Roving Diver Survey as a Rapid and Cost-Effective Methodology to Register Species Richness in Sub-Antarctic Kelp Forests
Abstract
1. Introduction
2. Materials and Methods
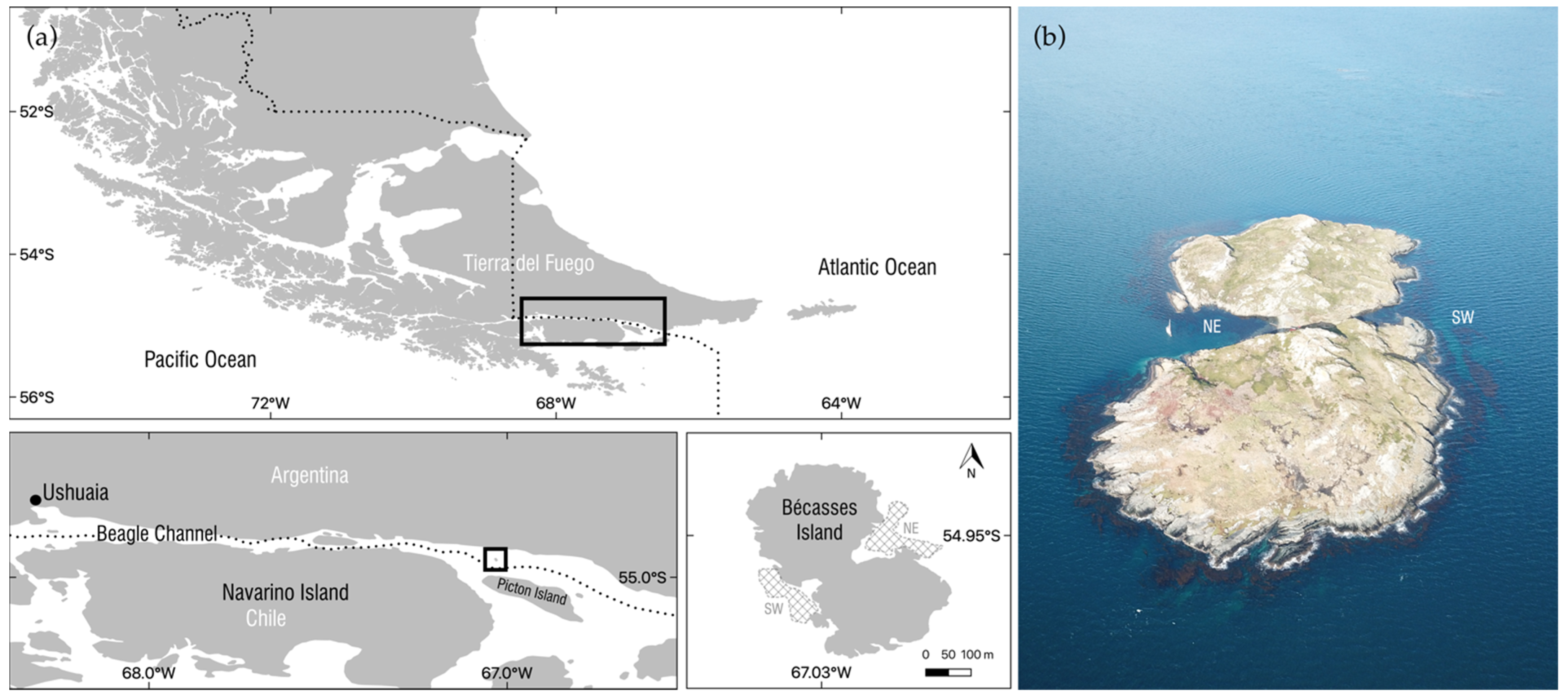
2.1. Study Site
2.2. Survey Method
2.3. Data Preparation and Quality Control
3. Results
3.1. Environment
3.2. Bécasses Checklist
3.3. Comparison with Other Studies
4. Discussion
4.1. Limitations of the Roving Diver Survey Methodology
4.2. Why Have We Found More “Unique” Species Than in Previous Studies?
4.3. Recommendations for Applying the Roving Diver Survey
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miloslavich, P.; Bax, N.J.; Simmons, S.E.; Klein, E.; Appeltans, W.; Aburto-Oropeza, O.; Andersen Garcia, M.; Batten, S.D.; Benedetti-Cecchi, L.; Checkley, D.M.; et al. Essential ocean variables for global sustained observations of biodiversity and ecosystem changes. Glob. Chang. Biol. 2018, 24, 2416–2433. [Google Scholar] [CrossRef]
- Heberling, J.M.; Isaac, B.L. iNaturalist as a tool to expand the research value of museum specimens. Appl. Plant Sci. 2018, 6, e01193. [Google Scholar] [CrossRef]
- Boone, M.E.; Basille, M. Using iNaturalist to contribute your nature observations to science. EDIS 2019, 2019, 5. [Google Scholar] [CrossRef]
- Hewitt, S.; Picton, B.; DuPont, A.; Zahner, T.; Salvador, R. New records of marine molluscs from Saba, Caribbean Netherlands. Basteria 2021, 85, 59–72. [Google Scholar]
- Rosa, R.M.; Cavallari, D.C.; Salvador, R.B. iNaturalist as a tool in the study of tropical molluscs. PLoS ONE 2022, 17, e0268048. [Google Scholar] [CrossRef]
- Miloslavich, P.; Klein, E.; Díaz, J.M.; Hernández, C.E.; Bigatti, G.; Campos, L.; Artigas, F.; Castillo, J.; Penchaszadeh, P.E.; Neill, P.E.; et al. Marine biodiversity in the Atlantic and Pacific coasts of South America: Knowledge and gaps. PLoS ONE 2011, 6, e14631. [Google Scholar] [CrossRef]
- Bigatti, G.; Signorelli, J. Marine invertebrate biodiversity from the Argentine Sea, South Western Atlantic. ZooKeys 2018, 791, 47–70. [Google Scholar] [CrossRef]
- Mora-Soto, A.; Palacios, M.; Macaya, E.C.; Gómez, I.; Huovinen, P.; Pérez-Matus, A.; Young, M.; Golding, N.; Toro, M.; Yaqub, M. A high-resolution global map of Giant kelp (Macrocystis pyrifera) forests and intertidal green algae (Ulvophyceae) with Sentinel-2 imagery. Remote Sens. 2020, 12, 694. [Google Scholar] [CrossRef]
- Santelices, B.; Ojeda, F. Effects of canopy removal on the understory algal community structure of coastal forests of Macrocystis pyrifera from southern South America. Mar. Ecol. Prog. Ser. 1984, 14, 165–173. [Google Scholar] [CrossRef]
- Adami, M.L.; Gordillo, S. Structure and dynamics of the biota associated with Macrocystis pyrifera (Phaeophyta) from the Beagle Channel, Tierra del Fuego. Sci. Mar. 1999, 63, 183–191. [Google Scholar] [CrossRef]
- Vanella, F.A.; Fernández, D.A.; Carolina Romero, M.; Calvo, J. Changes in the fish fauna associated with a sub-Antarctic Macrocystis pyrifera kelp forest in response to canopy removal. Polar Biol. 2007, 30, 449–457. [Google Scholar] [CrossRef]
- Hockey, P.A.R. Kelp gulls Larus dominicanus as predators in kelp Macrocystis pyrifera beds. Oecologia 1988, 76, 155–157. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, C.; Diez, M.J.; Torres, M.A.; Dellabianca, N.A. Habitat use of Peale’s dolphin Lagenorhynchus australis in the Beagle Channel, Tierra del Fuego, Argentina. Mastozool. Neotropical 2020, 27, 247–252. [Google Scholar] [CrossRef]
