Abstract
Lakes and wetlands provide top-priority hotspots that play a key role in maintaining and protecting threatened and migratory waterfowl. Identifying seasonal and spatial variations in aquatic bird communities and their responses to environmental factors is vital conservation efforts. However, there is a lack of information on waterfowl in Mongolian lakes and their associated wetlands. The purpose of this study was to investigate the spatial–seasonal changes in waterfowl assemblage structures in Mongolian lakes, as well as to determine how they respond to various environmental factors (lake surface area, elevation, and geographical distribution). Statistical analyses were performed using seasonal data (May, July, and September) for 28 waterfowl collected from 54 lakes across the country between 2016 and 2018. Seasonal heterogeneity in species richness and abundance was observed in lakes in each geographical region (Eastern, Central, and Western Mongolia). The composition of waterfowl in the lake was also relatively similar between May and September compared to July. This was due to the overlapping migration seasons in spring and autumn. Regionally, the number of waterfowl was much higher in the Eastern Mongolian lakes, followed by Central Mongolian lakes and then Western Mongolian lakes. This is likely due to differences in habitat availability and water levels between the regions. Eastern Mongolian lakes tend to have more wetlands and shallow water habitats, while Central and Western Mongolian lakes tend to have deeper open-water habitats. These differences in habitat types could contribute to the observed differences in waterfowl abundance among the regions. Additionally, some small lakes and the group of small lakes supported a greater abundance and diversity of waterfowl compared to some medium-sized and large lakes, suggesting that they are important for conservation. Indices of diversity (H’) and species dominance (D’) showed positive and negative correlations with lake surface area, respectively. Perhaps the increased surface area of the lake decreases the habitat overlap for designated waterfowl due to habitat heterogeneity. Accordingly, the indices (H’ and EH) increased as the waterfowl species composition became relatively equivalent in large lakes. Overall, spatial variations among the lakes were primarily attributable to the individual features of the lakes (shallowness, small lake groupings, and surface area), and seasonal variation in the lakes depended majorly on the compositional changes of the waterfowl due to migration.
1. Introduction
Lakes and their associated wetlands play a vital role in preserving aquatic ecosystems and supporting ecological diversity [1]. A wide variety of waterbird species utilize the habitats at different times during their annual life cycles for nesting, breeding, food, shelter, migration stops, and wintering [2,3]. The presence and availability of wetlands and lakes throughout the flyways is especially important in supporting the migration success of long-distance migrants such as waterfowl, waders, and shorebirds [1,4]. Intense human disturbances (such as land-use modification, water pollution, and heavy grazing) and climate change, however, majorly affect their availabilities (natural presence, surface size, and heterogeneity), leading to the high risk of waterbird decline worldwide [2,4,5]. It is accordingly imperative to attend to and protect these habitats as they are vital to the conservation of migratory waterbirds and other biodiversity.
There are a variety of environmental descriptors that influence the structure of waterbird assemblages locally, regionally, and seasonally. Local characteristics of lakes such as depth, surface area, sedimentation ratio, and trophic status are key factors in identifying the composition, abundance, and richness of aquatic birds [2,5,6,7]. Shallow and highly productive lakes with a high sedimentation ratio support a high abundance of waterbirds. However, species richness increases with the size of lakes and their associated wetlands due to habitat heterogeneity in large systems. The spatial and seasonal dynamics of waterbird assemblages can also be found in systems due to differences in migration patterns, such as period and corridor [2,8,9]. Accordingly, local environmental factors affecting spatial–seasonal differences in aquatic bird communities are the basis for ecological studies associated with conservation targets [10].
Mongolia is one of the top-priority countries for the research and conservation of migratory birds due to its location at the junction of three major migratory flyways: East Asia–Australia, Central Asia, and Eastern Africa–West Asia [11,12]. A number of pristine natural habitats are also found in Mongolia such as the Altai-Sayan and Daguur ecoregions, which are among the world’s most biologically outstanding regions and include sites noted for their conservation value, Ramsar sites, and important bird areas (IBAs) [11,12,13,14,15]. Moreover, the IBAs with wetlands are considered important breeding and stopover sites for migratory land and water birds [1,12,16,17]. Unfortunately, many lakes and wetlands across Mongolia have been rapidly shrinking and drying up in recent decades due to the continual decrease in river inputs and rapid water evaporation as a consequence of climate change (increasing temperature and decreasing precipitation) [12,18,19,20]. The acceleration of human activities is, moreover, a major cause for the loss of lake sizes in Mongolia and their deterioration, which is due to overgrazing, intense mining, and expanding agricultural practices [18,19,20]. Attending to their conservation is therefore valuable for supporting the ecological requirements of aquatic birds, including nesting, breeding, food, shelter, and migration stopovers, and has international significance. Despite the fact that several monitoring programs have been actively implemented in a few lakes and rivers and their associated wetlands in Mongolia, there is still a lack of information on waterfowl that is connected with spatial–seasonal variations and the impacts of the local characteristics of lakes and wetlands [12,15,18,19,21,22,23,24,25,26].
Based on biological data from 54 Mongolian lakes and their associated wetlands, the present study has investigated spatial–seasonal variations in the assemblages of migratory waterfowl along the different migration flyways. The influence of the size of lakes and the grouping of small lakes was also addressed as descriptors for the changes in the waterfowl community structures [4,27]. This is because of the lack of attention given by the government to small lakes and wetlands in Mongolia compared to the larger lakes. Moreover, several factors affecting waterfowl communities around the country were investigated, including the gradients of geographical locations and longitudes.
