Benthic Foraminifera Diversity of the Abyssal Northwest Atlantic
Abstract
1. Introduction
1.1. Importance of Abyssal Ecosystems and Their Foraminifera
1.2. What Are Foraminifera?
1.3. Previous Studies
1.4. Focus of This Study
2. Materials and Methods
2.1. Materials
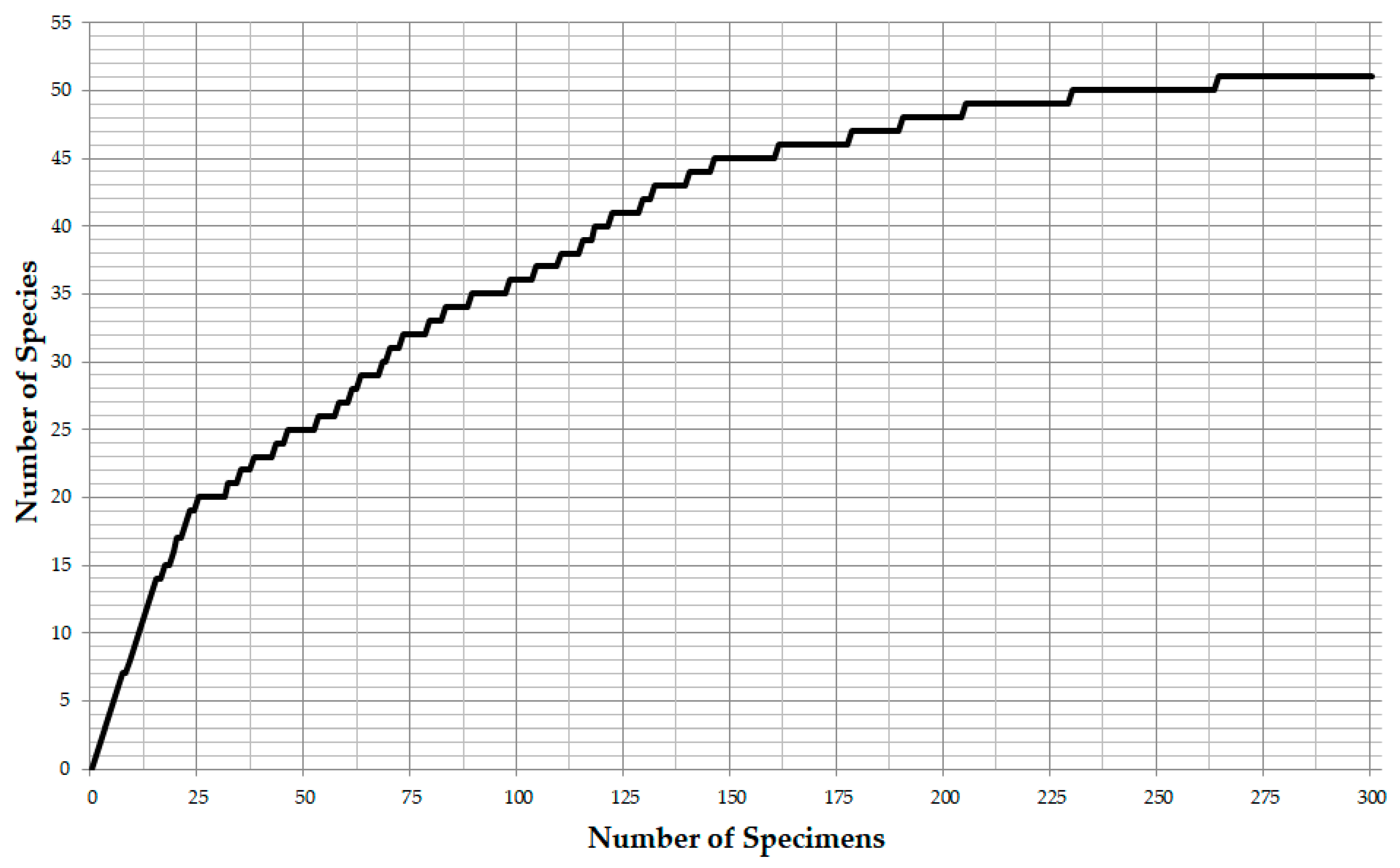
2.2. Methods
3. Results
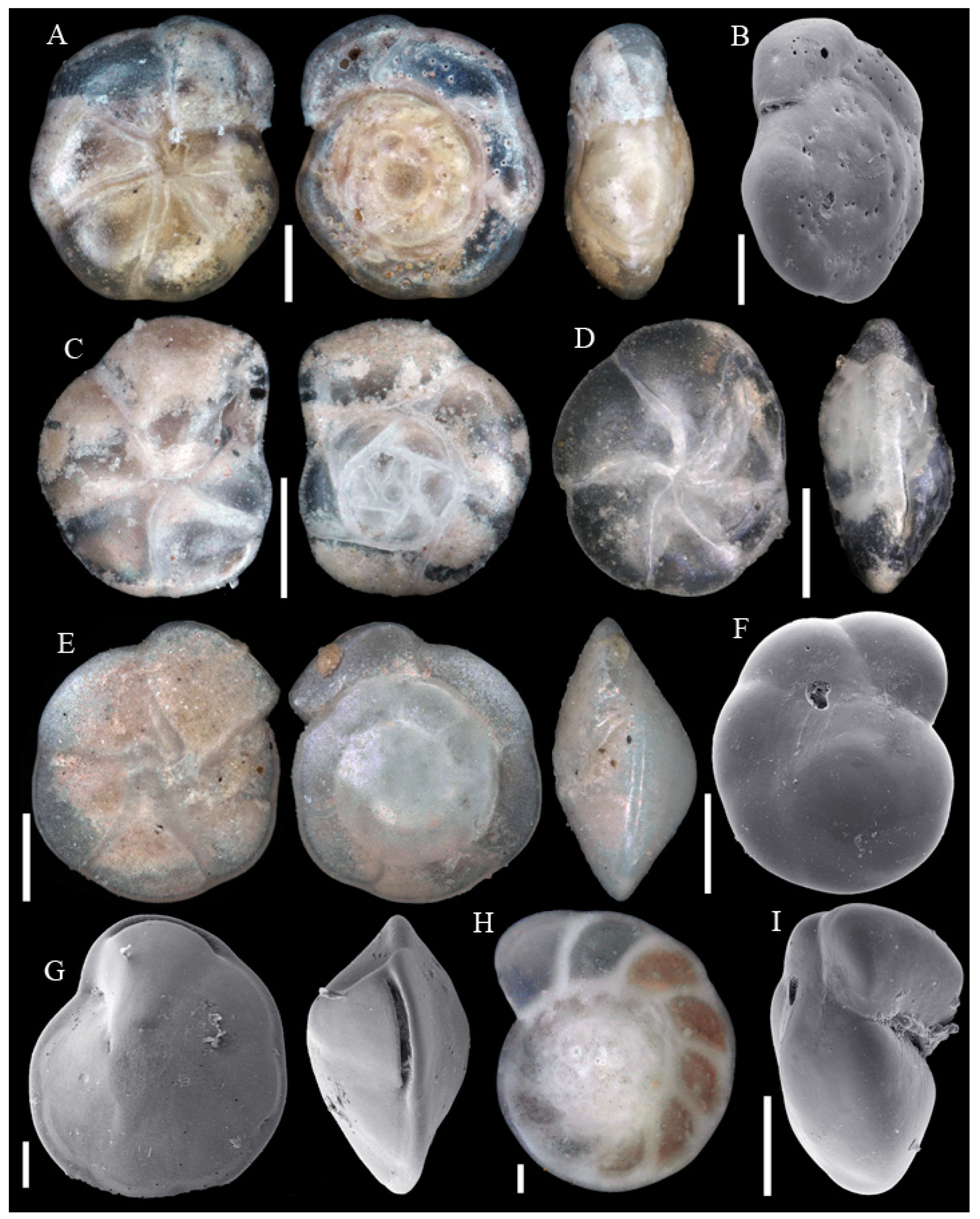
3.1. Identified Taxa
3.2. Identified Assemblages
3.3. Trends in the Assemblages down the Cores
4. Discussion
4.1. Limitations and Advantages of This Study
4.2. Benthic Foraminiferal Associations Dominated by Epistominella exigua
4.3. Benthic Foraminiferal Associations with High Evenness and Lacking Dominant Species
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

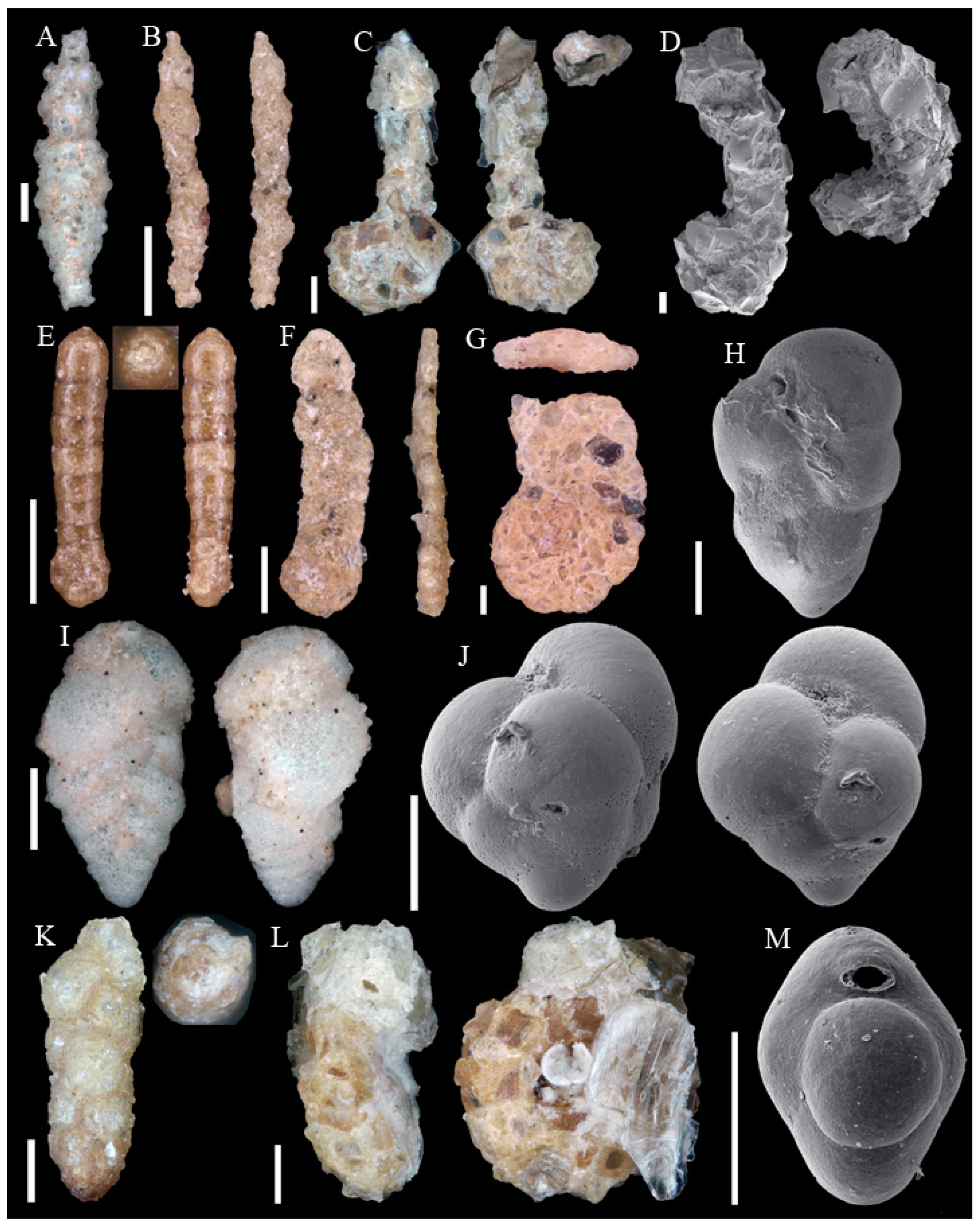



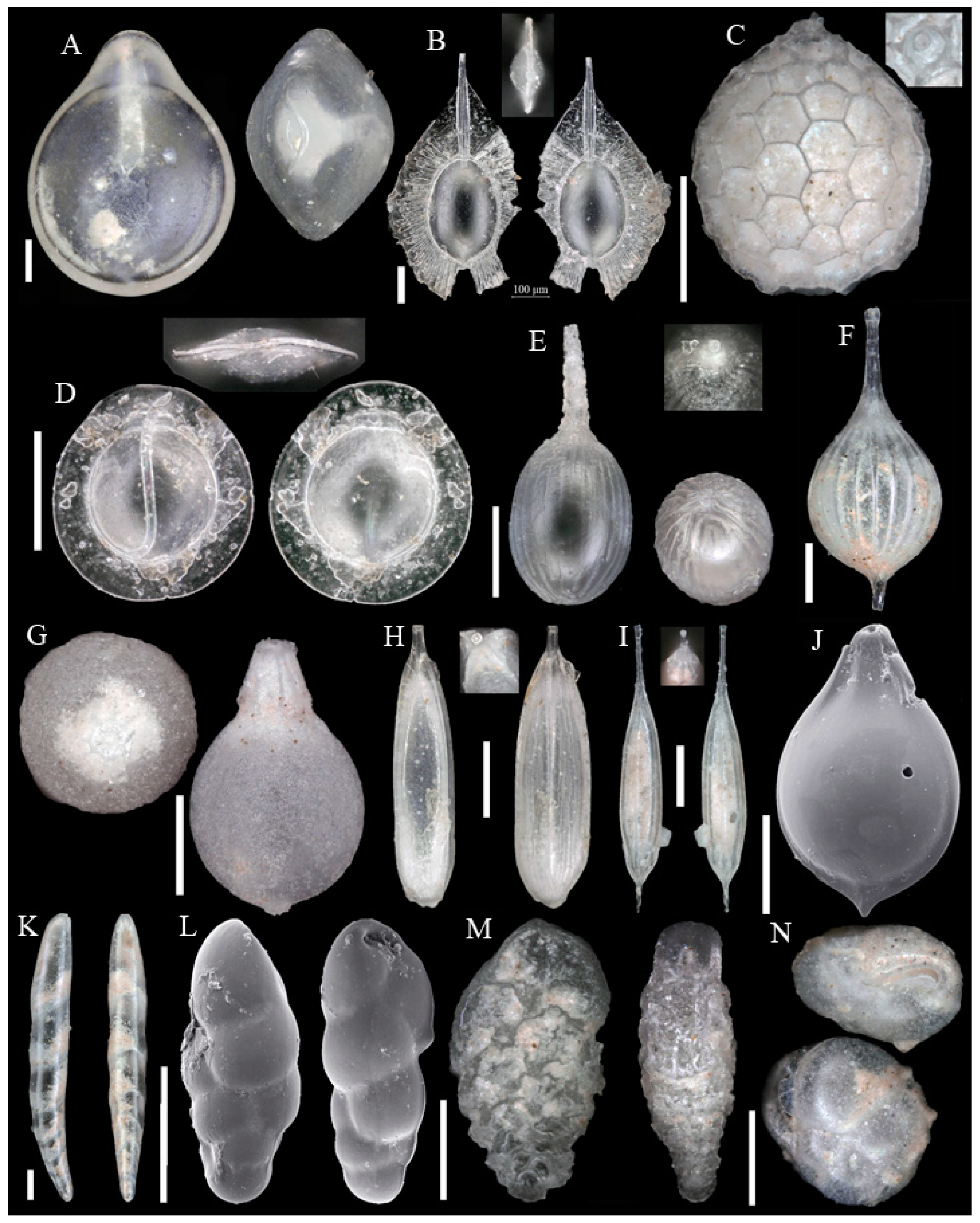
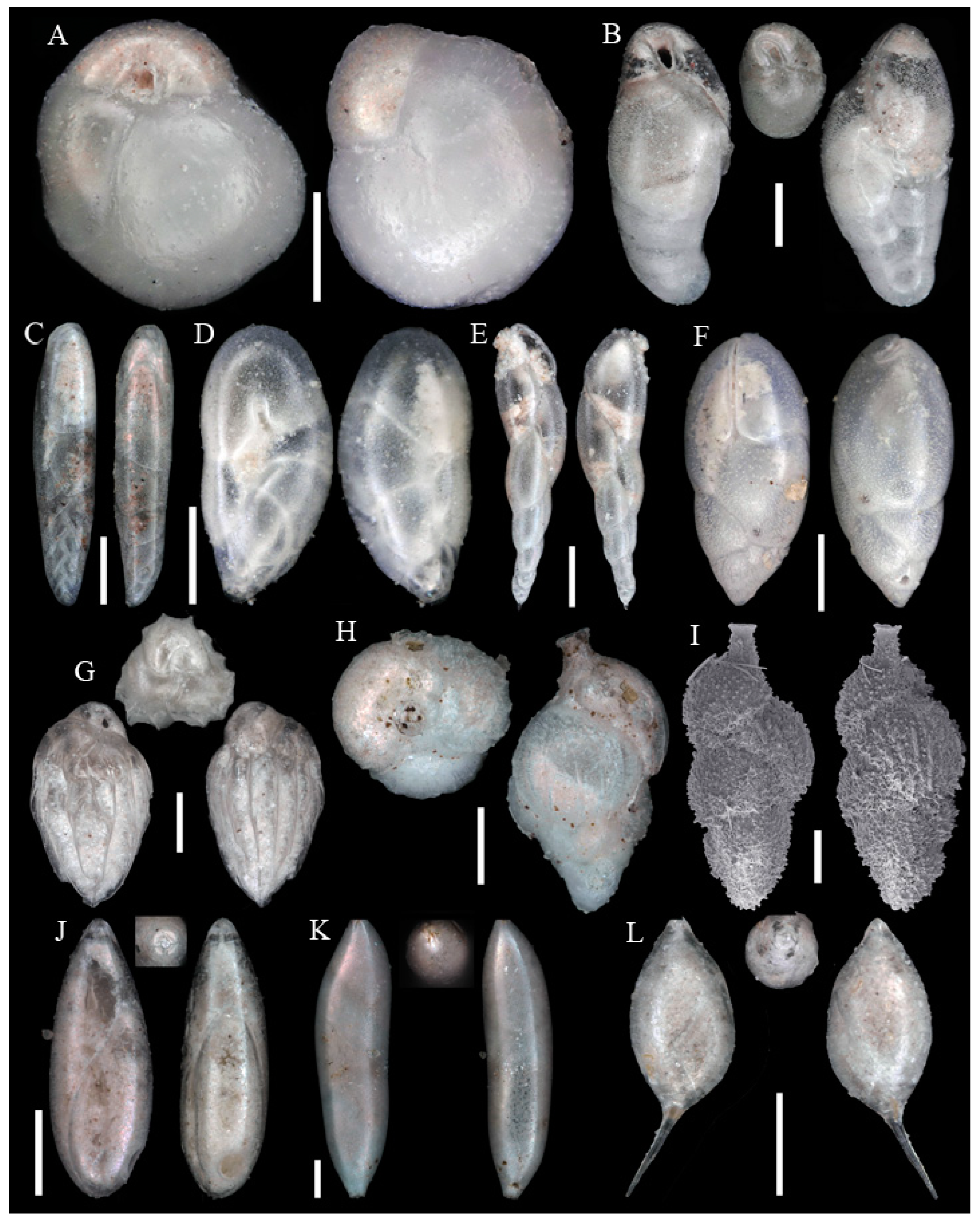
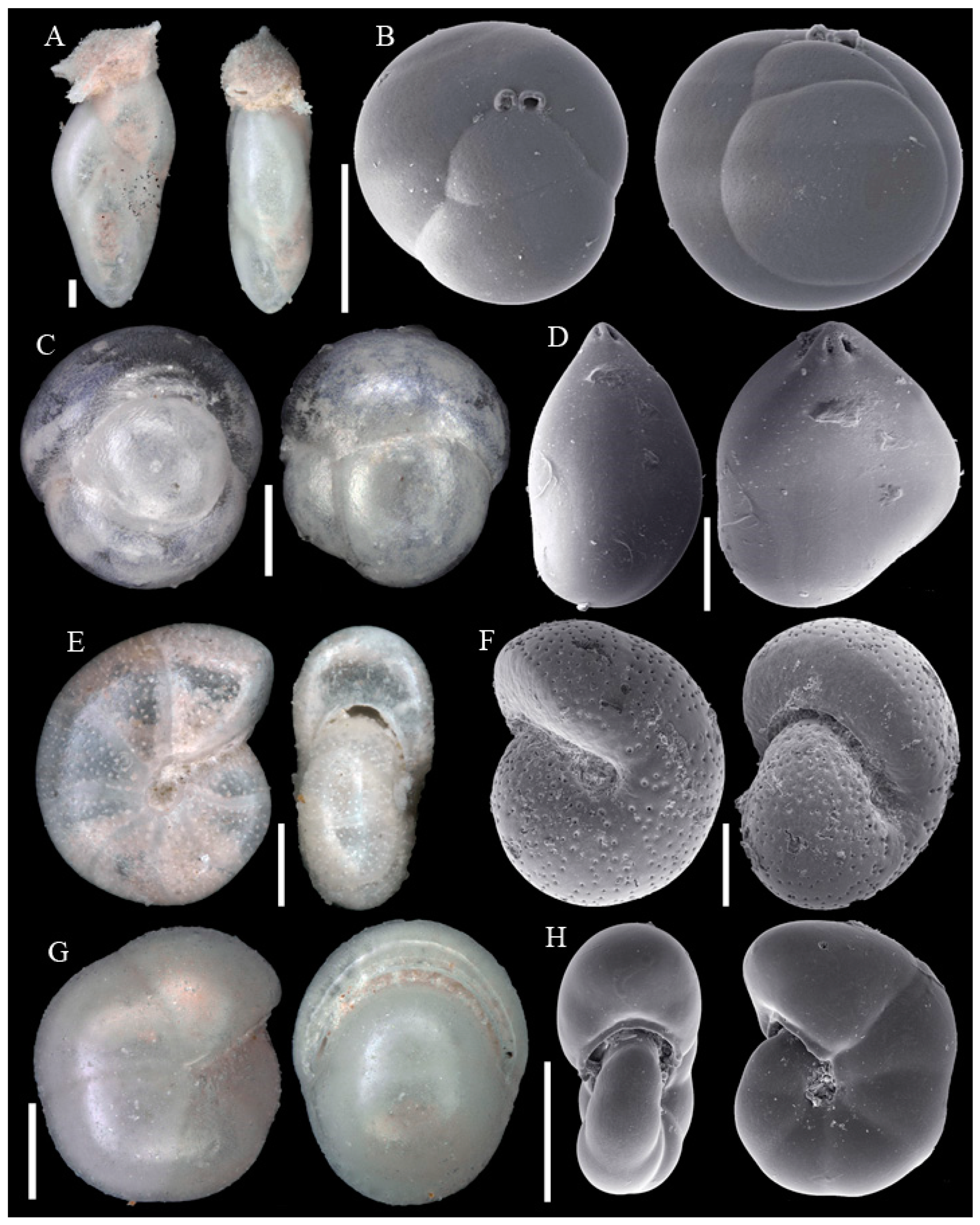
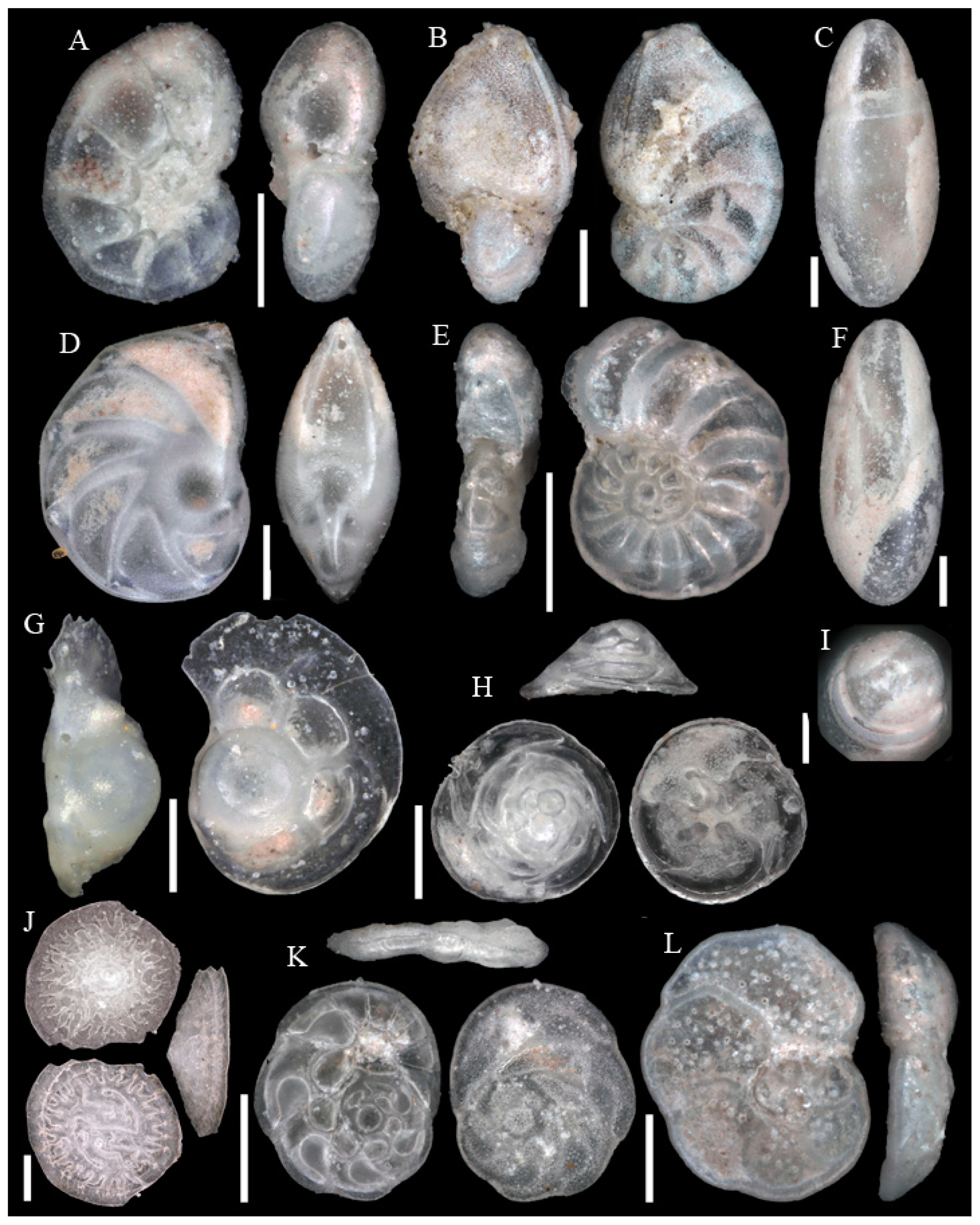


| Sample | Sta | C | Slice | WA | Lon | Lat | Depth | Dev | Sed | Facies |
|---|---|---|---|---|---|---|---|---|---|---|
| SO286_21_02 | 21 | 5 | 0–2 | LS | 58°14.422′ | 54°13.075′ | 3391 m | MUC | smu | Go |
| SO286_21_04 | 21 | 5 | 2–4 | LS | 58°14.422′ | 54°13.075′ | 3391 m | MUC | smu | Go |
| SO286_21_06 | 21 | 5 | 4–6 | LS | 58°14.422′ | 54°13.075′ | 3391 m | MUC | smu | Go |
| SO286_21_08 | 21 | 5 | 6–8 | LS | 58°14.422′ | 54°13.075′ | 3391 m | MUC | smu | Go |
| SO286_21_10 | 21 | 5 | 8–10 | LS | 58°14.422′ | 54°13.075′ | 3391 m | MUC | smu | Go |
| SO286_21_15 | 21 | 5 | 10–15 | LS | 58°14.422′ | 54°13.075′ | 3391 m | MUC | smu | Go |
| SO286_21_20 | 21 | 5 | 15–20 | LS | 58°14.422′ | 54°13.075′ | 3391 m | MUC | smu | Go |
| SO286_42_02 | 42 | 1 | 0–2 | LB | 51°58.255′ | 38°59.534′ | 3685 m | MUC | smu | Go |
| SO286_42_04 | 42 | 1 | 2–4 | LB | 51°58.255′ | 38°59.534′ | 3685 m | MUC | smu | Go |
| SO286_42_06 | 42 | 1 | 4–6 | LB | 51°58.255′ | 38°59.534′ | 3685 m | MUC | smu | Go |
| SO286_42_08 | 42 | 1 | 6–8 | LB | 51°58.255′ | 38°59.534′ | 3685 m | MUC | smu | Go |
| SO286_42_10 | 42 | 1 | 8–10 | LB | 51°58.255′ | 38°59.534′ | 3685 m | MUC | smu | Go |
| SO286_42_15 | 42 | 1 | 10–15 | LB | 51°58.255′ | 38°59.534′ | 3685 m | MUC | smu | Go |
| SO286_42_20 | 42 | 1 | 15–20 | LB | 51°58.255′ | 38°59.534′ | 3685 m | MUC | smu | Go |
| SO286_42_25 | 42 | 1 | 20–25 | LB | 51°58.255′ | 38°59.534′ | 3685 m | MUC | smu | Go |
| SO286_42_30 | 42 | 1 | 25–30 | LB | 51°58.255′ | 38°59.534′ | 3685 m | MUC | smu | Go |
| SO286_65_02 | 65 | 9 | full | ASW | 37°00.025′ | 35°29.491′ | 3193 m | MUC | smu | Go |
| SO286_65_02 | 65 | 11 | full | ASW | 37°00.025′ | 35°29.491′ | 3193 m | MUC | smu | Go |
| SO286_66_02 | 66 | 18 | full | ASW | 37°00.032′ | 35°29.441′ | 3192 m | MUC | smu | Go |
| SO286_67_02 | 67 | 11 | 0–2 | ASW | 37°00.037′ | 35°29.382′ | 3209 m | MUC | smu | Go |
| SO286_67_04 | 67 | 11 | 2–4 | ASW | 37°00.037′ | 35°29.382′ | 3209 m | MUC | smu | Go |
| SO286_67_06 | 67 | 11 | 4–6 | ASW | 37°00.037′ | 35°29.382′ | 3209 m | MUC | smu | Go |
| SO286_67_08 | 67 | 11 | 6–8 | ASW | 37°00.037′ | 35°29.382′ | 3209 m | MUC | smu | Go |
| SO286_67_10 | 67 | 11 | 8–10 | ASW | 37°00.037′ | 35°29.382′ | 3209 m | MUC | smu | Go |
| SO286_75_02 | 75 | 11 | 0–2 | SM | 37°13.922′ | 35°32.316′ | 2508 m | MUC | smu | Go |
| SO286_75_04 | 75 | 11 | 2–4 | SM | 37°13.922′ | 35°32.316′ | 2508 m | MUC | smu | Go |
