Threat Analysis of Forest Fragmentation and Degradation for Peruvian Primates
Abstract
1. Introduction
2. Materials and Methods
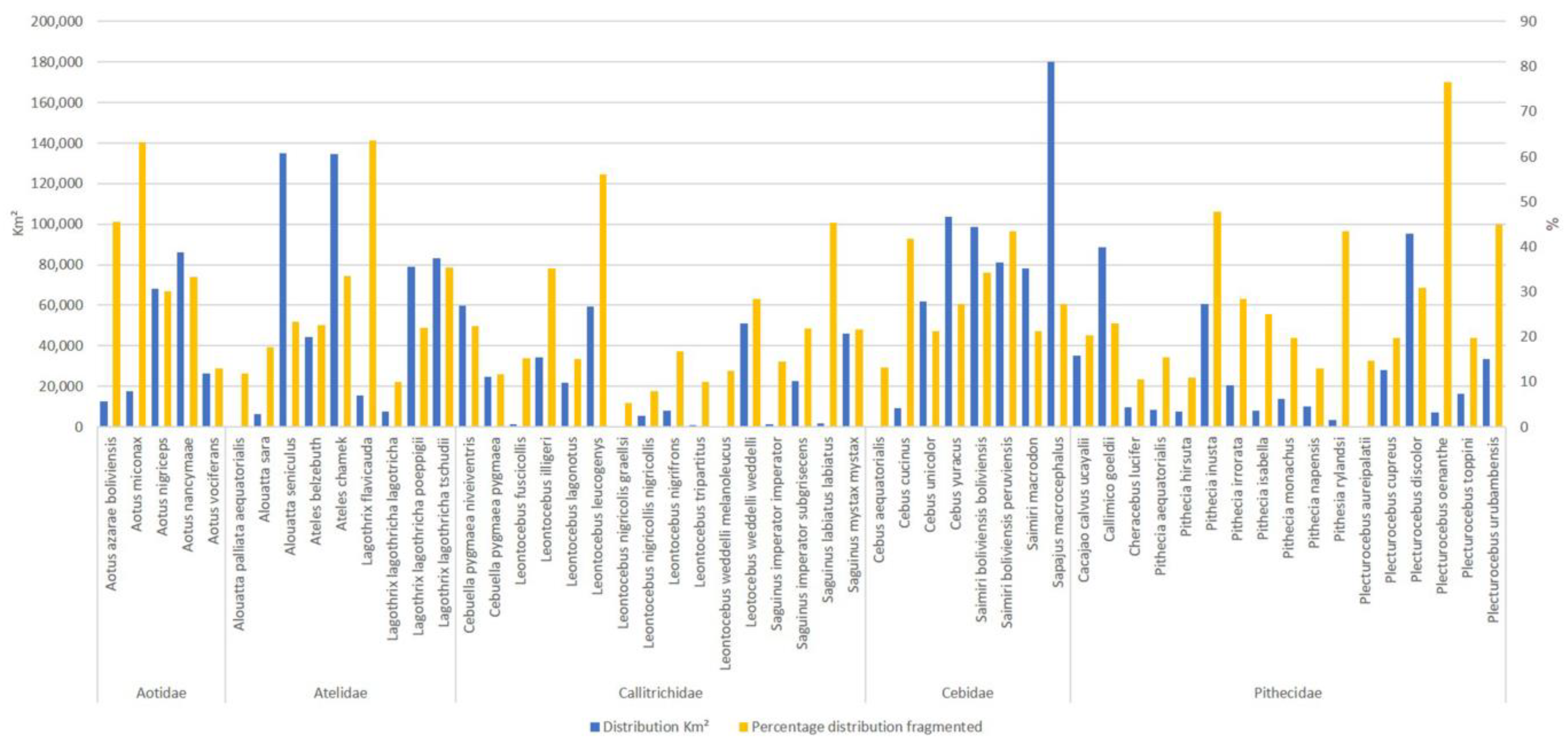
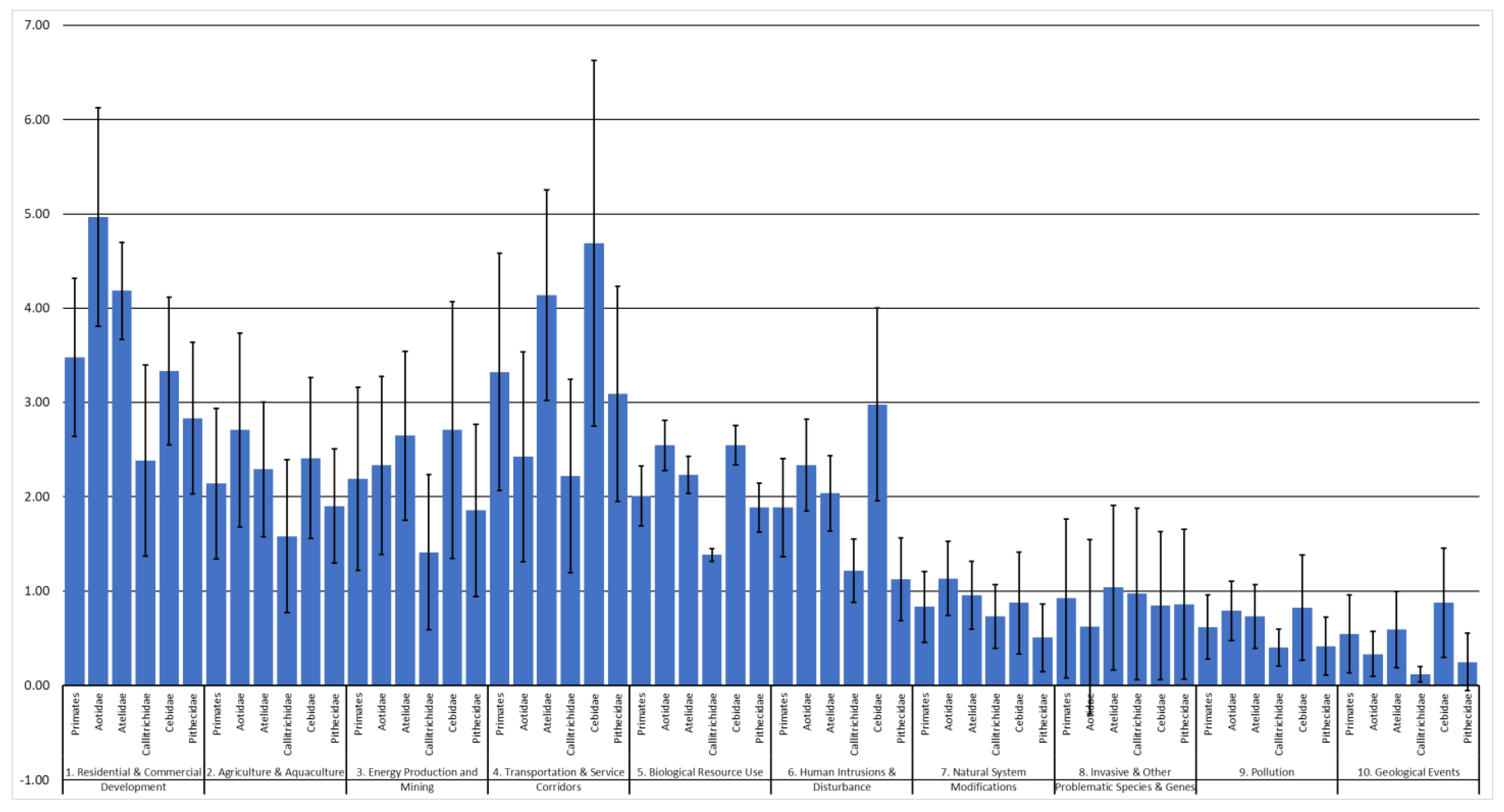
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IUCN. IUCN Red List of Threatened Species. Available online: https://www.redlist.org (accessed on 1 September 2021).
