Invading the Greek Seas: Spatiotemporal Patterns of Marine Impactful Alien and Cryptogenic Species
Abstract
1. Introduction
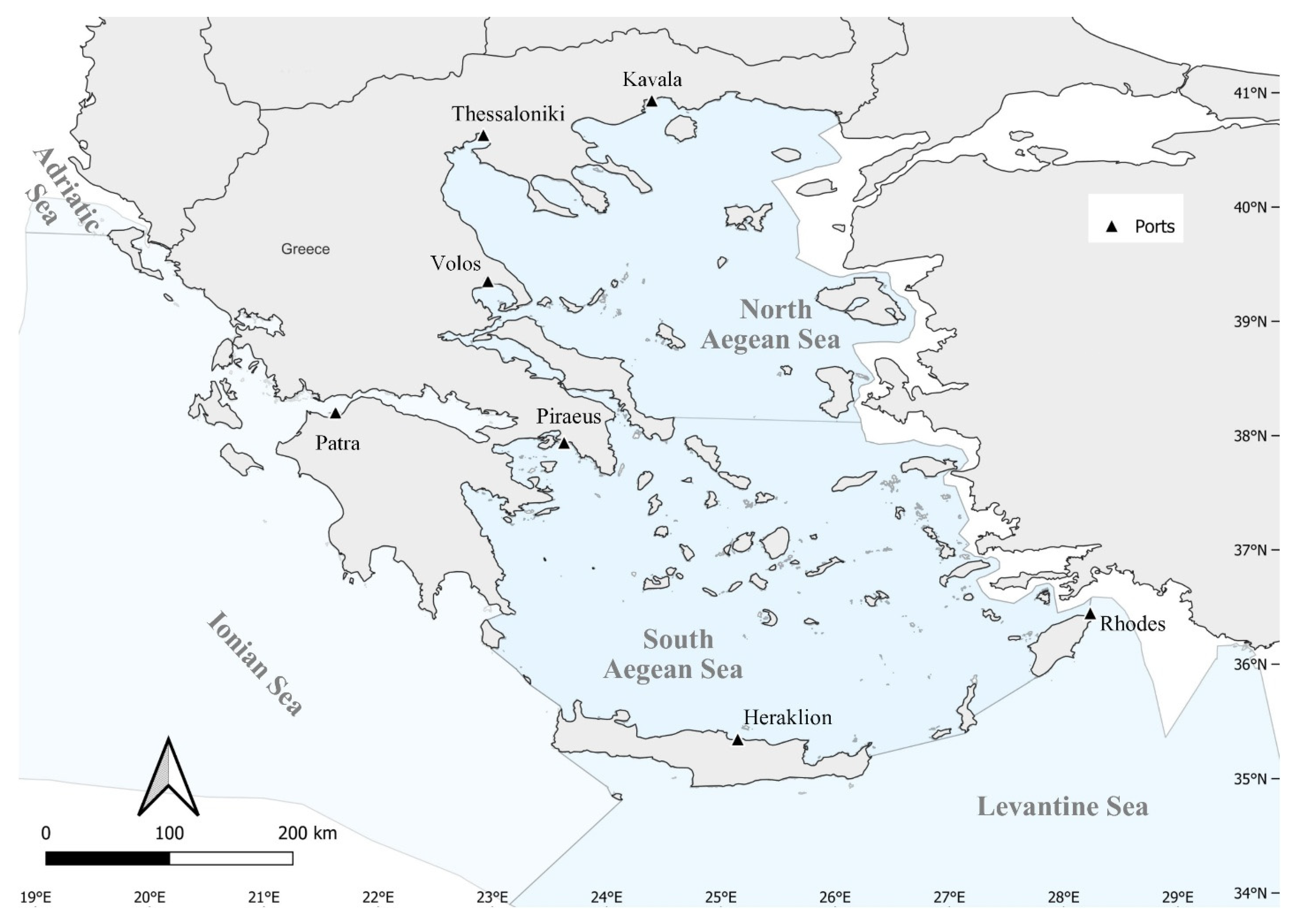
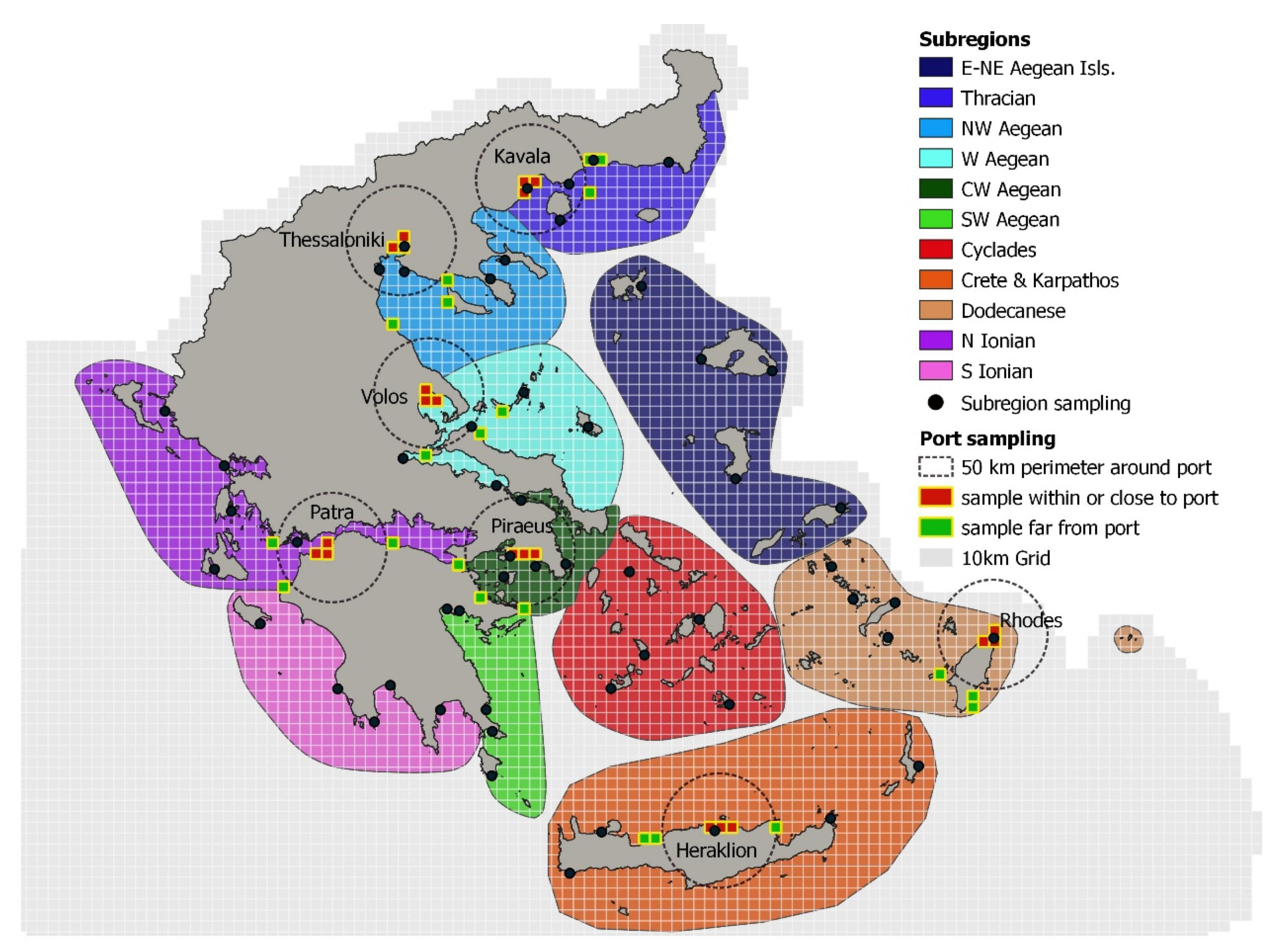
2. Materials and Methods
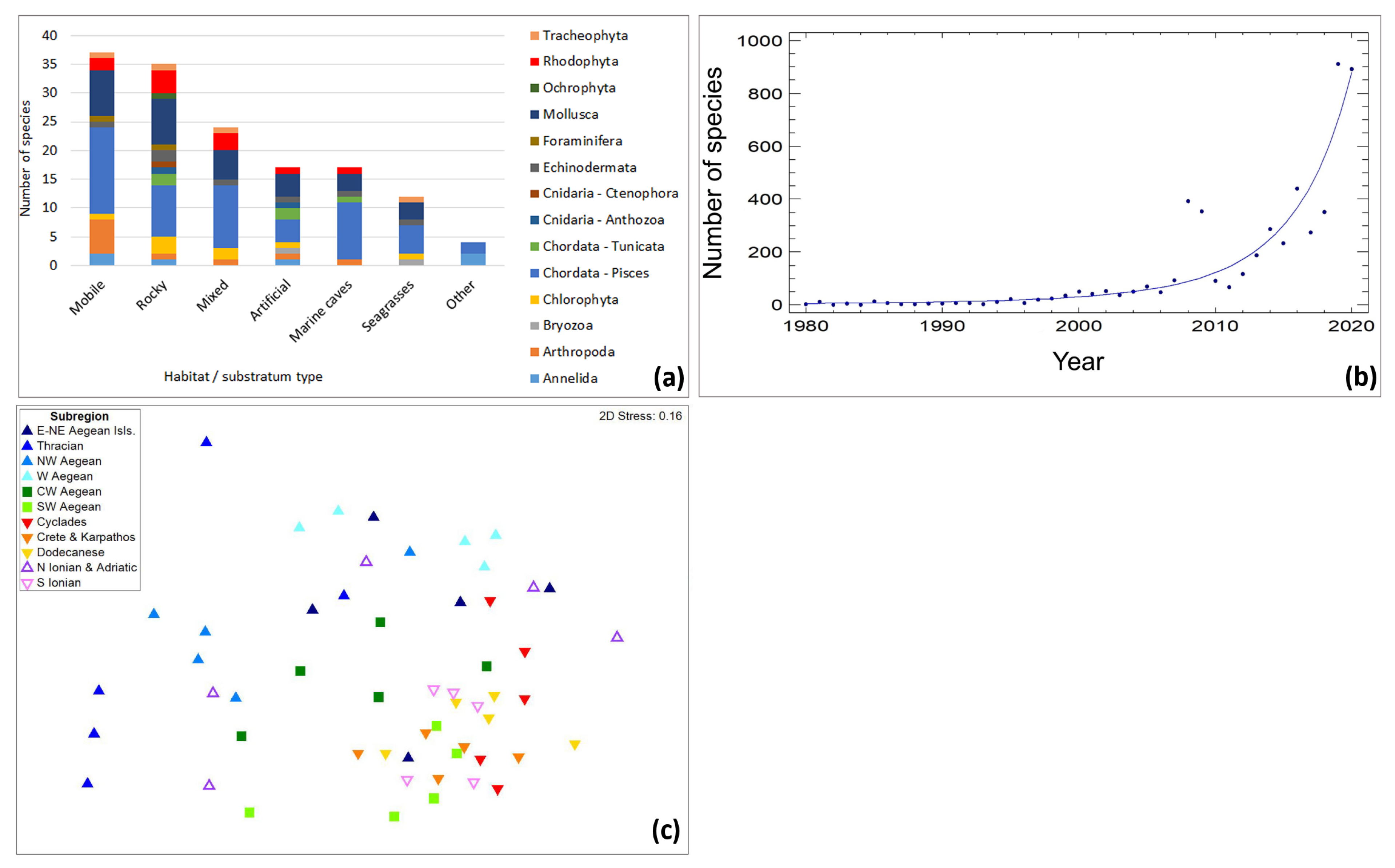
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brondizio, E.S.; Settele, J.; Díaz, S.; Ngo, H. Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services; IPBES Secretariat: Bonn, Germany, 2019; Available online: https://ipbes.net/global-assessment (accessed on 10 December 2022).