2. Materials and Methods
2.1. Background of Study Lakes
The lakes studied are distributed all over Mongolia, which is located in the transition zone between the boreal forests of Siberia and the deserts of Central Asia (Figure 1). The southern fringes of the Siberian Great Taiga (boreal forest) extend into northern Mongolia, whereas the vast Mongolian and Manchurian steppes spread into the country from the east [12,13]. The landscapes of southern Mongolia are dominated by the Gobi Desert, while the high Altai Mountains dominate the west and northwest. From north to south, the landscape changes from forested mountains through grassy steppe to barren, arid desert plains [12]. Meanwhile, the topography from the west to the east changes from a high mountainous terrain to boundless plains. Each region has its own specific floral and faunal characteristics. When combined, these characteristics comprise the unique biological richness of the country [12].
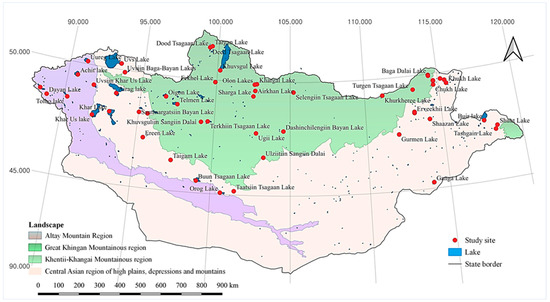
Figure 1.
Geographical distribution of the lakes studied throughout Mongolia.
There are three main watersheds: the Arctic Ocean Basin, the Pacific Ocean Basin, and the Central Asian Internal Drainage Basin [28]. The majority of lakes in this study address the third river watershed and include the western and south–central Mongolian lakes such as Uvs, Buun Tsagaan, and Khar Lake. Eastern Mongolian lakes (Khukh, Buir, and Tashgai Tavan Lakes, etc.) are in the Pacific Ocean Basin. Central–northern Mongolian lakes (Khuvsgul, Terkhiin Tsagaan, Ugii Lake, etc.) include the Arctic Ocean Basin [13]. Most lakes in the eastern region are fed by rainwater; however, some lakes are also fed by rivers and streams (e.g., Khukh and Buir Lake, etc.) [12,15]. The small- and medium-sized lakes in the Gobi-desert depend on rainfall levels. Most lakes in the western and north–central regions are fed by rivers and streams [12,23]. The surface area of the study lakes varied in the range of 1.17 km2 and 3350 km2 (<10 km2—twenty-six lakes, 10–20 km2—four lakes, 20–50 km2—four lakes, 50–100 km2—8 lakes, 100–500 km2—seven lakes, 500–1000 km2—two lakes, and 1000 km2 <—three lakes) (Table 1). The average depth of the study lakes ranged from 0.9–137.9 m (<5 m—twenty-four lakes, 5–10 m—twelve lakes, 10–20 m—four lakes, 20–30 m—three lakes, and 100 m <—one lake) (Table 1) [28,29,30].

Table 1.
Summary data of waterfowl community metrics in the lakes, showing lake surface area and depth.
2.2. Procedure of Field Observation
From 2016 to 2018, field surveys were conducted at all Mongolian important bird areas in May, July, and September. Observation data on twenty-eight waterfowl in fifty-four lakes were extracted from our collected data for this study. Observation sites were randomly selected along the shoreline within each lake and their associated wetlands, with high points being preferred. The waterfowl species were registered and counted from 525 sites using the point counting method and research tools (the visible eye encounter, field binoculars (Nikon Monarch HG, 10 × 42), and a spotting scope (CarlZeiss, 20—60 × 80)) [31]. The number of observation sites varied from five to twenty-four, depending on the size of the lakes and some difficult natural situations. The observations lasted approximately 10 min to diminish the probability of bird recount [31], and the distance between observers along the shores of the lakes varied from approximately 50 to 200 m.
2.3. Analytic Approach
Shannon–Weiner’s diversity (H’), evenness (EH), and Simpson’s dominance (D’) were applied to quantify the diversity of the waterfowl species because these indices provide more information about community composition than species richness [32,33,34]. These calculations were performed using PAST (version 4.3.0) [35]. Moreover, the following formula was utilized to compute the species’ probability of observation: P(species 1,2…) = (nl * nos)/(Nl * Nos), where P is the species probability to observe, nl is the number of observed lakes, nos is the number of observed sites, and Nl and Nos are the total numbers of study lakes and counting point sites, respectively. The relative abundance (RA) of a bird species was determined using the following expression: (number of individuals for species n/N total number of individuals) × 100%.
To illustrate seasonal variations in waterfowl community attributes (i.e., species richness and abundance), we performed an analysis of variance (ANOVA) and a Tukey’s post -hoc test using the function “aov” in R software (version 4.2.2) [36]. A non-metric multidimensional scaling (NMDS) [37] was performed to examine spatial variations in the waterfowl due to region and flyway differences across the country with the help of the function “metamds” in the R package vegan 2.6-2 [38]. Data on waterfowl abundance with total species were Hellinger-transformed using the R package vegan function “decostand” [39], which reduces the weights of abundant species but keeps the Bray–Curtis index between lakes [39]. Additionally, a permutational multivariate analysis of variance (PERMANOVA, using the “Adonis” function in R software [35]) was used to test for regional differences. A principal component analysis (PCA) was used to analyze the relationship between environmental variables and waterfowl community attributes, aided by the “FactoMiner” and “factoextra” packages in R software [40].
