Fragmentation Level Drives Local Fish Assemblage Diversity Patterns in Fragmented River Basins
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Fieldwork
2.2.1. Fish Sampling
2.2.2. Local Environmental Variables
2.3. Characterization of Fragmentation Status and Local Barriers
2.4. Statistical Analyses
2.4.1. Local Diversity Analysis
2.4.2. Dispersion Capacity Analysis
2.4.3. Assemblage Structure Analyses
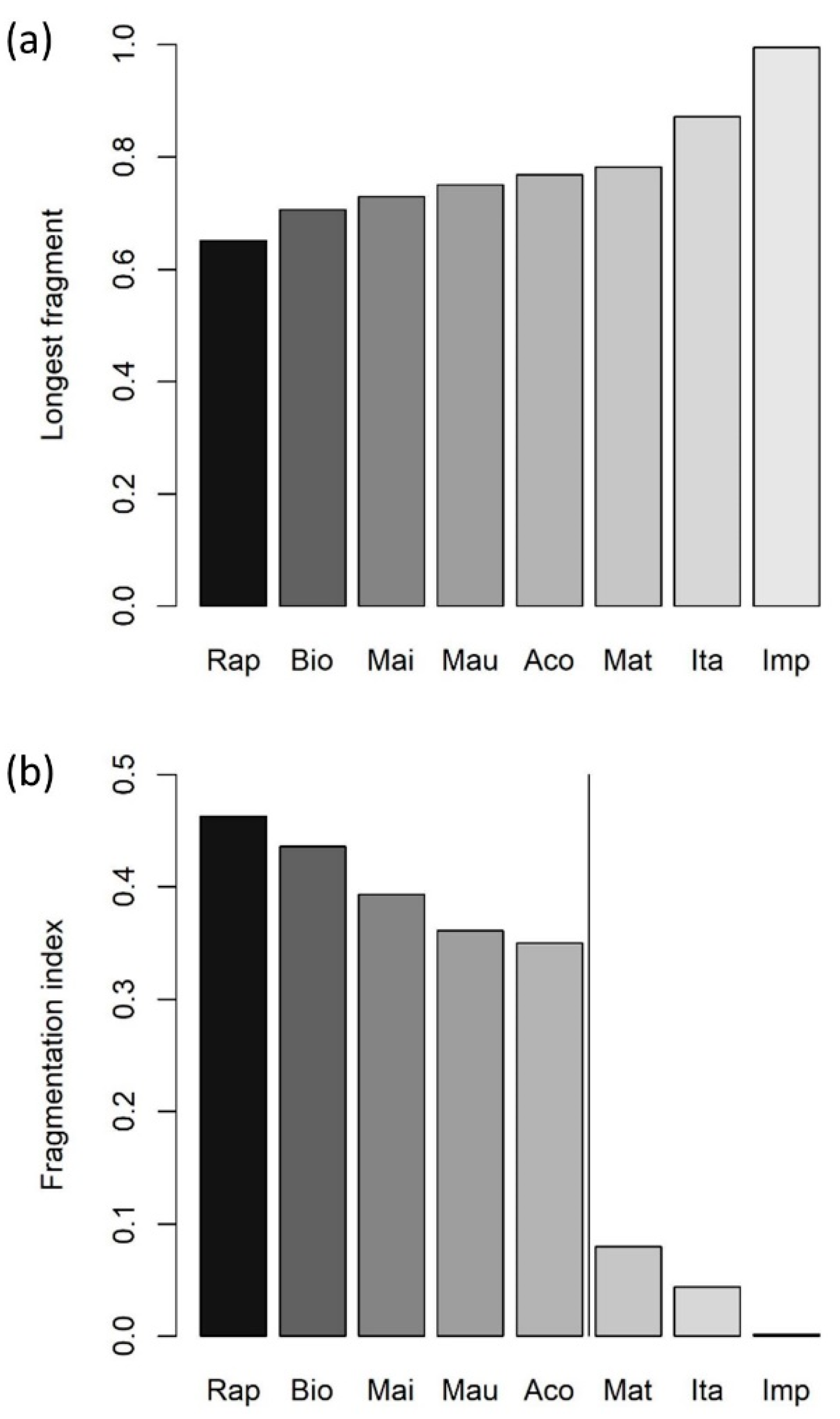
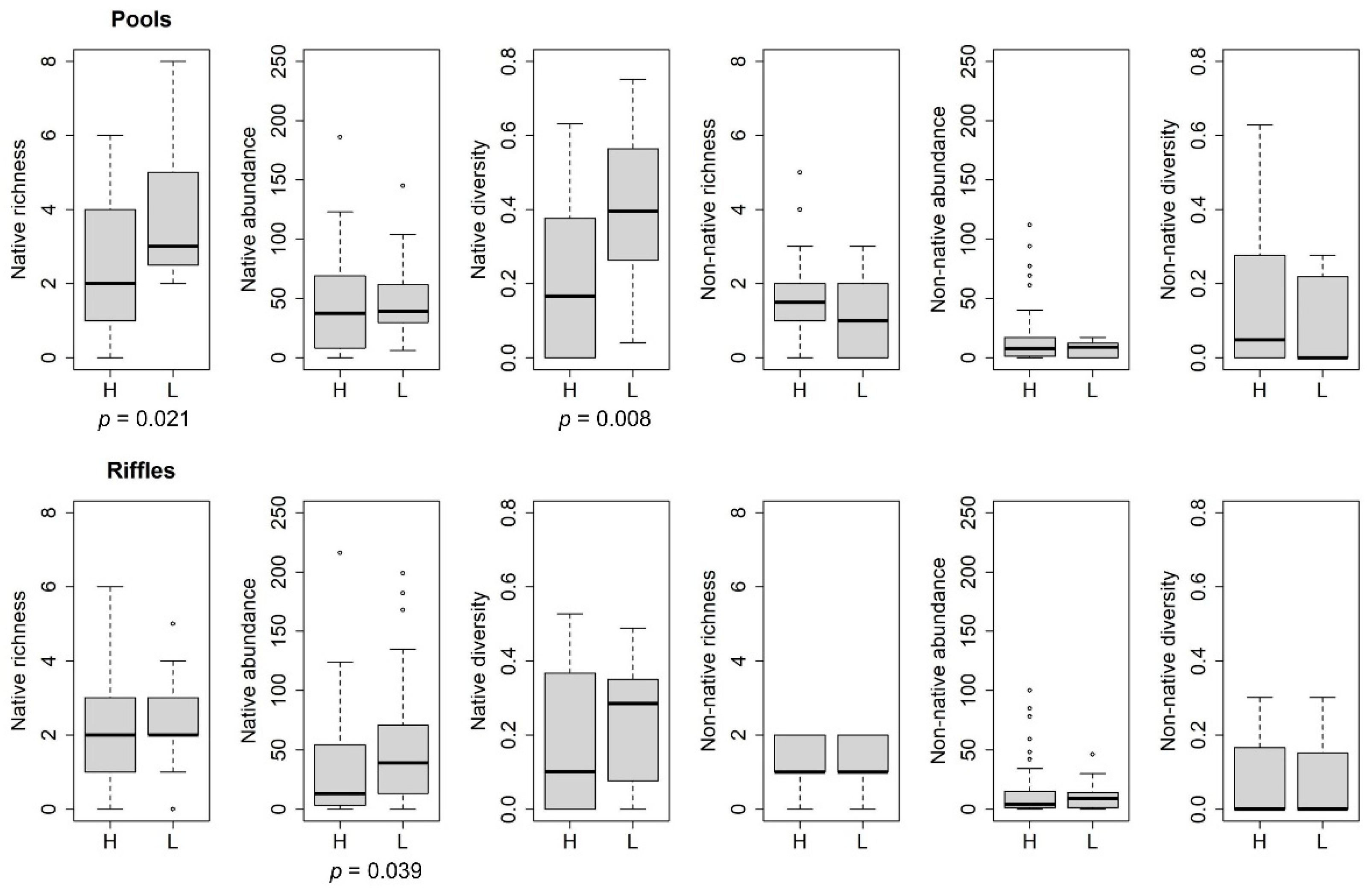
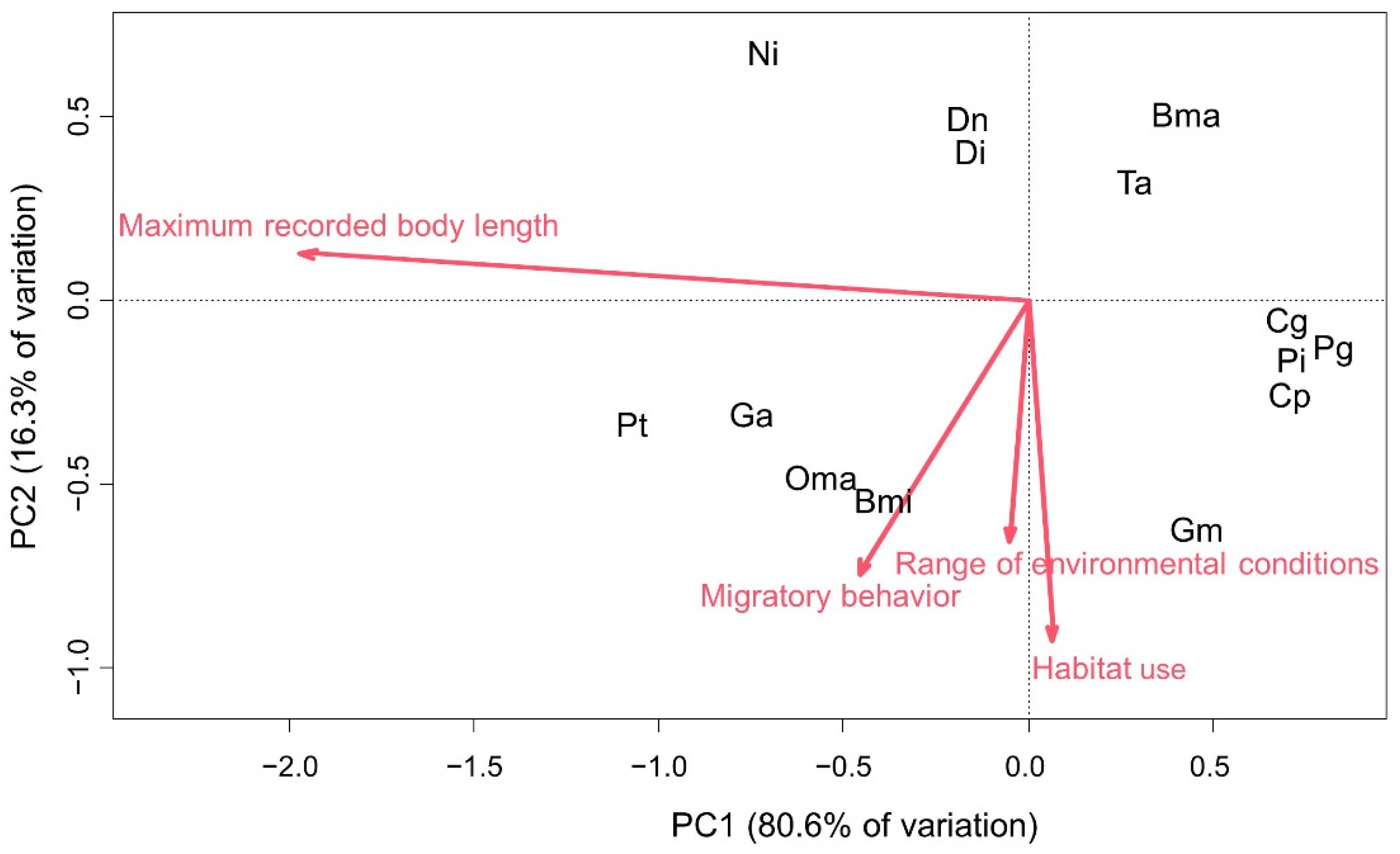
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strayer, D.L.; Dudgeon, D. Freshwater Biodiversity Conservation: Recent Progress and Future Challenges. J. N. Am. Benthol. Soc. 2010, 29, 344–358. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; McIntyre, P.B.; Gessner, M.O.; Dudgeon, D.; Prusevich, A.; Green, P.; Glidden, S.; Bunn, S.E.; Sullivan, C.A.; Liermann, C.R.; et al. Global Threats to Human Water Security and River Biodiversity. Nature 2010, 467, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Bunn, S.E.; Arthington, A.H. Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environ. Manag. 2002, 30, 492–507. [Google Scholar] [CrossRef] [PubMed]
- Thieme, M.L.; Tickner, D.; Grill, G.; Carvallo, J.P.; Goichot, M.; Hartmann, J.; Higgins, J.; Lehner, B.; Mulligan, M.; Nilsson, C.; et al. Navigating Trade-Offs between Dams and River Conservation. Glob. Sustain. 2021, 4, E17. [Google Scholar] [CrossRef]
- Couto, T.B.A.; Olden, J.D. Global Proliferation of Small Hydropower Plants—Science and Policy. Front. Ecol. Environ. 2018, 16, 91–100. [Google Scholar] [CrossRef]
- McCluney, K.E.; Poff, N.L.; Palmer, M.A.; Thorp, J.H.; Poole, G.C.; Williams, B.S.; Williams, M.R.; Baron, J.S. Riverine Macrosystems Ecology: Sensitivity, Resistance, and Resilience of Whole River Basins with Human Alterations. Front. Ecol. Environ. 2014, 12, 48–58. [Google Scholar] [CrossRef]
- Zarfl, C.; Lumsdon, A.E.; Berlekamp, J.; Tydecks, L.; Tockner, K. A Global Boom in Hydropower Dam Construction. Aquat. Sci. 2015, 77, 161–170. [Google Scholar] [CrossRef]
- Altermatt, F. Diversity in Riverine Metacommunities: A Network Perspective. Aquat. Ecol. 2013, 47, 365–377. [Google Scholar] [CrossRef]
