Assessment of Habitat Selection by Invasive Plants and Conditions with the Best Performance of Invasiveness Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. The Focal Invasion
2.2. Study Plots
2.3. Habitats on Plots
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vitousek, P.M.; D’Antonio, C.M.; Loope, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 12485. [Google Scholar] [CrossRef] [PubMed]
- Rumlerová, Z.; Vilà, M.; Pergl, J.; Nentwig, W.; Pyšek, P. Scoring environmental and socioeconomic impacts of alien plants invasive in Europe. Biol. Invasions 2016, 18, 3697–3711. [Google Scholar] [CrossRef]
- Grzędzicka, E.; Hanzelka, J.; Reif, J. The area of Caucasian hogweeds’ invasion impacts bird responses to habitats in a heterogeneous landscape. Ecol. Indic. 2022, 141, 109082. [Google Scholar] [CrossRef]
- Grainger, T.N.; Levine, J.M.; Gilbert, B. The invasion criterion: A common currency for ecological research. Trends Ecol. Evol. 2019, 34, 925–935. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Hardrath, A.; Jin, H.; van Kleuen, M. Increases in multiple resources promote competitive ability of naturalized non-native plants. Commun. Biol. 2022, 5, 1150. [Google Scholar] [CrossRef]
- Arroyo-Esquivel, J.; Hastings, A. Spatial dynamics and spread of ecosystem engineers: Two patch analysis. Bull. Math. Biol. 2020, 82, 149. [Google Scholar] [CrossRef]
- Bazzaz, F.A. Habitat selection in plants. Am. Nat. 1991, 137, 116–130. [Google Scholar] [CrossRef]
- Gersani, M.; Abramsky, Z.; Falik, O. Density-dependent habitat selection in plants. Evol. Ecol. 1998, 12, 223–234. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Weiner, J.; Mallory, E.B.; Kennedy, C. Growth and variability in crowded and uncrowded populations of dwarf marigolds (Tagetes patula). Ann. Bot. 1990, 65, 513–524. [Google Scholar] [CrossRef]
- Carranza, M.L.; Ricotta, C.; Carboni, M.; Acosta, A.T.R. Habitat selection by invasive alien plants: A bootstrap approach. Preslia 2011, 83, 529–536. [Google Scholar]
- Chytrý, M.; Jarošík, V.; Pyšek, P.; Hájek, O.; Knollová, I.; Tichý, L.; Danihelka, J. Separating habitat invasibility by alien plants from the actual level of invasion. Ecology 2008, 89, 1541–1553. [Google Scholar] [CrossRef] [PubMed]
- Essl, F.; Dullinger, S.; Kleinbauer, I. Changes in the spatio-temporal patterns and habitat preferences of Ambrosia artemisiifolia during its invasion of Austria. Preslia 2009, 81, 119–133. [Google Scholar]
- Goldstein, J.; Park, J.; Haran, M.; Liebhold, A.; Bjørnstad, O.N. Quantifying spatio-temporal variation of invasion spread. Proc. R. Soc. B 2019, 286, 20182294. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.M. Biological invasions: Lessons from ecology. Trends Ecol. Evol. 1993, 8, 133–136. [Google Scholar] [CrossRef]
- Lear, L.; Padfield, D.; Inamine, H.; Shea, K.; Buckling, A. Disturbance-mediated invasions are dependent on community resource abundance. Ecology 2022, 103, e3728. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Reyes, L.D.; Paz-Hernández, H.; Godínez-Álvarez, H.O.; del Coro Arizmendi, M.; Navarro-Sigüenza, A.G. Trait shifts in bird communities from primary forest to human settlements in Mexican seasonal forests. Are there ruderal birds? Perspect. Ecol. Conserv. 2022, 20, 117–125. [Google Scholar] [CrossRef]