- Ojeda, F.; Santelices, B. Invertebrate communities in holdfasts of the kelp Macrocystis pyrifera from southern Chile. Mar. Ecol. Prog. Ser. 1984, 16, 65–73. [Google Scholar] [CrossRef]
- Vásquez, J.; Castilla, J.; Santelices, B. Distributional patterns and diets of four species of sea urchins in giant kelp forest (Macrocystis pyrifera) of Puerto Toro, Navarino Island, Chile. Mar. Ecol. Prog. Ser. 1984, 19, 55–63. [Google Scholar] [CrossRef]
- Dayton, P.K. The Structure and Regulation of Some South American Kelp Communities. Ecol. Monogr. 1985, 55, 447–468. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Ballesteros, E.; Bell, T.W.; Giddens, J.; Henning, B.; Hüne, M.; Muñoz, A.; Salinas-de-León, P.; Sala, E. Marine biodiversity at the end of the world: Cape Horn and Diego Ramírez islands. PLoS ONE 2018, 13, e0189930. [Google Scholar] [CrossRef]
- Beaton, E.C.; Küpper, F.C.; van West, P.; Brewin, P.E.; Brickle, P. The influence of depth and season on the benthic communities of a Macrocystis pyrifera forest in the Falkland Islands. Polar Biol. 2020, 43, 573–586. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Ballesteros, E.; Bell, T.W.; Caselle, J.E.; Campagna, C.; Goodell, W.; Hüne, M.; Muñoz, A.; Salinas-de-León, P.; Sala, E.; et al. Kelp forests at the end of the earth: 45 years later. PLoS ONE 2020, 15, e0229259. [Google Scholar] [CrossRef]
- Cárdenas, C.A.; Montiel, A. The influence of depth and substrate inclination on sessile assemblages in subantarctic rocky reefs (Magellan region). Polar Biol. 2015, 38, 1631–1644. [Google Scholar] [CrossRef]
- Friedlander, A.M.; Ballesteros, E.; Goodell, W.; Hüne, M.; Muñoz, A.; Salinas-de-León, P.; Velasco-Charpentier, C.; Sala, E. Marine communities of the newly created Kawésqar National Reserve, Chile: From glaciers to the Pacific Ocean. PLoS ONE 2021, 16, e0249413. [Google Scholar] [CrossRef] [PubMed]
- Ríos, C.; Arntz, W.E.; Gerdes, D.; Mutschke, E.; Montiel, A. Spatial and temporal variability of the benthic assemblages associated to the holdfasts of the kelp Macrocystis pyrifera in the Straits of Magellan, Chile. Polar Biol. 2007, 31, 89–100. [Google Scholar] [CrossRef]
- Wartenberg, R. On the Underwater Visual Census of Western Indian Ocean Coral Reef Fishes. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 2012. [Google Scholar]
- Rassweiler, A.; Dubel, A.K.; Hernan, G.; Kushner, D.J.; Caselle, J.E.; Sprague, J.L.; Kui, L.; Lamy, T.; Lester, S.E.; Miller, R.J. Roving divers surveying fish in fixed areas capture similar patterns in biogeography but different estimates of density when compared with belt transects. Front. Mar. Sci. 2020, 7, 272. [Google Scholar] [CrossRef]
- Schmitt, E.; Sluka, R.; Sullivan-Sealey, K. Evaluating the use of roving diver and transect surveys to assess the coral reef fish assemblage off southeastern Hispaniola. Coral Reefs 2002, 21, 216–223. [Google Scholar] [CrossRef]
- Leite, T.S.; Haimovici, M.; Mather, J.; Oliveira, J.E.L. Habitat, distribution, and abundance of the commercial octopus (Octopus insularis) in a tropical oceanic island, Brazil: Information for management of an artisanal fishery inside a marine protected area. Fish. Res. 2009, 98, 85–91. [Google Scholar] [CrossRef]