- Carrara, F.; Altermatt, F.; Rodriguez-Iturbe, I.; Rinaldo, A. Dendritic Connectivity Controls Biodiversity Patterns in Experimental Metacommunities. Proc. Natl. Acad. Sci. USA 2012, 109, 5761–5766. [Google Scholar] [CrossRef]
- Fullerton, A.H.; Burnett, K.M.; Steel, E.A.; Flitcroft, R.L.; Pess, G.R.; Feist, B.E.; Torgersen, C.E.; Miller, D.J.; Sanderson, B.L. Hydrological Connectivity for Riverine Fish: Measurement Challenges and Research Opportunities. Freshw. Biol. 2010, 55, 2215–2237. [Google Scholar] [CrossRef]
- Heino, J. The Importance of Metacommunity Ecology for Environmental Assessment Research in the Freshwater Realm. Biol. Rev. 2013, 88, 166–178. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.; Meier, P.; Trautwein, C.; Schmid, M.; Robinson, C.T.; Weber, C.; Brodersen, J. Basin-Scale Effects of Small Hydropower on Biodiversity Dynamics. Front. Ecol. Environ. 2018, 16, 397–404. [Google Scholar] [CrossRef]
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish Conservation in Freshwater and Marine Realms: Status, Threats and Management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 838–857. [Google Scholar] [CrossRef]
- Benejam, L.; Saura-Mas, S.; Bardina, M.; Solà, C.; Munné, A.; García-Berthou, E. Ecological Impacts of Small Hydropower Plants on Headwater Stream Fish: From Individual to Community Effects. Ecol. Freshw. Fish 2016, 25, 295–306. [Google Scholar] [CrossRef]
- Fagan, W.F.; Aumann, C.; Kennedy, C.M.; Unmack, P.J. Rarity, Fragmentation, and the Scale Dependence of Extinction Risk in Desert Fishes. Ecology 2005, 86, 34–41. [Google Scholar] [CrossRef]
- van Puijenbroek, P.J.T.M.; Buijse, A.D.; Kraak, M.H.S.; Verdonschot, P.F.M. Species and River Specific Effects of River Fragmentation on European Anadromous Fish Species. River Res. Appl. 2019, 35, 68–77. [Google Scholar] [CrossRef]
- Clavero, M.; Hermoso, V. Reservoirs Promote the Taxonomic Homogenization of Fish Communities within River Basins. Biodivers. Conserv. 2011, 20, 41–57. [Google Scholar] [CrossRef]
- Liew, J.H.; Tan, H.H.; Yeo, D.C.J. Dammed Rivers: Impoundments Facilitate Fish Invasions. Freshw. Biol. 2016, 61, 1421–1429. [Google Scholar] [CrossRef]