3. Results
3.1. Waterfowl Diversity and Abundance in Lakes
During this study, 139,641 individual waterfowl were recorded, belonging to 28 waterfowl species from the families Anatidae and Anseriiformes. The richness varied from 2 to 21 among the lakes, with a range of 0.04–2.62 for species diversity (H’) (Table 1). The highest richness (21 species) was found in the group lake of Baga-Bayan in western Mongolia. On the contrary, in the central region, Khargal Lake showed the lowest richness (2 species). According to the evenness index (EH), the evenness of waterfowl in the lakes ranged between 0.16 and 0.81. The highest value was identified in Khuvsgul Lake and the smallest value was found in the Ulziitiin Sangiin Dalai lake in central Mongolia. Regarding the dominance index (D’), there was a high variation among lakes in the range of 0.09–0.99. The total abundance of the 28 waterfowl varied greatly between lakes. The most abundant lake (22219 individuals) was Khukh Lake in eastern Mongolia, and the lowest abundance was observed in Khargal Lake, which had 82 individuals (Table 1).
According to each species’ abundance, the most numerous species were Tadorna ferruginea (relative abundance: 19.35%, mean ± s.e.: 77.9 ± 10.2), Aythya ferina (10.14%, 80.9 ± 11.2), Anser indicus (8.87%, 129.0 ± 28.3), and Aythya fuligula (8.23%, 69.2 ± 8.6) (Table 2). The first two species were observed in the majority of the lakes in our study, which included 51 and 46 lakes, respectively. On the other hand, Anser indicus was mostly found in lakes in western and central Mongolia (31/54), and Anser cygnoid was commonly observed in the lakes of eastern and central Mongolia (35/54). Moreover, the least numerous species were Oxyure leucocephala (0.02%, 6.6 ± 0.9), Cygnus columbianus (0.1%, 2.5 ± 0.8), and Anas zonorhyncha (0.001%, 1.5 ± 0.5). Additionally, Tadorna ferruginea (62.42%), Cygnus cygnus (46.05%), Aythya ferina (28.40%), and Aythya fuligula (26.35%) were evaluated as having a higher possibility of observation than other species throughout Mongolia (Table 2).

Table 2.
The assemblage patterns of 28 waterfowl in lakes throughout the country.
Among the observed months, a statistical difference in species diversity and evenness (H’ and EH) was found during the study years (ANOVA, df = 122.2, F = 14.34 and 14.86, p < 0.001, respectively). The indexes and species richness were higher in the May surveys than in the July and September surveys (Figure 2A, Table 3). Waterfowl abundance and the species diversity of regional levels showed similar seasonal patterns (Table 3), with a lower abundance of birds in May, followed by a tendency to peak in July and September. The seasonal variation in waterfowl abundance was significantly different in the eastern region (May–July–September; one-way ANOVA, df = 98.78, F = 1.6, p = 0.1), central Region (ANOVA, df = 89.9, F = 7.6, p < 0.01) and the western region (ANOVA, df = 101.2, F = 6.3, p < 0.01) (Table 3).
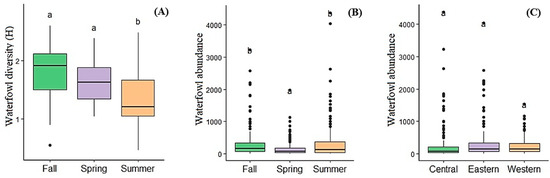
Figure 2.
Seasonal and regional differences in waterfowl diversity (H’) and abundance in the lakes. Different small letters indicate significant differences between seasons at p < 0.05 analyzed by the Tukey test.

Table 3.
The seasonal patterns in waterfowl assemblage structures are described by the regions and overall.
A Tukey test (all pairwise multiple comparisons) determined that there were differences in species diversity and the evenness of waterfowl surveys from May to September, whereas the diversity and evenness of surveys from July were statistically different from others and clearly lower than that of other months (Figure 2A and Table 3). By contrast, the abundance of twenty-eight waterfowl was higher in July than in other months (ANOVA, df = 302.8, F = 13.07, p < 0.001) (Figure 2B). Additionally, Tukey tests evaluated that the abundance recorded by the surveys in July and September was no different, although both of them differed statistically from the May survey with respect to the abundance of waterfowl.
3.2. Spatial–Seasonal Variations of Waterfowl Diversity and Abundance and with Species Similarity among Lakes
The lakes of eastern Mongolia observed a higher abundance of waterfowl than western and central Mongolian lakes, but not significantly (Table 1 and Figure 2C). In contrast, the species richness of the western and central Mongolian lakes was slightly higher than that of the eastern Mongolian lakes (Table 1 and Table 3). The ANOVA determined that the species richness in the three regions differed statistically (ANOVA, df = 33.6, F = 7.73, p < 0.05).
The NMDS analysis specified 28 species of waterfowl that were affected by regional variables (Figure 3). Tadorna tadorna and Anser cygnoid were highly abundant in the eastern lakes with low altitudes and small sizes. In contrast, Anser fabalis, Anser indicus, Anas acuta, Aythya marila, and Mergus merganser were found in western and central lakes with high altitudes and large lakes. Furthermore, an NMDS (stress = 0.22) analysis of waterfowl abundance based on Bray–Curtis revealed a distinct similar grouping in all three regions. There was no dramatic separation of waterfowl species according to the regional level (PERMANOVA: R2 = 0.15, F2,53 = 1.45, p = 0.1).
