Evidence of Coral Diseases, Phase Shift, and Stressors in the Atolls of Lakshadweep Islands, Arabian Sea—With Geographical Notes on Their Occurrence within the Indian EEZ and Contiguous International Waters
Abstract


Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthur, R. Coral bleaching and mortality in three Indian reef regions during El Nino southern oscillation event. Curr. Sci. 2000, 79, 1723–1729. [Google Scholar]
- Arthur, R.; Done, T.J.; Marsh, H.; Harriott, V. Local processes strongly influence post-bleaching benthic recovery in the Lakshadweep Islands. Coral Reefs 2006, 25, 427–440. [Google Scholar] [CrossRef]
- Harithsa, S.; Raghukumar, C.; Dalal, S.G. Stress response of two coral species in the Kavaratti atoll of the Lakshadweep Archipelago, India. Coral Reefs 2005, 24, 463–474. [Google Scholar] [CrossRef]
- Arthur, R.; Karkarey, R.; Lobo, A.S.; Alcoverro, T.; Kelkar, N. Coral Reef Resilience: Recovery and Resistance across Lakshadweep Archipelago; Ocean and coasts program; Nature Conservation Foundation: Mysuru, India, 2010; p. 24. [Google Scholar]
- Vinoth, R.; Gopi, M.; Kumar, T.T.A.; Thangaradjou, T.; Balasubramanian, T. Coral reef bleaching at Agatti Island of Lakshadweep Atolls, India. J. Ocean Univ. China 2012, 11, 105–110. [Google Scholar] [CrossRef]
- Hughes, T.P.; Anderson, K.; Conolly, S.R.; Heron, S.F.; Kerry, J.T.; Louch, J.M.; Baird, A.H.; Baum, J.K.; Berumen, M.L.; Bridge, T.C.; et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 2018, 359, 80–83. [Google Scholar] [CrossRef]
- Ravindran, J.; Raghukumar, C.; Raghukumar, S. Disease and stress-induced mortality of corals in Indian reefs and observations on bleaching of corals in the Andamans. Curr. Sci. 1999, 76, 233–237. [Google Scholar]
- Hazraty-Kari, S.; Tavakoli-Kolour, P.; Das, R.R.; Farhadi, M.; Barkhordari-Ahmadi, A.; Yahyavi, M.; Rezai, H. Baseline assessment of coral diseases in an environmentally extreme environment of the northern Persian Gulf. Mar. Poll. Bull. 2021, 171, 112707. [Google Scholar] [CrossRef]
- Croquer, A.; Weil, E. Changes in Caribbean coral disease prevalence after the 2005 bleaching event. Dis. Aquat. Org. 2009, 87, 33–43. [Google Scholar] [CrossRef]
- Ravindran, J.; Raghukumar, C. Pink line syndrome (PLS) in the scleractinian coral Porites lutea. Coral Reefs 2002, 21, 252. [Google Scholar] [CrossRef]
- Ranith, R.P.; Senthilnathan, L.; Machendiranathan, M.; Thangaradjou, T.; Sasamal, S.; Choudhury, S.B. Sources and threats of chronic tissue loss on coral reefs in the Lakshadweep Islands, Indian Ocean. Mar. Ecol. 2017, 38, e12436. [Google Scholar] [CrossRef]
- Rajasuriya, A.; Zahir, H.; Muley, E.V.; Subramanian, B.R.; Venkataraman, K.; Wafar, M.V.M.; Munjurul Hannan Khan, S.M.; Whittingham, E. Status of coral reefs in South Asia: Bangladesh, India, Maldives, and Sri Lanka. In Status of Coral Reefs of the World, Proceedings of the Ninth International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; Moosa, M.K., Soemodihardjo, S., Soegiarto, A., Romimohtarto, K., Nontji, A., Soekarno, S., Eds.; Global Coral Reef Monitoring Network and Reef and Rainforest Research Centre: Townsville, Australia; Volume 2, pp. 23–27.