- Sabdono, A.; Radjasa, O.K.; Trianto, A.; Sibero, M.T.; Kristiana, R.; Larasati, S.J.H. Comparative assessment of gorgonian abundance and diversity among Islands with different anthropogenic stressors in Karimunjawa Marine National Park, Java Sea. Int. J. Conserv. Sci. 2022, 13, 341–348. [Google Scholar]
- Iachetti, C.M.; Lovrich, G.; Alder, V.A. Temporal variability of the physical and chemical environment, chlorophyll and diatom biomass in the euphotic zone of the Beagle Channel (Argentina): Evidence of nutrient limitation. Prog. Oceanogr. 2021, 195, 102576. [Google Scholar] [CrossRef]
- Rabassa, J.; Coronato, A.; Bujalesky, G.; Salemme, M.; Roig, C.; Meglioli, A.; Heusser, C.; Gordillo, S.; Roig, F.; Borromei, A. Quaternary of Tierra del Fuego, southernmost South America: An updated review. Quat. Int. 2000, 68, 217–240. [Google Scholar] [CrossRef]
- Isla, F.; Bujalesky, G.; Coronato, A. Procesos estuáricos en el Canal Beagle, Tierra del Fuego. Rev. Asoc. Geol. Argent. 1999, 54, 307–318. [Google Scholar]
- Bujalesky, G.G. La Inundación del Valle Beagle (11.000 AÑOS AP), Tierra del Fuego. An. Inst. Patagon. 2011, 39, 5–21. [Google Scholar]
- Ponce, J.F.; Fernández, M. Climatic and Environmental History of Isla de los Estados, Argentina; Springer Briefs in Earth System Sciences; Springer: Dordrecht, The Netherlands, 2014; ISBN 978-94-007-4362-5. [Google Scholar]
- Giesecke, R.; Martín, J.; Piñones, A.; Höfer, J.; Garcés-Vargas, J.; Flores-Melo, X.; Alarcón, E.; Durrieu de Madron, X.; Bourrin, F.; González, H.E. General Hydrography of the Beagle Channel, a Subantarctic Interoceanic Passage at the Southern Tip of South America. Front. Mar. Sci. 2021, 8, 621822. [Google Scholar] [CrossRef]
- Almandoz, G.O.; Hernando, M.P.; Ferreyra, G.A.; Schloss, I.R.; Ferrario, M.E. Seasonal phytoplankton dynamics in extreme southern South America (Beagle channel, Argentina). J. Sea Res. 2011, 66, 47–57. [Google Scholar] [CrossRef]
- Iturraspe, R.; Sottini, R.; Schroeder, C.; Escobar, J. Hidrología y variables climáticas del Territorio de Tierra del Fuego. Información básica. Contrib. Cient. CADIC 1989, 7, 196. [Google Scholar]
- Balestrini, C.; Manzella, G.; Lovrich, G.A. Simulación de corrientes en el Canal Beagle y Bahía Ushuaia, mediante un modelo bidimensional. Serv. Hidrogr. Nav. 1998, 98, 1–58. [Google Scholar]
- Flores Melo, X.; Martín, J.; Kerdel, L.; Bourrin, F.; Colloca, C.B.; Menniti, C.; de Madron, X.D. Particle dynamics in Ushuaia Bay (Tierra del Fuego)-Potential effect on dissolved oxygen depletion. Water 2020, 12, 324. [Google Scholar] [CrossRef]
- Harris, S.; Samaniego, R.A.S.; Rey, A.R. Insights into diet and foraging behavior of imperial shags (Phalacrocorax atriceps) breeding at Staten and Becasses Islands, Tierra del Fuego, Argentina. Wilson J. Ornithol. 2016, 128, 811–820. [Google Scholar] [CrossRef]
- Schiavini, A.C.M.; Crespo, E.A.; Szapkievich, V. Status of the population of South American sea lion (Otaria flavescens Shaw, 1800) in southern Argentina. Mamm. Biol. 2004, 69, 108–118. [Google Scholar] [CrossRef]
- Bravo, G.; Kaminsky, J.; Bagur, M.; Alonso, C.P.; Rodríguez, M. INaturalist Project: “Biodiversidad Submarina Islas Bécasses”. Available online: https://www.inaturalist.org/projects/biodiversidad-submarina-islas-becasses (accessed on 10 December 2022).