- Liu, K.; Liang, T.; Qiang, W.; Du, G.; Baskin, J.M.; Baskin, C.C.; Bu, H.; Yang, H.; Xiao, S. Changes in seed germination strategy along the successional gradient from abandoned cropland to climax grassland in a subalpine meadow and some implications for rangeland restoration. Agric. Ecosyst. Environ. 2020, 289, 106746. [Google Scholar] [CrossRef]
- Grzędzicka, E. Impact of invasive weeds on the diversity and dissimilarity of bird communities in forested areas. Diversity 2022, 14, 229. [Google Scholar] [CrossRef]
- Lapin, K.; Oettel, J.; Steiner, H.; Langmaier, M.; Sustic, D.; Starlinger, F.; Kindermann, G.; Frank, G. Invasive alien plant species in unmanaged forest reserves, Austria. NeoBiota 2019, 48, 71–96. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef] [PubMed]
- Trombulak, S.C.; Frissell, C.A. Review of ecological effects of roads on terrestrial and aquatic communities. Conserv. Biol. 2000, 14, 18–30. [Google Scholar] [CrossRef]
- Fenner, M.; Thompson, K. The Ecology of Seeds; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Alpert, P.; Bone, E.; Holzapfel, C. Invasiveness, invasibility and the role of environmental stress in the spread of non-native plants. Perspect. Plant Ecol. 2000, 3, 52–66. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, H.; Wei, M.; Wang, S.; Wu, B.; Du, D. Plant height and leaf size: Which one is more important in affecting the successful invasion of Solidago canadensis and Conyza canadensis in urban ecosystems? Urban For. Urban Green. 2021, 59, 127033. [Google Scholar] [CrossRef]
- Catford, J.A.; Jansson, R.; Nilsson, C. Reducing redundancy in invasion ecology by integrating hypotheses into a single theoretical framework. Divers. Distrib. 2009, 15, 22–40. [Google Scholar] [CrossRef]
- Ricciardi, A.; Hoopes, M.F.; Marchetti, M.P.; Lockwood, J.L. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr. 2013, 83, 263–282. [Google Scholar] [CrossRef]
- Tomasetto, F.; Duncan, R.P.; Hulme, P.E. Environmental gradients shift the direction of the relationship between native and alien plant species richness. Divers. Distrib. 2013, 19, 49–59. [Google Scholar] [CrossRef]
- Grzędzicka, E. Invasion of the Giant Hogweed and the Sosnowsky’s Hogweed as a multidisciplinary problem with unknown future—A review. Earth 2022, 3, 287–312. [Google Scholar] [CrossRef]
- Pearson, D.E.; Ortega, Y.K.; Runyon, J.B.; Butler, J.C. Secondary invasion: The bane of weed management. Biol. Conserv. 2016, 197, 8–17. [Google Scholar] [CrossRef]
- Neve, P.; Barney, J.N.; Buckley, Y.; Cousens, R.D.; Graham, S.; Jordan, N.R.; Lawton-Rauh, A.; Liebman, M.; Mesgaran, M.B.; Schut, M.; et al. Reviewing research priorities in weed ecology, evolution and management: A horizon scan. Weed Res. 2018, 58, 250–258. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 February 2021).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra/ (accessed on 15 February 2021).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan/ (accessed on 27 December 2021).
- Peterson, R.A.; Cavanaugh, J.E. Ordered quantile normalization: A semiparametric transformation built for the cross-validation era. J. Appl. Stat. 2020, 47, 2312–2327. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.A. Finding Optimal Normalizing Transformations via bestNormalize. R J. 2021, 13, 310–329. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Maechler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Calcagno, V. glmulti: Model Selection and Multimodel Inference Made Easy. R Package Version 1.0.8. 2020. Available online: https://CRAN.R-project.org/package=glmulti/ (accessed on 14 July 2022).