- Díaz, G.; Górski, K.; Heino, J.; Arriagada, P.; Link, O.; Habit, E. The Longest Fragment Drives Fish Beta Diversity in Fragmented River Networks: Implications for River Management and Conservation. Sci. Total Environ. 2021, 766, 144323. [Google Scholar] [CrossRef]
- Legendre, P.; De Cáceres, M. Beta Diversity as the Variance of Community Data: Dissimilarity Coefficients and Partitioning. Ecol. Lett. 2013, 16, 951–963. [Google Scholar] [CrossRef]
- Perkin, J.S.; Gido, K.B. Fragmentation Alters Stream Fish Community Structure in Dendritic Ecological Networks. Ecol. Appl. 2012, 22, 2176–2187. [Google Scholar] [CrossRef]
- Holcomb, J.M.; Nichols, R.B.; Gangloff, M.M. Effects of Small Dam Condition and Drainage on Stream Fish Community Structure. Ecol. Freshw. Fish 2016, 25, 553–564. [Google Scholar] [CrossRef]
- Rolls, R.J.; Heino, J.; Chessman, B.C. Unravelling the Joint Effects of Flow Regime, Climatic Variability and Dispersal Mode on Beta Diversity of Riverine Communities. Freshw. Biol. 2016, 61, 1350–1364. [Google Scholar] [CrossRef]
- Habit, E.; García, A.; Díaz, G.; Arriagada, P.; Link, O.; Parra, O.; Thoms, M. River Science and Management Issues in Chile: Hydropower Development and Native Fish Communities. River Res. Appl. 2018, 35, 489–499. [Google Scholar] [CrossRef]
- Fierro, P.; Valdovinos, C.; Arismendi, I.; Díaz, G.; Ruiz De Gamboa, M.; Arriagada, L. Assessment of Anthropogenic Threats to Chilean Mediterranean Freshwater Ecosystems: Literature Review and Expert Opinions. Environ. Impact Assess. Rev. 2019, 77, 114–121. [Google Scholar] [CrossRef]
- Díaz, G.; Arriagada, P.; Górski, K.; Link, O.; Karelovic, B.; Gonzalez, J.; Habit, E. Fragmentation of Chilean Andean Rivers: Expected Effects of Hydropower Development. Rev. Chil. Hist. Nat. 2019, 92, 1. [Google Scholar] [CrossRef]
- Valenzuela-Aguayo, F.; McCracken, G.R.; Manosalva, A.; Habit, E.; Ruzzante, D.E. Human-Induced Habitat Fragmentation Effects on Connectivity, Diversity, and Population Persistence of an Endemic Fish, Percilia Irwini, in the Biobío River Basin (Chile). Evol. Appl. 2020, 13, 794–807. [Google Scholar] [CrossRef]
- Habit, E.; Zurita, A.; Díaz, G.; Manosalva, A.; Arriagada, P.; Link, O.; Górski, K. Latitudinal and Altitudinal Gradients of Riverine Landscapes in Andean Rivers. Water 2022, 14, 2614. [Google Scholar] [CrossRef]