- Ahyong, S.; Boyko, C.B.; Bailly, N.; Bernot, J.; Bieler, R.; Brandão, S.N.; Daly, M.; De Grave, S.; Gofas, S.; Hernandez, F.; et al. World Register of Marine Species (WoRMS). Available online: https://www.marinespecies.org (accessed on 10 December 2022).
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 10 December 2022).
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: www.fishbase.org (accessed on 10 December 2022).
- Häussermann, V.; Molinet, C.; Díaz Gómez, M.; Försterra, G.; Henríquez, J.; Espinoza Cea, K.; Matamala Ascencio, T.; Hüne, M.; Cárdenas, C.A.; Glon, H.; et al. Recent massive invasions of the circumboreal sea anemone Metridium senile in North and South Patagonia. Biol. Invasions 2022, 24, 3665–3674. [Google Scholar] [CrossRef]
- Fraysse, C.; Boy, C.; Ojeda, M.; Rodriguez, M.; Pérez, A. Distribution and development patterns in Asteroidea of the Southern Atlantic, including marine protected areas, Argentina. 2023; to be submitted. [Google Scholar]
- Willenz, P.; Hajdu, E.; Desqueyroux-Faúndez, R.; Lôbo-Hajdu, G.; Carvalho, M. Porifera-Sponges. In Marine Benthic Fauna of Chilean Patagonia; Nature in Focus: Santiago, Chile, 2009; Volume 1000, pp. 93–94. [Google Scholar]
- Ojeda, J.; Marambio, J.; Rosenfeld, S.; Contador, T.; Rozzi, R.; Mansilla, A. Seasonal changes of macroalgae assemblages on the rocky shores of the Cape Horn Biosphere Reserve, Sub-Antarctic Channels, Chile. Aquat. Bot. 2019, 157, 33–41. [Google Scholar] [CrossRef]
- Kaminsky, J.; Bagur, M.; Boraso, A.; Schloss, I.R.; Quartino, M.L. Centro Austral de Investigaciones Científicas (CADIC-CONICET), Ushuaia, Argentina. 2023; manuscript in preparation. [Google Scholar]
- Mendoza, M.L. State of knowledge of the Corallinales (Rhodophyta) of Tierra del Fuego and the Antarctic Peninsula. Sci. Mar. 1999, 63, 139–144. [Google Scholar] [CrossRef]
- Noss, R.F. Indicators for Monitoring Biodiversity: A Hierarchical Approach. Conserv. Biol. 1990, 4, 355–364. [Google Scholar] [CrossRef]
- Costello, M.J.; Tsai, P.; Wong, P.S.; Cheung, A.K.L.; Basher, Z.; Chaudhary, C. Marine biogeographic realms and species endemicity. Nat. Commun. 2017, 8, 1057. [Google Scholar] [CrossRef]
- Alonso, G.M. Amphipod crustaceans (Corophiidea and Gammaridea) associated with holdfasts of Macrocystis pyrifera from the Beagle Channel (Argentina) and additional records from the Southwestern Atlantic. J. Nat. Hist. 2012, 46, 1799–1894. [Google Scholar] [CrossRef]
- Häussermann, V.; Försterra, G. Marine Benthic Fauna of Chilean Patagonia; Nature in Focus: Santiago, Chile, 2009; Volume 1000. [Google Scholar]
- GBIF Secretariat; GBIF Backbone Taxonomy. Checklist Dataset. via GBIF.org. Available online: https://doi.org/10.15468/39omei (accessed on 10 July 2022).
- Rosenfeld, S.; Ojeda, J.; Hüne, M.; Mansilla, A.; Contador, T. Egg masses of the Patagonian squid Doryteuthis (Amerigo) gahi attached to giant kelp (Macrocystis pyrifera) in the sub-Antarctic ecoregion. Polar Res. 2014, 33, 21636. [Google Scholar] [CrossRef]
- Küpper, F.C.; Peters, A.F.; Kytinou, E.; Asensi, A.O.; Vieira, C.; Macaya, E.C.; De Clerck, O. Dictyota falklandica sp. nov. (Dictyotales, Phaeophyceae) from the Falkland Islands and southernmost South America. Phycologia 2019, 58, 640–647. [Google Scholar] [CrossRef]
- Lauretta, D.; Häussermann, V.; Brugler, M.R.; Rodríguez, E. Isoparactis fionae sp. nov. (Cnidaria: Anthozoa: Actiniaria) from Southern Patagonia with a discussion of the family Isanthidae. Org. Divers. Evol. 2014, 14, 31–42. [Google Scholar] [CrossRef]
- Cetra, N.; Gregoric, D.E.G.; Roche, A. A New Species of Placida (Gastropoda: Sacoglossa) from Southern South America. Malacologia 2021, 64, 109–120. [Google Scholar] [CrossRef]
- Asensi, A.; De Reviers, B. Illustrated catalogue of types of species historically assigned to Lessonia (Laminariales, Phaeophyceae) preserved at PC, including a taxonomic study of three South-American species with a description of L. searlesiana sp. nov. and a new lectotypification of L. flavicans. Cryptogam. Algol. 2009, 30, 209–249. [Google Scholar]
- UWIS Underwater Navigation System. Available online: https://uwis.fi/en (accessed on 15 July 2022).
- Hiscock, K. Marine Nature Conservation Review: Rationale and Methods; Coasts and Seas of the United Kingdom. MNCR Series; Joint Nature Conservation Committee: Peterborough, UK, 1996; ISBN 1-86107-410-7.