| SO286_75_06 | 75 | 11 | 4–6 | SM | 37°13.922′ | 35°32.316′ | 2508 m | MUC | smu | Go |
| SO286_75_08 | 75 | 11 | 6–8 | SM | 37°13.922′ | 35°32.316′ | 2508 m | MUC | smu | Go |
| SO286_75_10 | 75 | 11 | 8–10 | SM | 37°13.922′ | 35°32.316′ | 2508 m | MUC | smu | Go |
| SO286_75_15 | 75 | 11 | 10–15 | SM | 37°13.922′ | 35°32.316′ | 2508 m | MUC | smu | Go |
| SO286_75_20 | 75 | 11 | 15–20 | SM | 37°13.922′ | 35°32.316′ | 2508 m | MUC | smu | Go |
| SO286_75_25 | 75 | 11 | 20–25 | SM | 37°13.922′ | 35°32.316′ | 2508 m | MUC | smu | Go |
| Measure | Wg | Wg | Wg | Wg | Wg | Wg | Wg | Asa | Dsa | Wg | Ast | Dst | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Part | whole | Pa | Pr | Pa | Pr | Pa | Ssa | Ssa | Ssa | Sst | Sst | Sst | |
| Phase | a-4 | a-4 | a-4 | dry | dry | dry | dry | dry | dry | dry | dry | dry | |
| Fraction | full | full | full | full | full | wf | wf | wf | full | wf | wf | full | |
| Sample | Slice | c | m | m | c | m | m | m | m | c | m | m | c |
| Column | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| Calculation | 2 + 3 | 5/3*2 | 8/7*6/4 | 11/10*6/4 | |||||||||
| SO286_21_02 | 0–2 | 157.2 | 119.1 | 38.1 | 41.0 | 13.1 | 3.7 | 0.1 | 5 | 5 | 0.1 | 161 | 145 |
| SO286_21_04 | 2–4 | 153.3 | 134.9 | 18.4 | 41.1 | 5.6 | 3.5 | 0.1 | 2 | 2 | 0.1 | 153 | 130 |
| SO286_21_06 | 4–6 | 116.7 | 92.5 | 24.2 | 39.8 | 10.4 | 7.6 | 0.1 | 1 | 2 | 0.1 | 152 | 290 |
| SO286_21_08 | 6–8 | 203.9 | 167.7 | 36.2 | 90.8 | 19.6 | 4.7 | 0.1 | 0 | 0 | 0.1 | 161 | 83 |
| SO286_21_10 | 8–10 | 158.1 | 137.1 | 21.0 | 78.3 | 12.0 | 4.5 | 0.1 | 0 | 0 | 0.1 | 153 | 88 |
| SO286_21_15 | 10–15 | 372.6 | 334.3 | 38.3 | 178.9 | 20.5 | 9.6 | 0.1 | 0 | 0 | 0.1 | 163 | 87 |
| SO286_21_20 | 15–20 | 301.1 | 227.0 | 74.1 | 123.5 | 40.3 | 7.0 | 0.2 | 0 | 0 | 0.2 | 155 | 44 |
| SO286_42_02 | 0–2 | 126.0 | 85.8 | 40.2 | 43.5 | 20.4 | 24.1 | 0.2 | 1 | 3 | 0.2 | 158 | 438 |
| SO286_42_04 | 2–4 | 179.4 | 130.2 | 49.2 | 55.0 | 20.8 | 36.4 | 0.1 | 0 | 0 | 0.1 | 160 | 1059 |
| SO286_42_06 | 4–6 | 193.1 | 153.6 | 39.5 | 75.8 | 19.5 | 43.1 | 0.1 | 2 | 11 | 0.1 | 157 | 893 |
| SO286_42_08 | 6–8 | 174.1 | 147.5 | 26.6 | 70.4 | 12.7 | 43.6 | 0.1 | 0 | 0 | 0.1 | 153 | 948 |
| SO286_42_10 | 8–10 | 210.2 | 161.5 | 48.7 | 85.6 | 25.8 | 50.1 | 0.1 | 0 | 0 | 0.1 | 155 | 907 |
| SO286_42_15 | 10–15 | 326.8 | 278.5 | 48.3 | 148.2 | 25.7 | 84.7 | 0.1 | 0 | 0 | 0.1 | 154 | 880 |
| SO286_42_20 | 15–20 | 288.3 | 232.7 | 55.6 | 138.5 | 33.1 | 85.3 | 0.1 | 0 | 0 | 0.1 | 158 | 973 |
| SO286_42_25 | 20–25 | 418.1 | 349.2 | 68.9 | 198.2 | 39.1 | 63.4 | 0.1 | 0 | 0 | 0.1 | 159 | 1017 |
| SO286_42_30 | 25–30 | 191.9 | 156.5 | 35.4 | 81.8 | 18.5 | 47.6 | 0.2 | 0 | 0 | 0.2 | 152 | 442 |
| SO286_65_09 | full core | 178.2 | 160.9 | 17.3 | 79.1 | 8.5 | 49.0 | 0.3 | 1 | 2 | 0.3 | 152 | 314 |
| SO286_65_11 | full core | 220.8 | 185.7 | 35.1 | 98.9 | 18.7 | 56.3 | 0.4 | 4 | 6 | 0.4 | 154 | 219 |
| SO286_66 | full core | 602.1 | 568.5 | 33.6 | 311.3 | 18.4 | 167.4 | 0.5 | 0 | 0 | 0.5 | 156 | 168 |
| SO286_67_02 | 0–2 | 209.8 | 138.8 | 71.0 | 73.7 | 37.7 | 39.2 | 0.4 | 4 | 5 | 0.4 | 159 | 211 |
| SO286_67_04 | 2–4 | 258.9 | 215.3 | 43.6 | 114.6 | 23.2 | 66.4 | 0.3 | 0 | 0 | 0.3 | 157 | 303 |
| SO286_67_06 | 4–6 | 274.8 | 221.2 | 53.6 | 128.3 | 31.1 | 69.6 | 0.3 | 0 | 0 | 0.3 | 154 | 278 |
| SO286_67_08 | 6–8 | 180.9 | 138.3 | 42.6 | 68.8 | 21.2 | 45.2 | 0.4 | 0 | 0 | 0.4 | 150 | 246 |
