Prokaryotic Diversity and Dynamics during Dinoflagellate Bloom Decays in Coastal Tunisian Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sampling Procedures
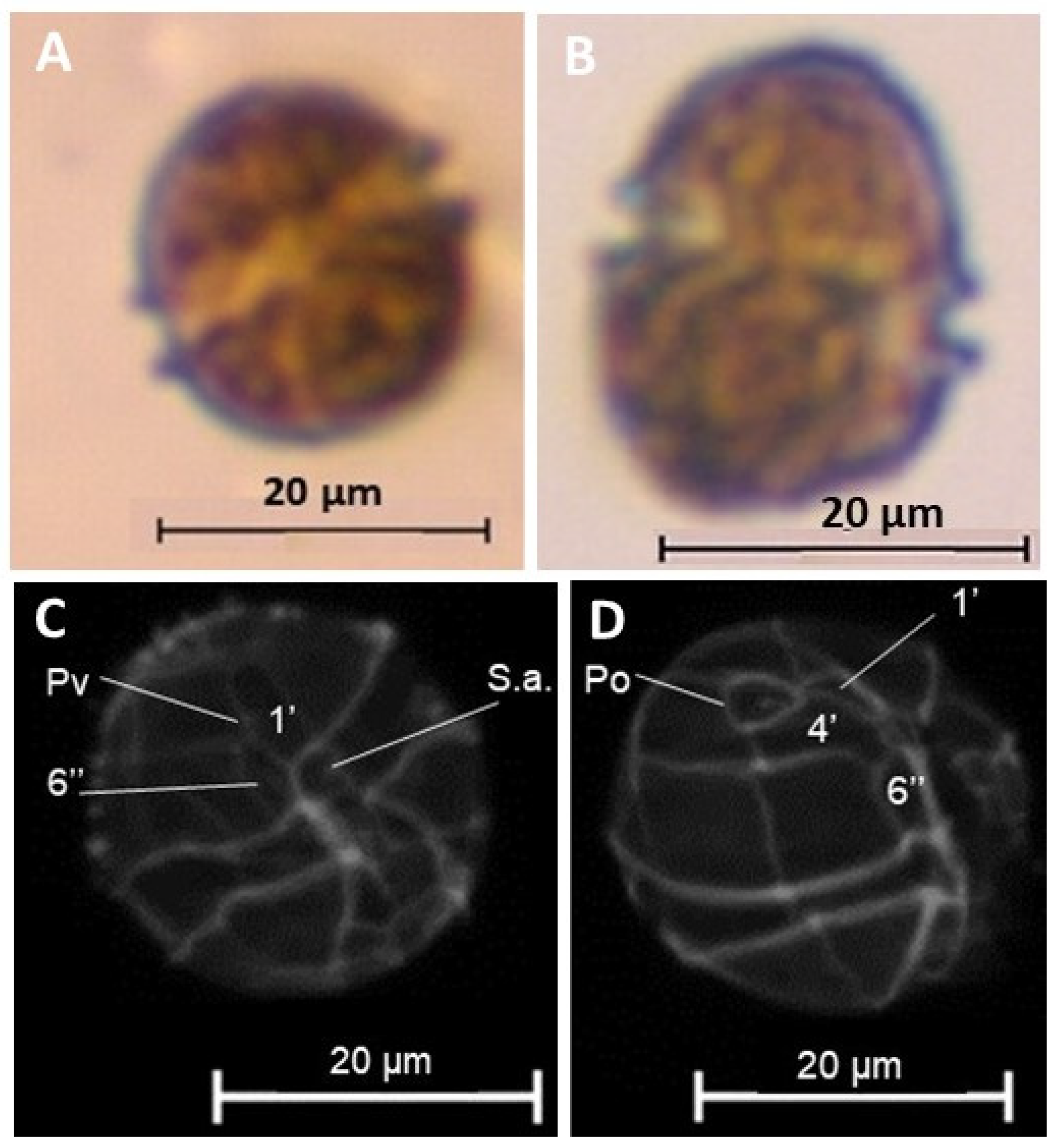
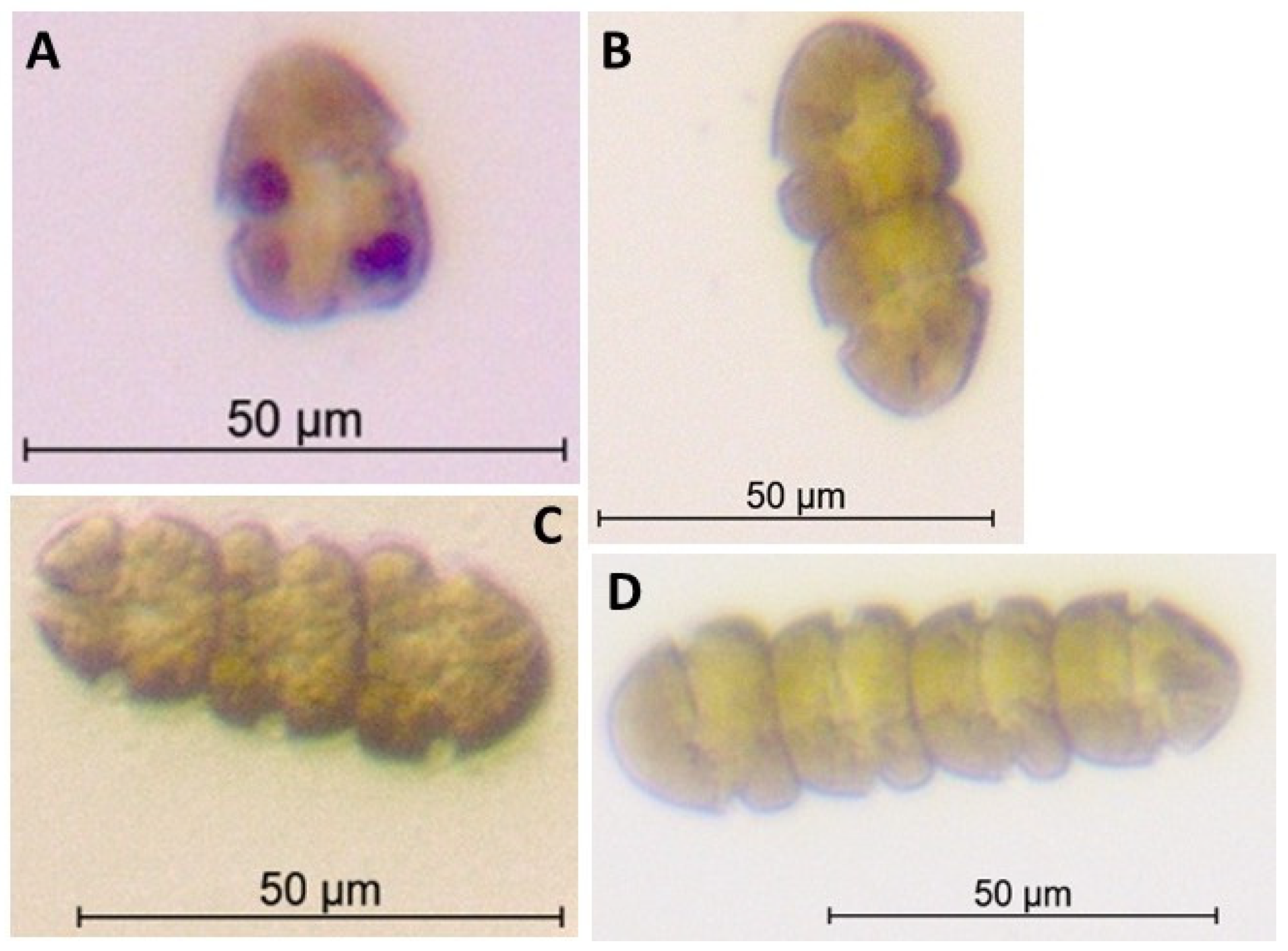
2.3. Dinoflagellate Enumeration, Cultures, and Microscopy Analyses
2.4. Environmental Parameters and Nutrient Analyses
2.5. Molecular Analyses
2.5.1. DNA Extraction
2.5.2. PCR and Sequencing of 16S rRNA Gene Fragments
2.5.3. PCR and Sequencing of ITS Regions and 28S rRNA Gene
2.6. Statistical Analyses
3. Results
3.1. Identification of HABs and Cell Abundances
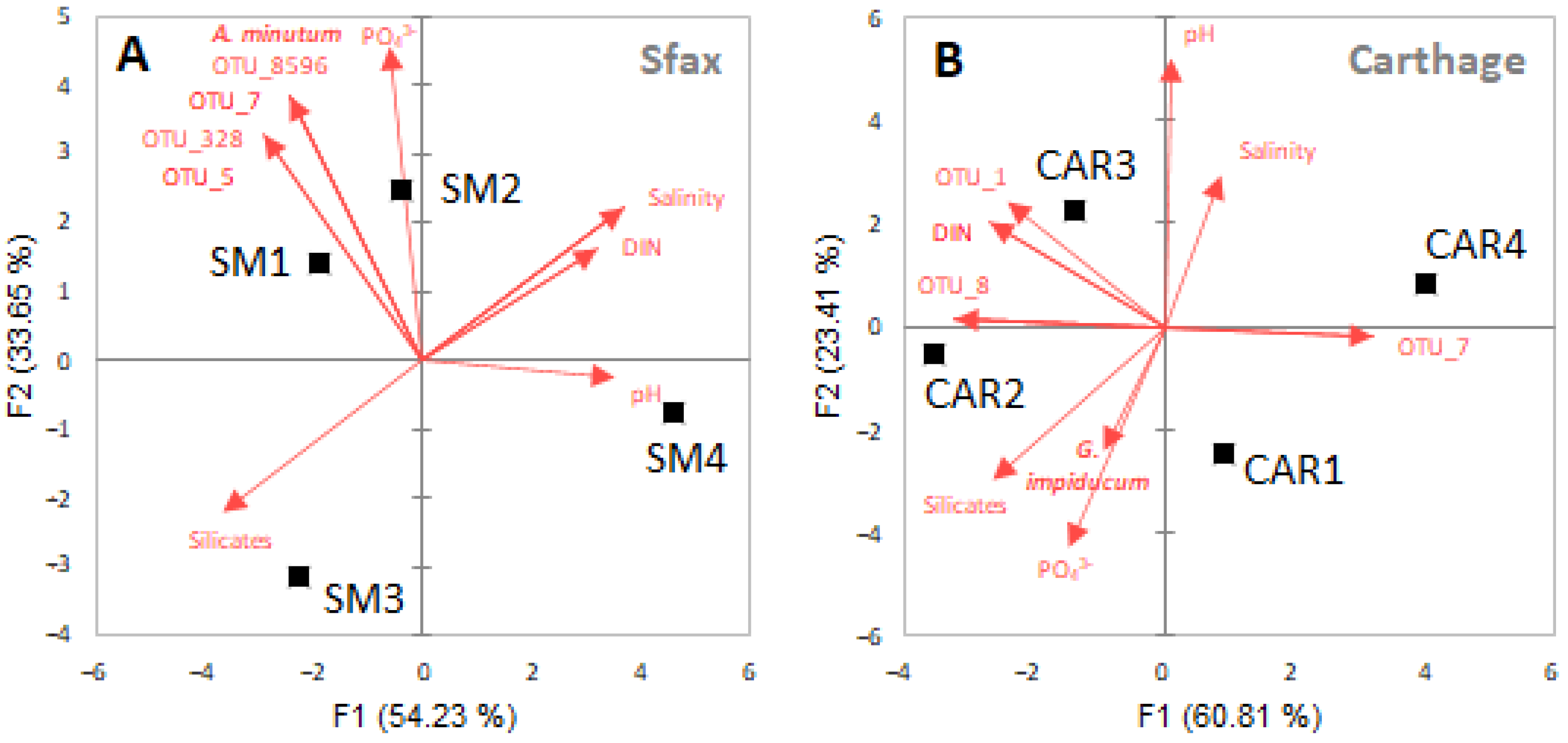
3.2. Environmental Variables and Correlations with HAB Cell Abundances
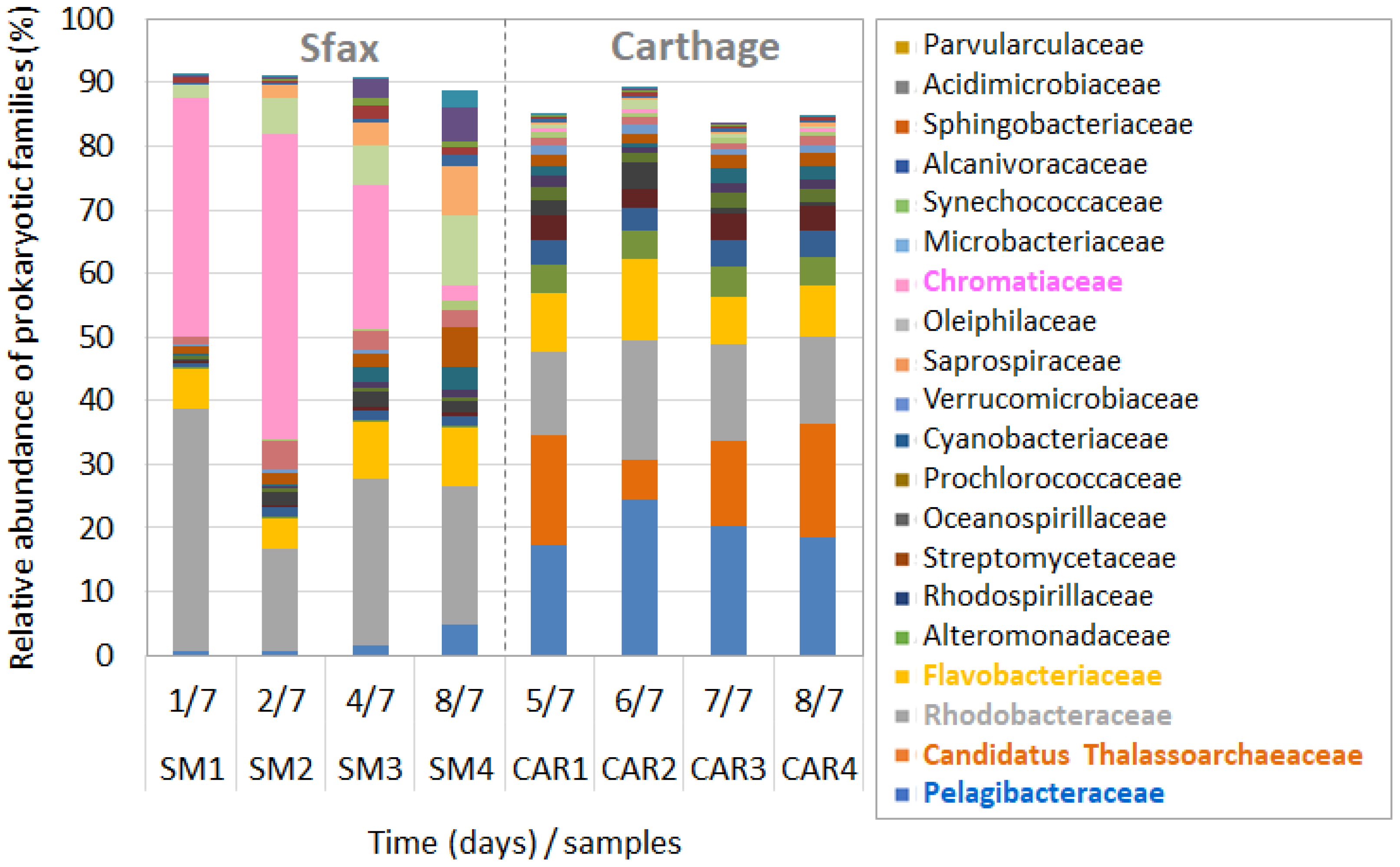
3.3. Prokaryotic Community Composition during Dinoflagellate Bloom Declines
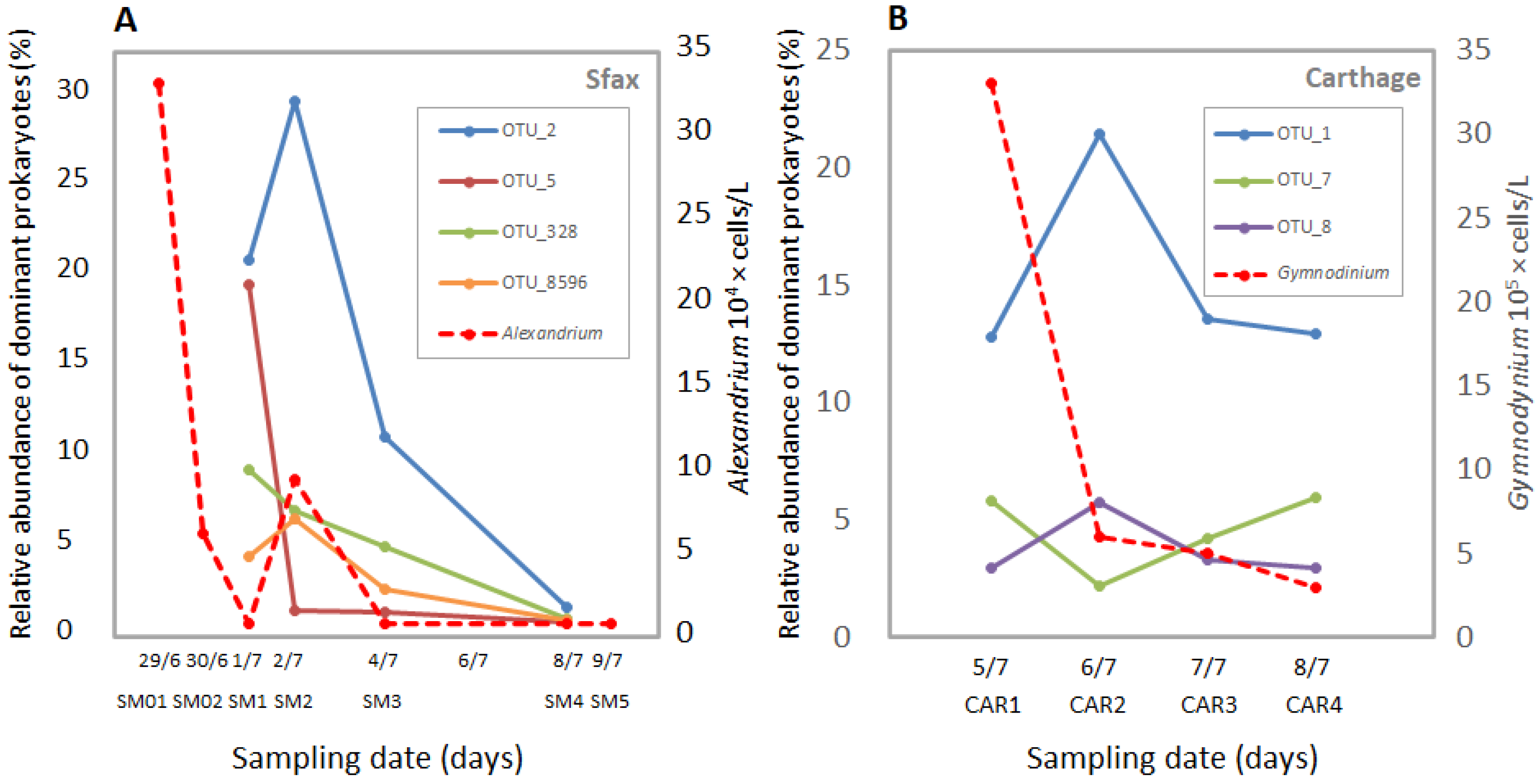
3.4. Dynamics of Abundant Prokaryotic Species during Dinoflagellate Bloom Declines
4. Discussion
4.1. HAB Identification and Densities
4.2. Relationships between HAB Species and Environmental Factors
4.3. Taxonomic Composition of the Prokaryotic Community during HAB Decays
4.4. Dominance of Gammaproteobacterial Chromatiaceae during A. minutum Decays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Käse, L.; Geuer, J.K. Phytoplankton Responses to Marine Climate Change—An Introduction. In YOUMARES 8—Oceans across Boundaries: Learning from Each Other; Jungblut, S., Liebich, V., Bode, M., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Falkowski, P.G. The role of phytoplankton photosynthesis in global biogeochemical cycles. Photosynth. Res. 1994, 39, 235–258. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fensin, E.; Gobler, C.J.; Hoeglund, A.E.; Hubbard, K.A.; Kulis, D.M. Marine harmful algal blooms (HABs) in the United States: History, current status and future trends. Harmful Algae 2021, 102, 101975. [Google Scholar] [CrossRef]
- McKenzie, C.H.; Bates, S.S.; Martin, J.L.; Haigh, N.; Howland, K.L.; Lewis, N.I.; Locke, A.; Peña, A.; Poulin, M.; Rochon, A.; et al. Three decades of Canadian marine harmful algal events: Phytoplankton and phycotoxins of concern to human and ecosystem health. Harmful Algae 2021, 102, 101852. [Google Scholar] [CrossRef] [PubMed]
- Smayda, T.J. Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 1997, 42, 1137–1153. [Google Scholar] [CrossRef]
- Sournia, A. Red tide and toxic marine phytoplankton of the world ocean: An inquiry into biodiversity. In Harmful. Marine. Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcailou-Le Baut, C., Eds.; UNESCO: Paris, France, 1995; pp. 103–112. [Google Scholar]
- Tester, P.A.; Steidinger, K.A. Gymnodinium breve red tide blooms: Initiation, transport, and consequences of surface circulation. Limnol. Oceanogr. 1997, 42, 1039–1051. [Google Scholar] [CrossRef]
- Bagatini, I.L.; Eiler, A.; Bertilsson, S.; Klaveness, D.; Tessarolli, L.P.; Vieira, A.A. Host-specificity and dynamics in bacterial communities associated with bloom-forming freshwater phytoplankton. PLoS ONE 2014, 9, e85950. [Google Scholar] [CrossRef] [PubMed]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 1016. [Google Scholar] [CrossRef] [PubMed]
- Gernez, P.; Antoine, D.; Huotb, Y. Diel cycles of the particulate beam attenuation coefficient under varying trophic conditions in the northwestern Mediterranean Sea: Observations and modeling. Limnol. Oceanogr. 2011, 56, 17–36. [Google Scholar] [CrossRef]
- Lasternas, S.; Tunin-Ley, A.; Ibañez, F.; Andersen, V.; Pizay, M.-D.; Lemée, R. Short-term dynamics of microplankton abundance and diversity in NW Mediterranean Sea during late summer conditions (DYNAPROC 2 cruise; 2004). Biogeosciences 2011, 8, 743–761. [Google Scholar] [CrossRef]