- Vila, I.; Fuentes, L.; Contreras, M. Peces Límnicos de Chile. Boletín Del Mus. Nac. De Hist. Nat. 1999, 48, 61–75. [Google Scholar]
- Myers, N.; Mittermeler, R.A.; Mittermeler, C.G.; Da Fonseca, G.A.B.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish, 2nd ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 1999. [Google Scholar]
- Dyer, B.S. Revisión sistemática de los Pejerreyes de Chile (Teleostei, Atheriniforme). Estud. Oceanol. 2000, 19, 99–127. [Google Scholar]
- Ruiz, V.H.; Marchant, M. Ictiofauna de Aguas Continentales Chilenas; Universidad de Concepción, Facultad de Ciencias Naturales y Oceanográficas: Concepción, Chile, 2004. [Google Scholar]
- Salas, D.; Véliz, D.; Scott, S. Diferenciación Morfológica En Especies Del Género Cheirodon (Ostariophysi: Characidae) Mediante Morfometría Tradicional y Geométrica. Gayana 2012, 76, 142–152. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package Vegan: Community Ecology Package; CRAN: Vienna, Austria, 2013. [Google Scholar]
- Dolédec, S.; Chessel, D.; Gimaret-Carpentier, C. Niche Separation in Community Analysis: A New Method. Ecology 2000, 81, 2914–2927. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Legendre, P.; Andersson, M.J. Distance-Based Redundancy Analysis: Testing Multispecies Responses in Multifactorial Ecological Experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- R-Team Computers. R: A Language and Environment for Statistical Computing, Version 3.6. 1; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Fuller, M.R.; Doyle, M.W.; Strayer, D.L. Causes and Consequences of Habitat Fragmentation in River Networks. Ann. N. Y. Acad. Sci. 2015, 1355, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Rolls, R.J. The Role of Life-History and Location of Barriers to Migration in the Spatial Distribution and Conservation of Fish Assemblages in a Coastal River System. Biol. Conserv. 2011, 144, 339–349. [Google Scholar] [CrossRef]
- Rolls, R.J.; Stewart-Koster, B.; Ellison, T.; Faggotter, S.; Roberts, D.T. Multiple Factors Determine the Effect of Anthropogenic Barriers to Connectivity on Riverine Fish. Biodivers. Conserv. 2014, 23, 2201–2220. [Google Scholar] [CrossRef]