- Sharma, D.; Ravindran, C. Diseases and pathogens of marine invertebrate corals in Indian reefs. J. Invertebr. Pathol. 2020, 173, 107373. [Google Scholar] [CrossRef]
- Montano, S.; Strona, G.; Seveso, D.; Galli, P. First report of coral diseases in the Republic of Maldives. Dis. Aquat. Org. 2012, 101, 159–165. [Google Scholar] [CrossRef]
- Raymundo, L.J.; Couch, C.S.; Harvell, C.D. Coral Disease Handbook: Guidelines for Assessment, Monitoring, and Management; Coral reef targeted research and capacity building for management program; University of Queensland: St. Lucia, QLD, Australia, 2008. [Google Scholar]
- Das, R.R.; Wada, H.; Masucci, G.D.; Singh, T.; Tavakoli-Kolour, P.; Wada, N.; Tang, S.L.; Yamashiro, H.; Reimer, J.D. Four-year field survey of black band disease and skeletal growth anomalies in encrusting Montipora spp. corals around Sesoko Island, Okinawa. Diversity 2022, 14, 32. [Google Scholar] [CrossRef]
- Sutherland, K.P.; Porter, J.W.; Torres, C. Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar. Ecol. Prog. Ser. 2004, 266, 273–302. [Google Scholar] [CrossRef]
- Bourne, D.G.; Ainsworth, T.D.; Pollock, F.J.; Willis, B.L. Towards a better understanding of white syndromes and their causes on Indo-Pacific coral reefs. Coral Reefs 2015, 34, 233–242. [Google Scholar] [CrossRef]
- Bythell, J.; Pantos, O.; Richardson, R. White Plague, White Band and other “White” Diseases. In Coral Health and Diseases; Rosenberg, E., Loya, Y., Eds.; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Adhavan, D.; Chandran, R.; Tikadar, S.; Sivakumar, K. Trematode infestation in coral colonies at Poshitra reef, Gulf of Kachchh marine national park, Gujarat, India. J. Threat. Taxa 2017, 9, 10345–10346. [Google Scholar] [CrossRef]
- Das, R.R.; Sreeraj, C.R.; Mohan, G.; Abhilash, K.R.; Samuel, V.K.D.; Ramachandran, P.; Ramachandran, R. Incursion of killer sponge Terpios hoshinota Rützler & Muzik, 1993 on the coral reefs of the Lakshadweep archipelago, Arabian Sea. J. Threat. Taxa 2020, 12, 17009–17013. [Google Scholar]
- Montano, S.; Seveso, D.; Strona, G.; Arrigoni, R.; Galli, P. Acropora muricata mortality associated with extensive growth of Caulerpa racemosa in Magoodhoo Island, Republic of Maldives. Coral Reefs 2012, 31, 793. [Google Scholar] [CrossRef][Green Version]