| SO286_67_10 | 8–10 | 228.1 | 187.8 | 40.3 | 101.6 | 21.8 | 63.4 | 0.6 | 0 | 0 | 0.6 | 157 | 163 |
| SO286_75_02 | 0–2 | 207.1 | 167.0 | 40.1 | 77.9 | 18.7 | 28.9 | 0.3 | 4 | 5 | 0.3 | 154 | 190 |
| SO286_75_04 | 2–4 | 182.4 | 145.1 | 37.3 | 78.2 | 20.1 | 26.7 | 0.3 | 0 | 0 | 0.3 | 154 | 175 |
| SO286_75_06 | 4–6 | 122.5 | 80.2 | 42.3 | 44.6 | 23.5 | 14.3 | 0.3 | 0 | 0 | 0.3 | 151 | 161 |
| SO286_75_08 | 6–8 | 172.4 | 134.1 | 38.3 | 70.4 | 20.1 | 23.5 | 0.4 | 0 | 0 | 0.4 | 159 | 133 |
| SO286_75_10 | 8–10 | 236.1 | 205.3 | 30.8 | 114.0 | 17.1 | 32.5 | 0.4 | 0 | 0 | 0.4 | 154 | 110 |
| SO286_75_15 | 10–15 | 433.8 | 361.1 | 72.7 | 246.4 | 49.6 | 65.3 | 0.3 | 0 | 0 | 0.3 | 157 | 139 |
| SO286_75_20 | 15–20 | 527.6 | 456.4 | 71.2 | 255.1 | 39.8 | 107.1 | 0.3 | 0 | 0 | 0.3 | 159 | 223 |
| SO286_75_25 | 20–25 | 453.4 | 366.1 | 87.3 | 205.1 | 48.9 | 118.2 | 0.2 | 0 | 0 | 0.2 | 155 | 447 |
| Abditodentrix pseudothalmanni (Boltovskoy & Guissani de Kahn, 1981) | Eggerella bradyi (Cushman, 1911) |
| Adercotryma glomeratum (Brady, 1878) | Epistominella exigua (Brady, 1884) |
| Alabaminella weddellensis (Earland, 1936) | Eponides repandus (Fichtel & Moll, 1798) |
| Ammobaculites agglutinans (d’Orbigny, 1846) | Eratidus foliaceus (Brady, 1881) |
| Ammobaculites crassaformis Zheng, 1988 | Eubuliminella exilis (Brady, 1884) |
| Ammobaculites filiformis Earland, 1934 | Eusphaeroidina inflata Ujiié, 1990 |
| Ammobaculites ? sp. | Favulina hexagona (Williamson, 1848) |
| Ammodiscus sp. * | Fissurina castanea (Flint, 1899) |
| Ammoglobigerina globulosa (Cushman, 1920) | Fissurina granifera trimarginata (Buchner, 1940) |
| Ammolagena clavata (Jones & Parker, 1860) | Fissurina orbignyana var. rhumbleri Buchner, 1940 |
| Ammomassilina alveoliniformis (Millett, 1898) | Fissurina staphyllearia Schwager, 1866 |
| Anomalinoides globulosus (Chapman & Parr, 1937) | Fissurina sp. 1 |
| Aschemonella scabra Brady, 1879 | Fissurina sp. 2 * |
| Bolivina sp. * | Fissurina sp. 3 * |
| Bulimina buchiana d’Orbigny, 1846 | Fissurina sp. 4 * |
| Buzasina galeata (Brady, 1881) | Fursenkoina texturata (Brady, 1884) |
| Buzasina ringens (Brady, 1879) | Galwayella trigonoornata (Brady, 1881) |
| Cassidulina reniforme Nørvang, 1945 | Gavelinopsis sp. |
| Cassidulina sp. * | Glaphyrammina americana (Cushman, 1910) |
| Chilostomella oolina Schwager, 1878 | Globocassidulina subglobosa (Brady, 1881) |
| Cibicides pachyderma (Rzehak, 1886) | Glomospira charoides (Jones & Parker, 1860) |
| Cibicides refulgens Montfort, 1808 | Glomospira gordialis (Jones & Parker, 1860) |
| Cibicidoides cicatricosus (Schwager, 1866) | Guttulina communis (d’Orbigny, 1826) |
| Cibicidoides mundulus (Brady, Parker & Jones, 1888) | Gyroidina sp. 1 |
| Cibicidoides wuellerstorfi (Schwager, 1866) | Gyroidina sp. 2 |
| Cibicidoides sp. 1 * | Hansenisca soldanii (d’Orbigny, 1826) |
| Cibicidoides sp. 2 * | Hoeglundina elegans (d’Orbigny, 1826) |
| Cibicidoides sp. 3 * | Hormosinelloides guttifer (Brady, 1884) |
| Cibicidoides sp. 4 * | Hyalinea balthica (Schröter, 1783) |
| Cornuloculina inconstans (Brady, 1879) | Hyperammina elongata Brady, 1878 |
| Cornuspira carinata (Costa, 1856) | Ioanella tumidula (Brady, 1884) |
| Cribrostomoides jeffreysii (Williamson, 1858) | Karreriella bradyi (Cushman, 1911) |
| Cribrostomoides sphaerilocula (Cushman, 1910) | Karrerulina conversa (Grzybowski, 1901) |