- Estrada, A.; Garber, P.A.; Chaudhary, A. Expanding global commodities trade and consumption place the world’s primates at risk of extinction. PeerJ 2019, 7, e7068. [Google Scholar] [CrossRef]
- Estrada, A.; Garber, P.A.; Rylands, A.B.; Roos, C.; Fernández-Duque, E.; Di Fiore, A.; Nekaris, K.A.-I.; Nijman, V.; Heymann, E.W.; Lambert, J.E.; et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 2017, 3, e1600946. [Google Scholar] [CrossRef] [PubMed]
- Crooks, K.R.; Burdett, C.L.; Theobald, D.M.; King, S.R.B.; Di Marco, M.; Rondinini, C.; Boitani, L. Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proc. Natl. Acad. Sci. USA 2017, 114, 7635–7640. [Google Scholar] [CrossRef] [PubMed]
- Palmeirim, A.F.; Santos-Filho, M.; Peres, C.A. Marked decline in forest-dependent small mammals following habitat loss and fragmentation in an Amazonian deforestation frontier. PLoS ONE 2020, 15, e0230209. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F.; Delamônica, P.; Laurance, S.G.; Vasconcelos, H.L.; Lovejoy, T.E. Rainforest fragmentation kills big trees. Nature 2000, 404, 836. [Google Scholar] [CrossRef] [PubMed]
- Jamhuri, J.; Samantha, L.D.; Tee, S.L.; Kamarudin, N.; Ashton-Butt, A.; Zubaid, A.; Lechner, A.M.; Azhar, B. Selective logging causes the decline of large-sized mammals including those in unlogged patches surrounded by logged and agricultural areas. Biol. Conserv. 2018, 227, 40–47. [Google Scholar] [CrossRef]
- Cudney-Valenzuela, S.J.; Arroyo-Rodríguez, V.; Morante-Filho, J.C.; Toledo-Aceves, T.; Andresen, E. Tropical forest loss impoverishes arboreal mammal assemblages by increasing tree canopy openness. Ecol. Appl. 2023, 33, e2744. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.G.; Ribeiro, M.C.; Hasui, É.; da Costa, C.A.; da Cunha, R.G.T. Patch Size, Functional Isolation, Visibility and Matrix Permeability Influences Neotropical Primate Occurrence within Highly Fragmented Landscapes. PLoS ONE 2015, 10, e0114025. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, K.; Paine, C.E.T.; Cornejo Valverde, F.H.; Pringle, E.G.; Beck, H.; Terborgh, J. Changes in tree community structure in defaunated forests are not driven only by dispersal limitation. Ecol. Evol. 2020, 10, 3392–3401. [Google Scholar] [CrossRef]
- Benchimol, M.; Peres, C.A. Predicting primate local extinctions within “real-world” forest fragments: A pan-neotropical analysis. Am. J. Primatol. 2014, 76, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, M.; Mace, G.M.; Jones, K.E.; Bielby, J.; Bininda-Emonds, O.R.P.; Sechrest, W.; Orme, C.D.L.; Purvis, A. Multiple causes of high extinction risk in large mammal species. Science 2005, 309, 1239–1241. [Google Scholar] [CrossRef]
- Carretero-Pinzón, X.; Defler, T.R.; McAlpine, C.A.; Rhodes, J.R. What do we know about the effect of patch size on primate species across life history traits? Biodivers. Conserv. 2016, 25, 37–66. [Google Scholar] [CrossRef]
- Galán-Acedo, C.; Arroyo-Rodríguez, V.; Cudney-Valenzuela, S.J.; Fahrig, L. A global assessment of primate responses to landscape structure. Biol. Rev. 2019, 94, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Benchimol, M.; Pere, C.A. Anthropogenic modulators of species-area relationships in Neotropical primates: A continental-scale analysis of fragmented forest landscapes. Divers. Distrib. 2013, 19, 1339–1352. [Google Scholar] [CrossRef]
- Programa Bosques. Bosque—no bosque y pérdida de bosque Húmedo Amazónico 2000–2014; Programa Nacional de Conservación de Bosques para la Mitigación del Cambio Climatico: Lima, Peru, 2015. [Google Scholar]
- Watch, G.F. World Resources Institute Open Data Porta. Available online: http://data.globalforestwatch.org/datasets/mining-concessions (accessed on 20 May 2020).