- Manikandan, B.; Ravindran, J. Differential response of coral communities to Caulerpa spp. bloom in the reefs of Indian Ocean. Environ. Sci. Poll. Res. 2017, 24, 3912–3922. [Google Scholar] [CrossRef]
- Thinesh, T.; Jose, P.A.; Ramasamy, P.; Meenatchi, R.; Selvan, K.M.; Selvin, J. Differential coral response to algae contact: Porites tissue loss, praise for Halimeda interaction at southeast coast of India. Environ. Sci. Poll. Res. 2019, 26, 17845–17852. [Google Scholar] [CrossRef]
- Nugues, M.M.; Smith, G.W.; Hooidonk, R.J.V.; Seabra, M.I.; Bak, R.P.M. Algal contact as a trigger for coral disease. Ecol. Lett. 2004, 7, 919–923. [Google Scholar] [CrossRef]
- Thaha, P.P.; Rathod, J.L. Report of coral diseases in the reef flats of Chetlat Island, Lakshadweep. J. Mar. Biol. Ass. India 2019, 61, 51–53. [Google Scholar] [CrossRef]
- Gopi, M.; Jeevamani, J.J.J.; Goutham, S.; Simon, N.T.; Samuel, V.D.; Abhilash, K.R.; Robin, R.S.; Hariharan, G.; Muruganandam, R.; Krishnan, P.; et al. Status of health and conservation classification of tropical coral reefs in Lakshadweep archipelago. Wetl. Ecol. Manag. 2021, 29, 653–668. [Google Scholar] [CrossRef]
- Ricci, F.; Leggat, W.; Page, C.E.; Ainsworth, T.D. Coral growth anomalies, neoplasms, and tumors in the Anthropocene. Trends Microbiol. 2022, 30, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; De, K.; Thomas, L.; Nagesh, R.; Mote, S.; Ingole, B. Prevalence of skeletal tissue growth anomalies in a scleractinian coral: Turbinaria mesenterina of Malvan marine sanctuary, eastern Arabian Sea. Dis. Aquat. Org. 2016, 121, 79–83. [Google Scholar] [CrossRef]
- Thinesh, T.; Mathews, G.; Patterson Edward, J.K. Coral disease prevalence in Mandapam group of islands, Gulf of Mannar, Southeastern India. Ind. J. Geo. Mar. Sci. 2009, 38, 444–450. [Google Scholar]
- Ramesh, C.H.; Mohanraju, R.; Murthy, K.N.; Karthick, P. Preliminary survey of diseases in the coral reefs of Burmanallah coast, Andaman’s. Ind. J. Geo. Mar. Sci. 2014, 43, 1972–1976. [Google Scholar]
- Kubomura, T.; Wee, H.B.; Reimer, J.D. Investigating incidence and possible cause of pink and purple pigmentation response in hard coral genus Porites around Okinawajima Island, Japan. Reg. Stud. Mar. Sci. 2021, 41, 101569. [Google Scholar] [CrossRef]
- Thinesh, T.; Mathews, G.; Diraviya-Raj, K.; Patterson-Edward, J.K. Variation in black and white band disease progression in corals of the Gulf of Mannar and Palk Bay, Southeastern India. Dis. Aquat. Org. 2014, 110, 227–234. [Google Scholar] [CrossRef]