| Cribrostomoides subglobosus (Cushman, 1910) | Laevidentalina haueri (Neugeboren, 1856) |
| Cribrostomoides sp. * | Laevidentalina sp. * |
| Cystammina pauciloculata (Brady, 1879) | Lagena striata (d’Orbigny, 1839) |
| Discorbinella complanata (Sidebottom, 1918) | Lagena sulcata (Walker & Jacob, 1798) |
| Lagena wiesneri Parr, 1950 | Pyrulina angusta (Egger, 1857) |
| Lagena sp. 1 | Pyrulina cylindroides (Roemer, 1838) |
| Lagena sp. 2 | Pyrulina fusiformis (Roemer, 1838) |
| Lagena sp. 3 * | Pyrulina sp. * |
| Lagena sp. 4 * | Quinqueloculina venusta Karrer, 1868 |
| Lagenammina arenulata (Skinner, 1961) | Quinqueloculina vulgaris d’Orbigny, 1826 |
| Lagenosolenia incomposita Patterson & Pettis, 1986 | Quinqueloculina sp. 1 * |
| Lagnea radiata (Seguenza, 1862) | Quinqueloculina sp. 2 * |
| Laticarinina pauperata (Parker & Jones, 1865) | Quinqueloculina sp. 3 * |
| Lenticulina convergens (Bornemann, 1855) | Quinqueloculina sp. 4 * |
| Lenticulina sp. 1 * | Recurvoides contortus Earland, 1934 |
| Lenticulina sp. 2 * | Reophax scorpiurus de Montfort, 1808 |
| Lobatula lobatula (Walker & Jacob, 1798) | Reophax sp. 1 |
| Melonis affinis (Reuss, 1851) | Reophax sp. 2 * |
| Melonis pompiloides (Fichtel & Moll, 1798) | Reophax sp. 3 |
| Miliolinella subrotunda (Montagu, 1803) | Rhizammina algaeformis Brady, 1879 |
| Nonionellina labradorica (Dawson, 1860) | Robertinoides bradyi (Cushman & Parker, 1936) |
| Nuttallides decorata (Phleger & Parker, 1951) | Rutherfordoides rotundatus (Parr, 1950) |
| Oolina globosa (Montagu, 1803) | Rutherfordoides rotundiformis (McCulloch, 1977) |
| Oridorsalis umbonatus (Reuss, 1851) | Saccorhiza ramosa (Brady, 1879) |
| Oridorsalis sp. * | Sigmoilopsis schlumbergeri (Silvestri, 1904) |
| Patellina corrugata Williamson, 1858 | Siphotextularia rolshauseni Phleger & Parker, 1951 |
| Patellina simplissima (McCulloch, 1977) | Sphaeroidina bulloides d’Orbigny in Deshayes, 1828 |
| Placopsilinella aurantiaca Earland, 1934 | Spiroloculina excavata d’Orbigny, 1846 |
| Portatrochammina sp. | Spirophthalmidium acutimargo (Brady, 1884) |
| Procerolagena gracilis (Williamson, 1848) | Spirosigmoilina pusilla (Earland, 1934) |
| Protoglobobulimina sp. | Subreophax aduncus (Brady, 1882) |
| Psammosphaera fusca Schulze, 1875 | Tolypammina schaudinni Rhumbler, 1904 |
| Pseudononion granuloumbilicatum Zheng, 1979 | Triloculina oblonga (Montagu, 1803) |
| Pseudopolymorphina novangliae (Cushman, 1923) | Triloculina trihedra Loeblich & Tappan, 1953 |
| Pullenia bulloides (d’Orbigny, 1826) | Triloculina sp. * |
| Pullenia quinqueloba (Reuss, 1851) | Tritaxis heronalleni Mikhalevich, 1972 |
| Pyrgo lucernula (Schwager, 1866) | Trochammina sp. 1 * |
| Pyrgo murrhina (Schwager, 1866) | Trochammina sp. 2 * |
| Pyrgo simplex (d’Orbigny, 1846) | Uvigerina sp. 1 |
| Pyrgo williamsoni (Silvestri, 1923) | Uvigerina sp. 2 |
| Pyrgo sp. * | Uvigerina sp. 3 * |
| Pyrgoella sp. | Agglutinated tube not assigned to the order Astrorhizida |
| Sample | Cluster | Number of Taxa | Fisher’s Alpha (α) | Equitability (J) | Shannon (H) | |||
|---|---|---|---|---|---|---|---|---|
| Single | Mean | Single | Mean | Single | Mean | |||
| SO286_21_02 | I | 40 | 17.05 | 14.60 | 0.93 | 0.83 | 3.44 | 2.98 |
| SO286_21_04 | I | 41 | 18.35 | 0.86 | 3.20 | |||
| SO286_21_06 | I | 39 | 16.97 | 0.88 | 3.23 | |||
| SO286_21_08 | I | 36 | 14.40 | 0.81 | 2.92 | |||