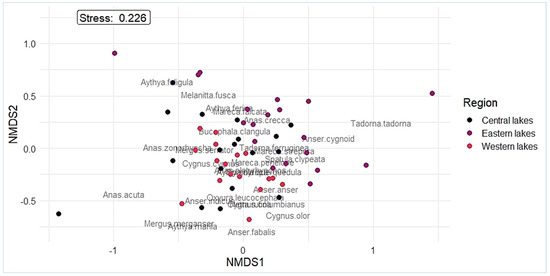
Figure 3.
Non-metric multidimensional scaling (NMDS) ordination of the waterfowl community structure under the different regions.
3.3. Species Diversity Metric-Lake Size Relationship Pattern
In order to confirm the diversity relationship results, we show the PCA of the diversity metric for the three regions together with the lake surface area, altitude, and longitude (Figure 4). As a general trend, the lakes of the same region are grouped together independently with respect to the environmental factor. For example, the dispersion of the lakes in central and western Mongolia are on the right side and the eastern lakes are on the left side. The dominance index (D’) was negatively correlated with the lake surface area (R = 0.33, p < 0.05) and positively explained the variations in the indices of waterfowl diversity (H’) (R = 0.29, p < 0.05). By contrast, the richness of waterfowl was significantly and positively correlated with the elevation (R = 0.38, p < 0.05). The longitude did not show a significant correlation with the diversity metric and total abundance (p > 0.05) (Figure 4).
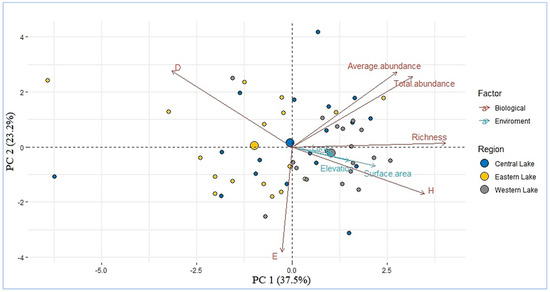
Figure 4.
Principal component analysis (PCA) correlation biplot of the environment and biological variables in the survey lakes.
4. Discussion and Conclusions
Spatial–seasonal variations and local environmental factors of the individual waterbodies are responsible for structuring aquatic bird assemblages [1,2,6,41]. Based on data collected from 54 Mongolian lakes between 2016 and 2018, this study identified several changes in the waterfowl community that were related to spatial–seasonal features and lake size (their associated wetlands). A high probability existed of observing Aythya fuligula, Aythya ferina, Cygnus Cygnus, and T. ferruginea throughout the lakes. Also, Tadorna ferruginea, Aythya ferina, Anser indicus, and Anser cygnoid were recorded more abundantly than other species during the study period. The first two species (T. ferruginea and A. ferina) were observed commonly throughout the country; however, the composition of waterfowl communities changed spatially with major contributions from A. indicus and A. cygnoid. A. indicus was abundantly observed in the lakes of western and central Mongolia and is mainly attributed to breeds in central Asia, from Kazakhstan and Kyrgyzstan in the extreme east, across southern Russia to western Mongolia and the Qinghai–Tibetan Plateau, south to Ladakh in India, and migrating along the Central Asian flyway [42,43]. By contrast, A. cygnoid were copiously observed in the lakes of eastern Mongolia and some central Mongolian lakes. Studies in the past have indicated a high prevalence of A. cygnoid in eastern Mongolia. In northeastern Mongolia, for instance, the small lakes of Mongol Daguur and Buir Lake support the largest population of A. cygnoid by serving as stopover sites and breeding habitats. Ganga Lake does the same in southeastern Mongolia [15,26,44,45,46]. The global population trend reports announced A. ferina, A. cygnoid, and A. indicus are in a downward trend, the trends of T. ferruginea and C. cygnus are unknown, and the other two species are experiencing an upward trend [22,47,48,49,50]. Accordingly, considering only the common and abundant species, the lakes and their associated wetlands in Mongolia can show a high importance value in waterfowl conservation, particularly for the species whose populations are globally and regionally decreased [15,22,25].
A large number of birds generally arrive in Mongolia by the East Asia—Australia flyway to breed and forage [12,15]. Similarly, according to our study, there appeared to be a higher abundance of waterfowl in Eastern Mongolia than in the western and central Mongolian lakes. This is likely due to differences in habitat availability and water levels between the regions. The PCA showed two main clusters of lake similarity, which comprised the most central Mongolian lakes and all western Mongolian lakes (right side), and some central Mongolian lakes and all Eastern Mongolian lakes (left side). Eastern Mongolian lakes tend to have more wetland and shallow water habitats, while central and western Mongolian lakes tend to have deeper open-water habitats [28]. For this study, these differences in habitat types could contribute more to the observed differences in waterfowl abundance among the regions than different flyways. Non-metric multidimensional scaling (NMDS) was used to demonstrate the geographic dissimilarity of waterfowl species. We did not find any differences in waterfowl community patterns between different lake sizes of lakes and regions in different latitudes. These findings show no significant difference between the species of waterfowl birds distributed in Mongolia; differences were observed only within seasonal and same regions.
The seasonal analysis of waterfowl data showed that the species diversity and evenness indices were higher in May and September than in July. There was no difference in species diversity and the evenness of waterfowl in the surveys from May–September, while the diversity and evenness in July were statistically different from others. A significant difference was, however, not found in the species richness among the study months (27 species in July and September and 28 species in May) for the chosen twenty-eight waterfowl. Another way to put it is that the composition of the selected species was more similar in May and September than in July. This can be explained in part by the seasonal movement of waterfowl during migratory periods. Several studies have found that the temporal variation in species diversity and the evenness of water birds is mainly driven by migration and movement in the northern hemisphere during spring and fall [2,9,51].