- Anderson, D.M.; Glibert, P.M.; Burkholder, J.M. Harmful algal blooms and eutrophication: Nutrients sources, composition and consequences. Estuaries 2002, 25, 704–726. [Google Scholar] [CrossRef]
- Cui, Y.; Chun, S.J.; Baek, S.S.; Baek, S.H.; Kim, P.J.; Cho, K.H.; Ahn, C.Y.; Oh, H.M. Unique microbial module regulates the harmful algal bloom (Cochlodinium polykrikoides) and shifts the microbial community along the Southern Coast of Korea. Sci. Total Environ. 2020, 721, 137725. [Google Scholar] [CrossRef]
- Park, Y.H.; Kim, S.; Kim, H.S.; Park, C.; Choi, Y.E. Adsorption strategy for removal of harmful cyanobacterial species Microcystis aeruginosa using chitosan fiber. Sustainability 2020, 12, 4587. [Google Scholar] [CrossRef]
- Higashi, A.; Fujitani, Y.; Nakayama, N.; Tani, A.; Ueki, S. Selective growth promotion of bloom-forming raphidophyte Heterosigma akashiwo by a marine bacterial strain. Harmful Algae 2016, 60, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lewitus, A.J.; Brown, P.; Wilde, S.B. Growth-promoting effects of a bacterium on raphidophytes and other phytoplankton. Harmful Algae 2008, 7, 1–10. [Google Scholar] [CrossRef]
- Liu, J.; Lewitus, A.J.; Kempton, J.W.; Wilde, S.B. The association of algicidal bacteria and raphidophyte blooms in South Carolina brackish detention ponds. Harmful Algae 2008, 7, 184–193. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, Q.; Chen, Q.; Yang, K.; Zhang, D.; Chen, Z.; Lu, S.; Shao, X.; Fan, Y.; Yao, L.; et al. Algicidal activity of novel marine bacterium Paracoccus sp. strain Y42 against a harmful algal-bloom-causing dinoflagellate, Prorocentrum donghaiense. Appl. Environ. Microbiol. 2018, 84, e01015-18. [Google Scholar] [CrossRef]
- Matcher, G.; Lemley, D.A.; Adams, J.B. Bacterial community dynamics during a harmful algal bloom of Heterosigma akashiwo. Aquat. Microb. Ecol. 2021, 86, 153167. [Google Scholar] [CrossRef]
- Zhou, J.; Richlen, M.L.; Sehein, T.R.; Kulis, D.M.; Anderson, D.M.; Cai, Z. Microbial community structure and associations during a marine dinoflagellate bloom. Front. Microbiol. 2018, 9, 1201. [Google Scholar] [CrossRef]
- Buchan, A.; LeCleir, G.R.; Gulvik, C.A.; Gonzalez, J.M. Master recyclers: Features and functions of bacteria associated with phytoplankton blooms. Nat. Rev. Microbiol. 2014, 12, 686–698. [Google Scholar] [CrossRef]
- Abdennadher, M.; Hamza, A.; Fekih, W.; Hannachi, I.; Bellaaj, A.Z.; Bradai, M.N.; Aleya, L. Factors determining the dynamics of toxic blooms of Alexandrium minutum during a 10-year study along the shallow southwestern Mediterranean coasts. Estuar. Coast. Shelf Sci. 2012, 106, 102–111. [Google Scholar] [CrossRef]
- Feki, W.; Hamza, A.; Frossard, V.; Abdennadher, M.; Hannachi, I.; Jacquot, M.; Bel Hassen, M.; Aleya, L. What are the potential drivers of blooms of the toxic dinoflagellate Karenia selliformis? A 10-year study in the Gulf of Gabes, Tunisia, southwestern Mediterranean Sea. Harmful Algae. 2013, 23, 8–18. [Google Scholar] [CrossRef]
- Daly Yahia-Kefi, O.; Daly Yahia, M.N. Le phytoplancton toxique dans trois milieux lagunaires tunisiens (Ghar El Melh, Lac Sud de Tunis et Bou Ghrara). In Actes Séminaire National sur la Gestion et la Conservation des Zones Humides Tunisiennes; The Minister of Agriculture: Tunis, Tunisia, 1997; pp. 27–29. [Google Scholar]
