chem. 2006, 99, 822–834. [Google Scholar] [CrossRef]
- Marinaş, I.C.; Oprea, E.; Geană, E.; Mihaela Luntraru, C.; Elena Gîrd, C.; Chifiriuc, M.-C. Chemical Composition, Antimicrobial and Antioxidant Activity of Phytolacca Americana L. Fruits and Leaves Extracts. Farmacia 2021, 69, 883–889. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of Flavonoids and Phenolic Acids in Greek Aromatic Plants: Investigation of Their Antioxidant Capacity and Antimicrobial Activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Kim, Y.O.; Johnson, J.D.; Lee, E.J. Phytotoxic Effects and Chemical Analysis of Leaf Extracts from Three Phytolaccaceae Species in South Korea. J. Chem. Ecol. 2005, 31, 1175–1186. [Google Scholar] [CrossRef]
- Saleri, F.D.; Chen, G.; Li, X.; Guo, M. Comparative Analysis of Saponins from Different Phytolaccaceae Species and Their Antiproliferative Activities. Molecules 2017, 22, 1077. [Google Scholar] [CrossRef]
- Beara, I.N.; Lesjak, M.M.; Orčić, D.Z.; Simin, N.D.; Četojević-Simin, D.D.; Božin, B.N.; Mimica-Dukić, N.M. Comparative Analysis of Phenolic Profile, Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Two Closely-Related Plantain Species: Plantago altissima L. and Plantago lanceolata L. LWT-Food Sci. Technol. 2012, 47, 64–70. [Google Scholar] [CrossRef]
- Nichita, C.; Neagu, G.; Cucu, A.; Vulturescu, V.; Berteșteanu, Ș.V.G. Antioxidative Properties of Plantago Lanceolata L. Extracts Evaluated by Chemiluminescence Method. AgroLife Sci. J. 2016, 5, 95–102. [Google Scholar]
- Grigore, A.; Bubueanu, C.; Pirvu, L.; Ionita, L.; Toba, G. Plantago Lanceolata L. Crops - Source of Valuable Raw Material for Various Industrial Applications. Sci. Pap. -Ser. A Agron. 2015, 58, 207–214. [Google Scholar]
- Guil-Guerrero, J.L. Nutritional Composition of Plantago Species (P. major L., P. lanceolata L., and P. media L.). Ecol. Food Nutr. 2010, 40, 481–495. [Google Scholar] [CrossRef]
- Nadgórska–Socha, A.; Kandziora-Ciupa, M.; Trzęsicki, M.; Barczyk, G. Air Pollution Tolerance Index and Heavy Metal Bioaccumulation in Selected Plant Species from Urban Biotopes. Chemosphere 2017, 183, 471–482. [Google Scholar] [CrossRef]
- Lukova, P.K.; Karcheva-Bahchevanska, D.P.; Nikolova, M.M.; Iliev, I.N.; Mladenov, R.D. Comparison of Structure and Antioxidant Activity of Polysaccharides Extracted from the Leaves of Plantago major L., P. media L. and P. lanceolata L. Bulg. Chem. Commun. 2017, 49, 282–288. [Google Scholar]
- Olaru, O.T.; Anghel, A.; Istudor, V.; Ancuceanu, R.; Dinu, M. Contributions to the Pharmacognostical and Phytobiological Study of Fallopia Aubertii (L. Henry) Holub. (Polygonaceae). Farmacia 2013, 61, 991–999. [Google Scholar]
- Zhang, F.-C.; Meng, Z.; Yin, D.; Su, L.; Chen, X. Study on the Differences in Chemical Compositions of Various Medicinal Parts of Rumex Patientia in Different Growth Years by High Performance Liquid Chromatography (HPLC). Afr. J. Biotechnol. 2009, 10, 15084–15088. [Google Scholar]
- Demirel, S.; Fen, Ü.; Dergisi, B.E.; Kaya, E.; Akbaş, P.; Ceyhan, G.; Karabekmez Erdem, T.; Alkan, H.; Sütcü, K. Determination the Fatty Acid Composition of the Rumex Patientia L. Leaves and in Vitro Antimicrobial Activity of Their Different Extracts. Süleyman Demirel Univ. J. Nat. Appl. Sci. 2020, 24, 362–367. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Dias, M.I.; Vasilakoglou, I.B.; Petrotos, K.; Barros, L.; Ferreira, I.C.F.R. Nutritional Value, Chemical Composition and Cytotoxic Properties of Common Purslane (Portulaca Oleracea L.) in Relation to Harvesting Stage and Plant Part. Antioxidant 2019, 8, 293. [Google Scholar] [CrossRef]
- Bishop, R.R.; Kubiak-Martens, L.; Warren, G.M.; Church, M.J. Getting to the Root of the Problem: New Evidence for the Use of Plant Root Foods in Mesolithic Hunter-Gatherer Subsistence in Europe. Veg. Hist. Archaeobot. 2022, 1–19. [Google Scholar] [CrossRef]
- Treutter, D.; Wang, D.; Farag, M.A.; Baires, G.D.A.; Rühmann, S.; Neumüller, M. Diversity of Phenolic Profiles in the Fruit Skin of Prunus Domestica Plums and Related Species. J. Agric. Food Chem. 2012, 60, 12011–12019. [Google Scholar] [CrossRef]