A number of studies have indicated that habitat heterogeneity with the increasing size of lakes and their associated wetlands is an influencing factor in the deviation of aquatic bird diversity [2,3,6,7,15,41,51,52,53]. Our study shows that the richness and abundance of waterfowl were not clearly related to the surface area of the lake. Regarding abundance, some small lakes supported a greater number of waterfowl than large ones during the migration terms. This might be attributable to the weak relationship between the lake size and the average abundance, which some papers reported [2,54]. The waterfowl diversity index was positively related to the surface area of the lake, whereas the species dominance index showed a negatively correlation. It might be due to the fact that the increased surface area of the lake decreases the habitat overlap for the designated waterfowl due to habitat heterogeneity [2,6,55]. In other words, there is more chance to select suitable habitats for the waterfowl seeking to breed, rest, and refuel. Accordingly, the composition of the waterfowl species might become relatively equivalent in large lakes, and these indices may increase. The richness in our study was restricted to a certain species of waterfowl; thus, it may not appropriately elucidate the relationship with the size of a lake.
Furthermore, shallow lakes with a large surface area had a tendency to support larger waterfowl assemblages during the migration seasons. The shallowness is also one of the main physical factors influencing the community structure of the water birds (richness and abundance) because the depth prerequisites of various feeding groups overlap [2,6,7,54,56]. Rich food availability also makes the waterfowl more flexible in foraging, and many duck species may have high diet overlap in such a type of “super-abundant” foraging habitat [57]. Generally, waterfowl utilize large amounts of protein during migration, nesting, and molting, and they fulfill this requirement by consuming aquatic invertebrates [58]. As lakes become shallower, they are more likely to be highly diverse in aquatic plants, which support a high number of aquatic invertebrates [54,59,60]. Therefore, the nutritional requirements of waterfowl have been met in shallow lakes and wetlands where diverse aquatic plant growth is abundant [60]. Additionally, the trophic state is a major factor that influences the abundance and richness of species on lakes with shallowness and a high primary productivity [5,6]. On one hand, some small lakes and the groups of small lakes also support the same number and more waterfowl species when compared with some medium-sized and large lakes. For instance, Erveekhii lake and Gun Tsengeleg lake, which was one of the smallest lakes in our study, demonstrated a higher richness than the Khoton, Dayan, Dood Tsagaan, and Telmen lakes, which were medium-sized and large-sized lakes. The four groups of small lakes, which were the Tsengeleg Lakes, Tashgain Tavan Lakes, Teshigiin Olon Lakes, and Uvsiin Baga-Bayan Lakes, had a greater abundance of waterfowl with the same richness compared to larger lakes with an equivalent area. Several studies suggested that a group of small lakes provides nearby alternative feeding sites and a suitably undisturbed breeding habitat for waterfowl and contain high habitat heterogeneity in the wetlands among small lakes [2,7].
Regardless of their size, all lakes and wetlands through certain flyways play a role during waterfowl migration as a stopover site (staging post). These sites are crucial to the success of the birds’ long migratory route, while water birds fly over and lack a direct way around large expanses of land that lack acceptable wetlands and lakes for resting and refueling, such as the Tibetan and Mongolian Plateau [2]. Therefore, the loss of any lakes and wetlands through flyways can have a harmful impact on migratory birds [1,16,61]. The lakes studied are internationally designed as wetlands of great conservation value for threatened species and migratory waterfowl, including the IBAs and the Ramsar sites in Mongolia. These can include, for instance, the lakes of Mongol Daguur, the Valley of Lakes, and Khar–Us Nuur National Park, located along the East Asia–Australia, Central Asian, and Eastern Africa–West Asian flyways [12,13].
Unfortunately, some valuable lakes and wetlands in Mongolia have rapidly shrunk and dried up due to intensive anthropogenic activities around the lakes (e.g., livestock overgrazing, mining, etc.) and climate changes, which have decreased precipitation, increased water evaporation, and increased the air temperature [12,18,19,20,21,22,23]. A rapid drying-out of lakes was reported around Mongolia from 1996 to 2011 in accordance with the study of [62]. Furthermore, Kang and Hong (2016) [18], who analyzed the surface area of 73 lakes in four different regions of Mongolia, identified that the lake area decreased by −9.3% at an annual rate of −53.7 km2 yr−1 from 2001 to 2011 for the lakes studied. The study also indicated slight-to-moderate decreases of lake surface area in semi-arid regions (desert steppe) and rapid reductions of lake surface area in arid regions (Gobi Desert). According to their study at Ugii lake, Sumiya et al. (2020) [20] revealed a significant decline in the total precipitation and inflow river discharge and an increase in the total annual evaporation and annual average temperature, as well as an abrupt diminishing of the lake surface area (which reduced by 13.8% over 32 years). However, there is a lack of information on the relationship between the lake surface area reduction and the water birds’ dynamics in Mongolian lakes, in addition to the physical and chemical variations associated with environmental conditions.
In summary, the presence of numerous migratory waterfowl recorded in our study shows that Mongolian lakes are a hotspot habitat for waterfowl birds and are used as breeding and stopover sites. We suggest that land-use changes and anthropogenic threats in Mongolia can have a significant impact on heterogeneity in waterfowl communities among lakes which may be greater than the impact of seasonal movements and migration patterns. Anthropogenic threats, such as overgrazing, mining, and expanding land use, have caused the degradation of wetland habitats, including the loss of vegetation and the degradation of water quality [18,19,21]. This can reduce the availability of food resources and nesting sites for waterfowl, as well as increase their vulnerability to predators and other threats [1,58,63]. There was little indication that changing wetlands and lakes resulted in a waterfowl habitat loss in Mongolia. A further monitoring program and frequent research on lakes and their associated wetlands are crucial for developing fundamental scientific resources for the conservation and management of waterbirds, with a priority on anthropogenically induced impacts on lakes and their surroundings. For instance, the factors should consider the degradation of land, water quality losses, and intense human activities near shorelines.