- Semmens, B.X.; Auster, P.J.; Paddack, M.J. Using Ecological Null Models to Assess the Potential for Marine Protected Area Networks to Protect Biodiversity. PLoS ONE 2010, 5, e8895. [Google Scholar] [CrossRef] [PubMed]
- Auster, P.J.; Semmens, B.X.; Barber, K. Pattern in the Co-Occurrence of Fishes Inhabiting the Coral Reefs of Bonaire, Netherlands Antilles. Environ. Biol. Fishes 2005, 74, 187–194. [Google Scholar] [CrossRef]
- Roberts, C.J.; Vergés, A.; Callaghan, C.T.; Poore, A.G.B. Many cameras make light work: Opportunistic photographs of rare species in iNaturalist complement structured surveys of reef fish to better understand species richness. Biodivers. Conserv. 2022, 31, 1407–1425. [Google Scholar] [CrossRef]


| Phylum | Class | Order | Family | Genus | Species | N (this study) | Beaton et al. 2020 [18] | Cárdenas and Montiel 2015 [20] | Friedlander et al. 2018 [17] | Friedlander et al. 2020 [19] | Friedlander et al. 2021 [21] | Santelices and Ojeda 1984 [9] | Vanella et al. 2007 [11] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Porifera | Calcarea | Leucosolenida | Syconidae | Sycon | 2 | * | * | * | |||||
| Demospongiae | 2 | ||||||||||||
| Haplosclerida | Chalinidae | Haliclona | 1 | * | * | * | * | ||||||
| Poecilosclerida | Hymedesmiidae | Phorbas | Phorbas ferrugineus | 9 | * | * | |||||||
| Cnidaria | Anthozoa | Actiniaria | 2 | * | * | ||||||||
| Actiniidae | Bunodactis | Bunodactis octoradiata | 1 | * | * | * | * | * | |||||
| Actinostolidae | Actinostola | 3 | |||||||||||
| Actinostolidae | Antholoba | Antholoba achates | 4 | * | * | * | |||||||
| Halcuriidae | Halcurias | 4 | |||||||||||
| Isanthidae | Isoparactis | Isoparactis fionae | 1 | ||||||||||
| Metridiidae | Metridium | Metridium senile | 24 | * | * | ||||||||
| Preactiniidae | Dactylanthus | Dactylanthus antarcticus | 4 | ||||||||||
| Alcyonacea | Alcyoniidae | Alcyonium | Alcyonium haddoni | 6 | |||||||||
| Clavulariidae | Incrustatus | 1 | |||||||||||
| Primnoidae | Primnoella | 4 | |||||||||||
| Primnoidae | Primnoella | Primnoella chilensis | 1 | * | * | ||||||||
| Hydrozoa | Leptothecata | Campanulariidae | Obelia | Obelia geniculata | 2 | * | * | * | |||||
| Staurozoa | Stauromedusae | Haliclystidae | Haliclystus | Haliclystus antarcticus | 1 | * | |||||||
| Annelida | Polychaeta | Chaetopteridae | Chaetopterus | 1 | |||||||||
| Chaetopteridae | Chaetopterus | Chaetopterus variopedatus | 1 | * | * | * | * | * | |||||
| Phyllodocida | Nereididae | 1 | |||||||||||
| Phyllodocidae | Eulalia | 1 | * | ||||||||||
| Phyllodocidae | 3 | ||||||||||||
| Polynoidae | 1 | ||||||||||||
| Terebellida | Cirratulidae | 1 | |||||||||||
| Mollusca | Bivalvia | Mytilida | Mytilidae | Aulacomya | Aulacomya atra | 3 | * | * | |||||
| Pectinida | Pectinidae | Austrochlamys | Austrochlamys natans | 2 | |||||||||
| Cephalopoda | Myopsida | Loliginidae | Doryteuthis | Doryteuthis gahi | 10 | ||||||||
| Octopoda | Enteroctopodidae | Enteroctopus | Enteroctopus megalocyathus | 4 | |||||||||
| Gastropoda | Limapontiidae | Placida | Placida sudamericana | 1 | |||||||||
| Nacellidae | Nacella | 2 | * | ||||||||||
| Nacellidae | Nacella | Nacella deaurata | 2 | ||||||||||
| Nacellidae | Nacella | Nacella mytilina | 3 | * | * | * | * | ||||||
| Plakobranchidae | Elysia | Elysia patagonica | 2 | ||||||||||
| Lepetellida | Fissurellidae | Fissurella | 1 | * | * | * | |||||||
| Fissurellidae | Fissurella | Fissurella oriens | 4 | * | |||||||||
| Fissurellidae | Fissurella | Fissurella picta | 1 | ||||||||||
| Littorinimorpha | Calyptraeidae | Crepidula | 1 | ||||||||||
| Calyptraeidae | Crepipatella | Crepipatella dilatata | 2 | * | * | ||||||||
| Cymatiidae | Fusitriton | Fusitriton magellanicus | 9 | * | * | * | |||||||
| Velutinidae | Lamellaria | 1 | * | * | |||||||||
| Neogastropoda | Cominellidae | Pareuthria | Pareuthria fuscata | 1 | * | * | * | * | |||||