- Romdhane, M.S.; Eilertsen, H.C.; Daly Yahia-Kéfi, O.; Daly Yahia, M.N. Toxic dinoflagellate blooms in Tunisian lagoons: Causes and consequences for aquaculture. In Harmful Algae; Reguera, B., Blanco, J., Fernandez, M.L., Wyatt, T., Eds.; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 1998; pp. 80–83. [Google Scholar]
- Daly Yahia-Kéfi, O.; Souissi, S.; Gómez, F.; Daly Yahia, M.N. Spatio-temporal distribution of the dominant diatom and dinoflagellate species in the Bay of Tunis (SW Mediterranean Sea). Mediterr. Mar. Sci. 2005, 6, 17–34. [Google Scholar] [CrossRef]
- Armi, Z.; Milandri, M.; Turki, S.; Hajjem, B. Alexandrium catenella and Alexandrium tamarense in the North Lake of Tunis: Bloom characteristics and occurrence of paralytic shellfish toxin. Afr. J. Aquat. Sci. 2011, 36, 36–41. [Google Scholar] [CrossRef]
- Turki, S.; Dhib, A.; Fertouna-Bellakhal, M.; Frossard, V.; Balti, N.; Kharrat, R.; Aleya, L. Harmful algal blooms (HABs) associated with phycotoxins in shellfish: What can be learned from five years of monitoring in Bizerte Lagoon (Southern Mediterranean Sea)? Ecol. Eng. 2014, 67, 39–347. [Google Scholar] [CrossRef]
- Sammari, C.; Koutitonsky, V.G.; Moussa, M. Sea level variability and tidal resonance in the Gulf of Gabes, Tunisia. Cont. Shelf Res. 2006, 26, 338–350. [Google Scholar] [CrossRef]
- Chifflet, S.; Tedetti, M.; Zouch, H.; Fourati, R.; Zaghden, H.; Elleuch, B.; Quéméneur, M.; Karray, F.; Sayadi, S. Dynamics of trace metals in a shallow coastal ecosystem: Insights from the Gulf of Gabès (southern Mediterranean Sea). AIMS Environ. Sci. 2019, 6, 277–297. [Google Scholar] [CrossRef]
- Zaghden, H.; Tedetti, M.; Sayadi, S.; Serbaji, M.M.; Elleuch, B.; Saliot, A. Origin and distribution of hydrocarbons and organic matter in the surficial sediments of the Sfax-Kerkennah channel (Tunisia, Southern Mediterranean Sea). Mar. Pollut. Bull. 2017, 117, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Utermöhl, H. Zur Vervolkommung der quantitativen Phytoplankton-methodik. Comm. Assoc. Int. Limnol. Theor. Appl. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Throndsen, J. Estimating cell numbers. In Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Enevoldsen, H.O., Eds.; IOCManual and Guides 33; UNESCO: Paris, France, 1995; pp. 63–80. [Google Scholar]
- Anderson, D.M.; Kulis, D.M.; Keafer, B.A.; Gribble, K.E.; Marin, R.; Scholin, C.A. Identification and enumeration of Alexandrium spp. from the Gulf of Maine using molecular probes. Deep Sea Res. Part II 2005, 52, 19–21. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Hargraves, P.E. Stichochrysis immobilis is a diatom, not a chrysophyte. Phycologia 1993, 32, 234–236. [Google Scholar] [CrossRef]
- Balech, E. The Genus Alexandrium Halim (Dinoflagellata); Sherkin Island Marine Station, Sherkin Island, Co.: Cork, Ireland, 1995; p. 151. [Google Scholar]
- Fritz, L.; Triemer, R.E. A rapid simple technique utilizing calcofluor white M2R for the visualization of dinoflagellates thecal plates. J. Phycol. 1985, 21, 662–664. [Google Scholar] [CrossRef]
- Tréguer, P.; Le Corre, P. Manuel d’analyse des sels nutritifs dans l’eau de mer; Université de Bretagne Occidentale: Brest, France, 1975; 110p. [Google Scholar]
- Takahashi, S.; Tomita, J.; Nishioka, K.; Hisada, T.; Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of Bacteria and Archaea using next-generation sequencing. PLoS ONE 2014, 9, e105592. [Google Scholar] [CrossRef] [PubMed]
- Dowd, S.E.; Callaway, T.R.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Hagevoort, R.G.; Edrington, T.S. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Adachi, M.; Sako, Y.; Ishida, Y. Restriction fragment length polymorphism of ribosomal DNA internal transcribed spacer and 5.8S regions in Japanese Alexandrium species (Dinophyceae). J. Phycol. 1994, 30, 857–863. [Google Scholar] [CrossRef]