- Montano, S.; Strona, G.; Seveso, D.; Galli, P. Prevalence, host range, and spatial distribution of black band disease in the Maldivian Archipelago. Dis. Aquat. Org. 2013, 105, 65–74. [Google Scholar] [CrossRef]
- Montano, S.; Strona, G.; Seveso, D.; Maggioni, D.; Galli, P. Widespread occurrence of coral diseases in the central Maldives. Mar. Freshw. Res. 2015, 67, 1253–1262. [Google Scholar] [CrossRef]
- Tkachenko, K.S. The northernmost coral frontier of the Maldives: The coral reefs of Ihavandippolu atoll under long-term environmental change. Mar. Environ. Res. 2012, 82, 40–42. [Google Scholar] [CrossRef]
- Montano, S.; Chou, W.-H.; Chen, C.A.; Galli, P.; Reimer, J.D. First record of the coral killing sponge Terpios hoshinota in the Maldives and the Indian Ocean. Bull. Mar. Sci. 2015, 91, 97–98. [Google Scholar] [CrossRef]
- Thinesh, T.; Mathews, G.; Raj, K.D.; Edward, J.K.P. Outbreaks of Acropora white syndrome and Terpios sponge overgrowth combined with coral mortality in Palk Bay, southeast coast of India. Dis. Aquat. Org. 2017, 126, 63–70. [Google Scholar] [CrossRef]
- Thinesh, T.; Arul Jose, P.; Hassan, S.; Muthamizh Selvan, K.; Selvin, J. Intrusion of coral-killing sponge (Terpios hoshinota) on the reefs of Palk Bay. Curr. Sci. 2015, 109, 1030–1032. [Google Scholar]
- Diraviya-Raj, K.; Selva Bharath, M.; Mathews, G.; Aeby, G.S.; Patterson Edward, J.K. Coral-killing sponge Terpios hoshinota invades the corals of Gulf of Mannar, Southeast India. Curr. Sci. 2018, 114, 117–119. [Google Scholar] [CrossRef]
- Wang, J.-T.; Hirose, E.; Hsu, C.-M.; Chen, Y.-Y.; Meng, P.-J.; Chen, C.A. A coral killing sponge, Terpios hoshinota, releases larvae harboring cyanobacterial symbionts: An implication of dispersal. Zool. Stud. 2012, 51, 314–320. [Google Scholar]
- Ashok, A.M.; Schonberg, C.H.L.; Diraviya-Raj, K.; Bhoopathi, M.; Selva Bharath, M.; Patterson Edward, J.K. A sponge of the Cliona viridis complex invades and excavates corals of the Gulf of Mannar, south-eastern India. Mar. Freshw. Res. 2018, 69, 874–882. [Google Scholar] [CrossRef]
- Ashok, A.M.; Calcinai, B.; Edward, J.K.P. The coral-killing sponge Clathria (Microciona) aceratoobtusa (Porifera: Demosponigiae) invades various coral communities of Gulf of Mannar Marine National Park, southeast India. Eur. Zool. J. 2020, 87, 1–11. [Google Scholar] [CrossRef]