- Pyšek, P.; Hulme, P.E.; Simberloff, D.; Bacher, S.; Blackburn, T.M.; Carlton, J.T.; Dawson, W.; Essl, F.; Foxcroft, L.C.; Genovesi, P. Scientists’ warning on invasive alien species. Biol. Rev. 2020, 95, 1511–1534. [Google Scholar] [CrossRef]
- Levine, J.M. Biological invasions. Curr. Biol. 2008, 18, R57–R60. [Google Scholar] [CrossRef] [PubMed]
- Vila, M.; Hulme, P.E. Impact of Biological Invasions on Ecosystem Services; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-45121-3. [Google Scholar] [CrossRef]
- Tsirintanis, K.; Azzurro, E.; Crocetta, F.; Dimiza, M.; Froglia, C.; Gerovasileiou, V.; Langeneck, J.; Mancinelli, G.; Rosso, A.; Stern, N.; et al. Bioinvasion impacts on biodiversity, ecosystem services, and human health in the Mediterranean Sea. Aquat Invasions 2022, 17, 308–352. [Google Scholar] [CrossRef]
- Convention on Biological Diversity Aichi Biodiversity Targets. Available online: https://www.cbd.int/sp/targets/ (accessed on 5 December 2022).
- Regulation, European Parliament. European Union Regulation No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the prevention and management of the introduction and spread of invasive alien species. Off. J. Eur. Union 2014, 317, 35–55. [Google Scholar]
- Directive, Strategy Framework. European Commission Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 establishing a framework for community action in the field of marine environmental policy. Off. J. Eur. Union L 2008, 164, 19–40. [Google Scholar]
- Katsanevakis, S.; Coll, M.; Piroddi, C.; Steenbeek, J.; Ben Rais Lasram, F.; Zenetos, A.; Cardoso, A.C. Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Front. Mar. Sci. 2014, 1, 32. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Poursanidis, D.; Hoffman, R.; Rizgalla, J.; Rothman, S.B.-S.; Levitt-Barmats, Y.; Hadjioannou, L.; Trkov, D.; Garmendia, J.M.; Rizzo, M.; et al. Unpublished Mediterranean records of marine alien and cryptogenic species. Bioinvasions Rec. 2020, 9, 165–182. [Google Scholar] [CrossRef]
- Clarke Murray, C.; Gartner, H.; Gregr, E.J.; Chan, K.; Pakhomov, E.; Therriault, T.W. Spatial distribution of marine invasive species: Environmental, demographic and vector drivers. Diversity Distrib. 2014, 20, 824–836. [Google Scholar] [CrossRef]
- Zenetos, A. Mediterranean Sea: 30 years of biological invasions (1988–2017). In Proceedings of the 1st Mediterranean Symposium on the Non-Indigenous Species, Antalya, Turkey, 17–18 January 2019; Langar, H., Ouerghi, A., Eds.; SPA/RAC Publication: Tunis, Tunis, 2019. 116p. [Google Scholar]
- Katsanevakis, S.; Wallentinus, I.; Zenetos, A.; Leppäkoski, E.; Çinar, M.E.; Oztürk, B.; Grabowski, M.; Golani, D.; Cardoso, A.C. Impacts of invasive alien marine species on ecosystem services and biodiversity: A pan-European review. Aquat. Invasions 2014, 9, 391–423. [Google Scholar] [CrossRef]
- Rilov, G.; Mazaris, A.D.; Stelzenmüller, V.; Helmuth, B.; Wahl, M.; Guy-Haim, T.; Mieszkowska, N.; Ledoux, J.-B.; Katsanevakis, S. Adaptive marine conservation planning in the face of climate change: What can we learn from physiological, ecological and genetic studies? Glob. Ecol. Conserv. 2019, 17, e00566. [Google Scholar] [CrossRef]
- Rilov, G.; Fraschetti, S.; Gissi, E.; Pipitone, C.; Badalamenti, F.; Tamburello, L.; Menini, E.; Goriup, P.; Mazaris, A.D.; Garrabou, J.; et al. A fast-moving target: Achieving marine conservation goals under shifting climate and policies. Ecol. Appl. 2020, 30, e02009. [Google Scholar] [CrossRef] [PubMed]
- Reichard, S.; White, P. Invasion biology: An emerging field of study. Ann. Missouri. Bot. Gard. 2003, 90, 64–66. [Google Scholar] [CrossRef]
- Steinmayer, A.G., Jr.; Turfa, J.M. Effects of shipworm on the performance of ancient Mediterranean warships. Int. J. Naut. Arch. 1996, 25, 104–121. [Google Scholar] [CrossRef]
- Bellard, C.; Cassey, P.; Blackburn, T.M. Alien species as a driver of recent extinctions. Biol. Lett. 2016, 12, 20150623. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Global sea warming and “tropicalization” of the Mediterranean Sea: Biogeographic and ecological aspects. Biogeogr. J. Integr. Biogeogr. 2003, 24, 319–327. [Google Scholar] [CrossRef]
- Pancucci-Papadopoulou, M.A.; Raitsos, D.E.; Corsini-Foka, M. Biological invasions and climatic warming: Implications for South Eastern Aegean ecosystem functioning. J. Mar. Biol. Assoc. United Kingd. 2012, 92, 777–789. [Google Scholar] [CrossRef]