| Muricidae | 1 | ||||||||||||
| Muricidae | Acanthina | Acanthina monodon | 1 | * | * | * | |||||||
| Muricidae | Trophon | Trophon geversianus | 1 | * | * | * | * | ||||||
| Volutidae | Adelomelon | Adelomelon ancilla | 6 | * | * | * | |||||||
| Volutidae | Odontocymbiola | Odontocymbiola magellanica | 6 | * | |||||||||
| Nudibranchia | Chromodorididae | Tyrinna | Tyrinna delicata | 3 | * | * | |||||||
| Coryphellidae | Coryphella | Coryphella falklandica | 5 | * | |||||||||
| Discodorididae | Diaulula | Diaulula hispida | 1 | * | * | * | |||||||
| Discodorididae | Diaulula | Diaulula punctuolata | 2 | * | * | * | |||||||
| Dorididae | Doris | Doris fontainii | 3 | * | * | * | |||||||
| Polyceridae | Thecacera | Thecacera darwini | 4 | * | * | * | |||||||
| Tritoniidae | Tritonia | Tritonia challengeriana | 10 | * | * | ||||||||
| Tritoniidae | Tritonia | Tritonia odhneri | 3 | ||||||||||
| Tritoniidae | Tritonia | Tritonia vorax | 1 | ||||||||||
| Pleurobranchida | Pleurobranchidae | Berthella | Berthella platei | 4 | * | ||||||||
| Trochida | 1 | ||||||||||||
| Calliostomatidae | Calliostoma | 1 | |||||||||||
| Calliostomatidae | Margarella | Margarella violacea | 3 | * | * | * | * | ||||||
| Polyplacophora | Chitonida | Chitonidae | Tonicia | 8 | * | * | |||||||
| Chitonidae | Chiton | Chiton magnificus | 3 | * | * | * | |||||||
| Chitonidae | Tonicia | Tonicia chilensis | 1 | * | |||||||||
| Chitonidae | Tonicia | Tonicia disjuncta | 5 | * | |||||||||
| Mopaliidae | Plaxiphora | Plaxiphora aurata | 2 | * | * | * | * | ||||||
| Arthropoda | Hexanauplia | 2 | * | * | |||||||||
| Balanomorpha | Balanidae | Austromegabalanus | Austromegabalanus psittacus | 4 | * | * | * | * | |||||
| Malacostraca | Amphipoda | Ampithoidae | 1 | ||||||||||
| Gammarellidae | cf. Austroregia | 2 | |||||||||||
| cf. Gammarellidae | 2 | ||||||||||||
| Decapoda | Campylonotidae | Campylonotus | Campylonotus vagans | 11 | * | * | * | ||||||
| Hippolytidae | Nauticaris | Nauticaris magellanica | 5 | * | * | * | * | ||||||
| Hymenosomatidae | Halicarcinus | Halicarcinus planatus | 2 | * | * | * | * | ||||||
| Inachidae | Eurypodius | Eurypodius longirostris | 7 | ||||||||||
| Lithodidae | Paralomis | Paralomis granulosa | 10 | * | * | * | * | ||||||
| Munididae | Grimothea | Grimothea gregaria | 7 | * | * | * | |||||||
| Paguridae | Pagurus | Pagurus comptus | 2 | * | * | * | * | ||||||
| Trichopeltariidae | Peltarion | Peltarion spinulosum | 1 | * | * | * | |||||||
| Brachiopoda | Rhynchonellata | Terebratulida | Terebratellidae | Magellania | Magellania venosa | 3 | * | * | * | * | |||
| Bryozoa | 4 | * | * | * | * | * | |||||||
| Gymnolaemata | Cheilostomatida | Beaniidae | Beania | Beania magellanica | 1 | * | * | * | * | ||||
| Cellariidae | Cellaria | Cellaria malvinensis | 2 | * | * | * | * | * | |||||
| Alcyonidiidae | Alcyonidium | Alcyonidium australe | 1 | * | |||||||||
| Stenolaemata | Cyclostomatida | Crisiidae | Crisia | 3 | * | * | |||||||
| Echinodermata | Asteroidea | Forcipulatida | Asteriidae | 1 | |||||||||
| Asteriidae | Anasterias | Anasterias antarctica | 4 | * | * | * | * | ||||||
| Asteriidae | Diplasterias | Diplasterias brandti | 12 | ||||||||||
| Heliasteridae | Labidiaster | Labidiaster radiosus | 21 | * | * | * | * | ||||||
| Stichasteridae | Allostichaster | Allostichaster capensis | 1 | ||||||||||
| Stichasteridae | Cosmasterias | Cosmasterias lurida | 16 | * | * | * | * | ||||||
| Spinulosida | Echinasteridae | Henricia | Henricia obesa | 4 | * | * | * | ||||||
| Valvatida | 1 | ||||||||||||
| Asterinidae | Asterina | Asterina fimbriata | 1 | * | * | * | * | ||||||
| Asterinidae | Cycethra | Cycethra verrucosa | 8 | * | * | * | * | ||||||
| Asterinidae | Ganeria | Ganeria falklandica | 9 | * | * | * | |||||||
| Odontasteridae | 2 | ||||||||||||
| Odontasteridae | Diplodontias | Diplodontias singularis | 3 | * | * | ||||||||