- Muñoz-Ramírez, C.P.; Victoriano, P.F.; Habit, E. Inter-Basin Dispersal through Irrigation Canals Explains Low Genetic Structure in Diplomystes Cf. Chilensis, an Endangered Freshwater Catfish from Central Chile. Limnologica 2015, 53, 10–16. [Google Scholar] [CrossRef]
- Oyanedel, A.; Habit, E.; Belk, M.C.; Solis-Lufí, K.; Colin, N.; Gonzalez, J.; Jara, A.; Muñoz-Ramírez, C.P. Movement Patterns and Home Range in Diplomystes camposensis (Siluriformes: Diplomystidae), an Endemic and Threatened Species from Chile. Neotrop. Ichthyol. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Victoriano, P.F.; Vera, I.; Olmos, V.; Dib, M.; Insunza, B.; Muñoz-Ramírez, C.; Montoya, R.; Jara, A.; Habit, E. Patrones Idiosincraticos de Diversidad Genética de Peces Nativos Del Río San Pedro (Cuenca Del Río Valdivia), Un Sistema de La Región Glaciada Del Sur de Chile. Gayana 2012, 76, 71–85. [Google Scholar] [CrossRef]
- Górski, K.; Habit, E.M.; Pingram, M.A.; Manosalva, A.J. Variation of the Use of Marine Resources by Galaxias Maculatus in Large Chilean Rivers. Hydrobiologia 2018, 814, 61–73. [Google Scholar] [CrossRef]
- Delgado, M.L.; Górski, K.; Habit, E.; Ruzzante, D.E. The Effects of Diadromy and Its Loss on Genomic Divergence: The Case of Amphidromous Galaxias Maculatus Populations. Mol. Ecol. 2019, 28, 5217–5231. [Google Scholar] [CrossRef]
- Ramírez-Álvarez, R.; Contreras, S.; Vivancos, A.; Reid, M.; López-Rodríguez, R.; Górski, K. Unpacking the Complexity of Longitudinal Movement and Recruitment Patterns of Facultative Amphidromous Fish. Sci. Rep. 2022, 12, 3164. [Google Scholar] [CrossRef]
- Valenzuela-Aguayo, F.; McCracken, G.R.; Diaz, G.; Manosalva, A.; Habit, E.; Ruzzante, D.E. Connectivity, Diversity, and Hybridization between Two Endemic Fish Species (Percilia Spp.) in a Complex Temperate Landscape. Conserv. Genet. 2022, 23, 23–33. [Google Scholar] [CrossRef]
- Vivancos, A.; Górski, K.; Manosalva, A.; Toledo, B.; Reid, M.; Habit, E. Hydrological Connectivity Drives Longitudinal Movement of Endangered Endemic Chilean Darter Percilia irwini (Eigenmann, 1927). J. Fish Biol. 2021, 98, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Loures, R.C.; Pompeu, P.S. Temporal Changes in Fish Diversity in Lotic and Lentic Environments along a Reservoir Cascade. Freshw. Biol. 2019, 64, 1806–1820. [Google Scholar] [CrossRef]