- Sisma-Ventura, G.; Yam, R.; Shemesh, A. Recent unprecedented warming and oligotrophy of the eastern Mediterranean Sea within the last millennium. Geophys. Res. Lett. 2014, 41, 5158–5166. [Google Scholar] [CrossRef]
- Pisano, A.; Marullo, S.; Artale, V.; Falcini, F.; Yang, C.; Leonelli, F.E.; Santoleri, R.; Nardelli, B.B. New evidence of Mediterranean climate change and variability from sea surface temperature observations. Remote Sens. 2020, 12, 132. [Google Scholar] [CrossRef]
- Novi, L.; Bracco, A.; Falasca, F. Uncovering marine connectivity through sea surface temperature. Sci. Rep. 2021, 11, 8839. [Google Scholar] [CrossRef]
- Essl, F.; Dullinger, S.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Katsanevakis, S.; Kühn, I.; Lenzner, B.; Pauchard, A.; Pyšek, P.; et al. A conceptual framework for range-expanding species that track human-induced environmental change. BioScience 2019, 69, 908–919. [Google Scholar] [CrossRef]
- Zenetos, A.; Gofas, S.; Morri, C.; Rosso, A.; Violanti, D.; García Raso, J.E.; Çinar, M.E.; Almogi-Labin, A.; Ates, A.S.; Azzurro, E.; et al. Alien species in the Mediterranean Sea by 2012. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part 2. Introduction trends and pathways. Mediterr. Mar. Sci. 2012, 13, 328–352. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Meditter. Mar. Sci. 2022, 23, 196–212. [Google Scholar] [CrossRef]
- Zenetos, A.; Albano, P.G.; Garcia, E.L.; Stern, N.; Tsiamis, K.; Galanidi, M. Corrigendum to the Review Article (Medit. Mar. Sci. 23/1 2022, 196-212) Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Meditter. Mar. Sci. 2022, 23, 876–878. [Google Scholar] [CrossRef]
- Garrabou, J.; Gómez-Gras, D.; Medrano, A.; Cerrano, C.; Ponti, M.; Schlegel, R.; Bensoussan, N.; Turicchia, E.; Sini, M.; Gerovasileiou; et al. Marine heatwaves drive recurrent mass mortalities in the Mediterranean Sea. Glob. Chang. Biol. 2022, 28, 5708–5725. [Google Scholar] [CrossRef]
- Edelist, D.; Rilov, G.; Golani, D.; Carlton, J.T.; Spanier, E. Restructuring the Sea: Profound shifts in the world’s most invaded marine ecosystem. Divers. Distrib. 2013, 19, 69–77. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Rilov, G.; Edelist, D. Impacts of marine invasive alien species on European fisheries and aquaculture—plague or boon? In Engaging Marine Scientists and Fishers to Share Knowledge and Perceptions—Early Lessons; Briand, F., Ed.; CIESM Monograph; CIESM: Villa Girasole, Monaco, 2018; Volume 50, pp. 125–132. [Google Scholar]
- Rilov, G. Multi-species collapses at the warm edge of a warming sea. Sci. Rep. 2016, 6, 36897. [Google Scholar] [CrossRef]
- Albano, P.G.; Steger, J.; Bošnjak, M.; Dunne, B.; Guifarro, Z.; Turapova, E.; Hua, Q.; Kaufman, D.S.; Rilov, G.; Zuschin, M. Native biodiversity collapse in the eastern Mediterranean. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202469. [Google Scholar] [CrossRef]
- Zenetos, A.; Karachle, P.K.; Corsini-Foka, M.; Gerovasileiou, V.; Simboura, N.; Xentidis, N.J.; Tsiamis, K. Is the trend in new introductions of marine non-indigenous species a reliable criterion for assessing good environmental status? The case study of Greece. Meditter. Mar. Sci. 2020, 21, 775–793. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Belchior, C.; Cardoso, A.C. Invading European Seas: Assessing pathways of introduction of marine aliens. Ocean Coast. Manag. 2013, 76, 64–74. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Zenetos, A.; Corsini-Foka, M.; Tsiamis, K. Biological invasions in the Aegean Sea: Temporal trends, pathways, and impacts. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–34. [Google Scholar]
- Pancucci-Papadopoulou, M.A.; Zenetos, A.; Corsini-Foka, M.; Politou, C.Y. Update of marine aliens in Hellenic waters. Mediterr. Mar. Sci. 2005, 6, 147–158. [Google Scholar] [CrossRef]
- Zenetos, A.; Pancucci-Papadopoulou, M.A.; Zogaris, S.; Papastergiadou, E.; Vardakas, L.; Aligizaki, K.; Economou, A.N. Aquatic alien species in Greece: Tracking sources, patterns and effects on the ecosystem. J. Biol. Res. 2009, 12, 135–172. [Google Scholar]
- Zenetos, A.; Katsanevakis, S.; Poursanidis, D.; Crocetta, F.; Damalas, D.; Apostolopoulos, G.; Gravili, C.; Vardala-Theodorou, E.; Malaquias, M. Marine alien species in Greek Seas: Additions and amendments by 2010. Mediterr. Mar. Sci. 2011, 12, 95–120. [Google Scholar] [CrossRef]