- GIZ. Cambio de Uso Actual de la Tierra en la Amazonía Peruana: Avances e Implementación en el Marco de la Ley Forestal y de Fauna Silvestre 29763; Cooperación Alemana: Lima, Peru, 2016; p. 28. [Google Scholar]
- Kahhat, R.; Parodi, E.; Larrea-Gallegos, G.; Mesta, C.; Vázquez-Rowe, I. Environmental impacts of the life cycle of alluvial gold mining in the Peruvian Amazon rainforest. Sci. Total Environ. 2019, 662, 940–951. [Google Scholar] [CrossRef]
- Oliveira, P.J.C.; Asner, G.P.; Knapp, D.E.; Almeyda, A.; Galván-Gildemeister, R.; Keene, S.; Raybin, R.F.; Smith, R.C. Land-Use Allocation Protects the Peruvian Amazon. Science 2007, 317, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Laurance, W.F. Conservation and the Global Infrastructure Tsunami: Disclose, Debate, Delay! Trends Ecol. Evol. 2018, 33, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.; Cornejo, F.; Cortés-Ortiz, L.; Encarnación, C.F.; Heymann, E.W.; Marsh, L.K.; Mittermeier, R.A.; Rylands, A.B.; Vermeer, J. Primates de Peru, Guia de Identificacion de Bolsillo; Conservation International: Arlington, VA, USA, 2015. [Google Scholar]
- Pacheco, V.; Graham-Angeles, L.; Dian Peña, S.R.; Hurtado, C.M.; Ruelas, D.; Cervantes Zevallos, O.K.; Serrano Villavicencio, J.E. Diversidad y distribución de los mamíferos del Perú por departamentos y ecorregiones I: Didelphimorphia, Paucituberculata, Sirenia, Cingulata, Pilosa, Primates, Lagomorpha, Eulipotyphla, Carnivora, Perissodactyla y Artiodactyla. Rev. Peru. Biol. 2020, 27, 289–328. [Google Scholar] [CrossRef]
- Hurtado, C.M.; Serrano-Villavicencio, J.; Pacheco, V. Densidad poblacional y conservación de los primates de la Reserva de Biosfera del Noroeste, Tumbes, Perú. Rev. Peru. Biol. 2016, 23, 151–158. [Google Scholar] [CrossRef]
- Boonratana, R. Asian primates in fragments: Understanding causes and consequences of fragmentation, and predicting primate population viability. Am. J. Primatol. 2020, 82, e23082. [Google Scholar] [CrossRef] [PubMed]
- Salafsky, N.; Salzer, D.; Stattersfield, A.J.; Hilton-Taylor, C.; Neugarten, R.; Butchart, S.H.M.; Collen, B.E.N.; Cox, N.; Master, L.L.; O’Connor, S.; et al. A Standard Lexicon for Biodiversity Conservation: Unified Classifications of Threats and Actions. Conserv. Biol. 2008, 22, 897–911. [Google Scholar] [CrossRef] [PubMed]
- Rondón, S.; Cavallero, S.; Renzi, E.; Link, A.; González, C.; D’Amelio, S. Parasites of Free-Ranging and Captive American Primates: A Systematic Review. Microorganisms 2021, 9, 2546. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Bilbao, G.; Martin-Solano, S.; Saegerman, C. Zoonotic Blood-Borne Pathogens in Non-Human Primates in the Neotropical Region: A Systematic Review. Pathogens 2021, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Solórzano-García, B.; Pérez-Ponce de León, G. Parasites of Neotropical Primates: A Review. Int. J. Primatol. 2018, 39, 155–182. [Google Scholar] [CrossRef]
- Rowe, N.; Myers, M. All the Worlds Primates; Pogonias Press: Charleston, RI, USA, 2016; p. 777. [Google Scholar]
- Ambiente, M.d. Estudio Para la Identificacion de Areas Degradadas y Propuesta de Monitoreo; Dirrecion General de Ordenamiento Territorial Ambiental—MINAM: Lima, Peru, 2018; p. 44. [Google Scholar]
- Rogelj, J.; Meinshausen, M.; Knutti, R. Global warming under old and new scenarios using IPCC climate sensitivity range estimates. Nat. Clim. Chang. 2012, 2, 248–253. [Google Scholar] [CrossRef]
- Kriticos, D.J.; Webber, B.L.; Leriche, A.; Ota, N.; Macadam, I.; Bathols, J.; Scott, J.K. CliMond: Global high-resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol. Evol. 2012, 3, 53–64. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Moss, R.H.; Edmonds, J.A.; Hibbard, K.A.; Manning, M.R.; Rose, S.K.; van Vuuren, D.P.; Carter, T.R.; Emori, S.; Kainuma, M.; Kram, T.; et al. The next generation of scenarios for climate change research and assessment. Nature 2010, 463, 747–756. [Google Scholar] [CrossRef]
- Allgas, N.; Shanee, S.; Shanee, N.; Collongues de Palomino, H. Rapid Survey of the Primate Density and Biomass at Katakari, Pacaya Samiria National Reserve, Peru. Primate Conserv. 2018, 32, 57–66. [Google Scholar]
- Terborgh, J. Five New World Primates: A Study in Comparative Ecology; Princeton University Press: Princeton, NJ, USA, 1983; p. 276. [Google Scholar]
- Finer, M.; Jenkins, C.N. Proliferation of Hydroelectric Dams in the Andean Amazon and Implications for Andes-Amazon Connectivity. PLoS ONE 2012, 7, e35126. [Google Scholar] [CrossRef]
- CIA. The World Fact Book. Available online: https://www.cia.gov/the-world-factbook/countries/peru/#people-and-society (accessed on 4 February 2021).