| Odontasteridae | Odontaster | Odontaster penicillatus | 4 | * | * | * | * | ||||||
| Poraniidae | Glabraster | Glabraster antarctica | 25 | * | * | * | |||||||
| Poraniidae | Poraniopsis | Poraniopsis echinaster | 6 | * | * | ||||||||
| Echinoidea | Arbacioida | Arbaciidae | Arbacia | Arbacia dufresnii | 23 | * | * | * | * | ||||
| Camarodonta | Parechinidae | Loxechinus | Loxechinus albus | 24 | * | * | * | * | |||||
| Temnopleuridae | Pseudechinus | Pseudechinus magellanicus | 11 | * | * | * | * | ||||||
| Holothuroidea | Apodida | Chiridotidae | Chiridota | Chiridota pisanii | 2 | * | * | ||||||
| Dendrochirotida | Cucumariidae | Cladodactyla | Cladodactyla crocea | 4 | * | ||||||||
| Cucumariidae | Trachythyone | Trachythyone lechleri | 1 | ||||||||||
| Psolidae | Psolus | Psolus patagonicus | 2 | * | |||||||||
| Ophiuroidea | Amphilepidida | Amphiuridae | Ophiophragmus | Ophiophragmus chilensis | 1 | ||||||||
| Ophiactidae | Ophiactis | Ophiactis asperula | 3 | * | * | * | * | ||||||
| Euryalida | Gorgonocephalidae | Gorgonocephalus | Gorgonocephalus chilensis | 16 | |||||||||
| Ophiacanthida | Ophiomyxidae | Ophiomyxa | Ophiomyxa vivipara | 1 | * | * | * | * | |||||
| Chordata | Actinopterygii | Perciformes | Bovichtidae | Cottoperca | Cottoperca trigloides | 2 | * | * | * | ||||
| Harpagiferidae | Harpagifer | Harpagifer bispinis | 1 | * | * | ||||||||
| Liparidae | Careproctus | Careproctus pallidus | 1 | * | * | ||||||||
| Nototheniidae | Patagonotothen | 9 | * | * | * | ||||||||
| Nototheniidae | Paranotothenia | Paranotothenia magellanica | 2 | * | * | * | * | ||||||
| Nototheniidae | Patagonotothen | Patagonotothen tessellata | 1 | * | * | * | * | ||||||
| Zoarcidae | Dadyanos | Dadyanos insignis | 3 | * | |||||||||
| Ascidiacea | 3 | * | * | ||||||||||
| Aplousobranchia | Didemnidae | 3 | * | ||||||||||
| Holozoidae | Sycozoa | Sycozoa gaimardi | 2 | * | * | * | * | ||||||
| Polyclinidae | Aplidium | 3 | * | * | * | * | |||||||
| Polyclinidae | Aplidium | Aplidium fuegiense | 5 | * | * | * | * | ||||||
| Stolidobranchia | Molgulidae | Paramolgula | Paramolgula gregaria | 1 | * | ||||||||
| Pyuridae | Pyura | Pyura legumen | 3 | * | * | * | * | ||||||
| Styelidae | Cnemidocarpa | 1 | * | * | * | ||||||||
| Styelidae | Cnemidocarpa | Cnemidocarpa verrucosa | 1 | * | * | * | |||||||
| Styelidae | Polyzoa | Polyzoa opuntia | 6 | * | * | * | |||||||
| Rhodophyta | Florideophyceae | Balliales | Balliaceae | Ballia | Ballia callitricha | 3 | * | * | |||||
| Bonnemaisoniales | Bonnemaisoniaceae | Ptilonia | Ptilonia magellanica | 9 | * | ||||||||
| Ceramiales | Delesseriaceae | Paraglossum | 1 | ||||||||||
| Delesseriaceae | Cladodonta | Cladodonta lyallii | 1 | ||||||||||
| Delesseriaceae | Hymenena | Hymenena falklandica | 2 | ||||||||||
| Delesseriaceae | Pseudophycodrys | Pseudophycodrys phyllophora | 6 | ||||||||||
| Rhodomelaceae | Lophurella | Lophurella hookeriana | 1 | ||||||||||
| Rhodomelaceae | Picconiella | Picconiella pectinata | 4 | ||||||||||
| Wrangeliaceae | Griffithsia | 1 | * | ||||||||||
| Corallinales | 3 | ||||||||||||
| Corallinaceae | Ellisolandia | Ellisolandia elongata | 1 | ||||||||||
| Gigartinales | 1 | ||||||||||||
| Gigartinaceae | Sarcopeltis | Sarcopeltis skottsbergii | 11 | * | * | ||||||||
| Kallymeniaceae | Callophyllis | Callophyllis atrosanguinea | 5 | ||||||||||
| Kallymeniaceae | Callophyllis | Callophyllis variegata | 2 | * | |||||||||
| Hildenbrandiales | Hildenbrandiaceae | Hildenbrandia | 3 | ||||||||||
| Plocamiales | Plocamiaceae | Plocamium | Plocamium secundatum | 1 | * | ||||||||
| Rhodymeniales | Rhodymeniaceae | Rhodymenia | Rhodymenia coccocarpa | 2 | |||||||||
| Rhodymeniaceae | Rhodymenia | Rhodymenia falklandica | 1 | * | |||||||||
| Ochrophyta | Phaeophyceae | Desmarestiales | Desmarestiaceae | Desmarestia | 5 | ||||||||