- Zenetos, A.; Corsini-Foka, M.; Crocetta, F.; Gerovasileiou, V.; Karachle, P.K.; Simboura, N.; Tsiamis, K.; Pancucci-Papadopoulou, M.A. Deep cleaning of alien species records in the Greek Seas (2018 update). Manag. Biol. Invasion 2018, 9, 209–226. [Google Scholar] [CrossRef]
- Zenetos, A.; Arianoutsou, M.; Bazos, I.; Balopoulou, S.; Corsini-Foka, M.; Dimiza, M.; Drakopoulou, P.; Katsanevakis, S.; Kondylatos, G.; Koutsikos, N.; et al. ELNAIS: A collaborative network on aquatic alien species in Hellas (Greece). Manag. Biol. Invasions 2015, 6, 185–196. [Google Scholar] [CrossRef]
- Giovos, I.; Kleitou, P.; Poursanidis, D.; Batjakas, I.; Bernardi, G.; Crocetta, F.; Doumpas, N.; Kalogirou, S.; Kampouris, T.E.; Keramidas, I.; et al. Citizen-science for monitoring marine invasions and stimulating public engagement: A case project from the eastern Mediterranean. Biol. Invasions 2019, 21, 3707–3721. [Google Scholar] [CrossRef]
- Crocetta, F.; Al Mabruk, S.A.A.; Azzurro, E.; Bakiu, R.; Bariche, M.; Batjakas, I.E.; Bejaoui, T.; Ben Souissi, J.; Cauchi, J.; Corsini-Foka, M.; et al. New alien Mediterranean biodiversity records (November 2021). Mediterr. Mar. Sci. 2021, 22, 724–746. [Google Scholar] [CrossRef]
- Digenis, M.; Ragkousis, M.; Vasileiadou, K.; Gerovasileiou, V.; Katsanevakis, S. New records of the Indo-Pacific shrimp Urocaridella pulchella Yokes and Galil, 2006 from the Eastern Mediterranean Sea. BioInvasions Rec. 2021, 10, 295–303. [Google Scholar] [CrossRef]
- Ragkousis, M.; Zenetos, A.; Ben Souissi, J.; Hoffman, R.; Ghanem, R.; Taşkın, E.; Muresan, M.; Karpova, E.; Slynko, E.; Dagli, E.; et al. Unpublished Mediterranean and Black Sea records of marine alien, cryptogenic, and neonative species. Bioinvasions Rec. 2023, 12, in press. [Google Scholar]
- Carlton, J.T. Biological invasions and cryptogenic species. Ecology 1996, 77, 1653–1655. [Google Scholar] [CrossRef]
- Essl, F.; Bacher, S.; Genovesi, P.; Hulme, P.E.; Jeschke, J.M.; Katsanevakis, S.; Kowarik, I.; Kühn, I.; Pyšek, P.; Rabitsch, W.; et al. Which taxa are alien? Criteria, applications, and uncertainties. BioScience 2018, 68, 496–509. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Deriu, I.; D’amico, F.; Nunes, A.L.; Sanchez, S.P.; Crocetta, F.; Arianoutsou, M.; Bazos, I.; Christopoulou, A.; Curto, G.; et al. European alien species information network (EASIN): Supporting European policies and scientific research. Manag. Biol. Invasions 2015, 6, 147–157. [Google Scholar] [CrossRef]
- Peyton, J.; Martinou, A.F.; Pescott, O.L.; Demetriou, M.; Adriaens, T.; Arianoutsou, M.; Bazos, I.; Bean, C.W.; Booy, O.; Botham, M. Horizon scanning for invasive alien species with the potential to threaten biodiversity and human health on a Mediterranean island. Biol. Invasions 2019, 21, 2107–2125. [Google Scholar] [CrossRef]
- Galanidi, M.; Zenetos, A.; Bacher, S. Assessing the socio-economic impacts of priority marine invasive fishes in the Mediterranean with the newly proposed SEICAT methodology. Mediterr. Mar. Sci. 2018, 19, 107–123. [Google Scholar] [CrossRef]
- Tsiamis, K.; Azzurro, E.; Bariche, M.; Çinar, M.E.; Crocetta, F.; De Clerck, O.; Galil, B.; Gómez, F.; Hoffman, R.; Jensen, K.R. Prioritizing marine invasive alien species in the European Union through horizon scanning. Aquat. Conserv. Mar. Freshwat. Ecosyst. 2020, 30, 794–845. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tsirintanis, K.; Sini, M.; Gerovasileiou, V.; Koukourouvli, N. Aliens in the Aegean—A sea under siege (ALAS). Res. Ideas Outcomes 2020, 6, e53057. [Google Scholar] [CrossRef]
- Tsiamis, K.; Zenetos, A.; Deriu, I.; Gervasini, E.; Cardoso, A.C. The native distribution range of the European marine non-indigenous species. Aquat. Invasions 2018, 13, 187–198. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, A.Z.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine ecoregions of the world: A bioregionalization of coastal and shelf areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Harrower, C.A.; Scalera, R.; Pagad, S.; Schonrogge, K.; Roy, H.E. Guidance for interpretation of CBD Categories on Introduction Pathways. Technical Note Prepared by IUCN for the European Commission. 2018. Available online: http://nora.nerc.ac.uk/id/eprint/519129/ (accessed on 2 November 2022).
- Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Plymouth Routines IN Multivariate Ecological Research (PRIMER-E): Plymouth, UK, 2001. [Google Scholar]
- Arianoutsou, M.; Adamopoulou, C.; Andriopoulos, P.; Bazos, I.; Christopoulou, A.; Galanidis, A.; Kalogianni, E.; Karachle, P.K.; Kokkoris, Y.; Martinou, A.F.; et al. HELLAS-ALIENS. The invasive alien species of Greece: Time traits, origin and pathways. NEOBIOTA, 2023; submitted. [Google Scholar]
- Nyberg, C.D.; Wallentinus, I. Can species traits be used to predict marine macroalgal introductions? Biol. Invasions 2015, 7, 265–279. [Google Scholar] [CrossRef]
- Azzurro, E.; Tuset, V.M.; Lombarte, A.; Maynou, F.; Simberloff, D.; Rodríguez-Pérez, A.; Solé, R.V. External morphology explains the success of biological invasions. Ecol. Let. 2014, 17, 1455–1463. [Google Scholar] [CrossRef]
- Karachle, P.K.; Oikonomou, A.; Pantazi, M.; Stergiou, K.I.; Zenetos, A. Can biological traits serve as predictors for fishes’ introductions, establishment, and Interactions? The Mediterranean Sea as a case study. Biology 2022, 11, 1625. [Google Scholar] [CrossRef]
- Raitsos, D.E.; Beaugrand, G.; Georgopoulos, D.; Zenetos, A.; Pancucci-Papadopoulou, A.M.; Theocharis, A.; Papathanassiou, E. Global climate change amplifies the entry of tropical species into the Eastern Mediterranean Sea. Limnol. Oceanogr. 2010, 55, 1478–1484. [Google Scholar] [CrossRef]
- Androulidakis, Y.S.; Krestenitis, Y.N. Sea Surface Temperature Variability and Marine Heat Waves over the Aegean, Ionian, and Cretan Seas from 2008–2021. J. Mar. Sci. Eng. 2022, 10, 42. [Google Scholar] [CrossRef]
- Zenetos, A.; Ovalis, P.; Giakoumi, S.; Kontadakis, C.; Lefkaditou, E.; Mpazios, G.; Simboura, N.; Tsiamis, K. Saronikos Gulf: A hotspot area for alien species in the Mediterranean Sea. BioInvasions Rec. 2020, 9, 873–889. [Google Scholar] [CrossRef]
- Cebrian, E.; Ballesteros, E. Invasion of Mediterranean benthic assemblages by red alga Lophocladia lallemandii (Montagne) F. Schmitz: Depth-related temporal variability in biomass and phenology. Aquat. Bot. 2010, 92, 81–85. [Google Scholar] [CrossRef]
- Piazzi, L.; Balata, D.; Bulleri, F.; Gennaro, P.; Ceccherelli, G. The invasion of Caulerpa cylindracea in the Mediterranean: The known, the unknown and the knowable. Mar. Biol. 2016, 163, 161. [Google Scholar] [CrossRef]
- Winters, G.; Beer, S.; Willette, D.A.; Viana, I.G.; Chiquillo, K.L.; Beca-Carretero, P.; Villamayor, B.; Azcárate-García, T.; Shem-Tov, R.; Mwabvu, B.; et al. The tropical seagrass Halophila stipulacea: Reviewing what we know from its native and invasive habitats, alongside identifying knowledge gaps. Front. Mar. Sci. 2020, 7, 300. [Google Scholar] [CrossRef]
- Lykousis, V.; Chronis, G.; Tselepides, A.; Price, N.B.; Theocharis, A.; Siokou-Frangou, I.; Van Wambeke, F.; Danovaro, R.; Stavrakakis, S.; Duineveld, G.; et al. Major outputs of the recent multidisciplinary biogeochemical researches undertaken in the Aegean Sea. J. Mar. Syst. 2002, 33, 313–334. [Google Scholar] [CrossRef]
- Zervakis, V.; Georgopoulos, D.; Karageorgis, A.P.; Theocharis, A. On the response of the Aegean Sea to climatic variability: A review. Int. J. Climatol. 2004, 24, 1845–1858. [Google Scholar] [CrossRef]
- Watkins, H.V.; Yan, H.F.; Dunic, J.C.; Côté, I.M. Research biases create overrepresented “poster children” of marine invasion ecology. Conserv. Lett. 2021, 14, e12802. [Google Scholar] [CrossRef]
- Colautti, R.I.; Grigorovich, I.A.; MacIsaac, H.J. Propagule pressure: A null model for biological invasions. Biol. Invasions 2006, 8, 1023–1037. [Google Scholar] [CrossRef]
- Holdgate, M.W. Summary and conclusions: Characteristics and consequences of biological invasions. Philos. Trans. R. Soc. B Biol. Sci. 1986, 314, 733–742. [Google Scholar] [CrossRef]