- Thomas, P.A. Boring sponges destructive to economically important molluscan beds and coral reefs in Indian seas. Ind. J. Fish. 1979, 26, 163–200. [Google Scholar]
- Venkataraman, K.; Rajan, P.T. Coral reefs of Mahatma Gandhi marine national park and crown of thorn starfish phenomenon. In Island Ecosystem and Sustainable Development; Gangwar, B., Chandra, K., Eds.; Andaman Science Association and Department of Science and Technology: Port Blair, India, 1998; pp. 124–132. [Google Scholar]
- Jeyabaskaran, R. Disturbances to coral reef communities of Andaman & Nicobar Islands. In National Symposium of Conservation and Valuation of Marine Biodiversity; The Director, ZSI, Ed.; Zoological Survey of India: Kolkata, India, 2007; pp. 117–124. [Google Scholar]
- Adam, M.S. Status Report and Survey Results; COT busters program, Marine research section; Ministry of Fisheries and Agriculture: Male’, Republic of Maldives, 1989; 12p. [Google Scholar]
- Saponari, L.; Montano, S.; Seveso, D.; Galli, P. The occurrence of an Acanthaster planci outbreak in Ari Atoll, Maldives. Mar. Biodivers. 2015, 45, 599–600. [Google Scholar] [CrossRef]
- Pernetta, J.; Wells, S. (Eds.) Marine Protected Area Needs in the South Asian Sea’s Region: Maldives; A Marine Conservation and Development Report; IUCN: Gland, Switzerland, 1993; Volume 3. [Google Scholar]
- Jaleel, A. The status of the coral reefs and the management approaches: The case of the Maldives. Ocean Coast. Manag. 2013, 82, 104–118. [Google Scholar] [CrossRef]
- Rajasuriya, A.; White, A.T. Coral reefs of Sri-lanka: Review of their extent, condition, and management status. Coast. Manag. 1995, 23, 77–90. [Google Scholar] [CrossRef]
- De Bruin, G.H.P. The crown-of-thorns starfish Acanthaster planci (Linne’) in Ceylon. Bull. Fish. Res. Stn. 1972, 23, 37–41. [Google Scholar]
- Kamalakannan, B.; Jeevamani, J.J.J.; Nagendran, N.A.; Pandiaraja, D.; Krishnan Kutty, N.; Chandrasekaran, S. Turbinaria sp. as victims to Kappaphycus alvarezii in reefs of Gulf of Mannar, India. Coral Reefs 2010, 29, 1077. [Google Scholar] [CrossRef]
- Krishnan, P.; Abhilash, K.R.; Sreeraj, C.R.; Samuel, V.D.; Purvaja, R.; Anand, A.; Mahapatra, M.; Sankar, R.; Raghuraman, R.; Ramesh, R. Balancing livelihood enhancement and ecosystem conservation in seaweed farmed areas: A case study from Gulf of Mannar Biosphere Reserve, India. Ocean Coast. Manag. 2021, 207, 105590. [Google Scholar] [CrossRef]