- Lüdecke, D.; Makowski, D.; Waggoner, P.; Patil, I. performance: Assessment of Regression Models Performance. 2020. Available online: https://CRAN.R-project.org/package=performance/ (accessed on 17 March 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Grzędzicka, E. Assessing the role of invasive weeds in the impact of successional habitats on the bird assemblage in overgrowing agriculture. J. Nat. Conserv. 2022, 72, 126352. [Google Scholar] [CrossRef]
- Sodhi, D.S.; Livingstone, S.W.; Carboni, M.; Cadotte, M.W. Plant invasion alters trait composition and diversity across habitats. Ecol. Evol. 2019, 9, 6199–6210. [Google Scholar] [CrossRef]
- Ni, M.; Deane, D.C.; Li, S.; Wu, Y.; Sui, X.; Xu, H.; Chu, C.; He, F.; Fang, S. Invasion success and impacts depend on different characteristics in non-native plants. Divers. Distrib. 2021, 27, 1194–1207. [Google Scholar] [CrossRef]
- Guarino, R.; Chytrý, M.; Attorre, F.; Landucci, F.; Marcenò, C. Alien plant invasions in Mediterranean habitats: An assessment for Sicily. Biol. Invasions 2021, 23, 3091–3107. [Google Scholar] [CrossRef]