- Zaiko, A.; Olenin, S.; Daunys, D.; Nalepa, T. Vulnerability of benthic habitats to the aquatic invasive species. Biol. Invasions 2007, 9, 703–714. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Tempera, F.; Teixeira, H. Mapping the impact of alien species on marine ecosystems: The Mediterranean Sea case study. Divers. Distrib. 2016, 22, 694–707. [Google Scholar] [CrossRef]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Hayes, K.R.; Barry, S.C. Are there any consistent predictors of invasion success? Biol. Invasions 2008, 10, 483–506. [Google Scholar] [CrossRef]
- Corriero, G.; Pierri, C.; Accoroni, S.; Alabiso, G.; Bavestrello, G.; Barbone, E.; Bastianini, M.; Bazzoni, A.M.; Aubry, F.B.; Boero, F.; et al. Ecosystem vulnerability to alien and invasive species: A case study on marine habitats along the Italian coast. Aquat. Conserv. Mar. Freshw. Ecosyst. 2005, 26, 392–409. [Google Scholar] [CrossRef]
- Mačić, V.; Albano, P.G.; Almpanidou, V.; Claudet, J.; Corrales, X.; Essl, F.; Evagelopoulos, A.; Giovos, I.; Jimenez, C.; Kark, S.; et al. Biological invasions in conservation planning: A global systematic review. Front. Mar. Sci. 2018, 5, 178. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Medina-Villar, S. Alien species of Mediterranean origin in the Baltic Sea Region: Current state and risk assessment. Environ. Rev. 2020, 28, 339–356. [Google Scholar] [CrossRef]
- Băncilă, R.I.; Skolka, M.; Ivanova, P.; Surugiu, V.; Stefanova, K.; Todorova, V.; Zenetos, A. Alien species of the Romanian and Bulgarian Black Sea coast: State of knowledge, uncertainties, and needs for future research. Aquat Invasions 2022, 17, 353–373. [Google Scholar] [CrossRef]
- Azzurro, E.; Bolognini, L.; Dragičević, B.; Drakulović, D.; Dulčić, J.; Fanelli, E.; Grati, F.; Kolitari, J.; Lipej, L.; Magaletti, E. Detecting the occurrence of indigenous and non-indigenous megafauna through fishermen knowledge: A complementary tool to coastal and port surveys. Mar. Pollut. Bull. 2019, 147, 229–236. [Google Scholar] [CrossRef]

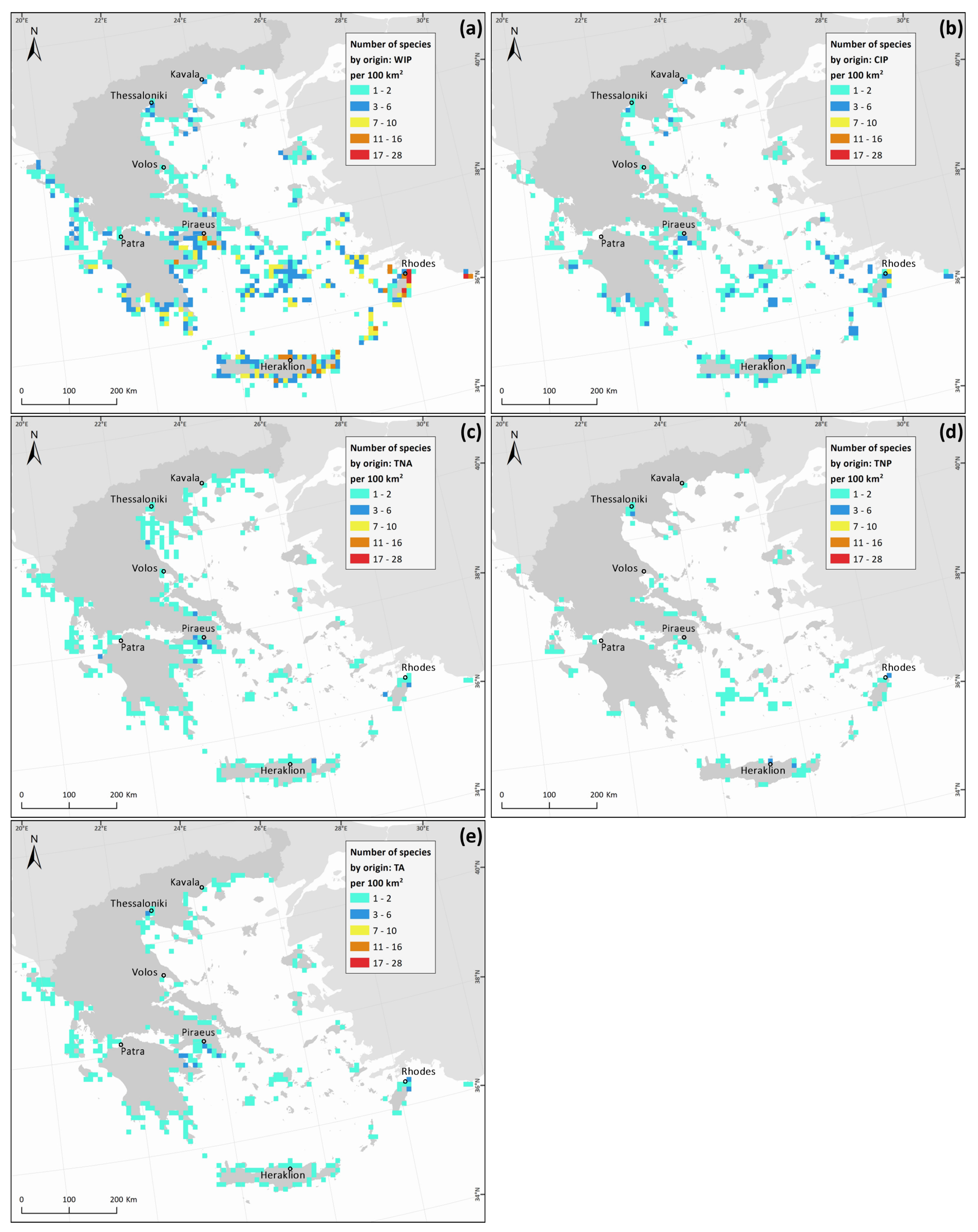


| Phylum, Class | Species | Status | Native Distribution Range | Pathway | Number of Records |
|---|---|---|---|---|---|
| Ochrophyta (66) | Stypopodium schimperi | Alien | WIP | CR | 66 |
| Chlorophyta (403) | Caulerpa cylindracea | Alien | TAu | EC/TS | 365 |
| Caulerpa taxifolia var. distichophylla | Alien | TAu | EC/TS | 15 | |
| Codium fragile subsp. fragile | Alien | TNP | TC/TS | 23 | |
| Rhodophyta (448) | Asparagopsis spp. | Alien | WIP/TAu | TS | 103 |
| Ganonema farinosum | Cryptogenic | WIP/TNA | CR/TS | 67 | |
| Lophocladia lallemandii | Alien | WIP/CIP/TNP | CR/TS | 243 | |
| Womersleyella setacea | Alien | WIP/CIP/EIP | EC/TS | 35 | |
| Tracheophyta (154) | Halophila stipulacea | Alien | WIP | CR/TS | 154 |
| Foraminifera (30) | Amphistegina lobifera | Alien | WIP | CR | 30 |
| Porifera (3) | Paraleucilla magna | Alien | TA | TS | 3 |
| Cnidaria–Anthozoa (71) | Oculina patagonica | Cryptogenic | TA/TNA | TS | 71 |
| Cnidaria–Scyphozoa (10) | Rhopilema nomadica | Alien | WIP | CR | 10 |
| Ctenophora (82) | Mnemiopsis leidyi * | Alien | TNA | TS/UNA | 82 |
| Bryozoa (21) | Amathia verticillata | Cryptogenic | WIP | TS | 20 |
| Tricellaria inopinata | Alien | TNP | TS | 1 | |
| Mollusca (559) | Anadara transversa | Alien | TA | TS | 1 |
| Brachidontes pharaonis | Alien | WIP | CR/TS | 34 | |
| Bursatella leachii | Alien | WIP/CIP/TA | CR | 72 | |
| Chama pacifica | Alien | WIP | CR/TS | 10 | |
| Conomurex persicus | Alien | WIP | TS | 152 | |
| Crepidula fornicata | Alien | TNA | TC/TS | 26 | |
| Dendostrea cf. folium | Alien | WIP | CR/TS | 33 | |