- Leaw, C.P.; Lim, P.T.; Ahmad, A.; Usup, G. Genetic diversity of Ostreopsis ovata (Dinophyceae) from Malaysia. Mar. Biotechnol. 2001, 3, 246–255. [Google Scholar]
- Scholin, C.A.; Herzog, M.; Sogin, M.; Anderson, D.M. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. J. Phycol. 1994, 30, 999–1011. [Google Scholar] [CrossRef]
- Abdennadher, M.; Bellaaj Zouari, A.; Medhioub, W.; Penna, A.; Hamza, A. Characterization of Coolia spp. (Gonyaucales, Dinophyceae) from Southern Tunisia: First record of Coolia malayensis in the Mediterranean Sea. Algae 2021, 6, 175–193. [Google Scholar] [CrossRef]
- Halim, Y. Alexandrium minutum nov. g. nov. sp. Dinoflagellate provocant des “eaux rouges”. Vie et Milieu. 1960, 11, 102–105. [Google Scholar]
- Smythe-Wright, D.; Daniel, A.; Boswell, S.; Purcell, D.; Hartman, M.; Hartman, S. Phytoplankton and pigment studies in the Bay of Biscay and English Channel. Deep Sea Res. Part II 2014, 106, 76–86. [Google Scholar] [CrossRef]
- Tas, S.; Yilmaz, I.N. Potentially harmful microalgae and algal blooms in a eutrophic estuary in Turkey. Mediterr. Mar. Sci. 2015, 16, 432–443. [Google Scholar] [CrossRef]
- Daly Yahia-Kefi, O.; Nézan, E.; Daly Yahia, M.N. Sur la présence du genre Alexandrium halim (Dinoflagellés) dans la baie de Tunis (Tunisie). Oceanol. Acta. 2001, 24, 17–25. [Google Scholar] [CrossRef]
- Fraga, S.; Bravo, I.; Delgado, M.; Franco, J.M.; Zapata, M. Gyrodinium impudicum sp. nov. (Dinophyceae), a non-toxic, chain forming, red tide dinoflagellate. Phycologia 1995, 34, 514–521. [Google Scholar] [CrossRef]
- Luo, Z.; Hu, Z.; Tang, Y.; Mertens, K.N.; Leaw, C.P.; Lim, P.T.; Teng, S.T.; Wang, L.; Gu, H. Morphology, ultrastructure, and molecular phylogeny of Wangodinium sinense gen. et sp. nov. (Gymnodiniales, Dinophyceae) and revisiting of Gymnodinium dorsalisulcum and Gymnodinium impudicum. J. Phycol. 2018, 54, 744. [Google Scholar] [CrossRef]
- Ranston, E.R.; Webber, D.F.; Larsen, J. The first description of the potentially toxic dinoflagellate, Alexandrium minutum in Hunts Bay, Kingston Harbour, Jamaica. Harmful Algae 2007, 6, 29–47. [Google Scholar] [CrossRef]
- Touzet, N.; Farrell, H.; Rathaille, A.N.; Rodriguez, P.; Alfonso, A.; Botana, L.M.; Raine, R. Dynamics of co-occurring Alexandrium minutum (Global Clade) and A. tamarense (West European) (Dinophyceae) during a summer bloom in Cork Harbour, Ireland (2006). Deep Sea Res. Part II 2010, 57, 268–278. [Google Scholar] [CrossRef]
- Labib, G.; Halim, Y. Diel vertical migration toxicity of Alexandrium minutum Halim red tide, in Alexandria. Egypt. Mar. Life 1995, 5, 11–17. [Google Scholar]
- Delgado, M.; Estrada, M.; Camp, J.; Fernandez, J.V.; Santmarti, M.; Lleti, C. Development of a toxic Alexandrium minutum Halim (Dinophyceae) bloom in the harbour of Sant Carles de la Ràpita (Ebro delta, northwestern Mediterranean). Scient. Mar. 1990, 54, 1–7. [Google Scholar]
- Garcés, E.; Bravo, I.; Vila, M.; Figueroa, R.I.; Maso, M.; Sampedro, N. Relationship between vegetative cells and cyst production during Alexandrium minutum bloom in Arenys de Mar harbor (NW Mediterranean). J. Plankton Res. 2004, 26, 637–645. [Google Scholar] [CrossRef]
- Chapelle, A.; Le Gac, M.; Labry, C.; Siano, R.; Quere, J.; Caradec, F.; Le Bec, C.; Nezan, E.; Doner, A.; Gouriou, J. The Bay of Brest (France), a new risky site for toxic Alexandrium minutum blooms and PSP shellfish contamination. Harmful Algae News 2015, 51, 4–5. [Google Scholar]