- Arulananthan, A.; Herath, V.; Kuganathan, S.; Upasantha, A.; Harishchandra, A. The status of the coral reefs of the Jaffna peninsula (Northern Sri Lanka), with 36 coral species to Sri Lanka confirmed by DNA bar-coding. Oceans 2021, 2, 509–529. [Google Scholar] [CrossRef]
- Rogers, C.S. Words matter: Recommendations for clarifying coral disease nomenclature and terminology. Dis. Aquat. Org. 2010, 167–175. [Google Scholar] [CrossRef]
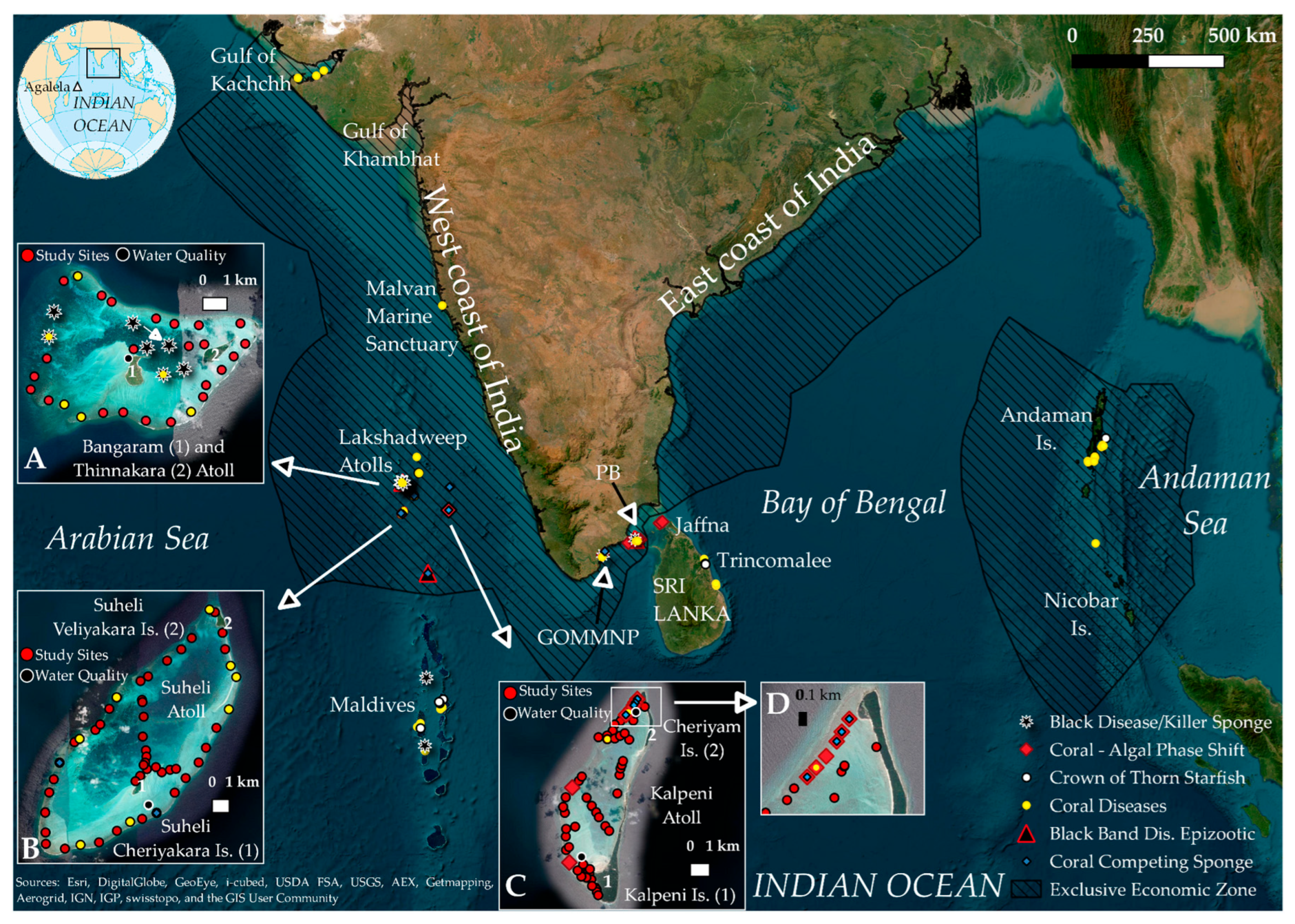

| Location (Islands) | Sea Surface Temperature (SST;°C) | pH | Dissolved Oxygen (mg/L) | Figures |
|---|---|---|---|---|
| Bangaram and Thinnakara (BT) | 29.97 (±1.29) | 7.63 (±0.04) | 6.82 (±1.2) | Figure 1A |
| Suheli (SUH) | 29.5 (±1.34) | 7.6 (0) | 7.72 (±0.5) | Figure 1B |
| Cheriyam (CHE) | 29.6 (±0.28) | 7.6 (±0.05) | 5.9 (±0.03) | Figure 1C |
| Kalpeni (KAL) | 29.1 (±1.34) | 7.5 (0) | 6.63 (±2.3) | Figure 1C |
| Location | GPS Coordinates | Genus/Sp. | Disease/Stressors/Transitions | Figures | Date of Survey | Additional Ref./Reading | Comments/Remarks |
|---|---|---|---|---|---|---|---|
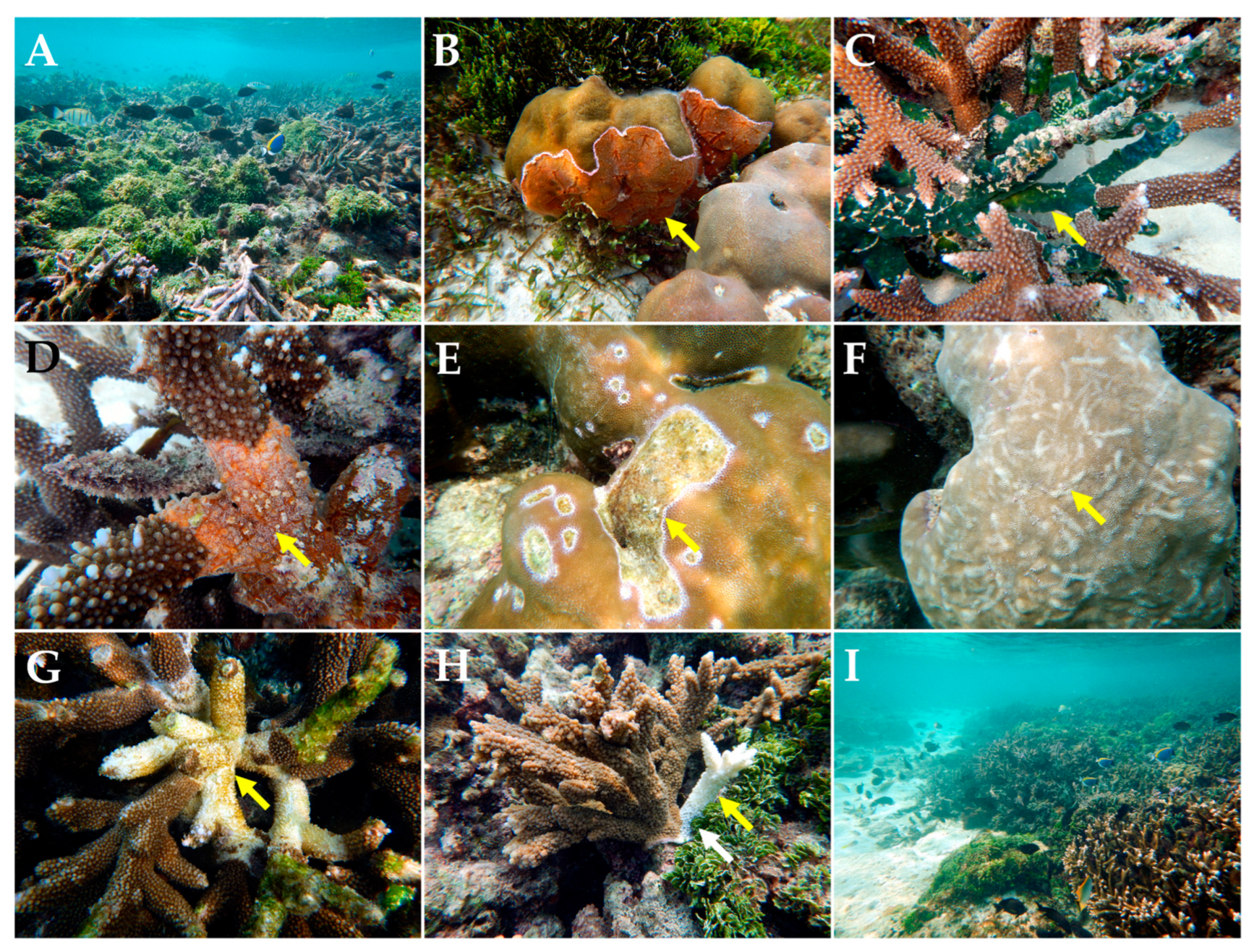
| Bangaram and Thinnakara (BT) | 10.94999 N, 72.29684 E | Goniastrea edwarsi | White syndrome | Figure 2A | 12 November 2016 | [17,18,19] | Following Bourne et al., [18] and Bythell et al., [19] this observation is placed under the general category of white syndrome |
| Hydnophora sp. | Black band disease (BBD) | Figure 2B | First report from the Arabian sea | ||||
| Goniastrea edwarsi | Tissue loss | Figure 2C | |||||
| Astreopora ocellata | Pink line syndrome (PLS) | Figure 2D | [10] | First report from Indian EEZ | |||
| Porites solida | Trematodiasis/pink spots (PS) | Figure 2E | [20] | Requires lab verification for confirmation | |||
| Isopora palifera; Cyphastrea spp.; Porites lutea | Black disease (BD)/T. hoshinota | [21] | |||||
| Porites cylindrica | Black disease (BD)/T. hoshinota | Figure 2F | [21] | BD presence overlooked at this site by Das et al., [21] (Figure 1A—White arrow) | |||
| Dipsastraea lizardensis | Black disease (BD)/T. hoshinota | Figure 2G | [21] | ||||
| Acropora spp. | Skeletal growth anomalies (GAs) | Figure 2H | |||||
| Fungia sp.; Herpolitha sp. | Compromised health signs (CHS) | Figure 2I | |||||