- Estrada, A.; Garber, P.A.; Chaudhary, A. Current and future trends in socio-economic, demographic and governance factors affecting global primate conservation. PeerJ 2020, 8, e9816. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A. Socioeconomic Contexts of Primate Conservation: Population, Poverty, Global Economic Demands, and Sustainable Land Use. Am. J. Primatol. 2013, 75, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Schjellerup, I. La Morada. A case study on the impact of human pressure on the environment in the Ceja de Selva, northeastern Peru. AMBIO J. Hum. Environ. 2000, 29, 451–454. [Google Scholar] [CrossRef]
- Shanee, N.; Shanee, S. Land trafficking, migration, and conservation in the “no-man’s land” of northeastern Peru. Trop. Conserv. Sci. 2016, 9, 1–16. [Google Scholar] [CrossRef]
- Gallice, G.R.; Larrea-Gallegos, G.; Vázquez-Rowe, I. The threat of road expansion in the Peruvian Amazon. Oryx 2017, 53, 284–292. [Google Scholar] [CrossRef]
- Lindshield, S.M. Protecting Nonhuman Primates in Peri-Urban Environments: A Case Study of Neotropical Monkeys, Corridor Ecology, and Coastal Economy in the Caribe Sur of Costa Rica. In Ethnoprimatology: Primate Conservation in the 21st Century; Waller, M.T., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 351–369. [Google Scholar]
- Bueno, C.; Sousa, C.O.M.; Freitas, S.R. Habitat or matrix: Which is more relevant to predict road-kill of vertebrates? Braz. J. Biol. 2015, 75, 228–238. [Google Scholar] [CrossRef]
- Chapman, C.A.; Twinomugisha, D.; Teichroeb, J.A.; Valenta, K.; Sengupta, R.; Sarkar, D.; Rothman, J.M. How Do Primates Survive Among Humans? Mechanisms Employed by Vervet Monkeys at Lake Nabugabo, Uganda. In Ethnoprimatology: Primate Conservation in the 21st Century; Waller, M.T., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 77–94. [Google Scholar]
- Estrada, A.; Raboy, B.E.; Oliveira, L.C. Agroecosystems and Primate Conservation in The Tropics: A Review. Am. J. Primatol. 2012, 74, 696–711. [Google Scholar] [CrossRef]
- Kalbitzer, U.; Chapman, C.A. Primate Responses to Changing Environments in the Anthropocene. In Primate Life Histories, Sex Roles, and Adaptability: Essays in Honour of Linda M. Fedigan; Kalbitzer, U., Jack, K.M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 283–310. [Google Scholar]
- Linder, J.M.; Palkovitz, R.E. The Threat of Industrial Oil Palm Expansion to Primates and Their Habitats. In Ethnoprimatology: Primate Conservation in the 21st Century; Waller, M.T., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 21–45. [Google Scholar]
- Gutiérrez-Vélez, V.H.; DeFries, R.; Pinedo-Vásquez, M.; Uriarte, M.; Padoch, C.; Baethgen, W.; Fernandes, K.; Lim, Y. High-yield oil palm expansion spares land at the expense of forests in the Peruvian Amazon. Environ. Res. Lett. 2011, 6, 044029. [Google Scholar] [CrossRef]
- Programa Bosques. Estrategia nacional Sobre bosques y cambio climático; Programa Nacional de Conservación de Bosques para la Mitigación del Cambio Climatico: Lima, Peru, 2016. [Google Scholar]
- Mayor, P.; Pérez-Peña, P.; Bowler, M.; Puertas, P.E.; Kirkland, M.; Bodmer, R. Effects of selective logging on large mammal populations in a remote indigenous territory in the northern Peruvian Amazon. Ecol. Soc. 2015, 20, 36. [Google Scholar] [CrossRef]
- Leberatto, A.C. Understanding the illegal trade of live wildlife species in Peru. Trends Organ. Crime 2016, 19, 42–66. [Google Scholar] [CrossRef]
- Finer, M.; Jenkins, C.N.; Sky, M.A.B.; Pine, J. Logging Concessions Enable Illegal Logging Crisis in the Peruvian Amazon. Sci. Rep. 2014, 4, 4719. [Google Scholar] [CrossRef] [PubMed]
- Endo, W.; Peres, C.A.; Salas, E.; Mori, S.; Sanchez-Vega, J.-L.; Shepard, G.H.; Pacheco, V.; Yu, D.W. Game Vertebrate Densities in Hunted and Nonhunted Forest Sites in Manu National Park, Peru. Biotropica 2010, 42, 251–261. [Google Scholar] [CrossRef]
- Mendoza, A.P.; Shanee, S.; Cavero, N.; Lujan-Vega, C.; Ibañez, Y.; Rynaby, C.; Villena, M.; Murillo, Y.; Olson, S.H.; Perez, A.; et al. Domestic networks contribute to the diversity and composition of live wildlife trafficked in urban markets in Peru. Glob. Ecol. Conserv. 2022, 37, e02161. [Google Scholar] [CrossRef]
- Shanee, N.; Mendoza, A.P.; Shanee, S. Diagnostic overview of the illegal trade in primates and law enforcement in Peru. Am. J. Primatol. 2017, 79, e22516. [Google Scholar] [CrossRef] [PubMed]