- Cosgrove, S.; Rathaille, A.N.; Raine, R. The influence of bloom intensity on the encystment rate and persistence of Alexandrium minutum in Cork harbor, Ireland. Harmful Algae 2014, 31, 114–124. [Google Scholar] [CrossRef]
- Guallar, C.; Bacher, C.; Chapelle, A. Global and local factors driving the phenology of Alexandrium minutum (Halim) blooms and its toxicity. Harmful Algae 2017, 67, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Maguer, J.F.; Wafar, M.; Madec, C.; Morin, P.; Erard-Le Denn, E. Nitrogen and phosphorus requirements of an Alexandrium minutum bloom in the Penze estuary, France. Limnol. Oceanogr. 2004, 49, 1108–1114. [Google Scholar] [CrossRef]
- Pitcher, G.C.; Cembella, A.D.; Joyce, L.B.; Larsen, J.; Probyn, T.A.; Ruiz Sebastian, C. The dinoflagellate Alexandrium minutum in Cape Town harbor (South Africa): Bloom characteristics, phylogenetic analysis and toxin composition. Harmful Algae 2007, 6, 823–836. [Google Scholar] [CrossRef]
- Santos, M.; Costa, P.R.; Porteiro, F.M.; Moita, M.T. First report of a massive bloom of Alexandrium minutum (Dinophyceae) in middle North Atlantic: A coastal lagoon in S. Jorge Island, Azores. Toxicon 2014, 90, 265–268. [Google Scholar] [CrossRef]
- Mikhail, S.; Khalil, N.; El-Hadary, M. A new recorded of red tide forming species; Heterocapsa triquetra, Gymnodinium impudicum, Heterosigma akashiwo and Thalassiosira rotula in Alexandria waters, Egypt. Egypt. J. Aquat. Biol. Fish. 2020, 24, 207–223. [Google Scholar] [CrossRef]
- Bravo, I.; Vila, M.; Maso, M.; Figueroa, R.I.; Ramilo, I. Alexandrium catenella and Alexandrium minutum blooms in the Mediterranean Sea: Toward the identification of ecological niches. Harmful Algae 2008, 7, 515–522. [Google Scholar] [CrossRef]
- Grzebyk, D.; Bechemin, C.; Ward, C.J.; Verite, C.; Codd, G.A.; Maestrini, S.Y. Effects of salinity and two coastal waters on the growth and toxin content of the dinoflagellate Alexandrium minutum. J. Plankton Res. 2003, 25, 1185–1199. [Google Scholar] [CrossRef]
- Lim, P.T.; Ogata, T. Salinity effect on growth and toxin production of four tropical Alexandrium species (Dinophyceae). Toxicon 2005, 45, 699–710. [Google Scholar] [CrossRef]
- Hwang, D.F.; Lu, Y.H. Influence of environmental and nutritional factors on growth, toxicity, and toxin profile of dinoflagellate Alexandrium minutum. Toxicon 2000, 38, 1491–1503. [Google Scholar] [CrossRef] [PubMed]
- Flores-Moya, A.; Rouco, M.; García-Sánchez, M.J.; García-Balboa, C.; González, R.; Costas, E.; López Rodas, V. Effects of adaptation, chance, and history on the evolution of the toxic dinoflagellate Alexandrium minutum under selection of increased temperature and acidification. Ecol. Evol. 2012, 2, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Maguer, J.F.; L’Helguen, S.; Madec, C.; Labry, C.; Le Corre, P. Nitrogen uptake and assimilation kinetics in Alexandrium minutum (Dynophyceae): Effect of N-limited growth rate on nitrate and ammonium interactions. J. Phycol. 2007, 43, 295303. [Google Scholar] [CrossRef]
- Giacobbe, M.G.; Oliva, F.D.; Maimone, G. Environmental factors and seasonal occurrence of the dinoflagellate Alexandrium minutum, a PSP potential producer, in a Mediterranean Lagoon. Estuar. Coast. Shelf Sci. 1996, 42, 539–549. [Google Scholar] [CrossRef]
- Le Bec, C.; Legendre, A.; Messiaen, G. Changes in the annual harmful algal blooms of Alexandrium minutum: Effects of environmental conditions and drainage basin inputs in the Rance estuary (Brittany, France). Aquat. Living Resour. 2016, 29, 104. [Google Scholar] [CrossRef]