- Liu, X.; Olden, J.D.; Wu, R.; Ouyang, S.; Wu, X. Dam Construction Impacts Fish Biodiversity in a Subtropical River Network, China. Diversity 2022, 14, 476. [Google Scholar] [CrossRef]
- Consuegra, S.; O’Rorke, R.; Rodriguez-Barreto, D.; Fernandez, S.; Jones, J.; Garcia de Leaniz, C. Impacts of Large and Small Barriers on Fish Assemblage Composition Assessed Using Environmental DNA Metabarcoding. Sci. Total Environ. 2021, 790, 148054. [Google Scholar] [CrossRef]
- Falke, J.A.; Fausch, K.D. From Metapopulations to Metacommunities: Linking Theory with Empirical Observations of the Spatial Population Dynamics of Stream Fishes. Am. Fish. Soc. Symp. 2010, 73, 207–233. [Google Scholar]
- Kuriqi, A.; Pinheiro, A.N.; Sordo-Ward, A.; Bejarano, M.D.; Garrote, L. Ecological Impacts of Run-of-River Hydropower Plants—Current Status and Future Prospects on the Brink of Energy Transition. Renew. Sustain. Energy Rev. 2021, 142, 110833. [Google Scholar] [CrossRef]
- Laborde, A.; Habit, E.; Link, O.; Kemp, P. Strategic Methodology to Set Priorities for Sustainable Hydropower Development in a Biodiversity Hotspot. Sci. Total Environ. 2020, 714, 136735. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, D.; Rojas, M.; Boisier, J.P.; Valdivieso, J. Climate Change Impacts on Hydroclimatic Regimes and Extremes over Andean Basins in Central Chile. Hydrol. Earth Syst. Sci. Discuss. 2017. [Google Scholar] [CrossRef]

| Species | Abbreviation | Endemic to Chile | Conservation Status | Riffle | Pool | |
|---|---|---|---|---|---|---|
| Native | Geotria australis | Ga | Vulnerable | x | x | |
| Cheirodon pisciculus | Cp | x | Vulnerable | x | x | |
| Cheirodon galusdae | Cg | x | Vulnerable | x | x | |
| Nematogenys inermis | Ni | x | Endangered | x | x | |
| Bullockia maldonadoi | Bma | x | Endangered | x | x | |
| Trichomycterus areolatus | Ta | Vulnerable | x | x | ||
| Diplomystes nahuelbutaensis | Dn | x | Endangered | x | ||
| Diplomystes incognitus | Di | x | Not assessed | x | x | |
| Galaxias maculatus | Gm | Vulnerable/Least Concern * | x | x | ||