| SO286_21_10 | I | 34 | 13.55 | 0.80 | 2.81 | |||
| SO286_21_15 | I | 31 | 11.35 | 0.78 | 2.69 | |||
| SO286_21_20 | I | 29 | 10.52 | 0.77 | 2.59 | |||
| SO286_42_02 | II | 35 | 13.93 | 14.14 | 0.77 | 0.73 | 2.74 | 2.60 |
| SO286_42_04 | II | 37 | 15.10 | 0.63 | 2.28 | |||
| SO286_42_06 | II | 40 | 17.33 | 0.74 | 2.73 | |||
| SO286_42_08 | II | 29 | 10.60 | 0.76 | 2.57 | |||
| SO286_42_10 | II | 33 | 12.84 | 0.71 | 2.47 | |||
| SO286_42_15 | II | 36 | 14.78 | 0.75 | 2.70 | |||
| SO286_42_20 | II | 33 | 12.70 | 0.70 | 2.45 | |||
| SO286_42_25 | II | 38 | 15.82 | 0.79 | 2.86 | |||
| SO286_42_30 | III | 40 | 17.69 | 23.26 | 0.88 | 0.87 | 3.24 | 3.37 |
| SO286_65_09 | III | 42 | 19.19 | 0.81 | 3.01 | |||
| SO286_65_11 | III | 45 | 21.38 | 0.84 | 3.19 | |||
| SO286_66 | III | 38 | 16.00 | 0.85 | 3.10 | |||
| SO286_67_02 | III | 48 | 23.36 | 0.87 | 3.36 | |||
| SO286_67_04 | III | 55 | 30.10 | 0.90 | 3.61 | |||
| SO286_67_06 | III | 50 | 25.72 | 0.88 | 3.44 | |||
| SO286_67_08 | III | 49 | 25.32 | 0.93 | 3.62 | |||
| SO286_67_10 | III | 48 | 23.58 | 0.89 | 3.45 | |||
| SO286_75_02 | IV | 49 | 24.81 | 25.86 | 0.90 | 0.92 | 3.49 | 3.62 |
| SO286_75_04 | IV | 58 | 33.84 | 0.92 | 3.72 | |||
| SO286_75_06 | IV | 49 | 25.19 | 0.93 | 3.62 | |||
| SO286_75_08 | IV | 49 | 24.21 | 0.93 | 3.62 | |||
| SO286_75_10 | IV | 48 | 23.92 | 0.92 | 3.57 | |||
| SO286_75_15 | IV | 47 | 22.73 | 0.93 | 3.60 | |||
| SO286_75_20 | IV | 50 | 25.09 | 0.94 | 3.68 | |||
| SO286_75_25 | III | 49 | 24.69 | ^ | 0.91 | ^ | 3.52 | ^ |
References
- Paulus, E. Shedding Light on Deep-Sea Biodiversity—A Highly Vulnerable Habitat in the Face of Anthropogenic Change. Front. Mar. Sci. 2021, 8, 667048. [Google Scholar] [CrossRef]
- Brix, S.; Taylor, J. Short Cruise Report, RV Sonne SO286; Senckenberg am Meer, Deutsches Zentrum für Marine Biodiversitätsforschung c/o Biocenter Grindel: Hamburg, Germany, 2021; pp. 1–6. [Google Scholar]
- Gooday, A.J.; Lejzerowicz, F.; Goineau, A.; Holzmann, M.; Kamenskaya, O.; Kitazato, H.; Lim, S.-C.; Pawlowski, J.; Radziejewska, T.; Stachowska, Z.; et al. The Biodiversity and Distribution of Abyssal Benthic Foraminifera and Their Possible Ecological Roles: A Synthesis Across the Clarion-Clipperton Zone. Front. Mar. Sci. 2021, 8, 634726. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: New York, NY, USA, 2006; ISBN 978-0-521-82839-0. [Google Scholar]
- Hayward, B.W.; Sabaa, A.T.; Grenfell, H.R.; Neil, H.; Bostock, H. Ecological Distribution of Recent Deep-Water Foraminifera around New Zealand. J. Foraminifer. Res. 2013, 43, 415–442. [Google Scholar] [CrossRef]
- Van Morkhoven, F.P.C.M.; Berggren, W.A.; Edwards, A.S.; Oertli, H.J. Cenozoic Cosmopolitan Deep-Water Benthic Foraminifera; Elf Aquitaine: Pau, France, 1986; ISBN 2901026206. [Google Scholar]
- Bellier, J.-P.; Mathieu, R.; Granier, B. Short Treatise on Foraminiferology (Essential on Modern and Fossil Foraminifera); Carnets de Géologie: Brest, France, 2010; ISBN 978-2-916733-07-4. [Google Scholar]
- Hayward, B.W.; Le Coze, F.; Vachard, D.; Gross, O. World Foraminifera Database. Available online: http://www.marinespecies.org/foraminifera (accessed on 15 January 2023).
- Hayward, B.W.; Coze, F.L.; Vandepitte, L.; Vanhoorne, B. Foraminifera in the World Register of Marine Species (Worms) Taxonomic Database. J. Foraminifer. Res. 2020, 50, 291–300. [Google Scholar] [CrossRef]