- Mayor, P.; El Bizri, H.R.; Morcatty, T.Q.; Moya, K.; Bendayán, N.; Solis, S.; Vasconcelos Neto, C.F.A.; Kirkland, M.; Arevalo, O.; Fang, T.G.; et al. Wild meat trade over the last 45 years in the Peruvian Amazon. Conserv. Biol. 2022, 36, e13801. [Google Scholar] [CrossRef] [PubMed]
- Daut, E.F.; Brightsmith, D.J.; Peterson, M.J. Role of non-governmental organizations in combating illegal wildlife–pet trade in Peru. J. Nat. Conserv. 2015, 24, 72–82. [Google Scholar] [CrossRef]
- Shanee, N.; Shanee, S. Denunciafauna–A social media campaign to evaluate wildlife crime and law enforcement in Peru. J. Political Ecol. 2021, 28, 533–552. [Google Scholar] [CrossRef]
- Wilkinson, D.A.; Marshall, J.C.; French, N.P.; Hayman, D.T.S. Habitat fragmentation, biodiversity loss and the risk of novel infectious disease emergence. J. R. Soc. Interface 2018, 15, 20180403. [Google Scholar] [CrossRef]
- Gibb, R.; Redding, D.W.; Chin, K.Q.; Donnelly, C.A.; Blackburn, T.M.; Newbold, T.; Jones, K.E. Zoonotic host diversity increases in human-dominated ecosystems. Nature 2020, 584, 398–402. [Google Scholar] [CrossRef]
- Rulli, M.C.; Santini, M.; Hayman, D.T.S.; D’Odorico, P. The nexus between forest fragmentation in Africa and Ebola virus disease outbreaks. Sci. Rep. 2017, 7, 41613. [Google Scholar] [CrossRef]
- White, L.A.; Forester, J.D.; Craft, M.E. Disease outbreak thresholds emerge from interactions between movement behavior, landscape structure, and epidemiology. Proc. Natl. Acad. Sci. USA 2018, 115, 7374. [Google Scholar] [CrossRef] [PubMed]
- Vittor, A.Y.; Gilman, R.H.; Tielsch, J.; Glass, G.; Shields, T.; Lozano, W.S.; Pinedo-Cancino, V.; Patz, J.A. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of Falciparum malaria in the Peruvian Amazon. Am. J. Trop. Med. Hyg. 2006, 74, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wenz, A.; Heymann, E.W.; Petney, T.N.; Taraschewski, H.F. The influence of human settlements on the parasite community in two species of Peruvian tamarin. Parasitology 2010, 137, 675–684. [Google Scholar] [CrossRef]
- Gillespie, T.R.; Chapman, C.A. Prediction of parasite infection dynamics in primate metapopulations based on attributes of forest fragmentation. Conserv. Biol. 2006, 20, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Rocklöv, J.; Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Scheffers, B.R.; De Meester, L.; Bridge, T.C.L.; Hoffmann, A.A.; Pandolfi, J.M.; Corlett, R.T.; Butchart, S.H.M.; Pearce-Kelly, P.; Kovacs, K.M.; Dudgeon, D.; et al. The broad footprint of climate change from genes to biomes to people. Science 2016, 354, aaf7671. [Google Scholar] [CrossRef]
- Sales, L.; Ribeiro, B.R.; Chapman, C.A.; Loyola, R. Multiple dimensions of climate change on the distribution of Amazon primates. Perspect. Ecol. Conserv. 2020, 18, 83–90. [Google Scholar] [CrossRef]
- Thomas, C.D. Climate, climate change and range boundaries. Divers. Distrib. 2010, 16, 488–495. [Google Scholar] [CrossRef]
- Graham, T.L.; Matthews, H.D.; Turner, S.E. A Global-Scale Evaluation of Primate Exposure and Vulnerability to Climate Change. Int. J. Primatol. 2016, 37, 158–174. [Google Scholar] [CrossRef]
- Ribeiro, B.R.; Sales, L.P.; De Marco, P., Jr.; Loyola, R. Assessing Mammal Exposure to Climate Change in the Brazilian Amazon. PLoS ONE 2016, 11, e0165073. [Google Scholar] [CrossRef] [PubMed]
- Strassburg, B.B.N.; Iribarrem, A.; Beyer, H.L.; Cordeiro, C.L.; Crouzeilles, R.; Jakovac, C.C.; Braga Junqueira, A.; Lacerda, E.; Latawiec, A.E.; Balmford, A.; et al. Global priority areas for ecosystem restoration. Nature 2020, 586, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Newmark, W.D.; Jenkins, C.N.; Pimm, S.L.; McNeally, P.B.; Halley, J.M. Targeted habitat restoration can reduce extinction rates in fragmented forests. Proc. Natl. Acad. Sci. USA 2017, 114, 9635. [Google Scholar] [CrossRef] [PubMed]
- Ferrier, S. Prioritizing where to restore Earth’s ecosystems. Nat. News Views 2020, 586, 680–681. [Google Scholar] [CrossRef]
| Threat | Threatens Primate | Threatens Primate Habitat | Causes Fragmentation | Exacerbates Fragmentation | Threatens Primate and Primate Habitats as a Consequence of Fragmentation | Row Total (Score Only) | |
|---|---|---|---|---|---|---|---|
| 1. | Residential and Commercial Development | 29/3.22 | 49/4.05 | 49/4.13 | 45/4.37 | 44/4.19 | 49/3.57 |