Author Contributions
This paper received individual contributions from each author as specified: Conceptualization: Z.P., N.J. and O.G.; data curation: Z.P., N.J. and O.G.; formal analysis: Z.P. and N.J.; funding acquisition: W.K.P. and J.W.L.; investigation: Z.P., N.J., O.G., M.M., E.P., A.J. and I.-H.P.; methodology: Z.P., N.J. and O.G.; software: Z.P. and N.J.; supervision: W.K.P. and J.W.L.; validation: Z.P., O.G., M.M., E.P., A.J., I.-H.P., W.K.P. and J.W.L.; visualization: Z.P. and N.J.; writing—original draft: Z.P. and N.J., writing—review & editing: Z.P., O.G. and J.W.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the research fund of the R&D Program for Forest Science Technology (Project No. 2021366B10-2223-BD 0131482092640102) provided by the Korea Forest Service (Korea Forestry Promotion Institute).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors appreciate the researchers and students of the Mongolian National University of Education who participated in carrying out the field work. We would also like to thank our research colleagues from the Mongolian National University of Education (MNUE), the National Science Museum of Korea, and the Cultural Heritage Administration of the Republic of Korea. This research was also sponsored by the National Research Foundation of Korea (NRF) and funded by the Korean government (Ministry of Science and ICT) (Grant No. 2022M3H9A1097213). The authors would also like to thank the Bio & Medical Technology Development Program of the National Research Foundation (NRF), funded by the Korean government (MSIT) (No.NRF-2017M3A9A5070202), for its support regarding the fieldwork.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kirby, J.S.; Stattersfield, A.J.; Butchart, S.H.; Evans, M.I.; Grimmett, R.F.; Jones, V.R.; O’Sullivan, J.; Tucker, G.M.; Newton, I. Key conservation issues for migratory land-and waterbird species on the world’s major flyways. Bird Conserv. Int. 2008, 18, S49–S73. [Google Scholar] [CrossRef]
- Ma, Z.; Cai, Y.; Li, B.; Chen, J. Managing wetland habitats for waterbirds: An international perspective. Wetlands 2010, 30, 15–27. [Google Scholar] [CrossRef]
- Nudds, T.D. Niche dynamics and organization of waterfowl guilds in variable environments. Ecology 1983, 64, 319–330. [Google Scholar] [CrossRef]
- Roach, J.; Griffith, B. Climate-induced lake drying causes heterogeneous reductions in waterfowl species richness. Landsc. Ecol. 2015, 30, 1005–1022. [Google Scholar] [CrossRef]
- Crozier, G.E.; Gawlik, D.E. Avian response to nutrient enrichment in an oligotrophic wetland, the Florida Everglades. Condor 2002, 104, 631–642. [Google Scholar] [CrossRef]
- Hoyer, M.V.; Canfield, D.E. Bird abundance and species richness on Florida lakes: Influence of trophic status, lake morphology, and aquatic macrophytes. In Aquatic Birds in the Trophic Web of Lakes; Springer: Dordrecht, The Netherlands, 1994; pp. 107–119. [Google Scholar] [CrossRef]
- Sebastián-González, E.; Green, A.J. Habitat use by waterbirds in relation to pond size, water depth, and isolation: Lessons from a restoration in southern Spain. Restor. Ecol. 2014, 22, 311–318. [Google Scholar] [CrossRef]
- Josens, M.L.; Haydee, E.A.; Favero, M. Seasonal variability of waterbird assemblages in relationship to habitat characteristics in a Pampas wetland. Waterbirds 2009, 32, 523–530. [Google Scholar] [CrossRef]
- Liao, J.T.; Ye, H.; Huang, T.F.; Peng, G.H. Seasonal waterbird population changes in Lashihai Lake in northwest Yunnan, China. J. Mt. Sci. 2017, 14, 1852–1862. [Google Scholar] [CrossRef]
- Pöysä, H.; Rintala, J.; Johnson, D.H.; Kauppinen, J.; Lammi, E.; Nudds, T.D.; Väänänen, V.M. Environmental variability and population dynamics: Do European and North American ducks play by the same rules? Ecol. Evol. 2016, 6, 7004–7014. [Google Scholar] [CrossRef]
- Gombobaatar, S.; Monks, E.M.; Seidler, R.; Sumiya, D.; Tseveenmyadag, N.; Bayarkhuu, S.; Baillie, J.; Boldbaatar, S.; Uuganbayar, C. Regional Red List; Series Birds; Zoological Society of London, National University of Mongolia and Mongolian Ornithological Society: London, UK, 2011; Volume 7. [Google Scholar]
- Nyambayar, B.; Tseveenmyadag, N. (Eds.) Directory of Important Bird Areas in Mongolia: Key Sites for Conservation; Wildlife Science and Conservation Center, Institute of Biology and BirdLife International: Ulaanbaatar, Mongolia, 2009. [Google Scholar]
- Batnasan, N. Freshwater issues in Mongolia. In Proceedings of the National Seminar on IRBM in Mongolia, Ulaanbaatar, Mongolia, 24–25 September 2003; p. 24. [Google Scholar]
- Batbayar, N.; Purev-Ochir, G. Biodiversity and Artisanal and Small-Scale Mining in Mongolia; Scoping High Biodiversity Values in Soums with Active Artisanal and Small-Scale Mining (ASM)within ESEC II Project Sites; Wildlife Science and Conservation of Mongolia: Ulaanbaatar, Mongolia, 2004. [Google Scholar] [CrossRef]
- Ganbold, O.; Bing, G.C.; Paik, I.H.; Purevee, E.; Munkhbayar, M.; Jargalsaikhan, A.; Paek, W.K. Avifauna of Mongol Daguur important bird area in Eastern Mongolia. Korean J. Ornithol. 2017, 24, 13–23. [Google Scholar] [CrossRef]
- BirdLife International. 2010. The Flyways Concept Can Help Coordinate Global Efforts to Conserve Migratory Birds. Available online: http://www.birdlife.org (accessed on 13 July 2020).