- Brady, H.B. Report on the Foraminifera Dredged by H.M.S. Challenger during the Years 1873–1876. Zoology 1884, 9 Pt 22, i–xxi, 1–814. [Google Scholar]
- Cushman, J.A. The Foraminifera of the Atlantic Ocean. Part I—Astrorhizidae. Bull. Am. Mus. Nat. Hist. 1918, 104 Pt 1, 1–111. [Google Scholar] [CrossRef]
- Cushman, J.A. The Foraminifera of the Atlantic Ocean. Part II—Lituolidae. Bull. Am. Mus. Nat. Hist. 1920, 104 Pt 2, 1–89. [Google Scholar] [CrossRef]
- Cushman, J.A. The Foraminifera of the Atlantic Ocean. Part III—Textulariidae. Bull. Am. Mus. Nat. Hist. 1922, 104 Pt 3, 1–143. [Google Scholar] [CrossRef]
- Cushman, J.A. The Foraminifera of the Atlantic Ocean. Part IV—Lagenidae. Bull. Am. Mus. Nat. Hist. 1923, 104 Pt 4, 1–178. [Google Scholar] [CrossRef]
- Cushman, J.A. The Foraminifera of the Atlantic Ocean. Part V—Chilostomellidae and Globigerinidae. Bull. Am. Mus. Nat. Hist. 1924, 104 Pt 5, 1–45. [Google Scholar] [CrossRef]
- Cushman, J.A. The Foraminifera of the Atlantic Ocean. Part VI—Miliolidae, Ophthalmidiidae and Fischerinidae. Bull. Am. Mus. Nat. Hist. 1929, 104 Pt 6, 1–101. [Google Scholar] [CrossRef]
- Cushman, J.A. The Foraminifera of the Atlantic Ocean. Part VII—Nonionidae, Camerinidae, Peneroplidae and Alveolinellidae. Bull. Am. Mus. Nat. Hist. 1930, 104 Pt 7, 1–55. [Google Scholar] [CrossRef]
- Cushman, J.A. The Foraminifera of the Atlantic Ocean. Part VIII—Rotaliidae, Amphisteginidae, Calcarinidae, Cymbaloporettidae, Globorotaliidae, Anomalinidae, Planorbulinidae, Rupertiidae, and Homotremidae. Bull. Am. Mus. Nat. Hist. 1931, 104 Pt 8, 1–144. [Google Scholar] [CrossRef]
- Flint, J.M. Recent Foraminifera. A Descriptive Catalogue of Specimens Dredged by the U.S. Fish Commission Steamer Albatross. Rep. U.S. Natl. Mus. 1899, 1897, 249–349. [Google Scholar] [CrossRef]
- Phleger, F.B.; Parker, F.L.; Pierson, J.F. North Atlantic Foraminifera. Rep. Swedish Deep Sea Exped. 1947–1948 1953, 7, 3–122. [Google Scholar]
- Schnitker, D. West Atlantic Abyssal Circulation during the Past 120,000 Years. Nature 1974, 248, 385–387. [Google Scholar] [CrossRef]
- Schnitker, D. Quaternary Deep-Sea Benthic Foraminifers and Bottom Water Masses. Annu. Rev. Earth Planet. Sci. 1980, 8, 343–370. [Google Scholar] [CrossRef]
- Schröder, C.J. Deep-Water Arenaceous Foraminifera in the Northwest Atlantic Ocean. Ph.D. Thesis, Dalhousie University, Halifax, NS, Canada, 1986. [Google Scholar]
- Bilodeau, G.; de Vernal, A.; Hillaire-Marcel, C. Benthic Foraminiferal Assemblages in Labrador Sea Sediments: Relations with Deep-Water Mass Changes since Deglaciation. Canad. J. Earth Sci. 1994, 31, 128–138. [Google Scholar] [CrossRef]
- Tikhonova, A.; Merenkova, S.; Korsun, S.; Matul, A. Image Dataset of Common Benthic Foraminiferal Taxa in the North Atlantic Seafloor Surface Sediments (59.5°N Transect) between the Labrador Sea and Faeroe-Shetland Sill. Data Brief 2019, 26, 104554. [Google Scholar] [CrossRef]
- Schönfeld, J. History and Development of Methods in Recent Benthic Foraminiferal Studies. J. Micropalaeontol. 2012, 31, 53–72. [Google Scholar] [CrossRef]
- Schmiedl, G. Rekonstruktion der Spätquartären Tiefenwasserzirkulation und Produktivität im Östlichen Südatlantik Anhand von Benthischen Foraminiferenvergesellschaftungen (Late Quaternary Benthic Foraminiferal Assemblages from the Eastern South Atlantic Ocean: Reconstruction of Deep Water Circulation and Productivity Changes. Ph.D. Thesis, University of Bremen, Bremen, Germany, 1995. [Google Scholar]