- Liu, W.; Nan, G.; Nisar, M.F.; Wan, C. Chemical Constituents and Health Benefits of Four Chinese Plum Species. J. Food Qual. 2020, 2020, 8842506. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G. Phenotypic Variation and Peel Contribution to Fruit Antioxidant Contents in European and Japanese Plums. Plants 2022, 11, 1338. [Google Scholar] [CrossRef] [PubMed]
- Krauze-Baranowska, M.; Głód, D.; Kula, M.; Majdan, M.; Hałasa, R.; Matkowski, A.; Kozłowska, W.; Kawiak, A. Chemical Composition and Biological Activity of Rubus Idaeus Shoots—A Traditional Herbal Remedy of Eastern Europe. BMC Complement Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef] [PubMed]
- Assafiri, O.; Abdallah, H.; El-Dakdouki, M. Antibacterial Effect and Phytochemical Analysis of the Shoot System of Rubus Canescens DC. Growing in Lebanon. BAU J.-Sci. Technol. 2020, 2, 9. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- Echavarría, A.; D´armas Regnault, H.; Nubia, L.; Matute, L.; Jaramillo, C.; Rojas-De-Astudillo, L.; Benítez, R. Evaluation of Antioxidant Capacity and Secondary Metabolites of Sixteen Medicinal Plants Extracts. Ciencia UNEMI 2016, 9, 29–35. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between Phenolic Content and Antioxidant Properties in Twenty-Four Plant Species of Traditional Ethnoveterinary Use in the Mediterranean Area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef]
- Repajić, M.; Cegledi, E.; Zorić, Z.; Pedisić, S.; Garofulić, I.E.; Radman, S.; Palčić, I.; Dragović-Uzelac, V. Bioactive Compounds in Wild Nettle (Urtica dioica L.) Leaves and Stalks: Polyphenols and Pigments upon Seasonal and Habitat Variations. Foods 2021, 10, 190. [Google Scholar] [CrossRef]
- Mzid, M.; Khedir, S.B.; Salem, M.B.; Regaieg, W.; Rebai, T. Antioxidant and Antimicrobial Activities of Ethanol and Aqueous Extracts from Urtica Urens. Pharm. Biol. 2017, 55, 775–781. [Google Scholar] [CrossRef]




| Plant Family | Number of Taxa | Number of Taxa Consumed Raw | Taxa Consumed Only Raw |
|---|---|---|---|
| Amaranthaceae | 10 | 5 | |
| Asteraceae | 10 | 4 | * Lapsana communis L. * Sonchus arvensis L. |
| Brassicaceae | 9 | 7 | * Cardamine pratensis L. * Cardamine amara L. * Lepidium campestre (L.) R. Br. * Lepidium latifolium L. * Lepidium perfoliatum L. |
| Polygonaceae | 4 | 4 | Fallopia aubertii (L. Henry) Holub |
| Urticaceae | 3 | 2 | Parietaria officinalis L. |
| Malvaceae | 3 | 2 | Malva sylvestris L. |
| Apiaceae | 3 | 3 | - |
| Caryophyllaceae | 3 | - | - |
| Rosaceae | 3 | 3 | - |
| Boraginaceae | 2 | - | - |
| Papaveraceae | 2 | - | - |
| Geraniaceae | 2 | - | - |
| Ranunculaceae | 2 | - | - |
| Plantaginaceae | 1 | - | - |
| Portulacaceae | 1 | 1 | - |
| Araceae | 1 | - | - |
| Fabaceae | 1 | 1 | - |
| Phytolaccaceae | 1 | - | - |
| Cannabaceae | 1 | - | - |
| Convolvulaceae | 1 | - | - |
| Adoxaceae | 1 | 1 | - |
| Equisetaceae | 1 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanova, T.; Marchev, A.; Chervenkov, M.; Bosseva, Y.; Georgiev, M.; Kozuharova, E.; Dimitrova, D. Catching the Green—Diversity of Ruderal Spring Plants Traditionally Consumed in Bulgaria and Their Potential Benefit for Human Health. Diversity 2023, 15, 435. https://doi.org/10.3390/d15030435
Ivanova T, Marchev A, Chervenkov M, Bosseva Y, Georgiev M, Kozuharova E, Dimitrova D. Catching the Green—Diversity of Ruderal Spring Plants Traditionally Consumed in Bulgaria and Their Potential Benefit for Human Health. Diversity. 2023; 15(3):435. https://doi.org/10.3390/d15030435
Chicago/Turabian StyleIvanova, Teodora, Andrey Marchev, Mihail Chervenkov, Yulia Bosseva, Milen Georgiev, Ekaterina Kozuharova, and Dessislava Dimitrova. 2023. "Catching the Green—Diversity of Ruderal Spring Plants Traditionally Consumed in Bulgaria and Their Potential Benefit for Human Health" Diversity 15, no. 3: 435. https://doi.org/10.3390/d15030435
APA StyleIvanova, T., Marchev, A., Chervenkov, M., Bosseva, Y., Georgiev, M., Kozuharova, E., & Dimitrova, D. (2023). Catching the Green—Diversity of Ruderal Spring Plants Traditionally Consumed in Bulgaria and Their Potential Benefit for Human Health. Diversity, 15(3), 435. https://doi.org/10.3390/d15030435