- Liu, H. Conservation of wetlands especially as waterfowl habitat in northeast China. Chin. Geogr. Sci. 1998, 8, 281–288. [Google Scholar] [CrossRef]
- Kang, S.; Hong, S.Y. Assessing seasonal and inter-annual variations of lake surface areas in Mongolia during 2000-2011 using minimum composite MODIS NDVI. PLoS ONE 2016, 11, e0151395. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Fang, J.; Zhao, X.; Zhao, S.; Shen, H.; Hu, H.; Tang, Z.; Wang, Z.; Guo, Q. Rapid loss of lakes on the Mongolian Plateau. Proc. Natl. Acad. Sci. USA 2015, 112, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Sumiya, E.; Dorjsuren, B.; Yan, D.; Dorligjav, S.; Wang, H.; Enkhbold, A.; Weng, B.; Qin, T.; Wang, K.; Gerelmaa, T.; et al. Changes in Water Surface Area of the Lake in the Steppe Region of Mongolia: A Case Study of Ugii Nuur Lake, Central Mongolia. Water 2020, 12, 1470. [Google Scholar] [CrossRef]
- Bayarjargal, Y.; Adyasuren, T.; Munkhtuya, S. Drought and vegetation monitoring in the arid and semi-arid regions of the Mongolia using remote sensing and ground data. In Proceedings of the 21st Asian Conference on Remote Sensing, Taipei, Taiwan, 4–8 December 2000; Volume 1, pp. 372–377. [Google Scholar]
- Ganbold, O.; Bing, G.C.; Lee, J.H.; Munkhbayar, M.; Paik, I.H.; Jargalsaikhan, A.; Purevee, E.; Purevdorj, Z.; Paek, W.K. An avifaunal survey of middle Mongolian wetlands: Important Bird Areas and threatened species. J. Asia-Pac. Biodivers. 2018, 11, 340–345. [Google Scholar] [CrossRef]
- Kang, S.; Lee, G.; Togtokh, C.; Jang, K. Characterizing regional precipitation-driven lake area change in Mongolia. J. Arid. Land 2014, 7, 146–158. [Google Scholar] [CrossRef]
- Nyambayar, B.; Tuvshinjargal, E.; David, W.; Otgonbayar, T.; Tseveenmyadag, N. For the migration survey of Mongolian birds. J. Toodog 2016, 2, 10–16. (In Mongolian) [Google Scholar]
- Purevdorj, Z.; Paek, W.K.; Munkhbayar, M.; Ganbold, O.; Bing, G.C.; Jargalsaikhan, A.; Purevee, E.; Paik, I.H.; Choi, W.S.; Jargal, N.; et al. The avifaunal survey at Important Bird Areas in western Mongolia. Korean J. Ornitol. 2019, 26, 7–15. [Google Scholar] [CrossRef]
- Tuvshintugs, S.; Gankhuyag, P.; Bolormunkh, E.; Tseveenmyadag, N. The avifauna and their conservation in Dariganga Nature Park, Mongolia. J. Toodog 2016, 2, 1–9. (In Mongolian) [Google Scholar]
- Tian, S.; Xu, X.L.; Liu, S.T.; Zhang, S.P. The influence of Dalai Lake area change on waterbird community. Sichuan J. Zool. 2016, 35, 201–209. [Google Scholar] [CrossRef]
- Limnological Gatalog of Mongolian Lakes 2015. Available online: http://oslo.geodata.es/mongolian_lakes/map/mongolia-map.php?lang=en (accessed on 15 May 2020).
- Mitamura, O.; Khadbaatar, D.; Ishida, N. Comparative investigation of chemical and biological characteristics in waters and trophic state of Mongolian lakes. Limnology 2010, 11, 17–30. [Google Scholar] [CrossRef]
- Tserensodnom, J. Catalog of Mongolian Lakes; The Institute of Geography, Mongolian Academy of Science: Ulaanbaatar, Mongolia, 2000; pp. 26–58. [Google Scholar]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A. Bird Census Techniques; Academic Press: London, UK, 1992. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Champaign, IL, USA, 1963. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Simpson, E. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Davis, J.C. Statistics and Data Analysis in Geology; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015; Available online: https://www.R-project.org/ (accessed on 22 January 2023).
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. 2019. Available online: https://cran.r-project.org/package=vegan (accessed on 5 January 2022).
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Francois, H.; Sebastien Le, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R, 2nd ed.; Chapman Hall/CRC: Boca Raton, FL, USA, 2017; Available online: http://factominer.free.fr/bookV2/index.html (accessed on 10 January 2023).