- Stefanoudis, P.V. Benthic Foraminiferal Responses to Mesoscale Environmental Heterogeneity at the Porcupine Abyssal Plain, NE Atlantic. Ph.D. Thesis, University of Southampton, School of Ocean & Earth Science Department,, Southampton, UK, 2016. [Google Scholar]
- Ellis, B.F.; Messina, A. Catalogue of Foraminifera; Micropaleontology Press; American Museum of Natural History: New York, NY, USA, 2020. [Google Scholar]
- Hammer, O.; Harper, D.; Ryan, P. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Hammer, O. PAST PAleontological STatistics Version 4.05. Reference Manual; Natural History Museum, University of Oslo: Oslo, Norway, 2021. [Google Scholar]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The Relation Between the Number of Species and the Number of Individuals in a RandomSample of an Animal Population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar] [CrossRef]
- Pielou, E.C. The Measurement of Diversity in Different Types of Biological Collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423; 623–656. [Google Scholar] [CrossRef]
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Loeblich, A.R.; Tappan, H. Foraminiferal Genera and Their Classification; Van Nostrand Reinhold Company: New York, NY, USA, 1987. [Google Scholar] [CrossRef]
- Corliss, B.H.; Chen, C. Morphotype Patterns of Norwegian Sea Deep-Sea Benthic Foraminifera and Ecological Implications. Geology 1988, 16, 716–719. [Google Scholar] [CrossRef]
- Schmiedl, G.; Mackensen, A.; Müller, P.J. Recent Benthic Foraminifera from the Eastern South Atlantic Ocean: Dependence on Food Supply and Water Masses. Mar. Micropaleontol. 1997, 32, 249–287. [Google Scholar] [CrossRef]
- Murray, J.W. Ecology and Palaeoecology of Benthic Foraminifera; Longman Scientific and Technical: Harlow, UK, 1991. [Google Scholar] [CrossRef]
- Carvalho, F.P.; Oliveira, J.M.; Soares, A.M.M. Sediment Accumulation and Bioturbation Rates in the Deep Northeast Atlantic Determined by Radiometric Techniques. ICES Mar. Sci. Symp. 2011, 68, 427–435. [Google Scholar] [CrossRef]
- Gooday, A. A response by benthic Foraminifera to the deposition of phytodetritus in the deep sea. Nature 1998, 332, 70–73. [Google Scholar] [CrossRef]
- Pawlowski, J.; Fahrni, J.; Lecroq, B.; Longet, D.; Cornelius, N.; Excoffier, L.; Cedhagen, T.; Gooday, A.J. Bipolar Gene Flow in Deep-Sea Benthic Foraminifera. Mol. Ecol. 2007, 16, 4089–4096. [Google Scholar] [CrossRef]
- PANGAEA®—Data Publisher for Earth & Environmental Science PANGAEA® Database. [CrossRef]
- Rönnfeld, W. Foraminifera: A catalogue of typical forms. Grzybowski Found. Spec. Pub. 2020, 24, 1–159. [Google Scholar]
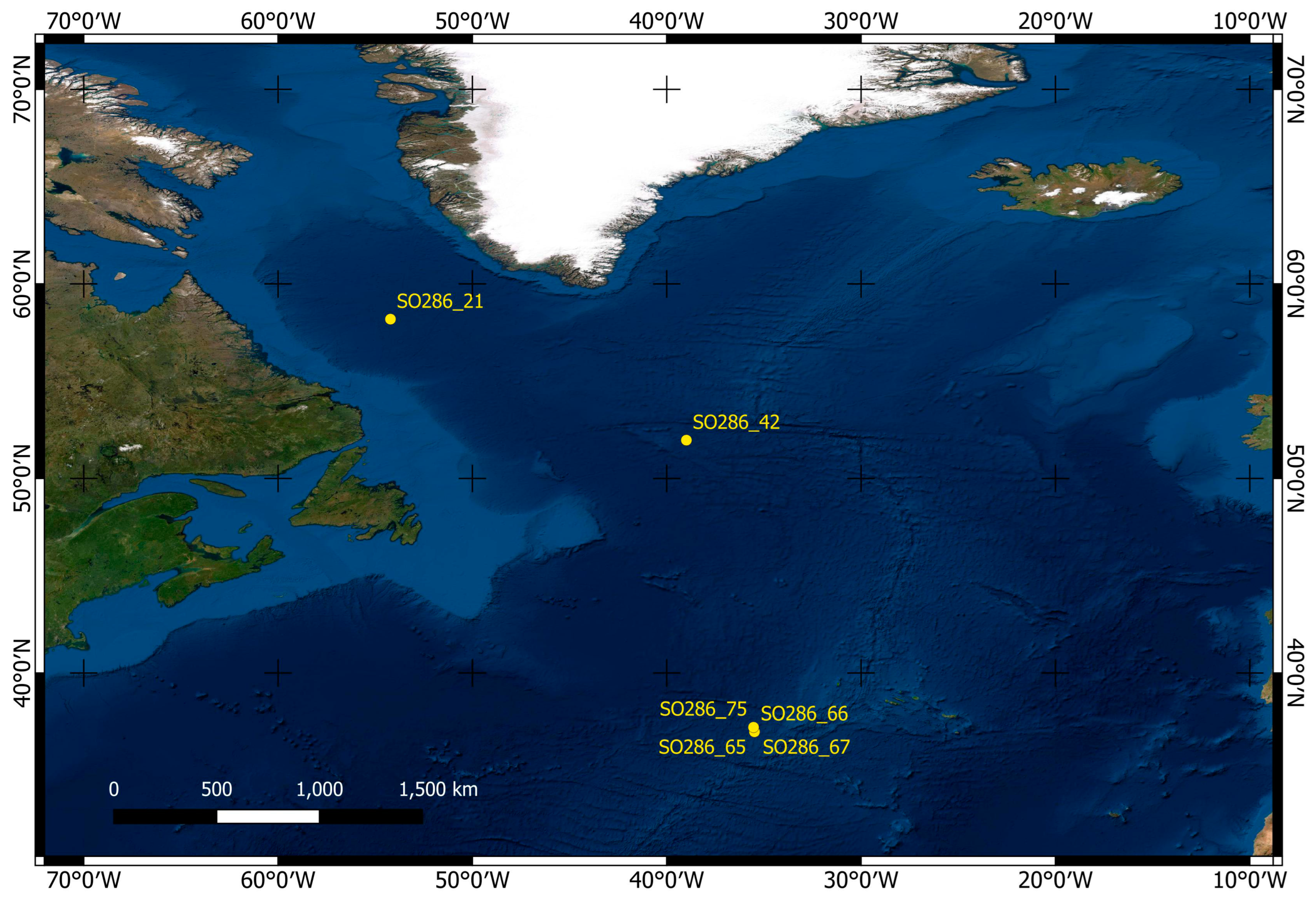
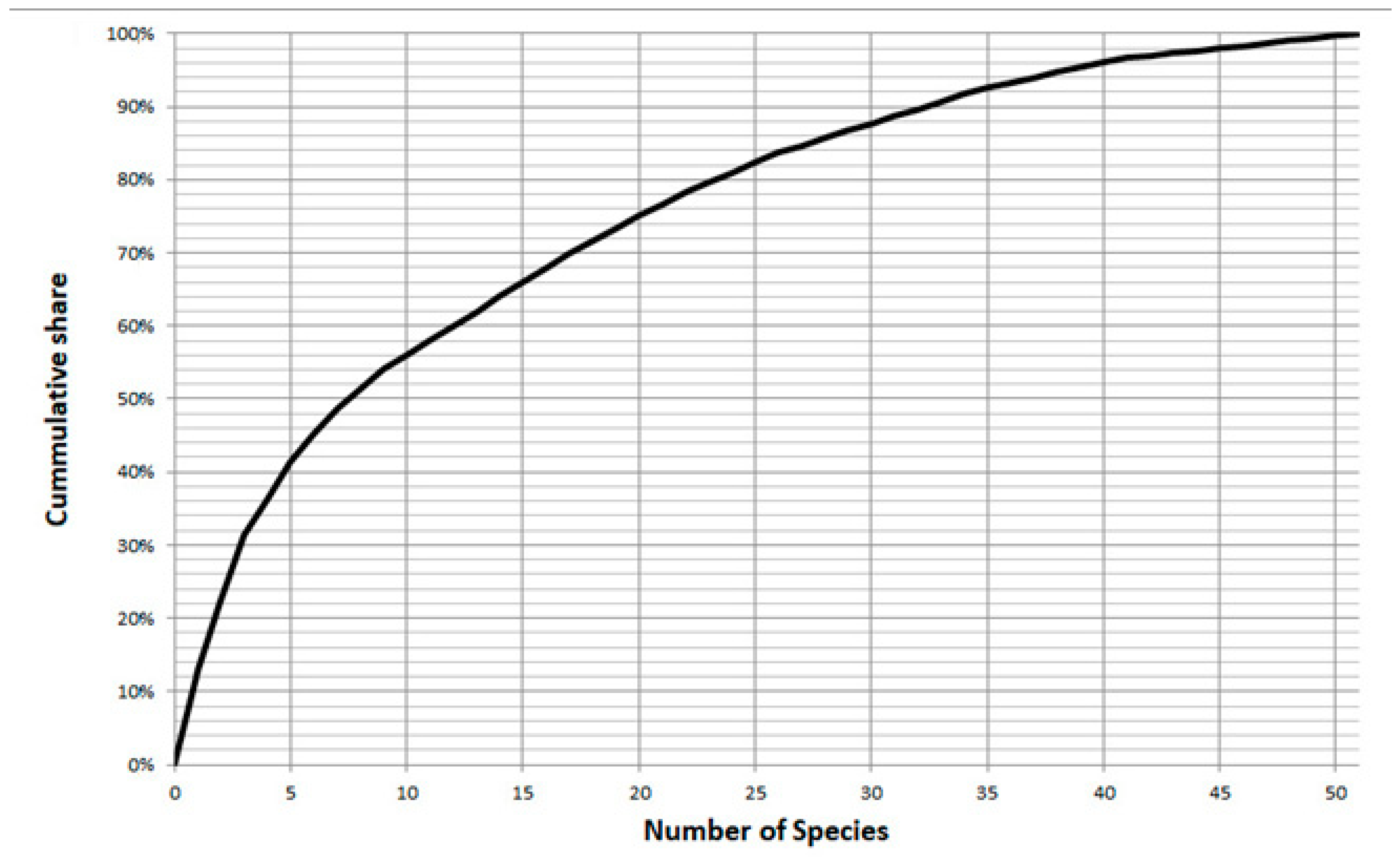


| Station | Working Area | Longitude | Latitude | Depth | Device | Sediment | Facies |
|---|---|---|---|---|---|---|---|
| SO286_21 | Labrador Sea | 58°14.422′ | 54°13.075′ | 3391 m | MUC | soft mud | globigerina ooze |
| SO286_42 | Labrador Basin | 51°58.255′ | 38°59.534′ | 3685 m | MUC | soft mud | globigerina ooze |
| SO286_65 | Azores SW | 37°00.025′ | 35°29.491′ | 3193 m | MUC | soft mud | globigerina ooze |
| SO286_66 | Azores SW | 37°00.032′ | 35°29.441′ | 3192 m | MUC | soft mud | globigerina ooze |
| SO286_67 | Azores SW | 37°00.037′ | 35°29.382′ | 3209 m | MUC | soft mud | globigerina ooze |