- Carrada, G.C.; Casotti, R.; Modigh, M.; Saggiomo, V. Presence of Gymnodinium catenatum (Dinophyceae) in a coastal Mediterranean lagoon. J. Plankton Res. 1991, 13, 229–238. [Google Scholar] [CrossRef]
- Friligos, N.; Gotsis-Skretas, O. Eutrophisation and red tide in Aegean coastal waters. Toxicol. Environ. Chem. 1989, 20, 171–180. [Google Scholar] [CrossRef]
- Oh, S.J.; Hyeon, K.K.; Hyeon, N.; Han-Soe, Y. Dissolved organic phosphorus utilization and alkaline phosphatase activity of the dinoflagellate Gymnodinium impudicum isolated from the South Sea of Korea. Ocean Sci. J. 2010, 45, 171–178. [Google Scholar] [CrossRef]
- Vautard, R.; Cattiaux, J.; You, P.; Thépaut, J.-N.; Ciais, P. Northern Hemisphere atmospheric stilling partly attributed to an increase in surface roughness. Nat. Geosci. 2010, 3, 756. [Google Scholar] [CrossRef]
- Deng, J.M.; Zhang, Y.L.; Qin, B.Q.; Shi, K. Long-term changes in surface solar radiation and their effects on air temperature in the Shanghai region. Int. J. Climatol. 2015, 35, 3385–3396. [Google Scholar] [CrossRef]
- Deng, J.; Paerl, H.W.; Qin, B.; Zhang, Y.; Zhu, G.; Jeppesen, E.; Cai, Y.; Xu, H. Climatically modulated decline in wind speed may strongly affect eutrophication in shallow lakes. Sci. Total. Environ. 2018, 645, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Brody, S.R.; Lozier, M. Changes in dominant mixing length scales as a driver of subpolar phytoplankton bloom initiation in the North Atlantic. Geophys. Res. Lett. 2014, 41, 3197–3203. [Google Scholar] [CrossRef]
- Brody, S.R.; Lozier, M.S. Characterizing upper-ocean mixing and its effect 603 on the spring phytoplankton bloom with in situ data. ICES. J. Mar. Sci. 2015, 72, 1961–1970. [Google Scholar] [CrossRef]
- Pellichero, V.; Boutin, J.; Claustre, H.; Merlivat, L.; Sallée, J.B.; Blain, S. Relaxation of wind stress drives the abrupt onsetof biological carbon uptake in the Kerguelen bloom: A multisensor approach. Geophys. Res. Lett. 2020, 47, e2019GL085992. [Google Scholar] [CrossRef]
- Merlivat, L.; Hemming, M.; Boutin, J.; Antoine, D.; Vincenzo Vellucci, V.; Golbol, M.; Lee, G.A.; Beaumon, L. Physical mechanisms for biological carbon uptake during the onset of the spring phytoplankton bloom in the northwestern Mediterranean Sea (BOUSSOLE site). Biogeosciences 2022, 19, 3911–3920. [Google Scholar] [CrossRef]
- Matteson, A.R.; Loar, S.N.; Pickmere, S.; DeBruyn, J.M.; Ellwood, M.J.; Boyd, P.W.; Hutchins, D.A.; Wilhelm, S.W. Production of viruses during a spring phytoplankton bloom in the South Pacific Ocean near of New Zealand. FEMS Microbiol. Ecol. 2012, 79, 709–719. [Google Scholar] [CrossRef]
- Kim, Y.S.; Son, H.J.; Jeong, S.Y. Isolation of an algicide from a marine bacterium and its effects against the toxic dinoflagellate Alexandrium catenella and other harmful algal bloom species. J. Microbiol. 2015, 53, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Fuhrman, J.A. Pronounced daily succession of phytoplankton, archaea and bacteria following a spring bloom. Nat. Microbiol. 2016, 1, 16005. [Google Scholar] [CrossRef]
- Rink, B.; Seeberger, S.; Martens, T.; Duerselen, C.D.; Simon, M.; Brinkhoff, T. Effects of phytoplankton bloom in a coastal ecosystem on the composition of bacterial communities. Aquat. Microb. Ecol. 2007, 48, 47–60. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.; Song, K.; Cassar, N. Estimation of phytoplankton size fractions based on spectral features of remote sensing ocean color data. J. Geophys. Res. Ocean. 2013, 118, 1445–1458. [Google Scholar] [CrossRef]