| 1.1 | Housing and urban | 26/5.38 | 49/6.38 | 49/6.48 | 45/6.56 | 44/6.31 | 49/5.48 |
| 1.2 | Commercial and industrial areas | 16/4.84 | 36/5.21 | 37/5.20 | 36/5.21 | 36/5.21 | 37/4.50 |
| 1.3 | Tourism and recreation | 13/4.81 | 19/5.00 | 20/4.88 | 22/4.89 | 18/4.86 | 23/3.91 |
| 2. | Agriculture and Aquaculture | 22/1.77 | 53/2.57 | 53/2.71 | 47/2.54 | 49/2.36 | 53/2.09 |
| 2.1 | Annual and Perennial Non-timber Crops | 16/3.71 | 52/4.12 | 52/4.13 | 45/4.25 | 47/3.99 | 52/3.34 |
| 2.1.1 | Shifting agriculture | 13/5.19 | 50/5.65 | 50/5.70 | 44/5.68 | 44/5.40 | 50/4.49 |
| 2.1.2 | Smallholder farming | 16/5.78 | 51/6.57 | 51/6.57 | 45/6.61 | 47/6.28 | 51/5.31 |
| 2.1.3 | Agro-industry farming | 14/5.36 | 38/6.12 | 38/6.12 | 35/6.14 | 36/5.97 | 38/5.11 |
| 2.1.4 | Scale unknown/unrecorded | 1/2.50 | 2/3.75 | 2/3.75 | 1/2.50 | 1/2.50 | 2/2.25 |
| 2.2 | Wood and Pulp Plantations | 10/3.00 | 34/3.38 | 34/3.43 | 32/3.26 | 32/3.10 | 34/2.74 |
| 2.2.1 | Smallholder plantations | 10/5.00 | 34/5.37 | 34/5.44 | 32/5.31 | 32/5.08 | 34/4.41 |
| 2.2.2 | Agro-Industry plantations | 8/4.69 | 31/5.16 | 31/5.24 | 27/5.19 | 26/5.10 | 31/4.08 |
| 2.2.3 | Scale unknown/unrecorded | 1/2.50 | 1/2.50 | 1/2.50 | 1/2.50 | 1/2.50 | 1/2.50 |
| 2.3 | Livestock Farming | 19/2.34 | 51/2.67 | 50/2.68 | 40/2.89 | 41/2.73 | 51/14.17 |
| 2.3.1 | Nomadic grazing | 2/3.75 | 3/4.17 | 3/3.33 | 3/3.33 | 3/3.33 | 3/3.33 |
| 2.3.2 | Smallholder grazing, ranching or farming | 17/5.44 | 49/6.63 | 48/6.72 | 38/7.04 | 39/6.54 | 49/5.15 |
| 2.3.3 | Agro-industry grazing, ranching or farming | 15/5.00 | 37/5.54 | 36/5.56 | 33/5.53 | 34/5.29 | 37/4.55 |
| 2.3.4 | Scale unknown/unrecorded | 1/2.50 | 1/2.50 | 1/2.50 | 1/2.50 | 1/2.50 | 1/2.50 |
| 2.4 | Marine and Freshwater Aquaculture | 5/2.50 | 24/2.50 | 2/2.50 | 2/2.50 | 25/1.65 | |
| 2.4.1 | Subsistence/artisanal aquaculture | 5/5.00 | 22/5.00 | 23/1.17 | |||
| 2.4.2 | Industrial aquaculture | 2/3.33 | 2/3.33 | 2/3.33 | 3/2.00 | ||
| 2.4.3 | Scale unknown/unrecorded | ||||||
| 3. | Energy Production and Mining | 12/2.71 | 47/4.63 | 47/4.68 | 44/4.35 | 43/3.95 | 47/3.54 |
| 3.1 | Oil and gas drilling | 4/5.00 | 39/5.77 | 41/5.61 | 34/5.51 | 31/5.24 | 41/4.02 |
| 3.2 | Mining | 9/5.00 | 36/5.83 | 35/6.00 | 33/5.91 | 325.55 | 36/4.65 |
| 3.3 | Renewable energy | ||||||
| 4. | Transportation and Service Corridors | 18/3.40 | 45/4.94 | 48/5.00 | 41/5.15 | 39/5.03 | 49/3.80 |
| 4.1 | Roads and railroads | 16/5.31 | 45/6.33 | 48/6.51 | 41/6.65 | 38/6.45 | 49/4.90 |
| 4.2 | Utility and service lines | 7/5.36 | 29/5.52 | 31/5.40 | 28/5.36 | 28/5.27 | 32/4.14 |
| 4.3 | Shipping lanes | ||||||
| 4.4 | Flight paths | ||||||
| 5. | Biological Resource Use | 55/1.85 | 54/2.03 | 54/2.07 | 47/2.65 | 49/2.27 | 55/2.03 |
| 5.1 | Hunting and Collecting of Terrestrial Animals | 55/3.95 | 14/2.74 | 10/2.75 | 44/3.75 | 38/3.73 | 55/2.15 |
| 5.1.1 | Intentional use | 55/6.59 | 12/5.42 | 9/5.28 | 44/6.65 | 38/6.51 | 55/3.69 |
| 5.1.2 | Unintentional effects | 43/5.35 | 8/5.00 | 5/5.00 | 31/5.16 | 27/5.28 | 43//2.78 |
| 5.1.3 | Persecution/Control | 11/5.45 | 2/5.00 | 2/5.00 | 8/5.31 | 7/5.00 | 11/2.86 |
| 5.1.4 | Motivation unknown/unrecorded | ||||||
| 5.2 | Gathering of Terrestrial Plants | 2/1.67 | 34/1.72 | 31/1.72 | 15/1.72 | 8/1.67 | 36/1.28 |
| 5.2.1 | Intentional use | ||||||
| 5.2.2 | Unintentional effects | 1/5.00 | 34/5.00 | 31/5.00 | 15/5.17 | 8/5.00 | 36/2.49 |
| 5.2.3 | Persecution/Control | 1/5.00 | 1/5.00 | 1/5.00 | 2/1.50 | ||
| 5.2.4 | Motivation unknown/unrecorded | ||||||
| 5.3 | Logging and Wood Harvesting | 25/3.40 | 54/4.26 | 54/4.15 | 44/4.17 | 47/3.79 | 54/3.34 |
| 5.3.1 | Intentional use: subsistence/small scale | ||||||
| 5.3.2 | Intentional use: large scale | ||||||
| 5.3.3 | Unintentional effects: subsistence/small scale | 24/5.21 | 54/6.57 | 54/6.39 | 44/6.31 | 47/5.85 | 54/5.10 |
| 5.3.4 | Unintentional effects: large scale | 24/5.31 | 47/7.07 | 47/6.91 | 43/6.28 | 43/5.99 | 47/5.59 |
| 5.3.5 | Motivation unknown/unrecorded | 1/2.50 | 1/2.50 | 1/2.50 | 1/2.50 | 1/2.50 | 1/2.50 |
| 5.4 | Fishing and Harvesting Aquatic Resources | 1/5.00 | 18/5.00 | 18/1.06 | |||
| 5.4.1 | Intentional use: subsistence/small scale | ||||||
| 5.4.2 | Intentional use: large scale | ||||||
| 5.4.3 | Unintentional effects: subsistence/small scale | 1/5.00 | 18/5.00 | 18/1.06 | |||
| 5.4.4 | Unintentional effects: large scale | ||||||