| SO286_75 | Seamount | 37°13.922′ | 35°32.316′ | 2508 m | MUC | soft mud | globigerina ooze |
| Order/Wall Material | Number of Genera | Number of Species |
|---|---|---|
| Astrorhizida | 6 | 7 |
| Hormosinida | 3 | 6 |
| Lituolida | 13 | 21 |
| Spirillinida (agglutinated) | 3 | 4 |
| Textulariida | 4 | 4 |
| Not assigned to a taxon | 1 | 1 |
| Subtotal Agglutinated | 30 | 43 |
| Miliolida | 12 | 23 |
| Subtotal Porcellaneous | 12 | 23 |
| Nodosariida | 3 | 10 |
| Polymorphinida | 9 | 19 |
| Robertinida | 2 | 2 |
| Rotaliida | 33 | 48 |
| Spirillinida (hyaline) | 1 | 2 |
| Vaginulinida | 1 | 3 |
| Subtotal Hyaline | 49 | 84 |
| Allogromiida | 1 | 1 |
| Subtotal Organic | 1 | 1 |
| Total | 92 | 150 |
| Cluster | I | II | III | IV | |||||
|---|---|---|---|---|---|---|---|---|---|
| Corresponding with station SO286_ | 21 | 42 | 65,66,67 | 75 | |||||
| Area | Labrador Sea | Labrador Basin | SW of Azores | Seamount | |||||
| Dominant species | E. exigua O. umbonatus | E. exigua O. umbonatus | E. exigua | None | |||||
| Subsidiary important species | H. elongata | G. communis | C. wuellerstorfi | A. pseudothalmanni | |||||
| C. wuellerstorfi | Melonis group | G. subglobosa | |||||||
| P. quinqueloba | |||||||||
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | ||
| Share in % | E. exigua | 23.3 | 6.8–32.9 | 39.0 | 30.4–50.6 | 22.7 | 13.3–29.9 | 5.9 | 3.2–9.1 |
| Share in % | O. umbonatus | 10.5 | 5.9–19.0 | 13.6 | 8.9–18.4 | 3.2 | 0.0–7.9 | 1.8 | 0.0–2.6 |
| Share in % | A. pseudothalmanni | 0.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.0–1.9 | 11.8 | 2.5–19.5 |
| Share in % | G. subglobosa | 0.1 | 0.0–0.6 | 0.0 | 0.0 | 1.9 | 0.0–9.7 | 10.4 | 6.5–15.1 |
| Diversity | Fisher’s alpha (α) | 14.6 | 10.5–18.4 | 14.1 | 10.6–17.3 | 23.3 | 16.0–30.1 | 25.9 | 22.7–33.8 |
| Evenness | Equitability (J) | 0.83 | 0.77–0.93 | 0.73 | 0.63–0.79 | 0.87 | 0.81–0.93 | 0.92 | 0.90–0.94 |
| Div. + Even. | Shannon (H) | 2.98 | 2.59–3.44 | 2.60 | 2.28–2.86 | 3.37 | 3.01–3.62 | 3.62 | 3.49–3.72 |
| Life Position | Epifaunal | 65% | 50%–76% | 80% | 70%–83% | 59% | 49%–67% | 37% | 33%–42% |
| Life Position | Infaunal | 15% | 6%–22% | 10% | 7%–17% | 25% | 17%–33% | 39% | 35%–44% |
| Life Position | Unknown/Both | 21% | 13%–32% | 10% | 8%–13% | 16% | 10%–22% | 23% | 16%–29% |
| Wall Material | Organic | 4% | 3%–7% | 1% | 0%–1% | 0% | 0%–1% | 0% | 0%–1% |
| Wall Material | Agglutinated | 27% | 19%–45% | 9% | 5%–17% | 7% | 3%–17% | 5% | 2%–8% |
| Wall Material | Porcelaneous | 4% | 2%–8% | 6% | 4%–8% | 11% | 7%–16% | 16% | 9%–24% |
| Wall Material | Calcareous | 65% | 48%–74% | 85% | 74%–90% | 82% | 68%–88% | 80% | 74%–88% |
| Density per g | 124 | 44–290 | 889 | 438–1059 | 279 | 163–447 | 189 | 110–223 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hesemann, M. Benthic Foraminifera Diversity of the Abyssal Northwest Atlantic. Diversity 2023, 15, 381. https://doi.org/10.3390/d15030381
Hesemann M. Benthic Foraminifera Diversity of the Abyssal Northwest Atlantic. Diversity. 2023; 15(3):381. https://doi.org/10.3390/d15030381
Chicago/Turabian StyleHesemann, Michael. 2023. "Benthic Foraminifera Diversity of the Abyssal Northwest Atlantic" Diversity 15, no. 3: 381. https://doi.org/10.3390/d15030381
APA StyleHesemann, M. (2023). Benthic Foraminifera Diversity of the Abyssal Northwest Atlantic. Diversity, 15(3), 381. https://doi.org/10.3390/d15030381