- Fandino, L.B.; Riemann, L.; Steward, G.F.; Long, R.A.; Azam, F. Variations in bacterial community structure during dinoflagellate bloom analyzed by DGGE and 16S rDNA sequencing. Aquat. Microb. Ecol. 2001, 23, 119–130. [Google Scholar] [CrossRef]
- Quéméneur, M.; Bel Hassen, M.; Armougom, F.; Khammeri, Y.; Lajnef, R.; Bellaaj-Zouari, A. Prokaryotic diversity and distribution along physical and nutrient gradients in the Tunisian coastal waters (South Mediterranean Sea). Front. Microbiol. 2020, 11, 593540. [Google Scholar] [CrossRef] [PubMed]
- Pernthaler, A.; Pernthaler, J.; Amann, R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 2002, 68, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Lima-Mendez, G.; Faust, K.; Henry, N.; Decelle, J.; Colin, S.; Carcillo, F. Determinants of community structure in the global planktoninteractome. Science 2015, 348, 1262073. [Google Scholar] [CrossRef]
- Martin-Cuadrado, A.B.; Garcia-Heredia, I.; Moltó, A.G.; López-Úbeda, R.; Kimes, N.; López-García, P.; Moreira, D.; Rodriguez-Valera, F. A new class of marine Euryarchaeota group II from the mediterranean deep chlorophyll maximum. ISME J. 2015, 9, 1619–1634. [Google Scholar] [CrossRef] [PubMed]
- Hugoni, M.; Taib, N.; Debroas, D. Structure of the rare archaeal biosphere and seasonal dynamics of active ecotypes in surface coastal waters. Proc. Natl. Acad. Sci. USA 2013, 110, 6004–6009. [Google Scholar] [CrossRef] [PubMed]
- Garcés, E.; Vila, M.; Reñé, A.; Alonso-Sáez, L.; Anglès, S.; Lugliè, A.; Masó, M.; Gasol, J.M. Natural bacterioplankton assemblage composition during blooms of Alexandrium spp. (Dinophyceae) in NW Mediterranean coastal waters. Aquat. Microb. Ecol. 2007, 46, 55–70. [Google Scholar] [CrossRef]
- Jasti, S.; Sieracki, M.E.; Poulton, N.J.; Giewat, M.W.; Rooney-Varga, J.N. Phylogenetic diversity and specificity of bacteria closely associated with Alexandrium spp. and other phytoplankton. Appl. Environ. Microbiol. 2005, 71, 3483–3494. [Google Scholar] [CrossRef]
- Zouch, H.; Karray, F.; Armougom, F.; Chifflet, S.; Hirschler-Réa, A.; Kharrat, H.; Kamoun, L.; Ben Hania, W.; Ollivier, B.; Sayadi, S.; et al. Microbial diversity in sulfate-reducing marine sediment enrichment cultures associated with anaerobic biotransformation of coastal stockpiled phosphogypsum (Sfax, Tunisia). Front. Microbiol. 2017, 8, 1583. [Google Scholar] [CrossRef] [PubMed]
- Zouch, H.; Cabrol, L.; Chifflet, S.; Tedetti, M.; Karray, F.; Zaghden, H.; Sayadi, S.; Quéméneur, M. Effect of acidic industrial effluent release on microbial diversity and trace metal dynamics during resuspension of coastal sediment. Front. Microbiol. 2018, 9, 3103. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, A.; Nagao, N.; Yonekawa, C.; Umekage, S.; Kikuchi, Y.; Eki, T.; Hirose, Y. Distribution of phototrophic purple nonsulfur bacteria in massive blooms in coastal and wastewater ditch environments. Microorganisms 2020, 8, 150. [Google Scholar] [CrossRef] [PubMed]
- Caumette, P.; Baleux, B. Etude des eaux rouges dues à la prolifération des bactéries photosynthétiques sulfo-oxydantes dans l’étang du Prévost, lagune saumâtre méditerranéenne. Mar. Biol. 1980, 56, 183–194. [Google Scholar] [CrossRef]
- Caumette, P. Phototrophic sulfur bacteria and sulfate-reducing bacteria causing red waters in a shallow brackish coastal lagoon (Prévost Lagoon, France). FEMS. Microbiol. Lett. 1986, 38, 113–124. [Google Scholar] [CrossRef]
- Belila, A.; Abbas, B.; Fazaa, I.; Saidi, N.; Snoussi, M.; Hassen, A.; Muyzer, G. Sulfur bacteria in wastewater stabilization ponds periodically affected by the red-water phenomenon. Appl. Microbiol. Biotechnol. 2013, 97, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Hampton, S.E.; Gray, D.K.; Izmest’eva, L.R.; Moore, M.V.; Ozersky, T. The rise and fall of plankton: Long-term changes in the vertical distribution of algae and grazers in Lake Baikal, Siberia. PLoS ONE 2014, 9, e88920. [Google Scholar] [CrossRef] [PubMed]