| Pocillopora sp. | Invertebrate galls (IGs) | No reports from the Indian EEZ, Maldives, or Sri Lanka. | |||||
| Goniopora sp. | Black band disease (BBD) | ||||||
| Cheriyam (CHE) | 10.14353 N, 73.65811 E | Acropora dominated reef | Massive overgrowth of Caulerpa racemosa | Figure 3A | 7 November 2016 | [22,23] | Similar opportunistic invasion reported in Maldives [22] and mainland India [23]. |
| Porites sp. | Coral competing sponge (CCS) overgrowth | Figure 3B | |||||
| Acropora muricata | Overgrowth of colonial asicidian | Figure 3C | |||||
| Acropora muricata | Coral competing sponge (CCS) overgrowth | Figure 3D | |||||
| Porites solida | Tissue loss | Figure 3E | |||||
| Porites sp. | Predation/fish bites | Figure 3F | [11] | ||||
| Acropora sp. | Multifocal tissue loss/white syndrome followed by growth of turf algae. | Figure 3G | [17,18,19] | Further studies related to white syndrome, involving multiple techniques remains necessary [18]. | |||
| Acropora sp. | Association with H. opuntia leading to tissue loss (white syndrome?) | Figure 3H | [17,18,19,24,25] | Necessary to study interaction and pathogen reservoir potential of H. opuntia [25]. | |||
| Reef habitat | C. racemosa overgrowing Acropora spp. dominated reef habitat | Figure 3I | [22] | ||||
| Kalpeni (KAL) | 10.08134 N, 73.63442 E | Isopora palifera | White syndrome in association with Halimeda opuntia | Figure 4A | 7 November 2016 | [17,18,19] | Reported as white band disease by Thaha and Rathod [26], needs lab verification/confirmation. |
| Porites spp. | Caulerpa racemosa overgrowth | Figure 4B | [23] | ||||
| Pavona varians | Black band disease (BBD) | Figure 4C | [26] | ||||
| Acropora spp. | C. racemosa overgrowth | Figure 4D | [22] | ||||
| Porites sp. | Coral competing sponge (CCS) | Figure 4E | |||||
| Porites sp. | Skeletal growth anomalies (GAs) | Figure 4F | |||||
| Reef habitat | Macroalgae causing coral mortality | Figure 4G | |||||
| Acropora spp. | Multifocal tissue loss, followed by algal overgrowth | Figure 4H | |||||
| Isopora palifera | White syndrome following algal overgrowth. Presence of H. opuntia noted. | Figure 4I | [17,18,19] | ||||
| Suheli (SUH) | 10.07667 N, 72.29111 E | Pocillopora damicornis | Invertebrate galls (IGs) | Figure 5A | 9–10 November 2016 | Not reported from the Indian EEZ, Maldives, or Sri Lanka. | |