- Ariori, C.; Aiello-Lammens, M.E.; Silander, J.A. Plant invasion along an urban-to-rural gradient in northeast Connecticut. J. Urban Ecol. 2017, 3, 1–13. [Google Scholar] [CrossRef]
- Huebner, C.D. Patterns of invasive plant abundance in disturbed versus undisturbed forests within three land types over 16 years. Divers. Distrib. 2021, 27, 130–143. [Google Scholar] [CrossRef]
- Melbourne, B.A.; Cornell, H.A.; Davies, K.F.; Dugaw, C.J.; Elmendorf, S.; Freestone, A.L.; Hall, R.J.; Harrison, S.; Hastings, A.; Holland, M.; et al. Invasion in a heterogeneous world: Resistance, coexistence or hostile takeover? Ecol. Lett. 2007, 10, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.W.; Lundberg, P.; Brown, J.S. On strategies of plant behaviour: Evolutionary games of habitat selection, defence, and foraging. Evol. Ecol. Res. 2016, 17, 619–636. [Google Scholar]


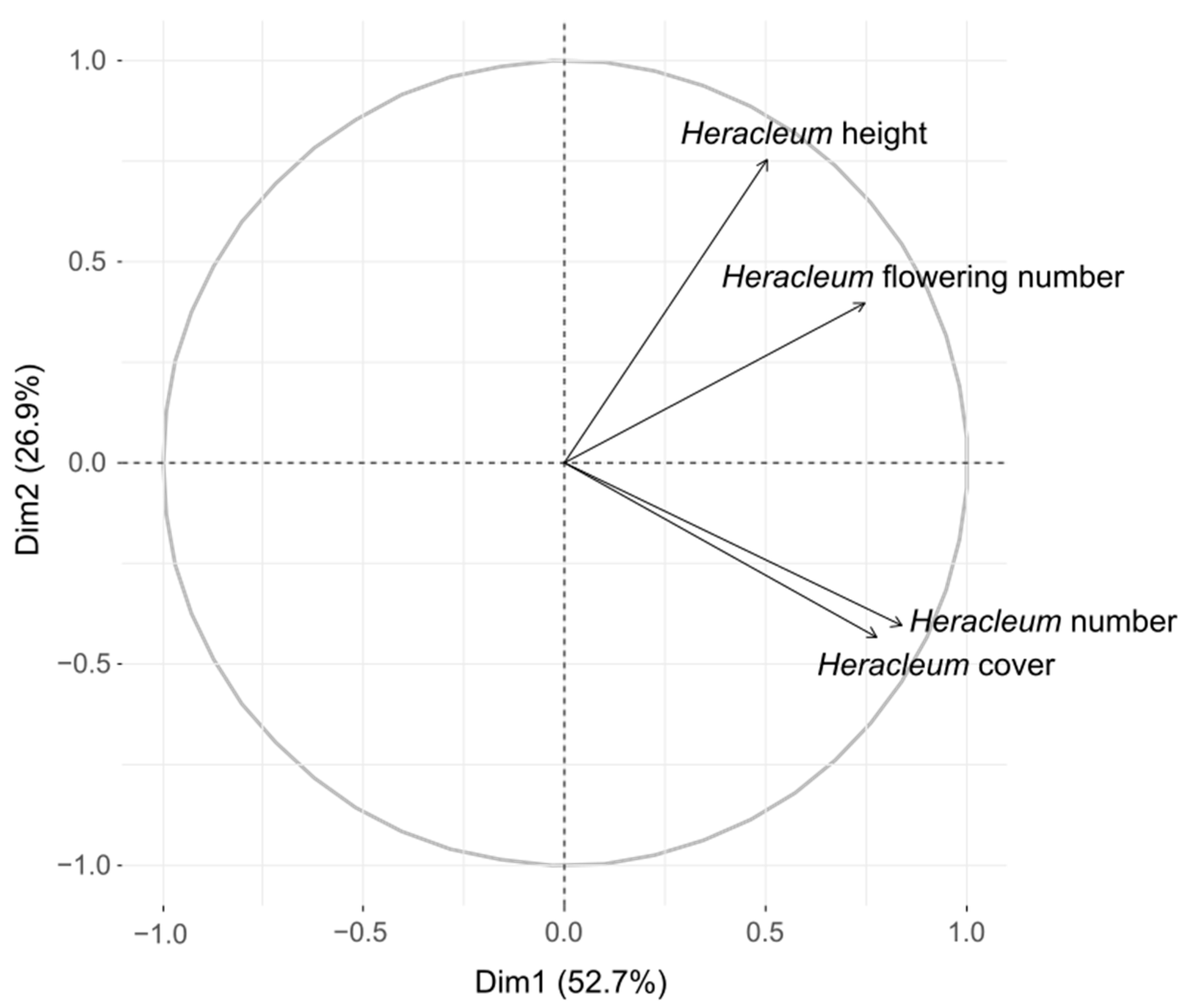
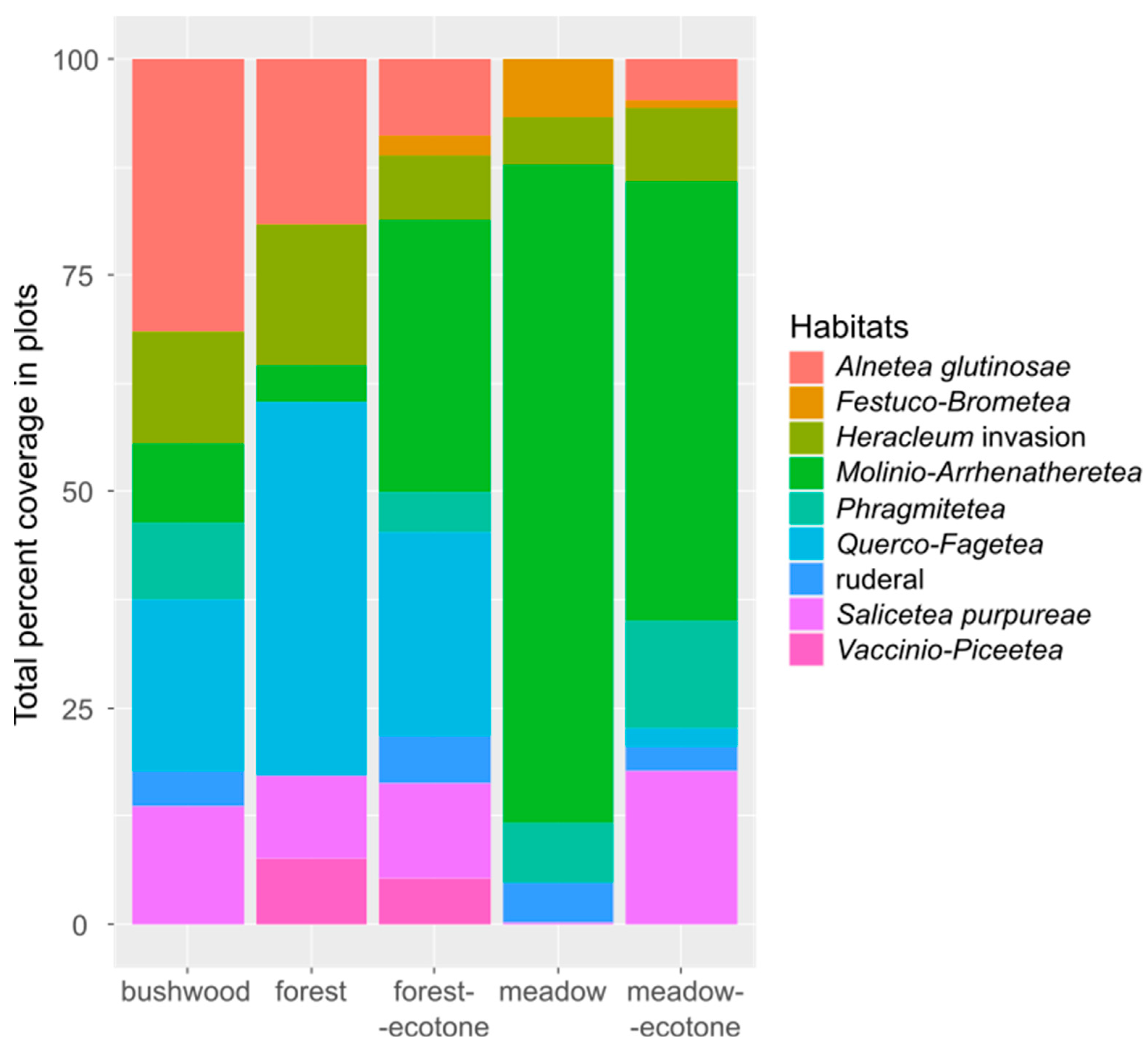


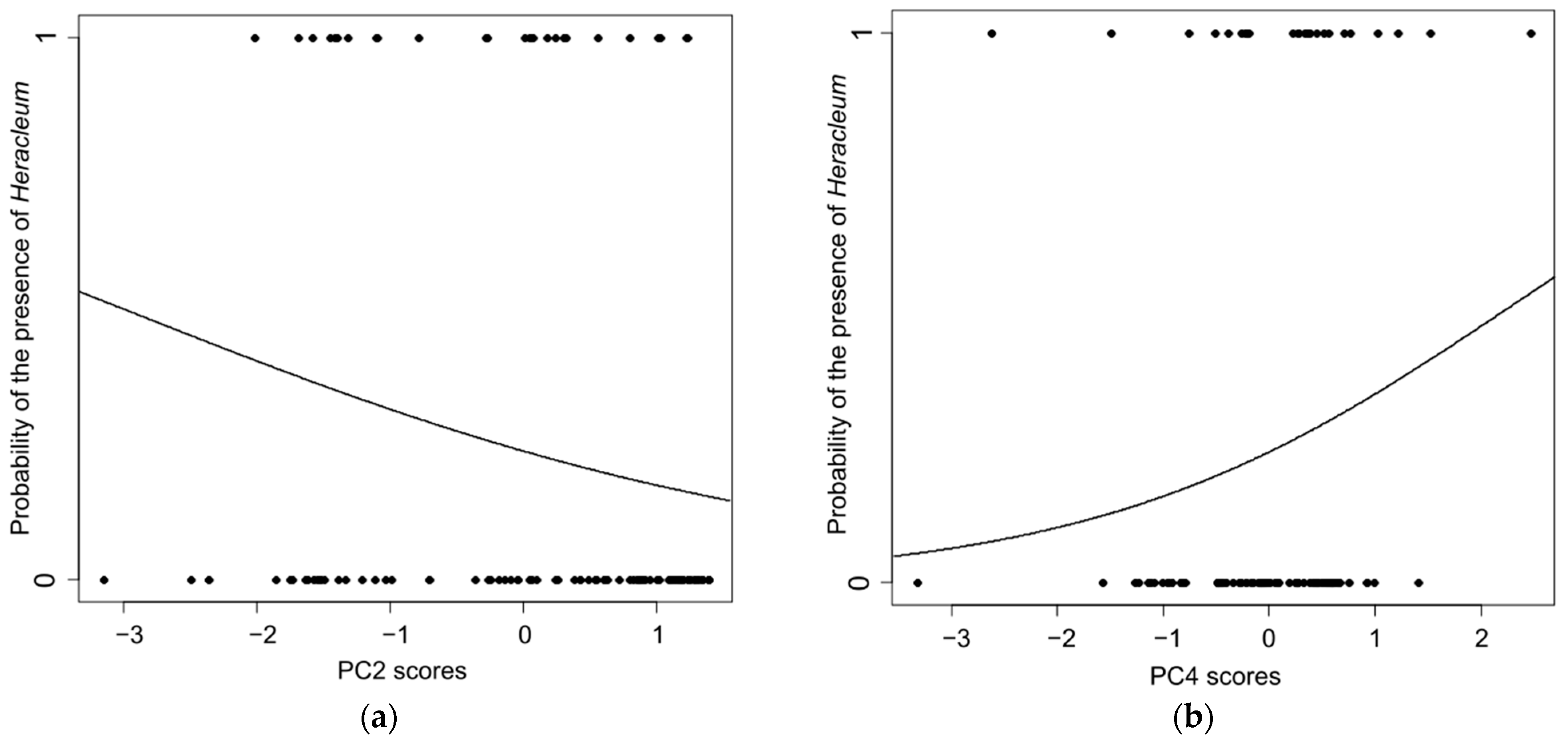
| Heracleum Traits | 1st Field Visit | 2nd Field Visit | 3rd Field Visit |