- Frenken, T.; Velthuis, M.; de Senerpont Domis, L.N.; Stephan, S.; Aben, R.; Kosten, S.; van Donk, E.; Van de Waal, D.B. Warming accelerates termination of a phytoplankton spring bloom by fungal parasites. Glob Chang. Biol. 2016, 22, 299–309. [Google Scholar] [CrossRef] [PubMed]


| Stations | Sfax (Gulf of Gabès) | Carthage (Gulf of Tunis) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | SM01 | SM02 | SM1 2 | SM2 2 | SM3 2 | SM4 2 | SM5 | CAR1 3 | CAR2 3 | CAR3 3 | CAR4 3 |
| Sampling Date (dd/mm) | 29/06 | 30/06 | 01/07 | 02/07 | 04/07 | 08/07 | 09/07 | 05/07 | 06/07 | 07/07 | 08/07 |
| Temperature (°C) | 32 | 33 | 31.2 | 33 | 31 | 34.4 | 33 | 25.7 | 25 | 25.5 | 26.1 |
| Air Temp Max (°C) | 36 | 33 | 31 | 32 | 34 | 39 | 37 | 37 | 39 | 38 | 40 |
| Air Temp Min (°C) | 23.5 | 23.5 | 24 | 23 | 24.5 | 24.5 | 25 | 24 | 22 | 25 | 26 |
| Sea-Level Pressure (hPa) | 1013 | 1015 | 1016 | 1015 | 1015 | 1013 | 1013 | 1015.5 | 1015.52 | 1013.5 | 1014.5 |
| Wind Max (km/h) | 8 | 11 | 21 | 18 | 11 | 9 | 11 | 24 | 15 | 13 | 26 |
| Wind Direction (°) | 130 | 137 | 135 | 135 | 135 | 135 | 135 | 23 | 23 | 1 | 180 |
| Day Length (h) | 14:29 | 14:28 | 14:28 | 14:26 | 14:25 | 14:23 | 14:22 | 14:36 | 14:35 | 14:34 | 14:34 |
| Sun Hours | 8 | 8 | 8 | 8 | 7 | 7 | 7 | 13 | 13 | 11 | 10 |
| pH | 7.59 | 7.90 | 7.95 | 8.10 | 8.00 | 8.23 | 8.40 | 7.90 | 7.95 | 8.20 | 8.00 |
| Salinity (psu) | 39.1 | 39.1 | 38.8 | 39.0 | 38.6 | 39.1 | 38.8 | 37.9 | 37.8 | 38.2 | 37.9 |
| NO2− (µM) | 0.369 | 0.912 | 0.782 | 0.956 | 0.391 | 0.999 | 0.304 | 9.281 | 14.193 | 15.84 | 7.651 |
| NO3− (µM) | 3.21 | 11.27 | 8.05 | 5.02 | 3.58 | 15.60 | 1.95 | 92.19 | 114.90 | 120.40 | 66.04 |
| NH4+ (µM) | 96.79 | 1.72 | 4.93 | 6.04 | 0.89 | 5.65 | 44.57 | 328.08 | 1283.26 | 826.51 | 257.39 |
| PO43− (µM) | 1.095 | 0.705 | 2.074 | 1.653 | 0.031 | 0.063 | 0.031 | 11.118 | 10.465 | 3.274 | 1.958 |
| Silicates (µM) | 0.721 | 0.434 | 2.306 | 0.757 | 2.45 | 0.508 | 0.577 | 4.923 | 5.302 | 4.292 | 4.04 |
| DIN (µM) 1 | 100.37 | 13.90 | 13.76 | 12.01 | 4.86 | 22.25 | 46.83 | 429.54 | 1412.34 | 962.75 | 331.09 |
| Microphytoplankton (103 cells/L) 2 | 300 | 50 | 72.2 | 80 | 65 | 10.2 | 4.7 | 3385 | 600 | 756 | 259.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajnef, R.; Quéméneur, M.; Abdennadher, M.; Dammak Walha, L.; Hamza, A.; Belhassen, M.; Bellaaj Zouari, A. Prokaryotic Diversity and Dynamics during Dinoflagellate Bloom Decays in Coastal Tunisian Waters. Diversity 2023, 15, 273. https://doi.org/10.3390/d15020273
Lajnef R, Quéméneur M, Abdennadher M, Dammak Walha L, Hamza A, Belhassen M, Bellaaj Zouari A. Prokaryotic Diversity and Dynamics during Dinoflagellate Bloom Decays in Coastal Tunisian Waters. Diversity. 2023; 15(2):273. https://doi.org/10.3390/d15020273
Chicago/Turabian StyleLajnef, Rim, Marianne Quéméneur, Moufida Abdennadher, Lamia Dammak Walha, Asma Hamza, Malika Belhassen, and Amel Bellaaj Zouari. 2023. "Prokaryotic Diversity and Dynamics during Dinoflagellate Bloom Decays in Coastal Tunisian Waters" Diversity 15, no. 2: 273. https://doi.org/10.3390/d15020273
APA StyleLajnef, R., Quéméneur, M., Abdennadher, M., Dammak Walha, L., Hamza, A., Belhassen, M., & Bellaaj Zouari, A. (2023). Prokaryotic Diversity and Dynamics during Dinoflagellate Bloom Decays in Coastal Tunisian Waters. Diversity, 15(2), 273. https://doi.org/10.3390/d15020273