| Fulvia fragilis | Alien | WIP | CR/TS | 27 | |
| Magallana sp. | Alien | TNP | EC | 3 | |
| Mya arenaria | Alien | TNA | TC/TS | 4 | |
| Petricolaria pholadiformis | Alien | TA/TNA | TS | 5 | |
| Pinctada radiata | Alien | WIP | CR/REL | 187 | |
| Rapana venosa | Alien | TNP | TC/UNA | 3 | |
| Spondylus spinosus | Alien | WIP | CR/TS | 2 | |
| Annelida (46) | Ficopomatus enigmaticus | Alien | TAu | TS | 19 |
| Hydroides elegans | Alien | WIP/CIP/TAu | TS | 27 | |
| Arthropoda (523) | Callinectes sapidus | Alien | TA/TNA | TS | 132 |
| Matuta victor | Alien | CIP/EIP | CR | 3 | |
| Paracerceis sculpta | Alien | TEP/TNP | TC/TS | 6 | |
| Penaeus aztecus | Alien | TA/TNA | EC/TS | 67 | |
| Penaeus pulchricaudatus | Alien | WIP/CIP/TNP | CR | 10 | |
| Percnon gibbesi | Cryptogenic | TA/TEP/TNA | UNK | 283 | |
| Portunus segnis | Alien | WIP | CR | 22 | |
| Echinodermata (202) | Diadema setosum | Alien | WIP/CIP | CR | 136 |
| Synaptula reciprocans | Alien | WIP | CR | 66 | |
| Chordata–Ascidiacea (42) | Ciona robusta | Alien | UNK | TS | 2 |
| Herdmania momus | Alien | WIP/CIP | CR/TS | 12 | |
| Styela plicata | Alien | TNP | TS | 28 | |
| Chordata–Teleostei (2818) | Apogonichthyoides pharaonis | Alien | WIP | CR | 11 |
| Atherinomorus forskali | Alien | WIP | CR | 6 | |
| Etrumeus golanii | Alien | WIP | CR | 47 | |
| Fistularia commersonii | Alien | WIP/CIP/EIP | CR | 192 | |
| Lagocephalus sceleratus | Alien | WIP/CIP | CR | 265 | |
| Nemipterus randalli | Alien | WIP | CR | 2 | |
| Parupeneus forsskalli | Alien | WIP | CR | 61 | |
| Pempheris rhomboidea | Alien | WIP | CR | 30 | |
| Pterois miles | Alien | WIP | CR | 511 | |
| Sargocentron rubrum | Alien | WIP/CIP | CR | 97 | |
| Saurida lessepsianus | Alien | WIP | CR | 6 | |
| Siganus luridus | Alien | WIP | CR | 849 | |
| Siganus rivulatus | Alien | WIP | CR | 532 | |
| Torquigener flavimaculosus | Alien | WIP | CR | 172 | |
| Upeneus moluccensis | Alien | WIP | CR | 14 | |
| Upeneus pori | Alien | WIP | CR | 19 |
| Subregions | Cyclades | Crete and Karpathos | Dodecanese | E-NE Aegean Isls. | Thracian | NW Aegean | W Aegean | CW Aegean | SW Aegean | N Ionian | S Ionian |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclades | * | ** | * | ||||||||
| Crete and Karpathos | * | ** | ** | * | |||||||
| Dodecanese | ** | ** | ** | * | * | * | |||||
| E-NE Aegean Isls. | * | * | |||||||||
| Thracian | * | * | ** | * | * | ** | |||||
| NW Aegean | ** | ** | ** | * | * | * | ** | ** | |||
| W Aegean | * | ** | ** | * | * | ** | ** | ||||
| CW Aegean | * | * | |||||||||
| SW Aegean | * | * | ** | ** | |||||||
| N Ionian | * | * | * | ||||||||
| S Ionian | * | ** | ** | ** | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragkousis, M.; Sini, M.; Koukourouvli, N.; Zenetos, A.; Katsanevakis, S. Invading the Greek Seas: Spatiotemporal Patterns of Marine Impactful Alien and Cryptogenic Species. Diversity 2023, 15, 353. https://doi.org/10.3390/d15030353
Ragkousis M, Sini M, Koukourouvli N, Zenetos A, Katsanevakis S. Invading the Greek Seas: Spatiotemporal Patterns of Marine Impactful Alien and Cryptogenic Species. Diversity. 2023; 15(3):353. https://doi.org/10.3390/d15030353
Chicago/Turabian StyleRagkousis, Michail, Maria Sini, Nikoletta Koukourouvli, Argyro Zenetos, and Stelios Katsanevakis. 2023. "Invading the Greek Seas: Spatiotemporal Patterns of Marine Impactful Alien and Cryptogenic Species" Diversity 15, no. 3: 353. https://doi.org/10.3390/d15030353
APA StyleRagkousis, M., Sini, M., Koukourouvli, N., Zenetos, A., & Katsanevakis, S. (2023). Invading the Greek Seas: Spatiotemporal Patterns of Marine Impactful Alien and Cryptogenic Species. Diversity, 15(3), 353. https://doi.org/10.3390/d15030353