| Basilichthys microlepidotus | Bmi | x | Vulnerable/Near Threatened * | x | x | |
| Odontesthes mauleanum | Oma | x | Vulnerable | x | ||
| Percichthys trucha | Pt | Near Threatened/Least Concern ** | x | x | ||
| Percilia gillissi | Pg | x | Endangered | x | x | |
| Percilia irwini | Pi | x | Endangered | x | x | |
| Non-native | Cyprinus carpio | Cc | x | x | ||
| Carassius auratus | Ca | x | ||||
| Tinca tinca | Tt | x | ||||
| Cheirodon interruptus | Ci | x | x | |||
| Salmo trutta | St | x | x | |||
| Oncorhynchus mykiss | Omy | x | x | |||
| Gambusia holbrooki | Gh | x | x | |||
| Cnesterodon decemmaculatus | Cd | x | x | |||
| Australoheros facetus | Af | x | x |
| Habitat | Species | Frequency of Occurrence in Low-Fragmented Basins | Frequency of Occurrence in High-Fragmented Basins |
|---|---|---|---|
| Pools | Percilia gillissi * | 1.000 | 0.148 |
| Trichomycterus areolatus | 0.545 | 0.629 | |
| Percilia irwini | 0.000 | 0.518 | |
| Percichthys trucha | 0.454 | 0.296 | |
| Basilichthys microlepidotus | 0.363 | 0.481 | |
| Cheirodon pisciculus | 0.272 | 0.111 | |
| Galaxias maculatus * | 0.363 | 0.111 | |
| Cheirodon galusdae | 0.181 | 0.259 | |
| Geotria australis * | 0.363 | 0.000 | |
| Bullockia maldonadoi * | 0.272 | 0.037 | |
| Diplomystes incognitus * | 0.090 | 0.000 | |
| Odontesthes mauleanum | 0.000 | 0.111 | |
| Nematogenys inermis * | 0.090 | 0.000 |
| Habitat | Species | Average Abundance in Low-Fragmented Basins | Average Abundance in High-Fragmented Basins |
|---|---|---|---|
| Pools | Percilia gillissi * | 4.170 | 0.636 |
| Basilichthys microlepidotus | 1.612 | 2.340 | |
| Percilia irwini | 0.000 | 2.190 | |
| Trichomycterus areolatus | 1.569 | 1.483 | |
| Percichthys trucha | 1.505 | 1.077 | |
| Cheirodon pisciculus | 0.765 | 0.379 | |
| Cheirodon galusdae | 0.639 | 0.829 | |
| Galaxias maculatus * | 1.002 | 0.168 | |
| Geotria australis * | 0.791 | 0.000 | |
| Odontesthes mauleanum | 0.000 | 0.490 | |
| Bullockia maldonadoi | 0.363 | 0.104 | |
| Diplomystes incognitus * | 0.090 | 0.000 | |
| Nematogenys inermis * | 0.128 | 0.000 | |
| Riffles | Trichomycterus areolatus | 5.312 | 3.643 |
| Percilia gillissi * | 3.247 | 1.009 | |
| Percilia irwini | 0.000 | 1.738 | |
| Diplomystes incognitus | 0.598 | 0.300 | |