| 5.4.5 | Persecution/Control | ||||||
| 5.4.6 | Motivation unknown/unrecorded | ||||||
| 6. | Human Intrusions and Disturbance | 28/2.11 | 35/3.21 | 35/3.07 | 343.19 | 26/2.88 | 39/2.37 |
| 6.1 | Recreational Activities | 28/5.27 | 28/5.18 | 28/5.00 | 30/5.25 | 26/5.38 | 36/4.06 |
| 6.2 | War, Civil Unrest and Military Exercises | 3/5.00 | 13/5.38 | 13/5.00 | 13/5.38 | 7/5.00 | 16/3.19 |
| 6.3 | Work and Other Activities | 3/5.00 | 23/5.33 | 22/5.34 | 18/5.42 | 9/5.56 | 23/3.50 |
| 7. | Natural System Modifications | 18/0.83 | 38/1.60 | 32/1.91 | 28/1.37 | 24/1.39 | 38/1.10 |
| 7.1 | Fire and Fire Suppression | 18/5.00 | 36/5.90 | 30/5.83 | 27/5.93 | 24/6.04 | 36/4.35 |
| 7.1.1 | Increase in fire frequency/intensity | 18/5.00 | 36/5.90 | 30/5.83 | 27/5.93 | 24/6.04 | 36/4.35 |
| 7.1.2 | Suppression in fire frequency/intensity | ||||||
| 7.1.3 | Trend unknown/unrecorded | ||||||
| 7.2 | Dams and Water Management/Use | 20/1.53 | 26/1.48 | 8/1.75 | 5/2.20 | 31/1.21 | |
| 7.2.1 | Abstraction of surface water (domestic use) | 1/5.00 | 1/5.00 | 1/2.00 | |||
| 7.2.2 | Abstraction of surface water (commercial use) | 12/5.21 | 7/5.36 | 4/5.00 | 4/5.00 | 12/2.33 | |
| 7.2.3 | Abstraction of surface water (agricultural use) | 5/6.00 | 4/6.25 | 4/6.25 | 3/6.67 | 5/4.00 | |
| 7.2.4 | Abstraction of surface water (unknown use) | ||||||
| 7.2.5 | Abstraction of ground water (domestic use) | 1/5.00 | 4/5.00 | 4/2.00 | |||
| 7.2.6 | Abstraction of ground water (commercial use) | ||||||
| 7.2.7 | Abstraction of ground water (agricultural use) | ||||||
| 7.2.8 | Abstraction of ground water (unknown use) | ||||||
| 7.2.9 | Small dams | 10/5.00 | 23/5.22 | 5.5/00 | 3/5.00 | 24/1.75 | |
| 7.2.10 | Large dams | ||||||
| 7.2.11 | Dams (size unknown) | ||||||
| 7.3 | Other Ecosystem Modifications | ||||||
| 8. | Invasive and Other Problematic Species and Genes | 52/1.36 | 2/0.56 | 2/0.56 | 52/1.36 | 54/1.36 | 55/0.79 |
| 8.1 | Invasive Non-Native/Alien Species | 1/0.00 | |||||
| 8.1.1 | Unspecified species | 1/0.00 | |||||
| 8.1.2 | Named species | ||||||
| 8.2 | Problematic Native Species | 2/2.50 | 2/2.50 | 2/2.50 | 2/2.50 | 2/2.50 | 2/2.75 |
| 8.2.1 | Unspecified species | 2/2.50 | 2/2.50 | 2/2.50 | 2/2.50 | 2/2.50 | 2/2.50 |
| 8.2.2 | Named species | 2/2.50 | 2/2.50 | 2/2.50 | 2/2.50 | 2/2.50 | 2/2.50 |
| 8.3 | Introduced Genetic Material | 1/5.00 | 1/1.00 | ||||
| 8.4 | Pathogens and Microbes | 52/2.38 | 52/2.45 | 52/2.48 | 55/1.38 | ||
| 8.4.1 | Unspecified species | 51/2.50 | 52/2.50 | 52/2.50 | 54/1.44 | ||
| 8.4.2 | Named species | 46/2.61 | 50/2.50 | 51/2.50 | 54/1.38 | ||
| 8.5 | Viral/Prion-induced Diseases | 52/2.45 | 52/2.48 | 54/2.55 | 55/1.43 | ||
| 8.5.1 | Unspecified species (disease) | 51/2.50 | 52/2.50 | 54/2.50 | 54/1.44 | ||
| 8.5.2 | Named species (disease) | 51/2.50 | 51/2.50 | 53/2.64 | 54/1.45 | ||
| 8.6 | Disease of Unknown Cause | 46/2.61 | 46/2.50 | 48/2.50 | 48/0.00 | ||
| 9. | Pollution | 17/0.57 | 48/1.08 | 42/0.86 | 39/0.96 | 26/0.72 | 48/0.64 |
| 9.1 | Household Sewage and Urban Waste Water | 8/2.81 | 2/3.75 | 8/0.75 | |||
| 9.1.1 | Sewage | 8/4.38 | 2/5.00 | 8/1.13 | |||
| 9.1.2 | Run-off | 2/5.00 | 1/5.00 | 2/1.50 | |||
| 9.1.3 | Type unknown/unrecorded | ||||||
| 9.2 | Industrial and Military Effluents | 10/2.50 | 45/3.53 | 39/3.33 | 35/3.64 | 18/3.47 | 46/2.19 |
| 9.2.1 | Oil spills | 37/4.39 | 30/4.33 | 27/4.54 | 12/4.17 | 37/2.51 | |
| 9.2.2 | Seepage from mining | 10/5.00 | 32/4.84 | 29/4.48 | 28/4.73 | 15/5.00 | 33/3.29 |
| 9.2.3 | Type unknown/unrecorded | ||||||
| 9.3 | Agricultural and Forestry Effluents | 3/1.67 | 23/2.25 | 19/1.75 | 16/1.77 | 8/1.88 | 23/1.16 |
| 9.3.1 | Nutrient loads | 5/5.00 | 5/1.00 | ||||
| 9.3.2 | Soil erosion | 23/5.22 | 19/5.26 | 16/5.31 | 8/5.63 | 23/3.04 | |
| 9.3.3 | Herbicides and pesticides | 3/5.00 | 2/5.00 | 5/1.00 | |||
| 9.3.4 | Type unknown/unrecorded | ||||||
| 9.4 | Garbage and Solid Waste | 1/5.00 | 3/5.83 | 1/5.00 | 2/5.00 | 1/5.00 | 4/0.00 |
| 9.5 | Airborne Pollutants | ||||||
| 9.5.1 | Acid rain | ||||||
| 9.5.2 | Smog | ||||||
| 9.5.3 | Ozone | ||||||
| 9.5.4 | Type unknown/unrecorded | ||||||
| 9.6 | Excess Energy | 9/1.81 | 5/1.75 | 5/1.75 | 10/1.35 | ||
| 9.6.1 | Light pollution | 6/2.50 | 3/2.50 | 4/2.50 | 6/1.08 | ||
| 9.6.2 | Thermal pollution | ||||||
| 9.6.3 | Noise pollution | 6/2.92 | 2/5.00 | 2/3.75 | 7/1.00 | ||