| Dictyotales | Dictyotaceae | Dictyota | Dictyota falklandica | 5 | |||||||||
| Ectocarpales | Adenocystaceae | Adenocystis | Adenocystis utricularis | 1 | * | * | |||||||
| Adenocystaceae | Caepidium | Caepidium antarcticum | 2 | * | |||||||||
| Scytosiphonaceae | Colpomenia | 1 | |||||||||||
| Laminariales | Laminariaceae | Macrocystis | Macrocystis pyrifera | 9 | * | * | * | * | * | * | |||
| Lessoniaceae | Lessonia | Lessonia flavicans | 9 | * | * | ||||||||
| Lessoniaceae | Lessonia | Lessonia searlesiana | 4 | ||||||||||
| Sphacelariales | Stypocaulaceae | Halopteris | 1 | ||||||||||
| Syringodermatales | Syringodermataceae | Microzonia | Microzonia velutina | 3 | |||||||||
| Chlorophyta | Ulvophyceae | Bryopsidales | Bryopsidaceae | Bryopsis | 1 | ||||||||
| Codiaceae | Codium | Codium subantarcticum | 8 | ||||||||||
| Ulvales | Ulvaceae | Ulva | 4 | * |
| Study | Geographic Area | Method | Depth Range (m) | Porifera | Cnidaria | Annelida | Mollusca | Arthropoda | Bryozoa | Echinodermata | Tunicates | others invertebrates | Fish | Macroalgae | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Santelices and Ojeda 1984 [9] | Puerto Toro, Navarino Island, Chile (55°) | Extractive quadrat in transects | 4–10 | 39 | 39 * | ||||||||||
| Vanella et al. 2007 [11] | Despard Island, Beagle Channel, Argentina (54°) | Trammel nets and holdfast removal | 6 | 11 | 11 * | ||||||||||
| Cárdenas and Montiel 2015 [20] | Santa Ana, Magellan Strait, Chile (53°) | Photoquadrats in vertical walls | 0–30 | 5 | 5 | 3 | 3 | 2 | 9 | 2 | 6 | 1 | 31 | 67 | |
| Friedlander et al. 2018 [17] | Francisco Coloane Reserve, Cape Horn, Diego Ramirez, Chile (53°–56°) | Visual transect survey | 7–15 | 13 | 12 | 2 | 31 | 15 | 8 | 20 | 11 | 2 | 14 | 2 * | 130 |
| Friedlander et al. 2020 [19] | Península Mitre and Isla de los Estados, Argentina (54°) | Visual transect survey | 3.7–17 | 20 | 14 | 4 | 33 | 14 | 10 | 28 | 14 | 1 | 21 | 3 * | 162 |
| Friedlander et al. 2021 [21] | Kawésqar Reserve, Chile (50°–54°) | Visual transect survey | 3.5–10 | 19 | 20 | 6 | 43 | 18 | 13 | 32 | 19 | 4 | 19 | 2 * | 195 |
| Beaton et al. 2020 [18] | Malvinas Islands, Argentina (51°) | Photoquadrats along transects | 5–20 | 19 | 11 | 8 | 31 | 9 | 2 | 21 | 13 | 2 | 2 * | 118 | |
| This study | Bécasses Island, Argentina (54°) | Roving diver survey | 2–33 | 4 | 14 | 7 | 40 | 13 | 5 | 27 | 10 | 1 | 7 | 32 | 160 |
| SD | 7.28 | 4.89 | 2.37 | 14.2 | 5.64 | 3.87 | 10.63 | 4.36 | 1.17 | 5.73 | 4.36 | 44.3 | |||
| CV | 54.64 | 38.57 | 47.33 | 47.09 | 47.63 | 49.39 | 49.08 | 35.8 | 63.77 | 39.77 | 12.82 | 31.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo, G.; Kaminsky, J.; Bagur, M.; Alonso, C.P.; Rodríguez, M.; Fraysse, C.; Lovrich, G.; Bigatti, G. Roving Diver Survey as a Rapid and Cost-Effective Methodology to Register Species Richness in Sub-Antarctic Kelp Forests. Diversity 2023, 15, 354. https://doi.org/10.3390/d15030354
Bravo G, Kaminsky J, Bagur M, Alonso CP, Rodríguez M, Fraysse C, Lovrich G, Bigatti G. Roving Diver Survey as a Rapid and Cost-Effective Methodology to Register Species Richness in Sub-Antarctic Kelp Forests. Diversity. 2023; 15(3):354. https://doi.org/10.3390/d15030354
Chicago/Turabian StyleBravo, Gonzalo, Julieta Kaminsky, María Bagur, Cecilia Paula Alonso, Mariano Rodríguez, Cintia Fraysse, Gustavo Lovrich, and Gregorio Bigatti. 2023. "Roving Diver Survey as a Rapid and Cost-Effective Methodology to Register Species Richness in Sub-Antarctic Kelp Forests" Diversity 15, no. 3: 354. https://doi.org/10.3390/d15030354
APA StyleBravo, G., Kaminsky, J., Bagur, M., Alonso, C. P., Rodríguez, M., Fraysse, C., Lovrich, G., & Bigatti, G. (2023). Roving Diver Survey as a Rapid and Cost-Effective Methodology to Register Species Richness in Sub-Antarctic Kelp Forests. Diversity, 15(3), 354. https://doi.org/10.3390/d15030354