- Paracuellos, M.; Tellería, J.L. Factors affecting the distribution of a waterbird community: The role of habitat configuration and bird abundance. Waterbirds 2004, 27, 446–453. [Google Scholar] [CrossRef]
- Batbayar, N.; Takekawa, J.Y.; Natsagdorj, T.; Spragens, K.A.; Xiao, X. Site selection and nest survival of the Bar-headed Goose (Anser indicus) on the Mongolian Plateau. Waterbirds 2014, 37, 381–393. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, G.; Li, F.; Ma, T.; Lu, J.; Qian, F. A revised species population estimate for the bar-headed goose (Anser indicus). Avian Res. 2017, 8, 7. [Google Scholar] [CrossRef]
- Batbayar, N.; Takekawa, J.Y.; Newman, S.H.; Prosser, D.J.; Natsagdorj, T.; Xiao, X. Migration strategies of Swan Geese Anser cygnoides from northeast Mongolia. Wildfowl 2011, 61, 90–109. [Google Scholar]
- Goroshko, O.A. Extremely unfavourable year (2003) for Swan Geese in Dauria trans-boundary region (Russia and Mongolia). In Proceedings of the International Anatidae Symposium in East Asia & Siberia Region, Seosan, Korea, 31 October–3 November 2003; pp. 83–92. [Google Scholar]
- Goroshko, O.A. Data for waterbirds at Buyr-Nuur (Eastern Mongolia). Mong. J. Biol. Sci. 2004, 2, 67–68. [Google Scholar] [CrossRef]
- BirdLife International. Tadorna ferruginea. The IUCN Red List of Threatened Species 2016:e.T22680003A86011049. 2016. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22680003A86011049.en (accessed on 19 August 2021).
- BirdLife International. Anser cygnoid. The IUCN Red List of Threatened Species 2016: E.T22679869A92832782. 2016. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22679869A92832782.en (accessed on 19 August 2021).
- BirdLife International. Cygnus cygnus. The IUCN Red List of Threatened Species 2016: E. T22679856A85965262. 2016. Available online: https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22679856A85965262.en (accessed on 19 August 2021).
- BirdLife International. Anser indicus. The IUCN Red List of Threatened Species 2018: E.T22679893A131908564. 2018. Available online: https://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22679893A131908564.en (accessed on 19 August 2021).
- Hattori, A.; Mae, S. Habitat use and diversity of waterbirds in a coastal lagoon around Lake Biwa, Japan. Ecol. Res. 2001, 16, 543–553. [Google Scholar] [CrossRef]
- Colwell, M.A.; Taft, O.W. Waterbird communities in managed wetlands of varying water depth. Waterbirds 2000, 23, 45–55. [Google Scholar]
- Guadagnin, D.L.; Maltchik, L. Habitat and landscape factors associated with neotropical waterbird occurrence and richness in wetland fragments. Biodivers. Conserv. 2007, 16, 1231–1244. [Google Scholar] [CrossRef]
- Scheffer, M.; Van Geest, G.J.; Zimmer, K.; Jeppesen, E.; Søndergaard, M.; Butler, M.G.; Hanson, M.A.; Declerck, S.; De Meester, L. Small habitat size and isolation can promote species richness: Second-order effects on biodiversity in shallow lakes and ponds. Oikos 2006, 112, 227–231. [Google Scholar] [CrossRef]
- Elmberg, J.; Nummi, P.; Poysa, H.; Sjoberg, K. Relationships between species number, lake size and resource diversity in assemblages of breeding waterfowl. J. Biogeogr. 1994, 21, 75–84. [Google Scholar] [CrossRef]
- Tavares, D.C.; Guadagnin, D.L.; de Moura, J.F.; Siciliano, S.; Merico, A. Environmental and anthropogenic factors structuring waterbird habitats of tropical coastal lagoons: Implications for management. Biol. Conserv. 2015, 186, 12–21. [Google Scholar] [CrossRef]
- Nummi, P.; Väänänen, V.M. High overlap in diets of sympatric dabbling ducks—An effect of food abundance? In Annales Zoologici Fennici; Finnish Zoological and Botanical Publishing Board: Helsinki, Finland, 2001; pp. 123–130. Available online: https://www.jstor.org/stable/23735758 (accessed on 31 May 2020).
- Fredrickson, L.H.; Reid, F.A. Nutritional Values of Waterfowl Foods. In Waterfowl Management Handbook; U.S. Fish and Wildlife Service: Fort Collins, CO, USA, 1988. [Google Scholar]
- Gregg, W.W.; Rose, F.L. Influences of aquatic macrophytes on invertebrate community structure, guild structure, and microdistribution in streams. Hydrobiologia 1985, 128, 45–56. [Google Scholar] [CrossRef]
- Wersal, R.M.; Getsinger, K.D. Chapter 3: Impacts of invasive aquatic plants on waterfowl. In Biology and Control of Aquatic Plants: A Best Management Practices Handbook; Aquatic Ecosystem Restoration Foundation: Marietta, GA, USA, 2009; Volume 210, pp. 19–24. [Google Scholar]
- Hutto, R.L. On the importance of stopover sites to migrating birds. Auk 1998, 115, 823–825. [Google Scholar] [CrossRef]
- Ministry of Environment and Green Development. Mongolian Second Assessment Report on Climate Change; Ministry of Environment and Tourism: Ulaanbaatar, Mongolia, 2014; pp. 109–126. [Google Scholar]
- Fredrickson, L.H.; Drobney, R.D. Habitat utilization by post breeding waterfowl. In Waterfowl and Wetlands: An Integrated Review; Midwest Fish & Wildlife Conference: Overland Park, KS, USA, 1979; pp. 119–127. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).