| 9.6.4 | Type unknown/unrecorded | ||||||
| 10. | Geological Events | 2/2.50 | 22/2.61 | 20/2.50 | 9/2.50 | 6/2.50 | 22/1.36 |
| 10.1 | Volcanoes | ||||||
| 10.2 | Earthquakes, tsunamis | 2/2.50 | 3/3.33 | 2/2.50 | 2/2.50 | 2/2.50 | 3/2.00 |
| 10.3 | Avalanches, landslides | 2/2.50 | 22/4.77 | 20/4.75 | 20/4.44 | 9/4.17 | 22/2.45 |
| 11. | Climate Change and Severe Weather | 55/6.41 | 55/6.49 | 55/6.42 | 55/6.49 | 55/6.48 | 55/6.46 |
| 11.1 | Habitat Shifting and alteration | 53/7.17 | 55/7.00 | 55/7.00 | 55/7.00 | 55/7.00 | 55/6.98 |
| 11.2 | Droughts | 55/5.91 | 55/6.00 | 55/5.86 | 55/6.00 | 55/5.95 | 55/5.95 |
| 11.3 | Temperature excess | 54/7.13 | 54/7.22 | 54/7.13 | 54/7.22 | 54/7.22 | 54/7.19 |
| 11.4 | Storms and flooding | 54/5.93 | 54/5.97 | 54/5.93 | 54/5.97 | 54/5.97 | 54/5.95 |
| 11.5 | Other impacts |
| Threat | Threatens Primate (1) | Threat | Threatens Primate Habitat (2) | Threat | Causes Fragmentation (3) | Threat | Exacerbates Fragmentation (4) | Threat | Threatens Primate and Primate Habitats as a Consequence of Fragmentation (5) | Threat | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hunting intentional use | 55/ 6.59 | Logging unintentional effects: subsistence/ small-scale | 54/ 6.57 | Logging unintentional effects: subsistence/ small-scale | 54/ 6.39 | Housing and urban | 45/ 6.56 | Smallholder farming | 47/ 6.28 | Logging unintentional effects: subsistence/ small-scale | 54/ 5.10 |
| Hunting unintentional effects | 43/ 5.35 | Smallholder farming | 51/ 6.57 | Smallholder farming | 51/ 6.57 | Smallholder farming | 45/ 6.61 | Logging unintentional effects: subsistence/ small-scale | 47/ 5.85 | Smallholder farming | 51/ 5.31 |
| Housing and urban | 26/ 5.38 | Housing and urban | 49/ 6.38 | Housing and urban | 49/ 6.48 | Hunting intentional use | 44/ 6.65 | Housing and urban | 44/ 6.31 | Housing and urban | 49/ 5.48 |
| Logging unintentional effects: large-scale | 24/ 5.31 | Smallholder grazing, ranching or farming | 49/ 6.63 | Smallholder grazing, ranching or farming | 48/ 6.72 | Logging unintentional effects: subsistence/ small-scale | 44/ 6.31 | Logging unintentional effects: large-scale | 43/ 5.99 | Smallholder grazing, ranching or farming | 49/ 5.15 |
| Smallholder grazing, ranching or farming | 17/ 5.44 | Logging unintentional effects: large-scale | 47/ 7.07 | Roads and railroads | 48/ 6.51 | Logging unintentional effects: large-scale | 43/ 6.28 | Smallholder grazing, ranching or farming | 39/ 6.54 | Roads and railroads | 49/ 4.90 |
| Smallholder farming | 16/ 7.78 | Roads and railroads | 45/ 6.33 | Logging unintentional effects: large-scale | 47/ 6.91 | Roads and railroads | 41/ 6.65 | Roads and railroads | 38/ 6.45 | Logging unintentional effects: large-scale | 47/ 5.59 |
| Agro-industry farming | 38/ 6.12 | Agro-industry farming | 38/ 6.12 | Smallholder grazing, ranching or farming | 38/ 7.04 | Hunting intentional use | 38/ 6.51 | ||||
| Agro-industry farming | 35/ 6.14 | Agro-industry farming | 36/ 5.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanee, S.; Fernández-Hidalgo, L.; Allgas, N.; Vero, V.; Bello-Santa Cruz, R.; Bowler, M.; Erkenswick Watsa, M.; García Mendoza, G.; García-Olaechea, A.; Hurtado, C.; et al. Threat Analysis of Forest Fragmentation and Degradation for Peruvian Primates. Diversity 2023, 15, 276. https://doi.org/10.3390/d15020276
Shanee S, Fernández-Hidalgo L, Allgas N, Vero V, Bello-Santa Cruz R, Bowler M, Erkenswick Watsa M, García Mendoza G, García-Olaechea A, Hurtado C, et al. Threat Analysis of Forest Fragmentation and Degradation for Peruvian Primates. Diversity. 2023; 15(2):276. https://doi.org/10.3390/d15020276
Chicago/Turabian StyleShanee, Sam, Lorena Fernández-Hidalgo, Nestor Allgas, Veronica Vero, Raul Bello-Santa Cruz, Mark Bowler, Mrinalini Erkenswick Watsa, Gabriel García Mendoza, Alvaro García-Olaechea, Cindy Hurtado, and et al. 2023. "Threat Analysis of Forest Fragmentation and Degradation for Peruvian Primates" Diversity 15, no. 2: 276. https://doi.org/10.3390/d15020276
APA StyleShanee, S., Fernández-Hidalgo, L., Allgas, N., Vero, V., Bello-Santa Cruz, R., Bowler, M., Erkenswick Watsa, M., García Mendoza, G., García-Olaechea, A., Hurtado, C., Vega, Z., Marsh, L., Boonratana, R., & Mendoza, A. P. (2023). Threat Analysis of Forest Fragmentation and Degradation for Peruvian Primates. Diversity, 15(2), 276. https://doi.org/10.3390/d15020276