| Porites solida | Pink line syndrome (PLS)/pink spot (PS)-like condition | Figure 5B | [10,20] | ||||
| Porites sp. | Fish bites and pink spots (PS) | Figure 5C | [11] | ||||
| Platygyra pini | Skeletal growth anomalies (GAs) | Figure 5D | |||||
| Herpolitha sp. | Pink line syndrome (PLS) | Figure 5E | |||||
| Acropora sp. | Coral competing sponge (CCS) overgrowth | Figure 5F | |||||
| Porites cylindrica | Skeletal growth anomalies (GAs) | Figure 5G | First report from the Indian EEZ | ||||
| Pocillopora grandis | CCA-related partial mortality in association with H. opuntia | Figure 5H | |||||
| Acropora spp. | Tips of Acropora showing algal overgrowth. A possible scenario following tissue loss. | Figure 5I |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, R.R.; Sreeraj, C.R.; Mohan, G.; Simon, N.T.; Ramachandran, P.; Ramachandran, R.; Krishnan, P.; Kumar, D.S.V. Evidence of Coral Diseases, Phase Shift, and Stressors in the Atolls of Lakshadweep Islands, Arabian Sea—With Geographical Notes on Their Occurrence within the Indian EEZ and Contiguous International Waters. Diversity 2023, 15, 382. https://doi.org/10.3390/d15030382
Das RR, Sreeraj CR, Mohan G, Simon NT, Ramachandran P, Ramachandran R, Krishnan P, Kumar DSV. Evidence of Coral Diseases, Phase Shift, and Stressors in the Atolls of Lakshadweep Islands, Arabian Sea—With Geographical Notes on Their Occurrence within the Indian EEZ and Contiguous International Waters. Diversity. 2023; 15(3):382. https://doi.org/10.3390/d15030382
Chicago/Turabian StyleDas, Rocktim Ramen, Chemmencheri Ramakrishnan Sreeraj, Gopi Mohan, Nina Tabitha Simon, Purvaja Ramachandran, Ramesh Ramachandran, Pandian Krishnan, and Deepak Samuel Vijay Kumar. 2023. "Evidence of Coral Diseases, Phase Shift, and Stressors in the Atolls of Lakshadweep Islands, Arabian Sea—With Geographical Notes on Their Occurrence within the Indian EEZ and Contiguous International Waters" Diversity 15, no. 3: 382. https://doi.org/10.3390/d15030382
APA StyleDas, R. R., Sreeraj, C. R., Mohan, G., Simon, N. T., Ramachandran, P., Ramachandran, R., Krishnan, P., & Kumar, D. S. V. (2023). Evidence of Coral Diseases, Phase Shift, and Stressors in the Atolls of Lakshadweep Islands, Arabian Sea—With Geographical Notes on Their Occurrence within the Indian EEZ and Contiguous International Waters. Diversity, 15(3), 382. https://doi.org/10.3390/d15030382