| Diplomystes nahuelbutaensis | 0.000 | 0.514 | |
| Percichthys trucha | 0.473 | 0.241 | |
| Bullockia maldonadoi | 0.305 | 0.196 | |
| Basilichthys microlepidotus | 0.118 | 0.329 | |
| Cheirodon galusdae | 0.000 | 0.192 | |
| Cheirodon pisciculus | 0.104 | 0.065 | |
| Nematogenys inermis | 0.075 | 0.033 | |
| Geotria australis * | 0.086 | 0.000 | |
| Galaxias maculatus * | 0.150 | 0.000 |
| Habitat | Approach | Model Variables | Cum R2 Adj | Df | F | p |
|---|---|---|---|---|---|---|
| Pools | Abundance | Elevation | 0.051 | 1 | 3.027 | 0.002 |
| Conductivity | 0.093 | 1 | 2.668 | 0.002 | ||
| Number of barriers upstream | 0.136 | 1 | 2.706 | 0.002 | ||
| Type of barrier | 0.158 | 1 | 1.908 | 0.006 | ||
| All variables | 0.197 | |||||
| Occurrence | Elevation | 0.040 | 1 | 2.556 | 0.002 | |
| Number of barriers upstream | 0.068 | 1 | 2.080 | 0.004 | ||
| Conductivity | 0.098 | 1 | 2.162 | 0.002 | ||
| Capacity | 0.111 | 1 | 1.499 | 0.030 | ||
| All variables | 0.123 | |||||
| Riffles | Abundance | Elevation | 0.022 | 1 | 2.478 | 0.002 |
| Number of barriers upstream | 0.039 | 1 | 2.123 | 0.004 | ||
| Conductivity | 0.060 | 1 | 2.404 | 0.002 | ||
| Temperature | 0.067 | 1 | 1.431 | 0.048 | ||
| All variables | 0.095 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, G.; Górski, K.; Manosalva, A.; Toledo, B.; Habit, E. Fragmentation Level Drives Local Fish Assemblage Diversity Patterns in Fragmented River Basins. Diversity 2023, 15, 352. https://doi.org/10.3390/d15030352
Díaz G, Górski K, Manosalva A, Toledo B, Habit E. Fragmentation Level Drives Local Fish Assemblage Diversity Patterns in Fragmented River Basins. Diversity. 2023; 15(3):352. https://doi.org/10.3390/d15030352
Chicago/Turabian StyleDíaz, Gustavo, Konrad Górski, Aliro Manosalva, Bárbara Toledo, and Evelyn Habit. 2023. "Fragmentation Level Drives Local Fish Assemblage Diversity Patterns in Fragmented River Basins" Diversity 15, no. 3: 352. https://doi.org/10.3390/d15030352
APA StyleDíaz, G., Górski, K., Manosalva, A., Toledo, B., & Habit, E. (2023). Fragmentation Level Drives Local Fish Assemblage Diversity Patterns in Fragmented River Basins. Diversity, 15(3), 352. https://doi.org/10.3390/d15030352