|---|---|---|---|
| Heracleum cover | 49.23% (25–90%) | 54.33% (25–90%) | 55.38% (25–90%) |
| Heracleum number | 172 (10–1000) | 176.5 (10–1000) | 183 (10–1000) |
| Heracleum height | 48.3 cm (20–100 cm) | 130 cm (50–150 cm) | 202 cm (50–250 cm) |
| Heracleum flowering number | 0 | 0 | 94 (0–600) |
| Heracleum Traits | Meadow | Meadow Ecotone | Bushwood | Forest Ecotone | Forest |
|---|---|---|---|---|---|
| Heracleum cover | 65.62% | 56.82% | 55.45% | 47.73% | 50% |
| Heracleum number | 305 | 197 | 110 | 152 | 87 |
| Heracleum height | 220 cm | 210 cm | 177 cm | 200 cm | 200 cm |
| Heracleum flowering number | 179 | 125 | 42 | 48 | 40 |
| Habitat Variables | Estimate ± SE | z | p |
|---|---|---|---|
| (Intercept) | −0.340 ± 0.395 | −0.861 | 0.389 |
| Forest | 0.763 ± 0.329 | 2.323 | 0.020 |
| Forest ecotone | 0.099 ± 0.312 | 0.318 | 0.751 |
| Meadow | 1.073 ± 0.332 | 3.231 | 0.001 |
| Meadow ecotone | 0.057 ± 0.307 | 0.187 | 0.852 |
| Habitat Gradients (PC Scores) | Estimate ± SE | z | p |
|---|---|---|---|
| (Intercept) | −1.126 ± 0.234 | −4.823 | <0.001 |
| PC2 scores (overgrown–open) | −0.491 ± 0.225 | −2.182 | 0.029 |
| PC3 scores (forest–ruderal) | 0.514 ± 0.289 | 1.781 | 0.075 |
| PC4 scores (bushes–ruderal) | 0.654 ± 0.332 | 1.967 | 0.049 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzędzicka, E. Assessment of Habitat Selection by Invasive Plants and Conditions with the Best Performance of Invasiveness Traits. Diversity 2023, 15, 333. https://doi.org/10.3390/d15030333
Grzędzicka E. Assessment of Habitat Selection by Invasive Plants and Conditions with the Best Performance of Invasiveness Traits. Diversity. 2023; 15(3):333. https://doi.org/10.3390/d15030333
Chicago/Turabian StyleGrzędzicka, Emilia. 2023. "Assessment of Habitat Selection by Invasive Plants and Conditions with the Best Performance of Invasiveness Traits" Diversity 15, no. 3: 333. https://doi.org/10.3390/d15030333
APA StyleGrzędzicka, E. (2023). Assessment of Habitat Selection by Invasive Plants and Conditions with the Best Performance of Invasiveness Traits. Diversity, 15(3), 333. https://doi.org/10.3390/d15030333







